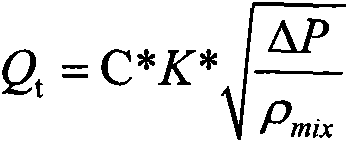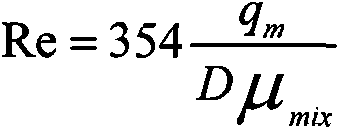Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
135 results about "Phase fraction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
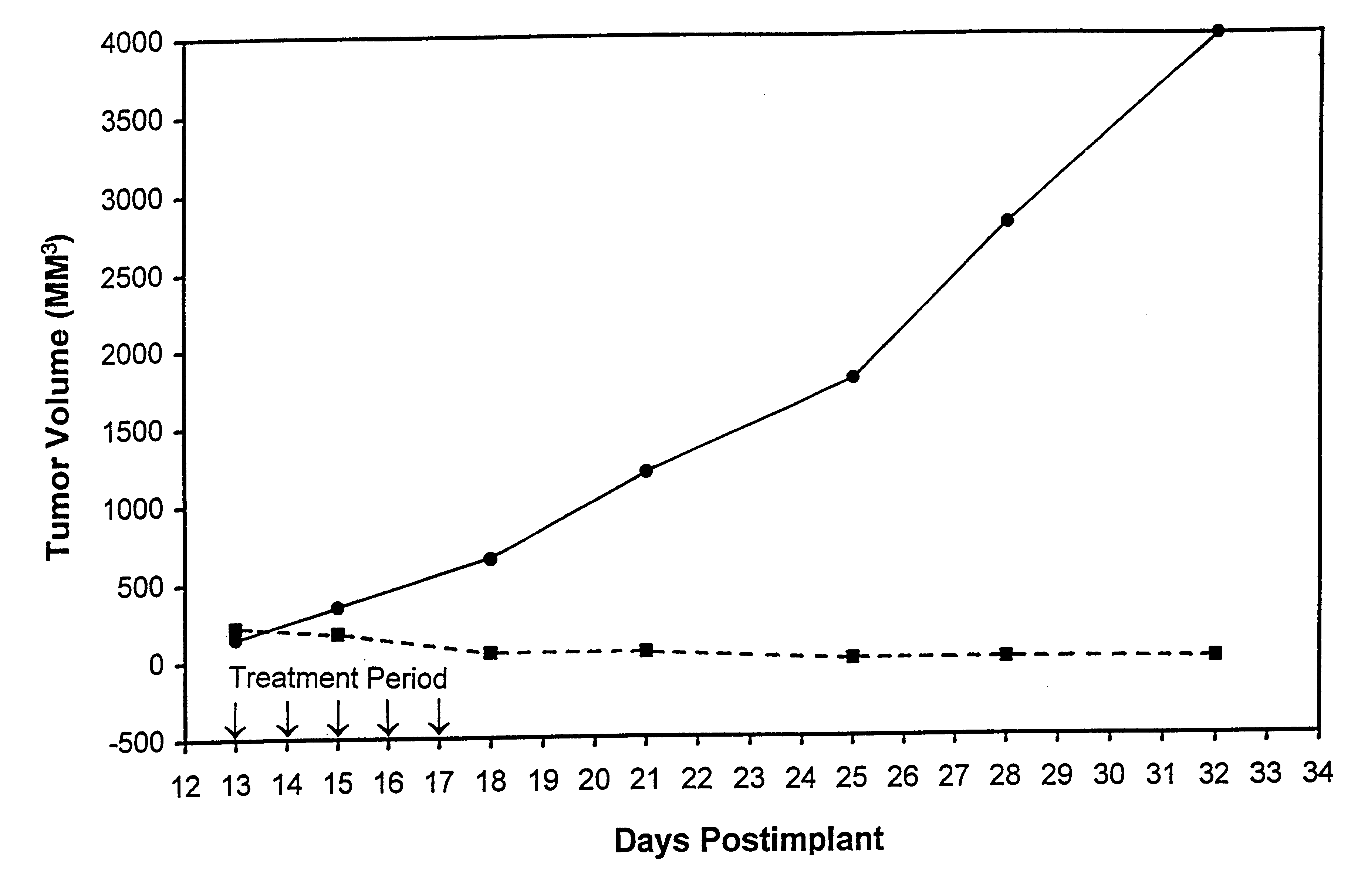
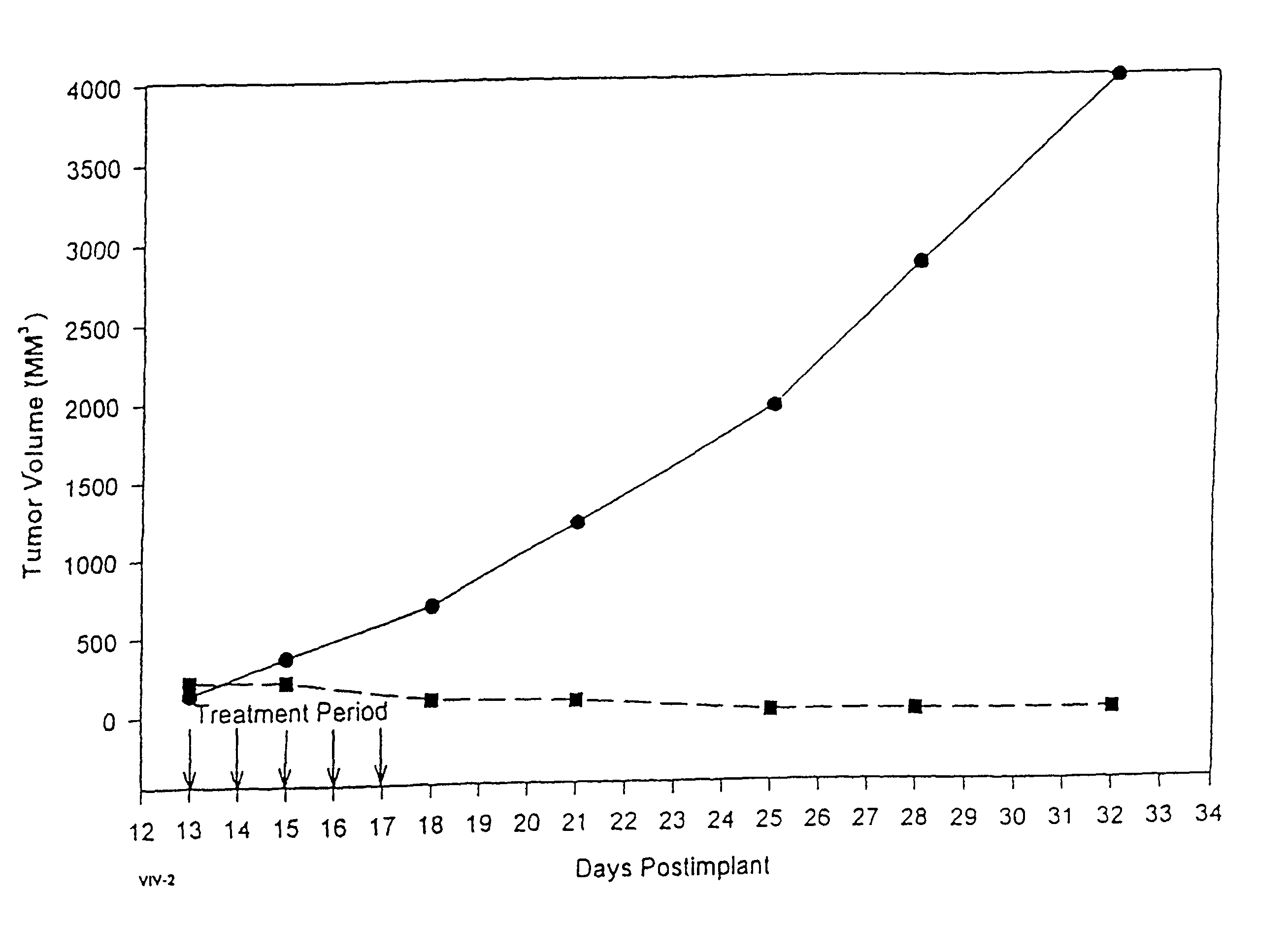
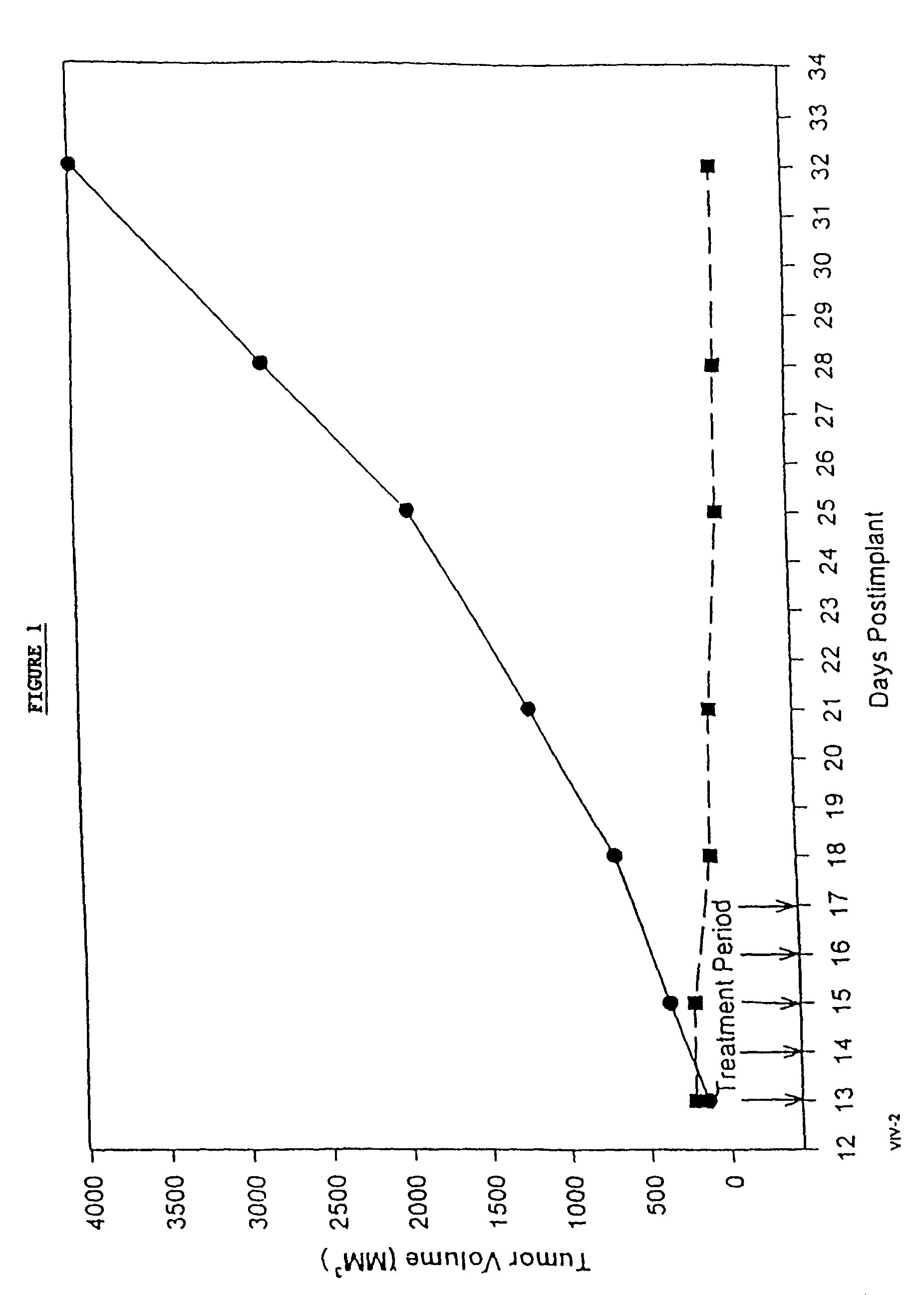
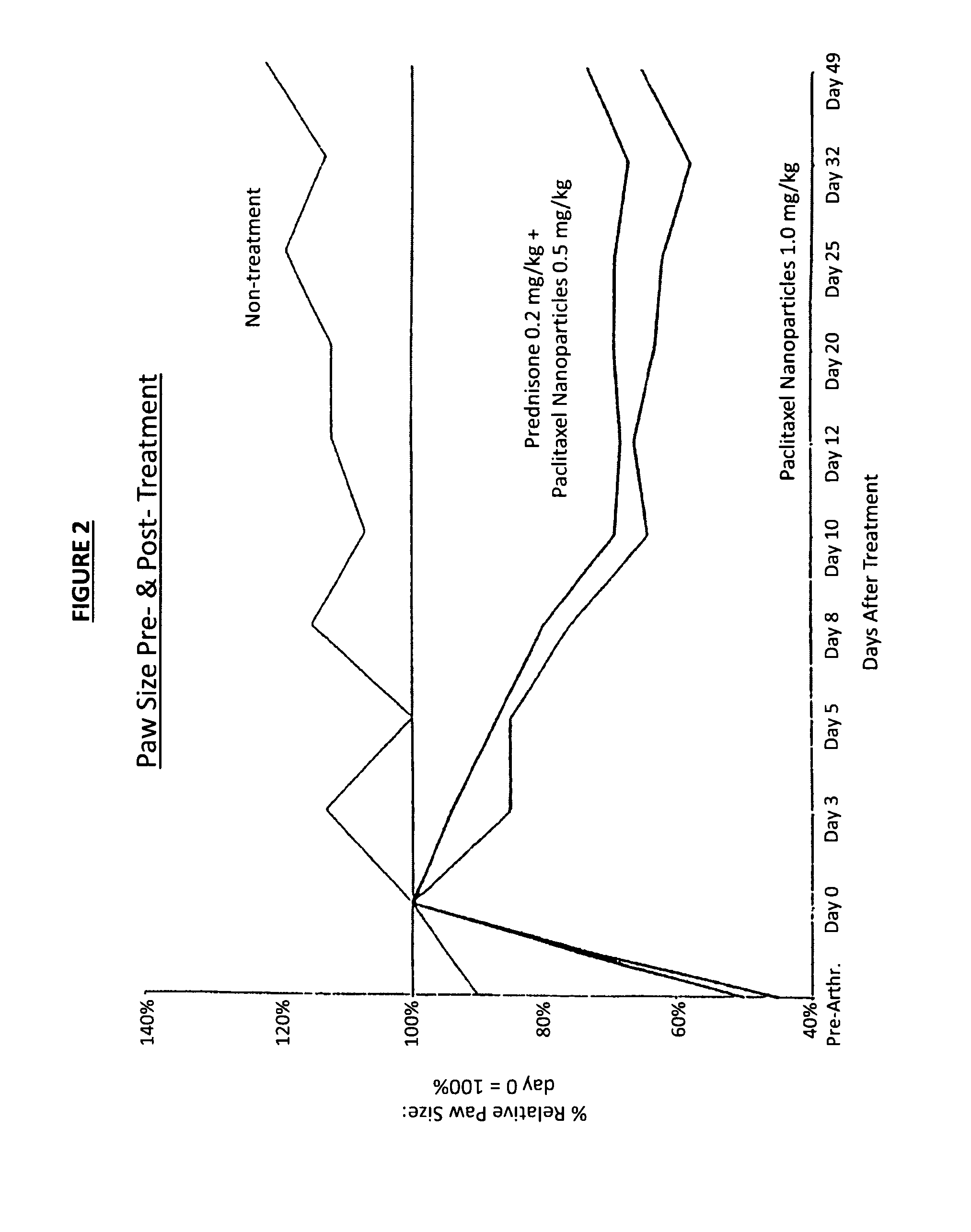
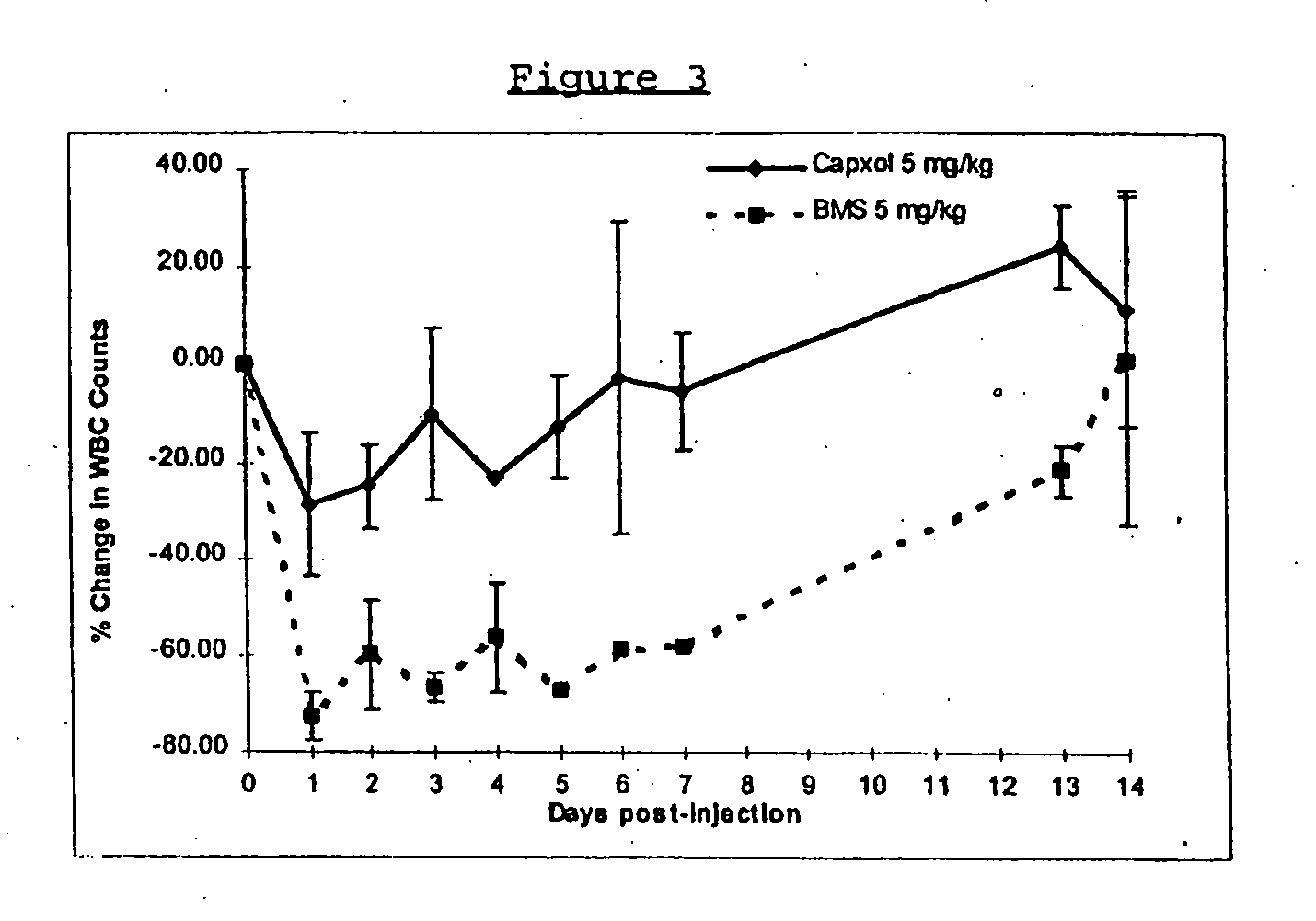
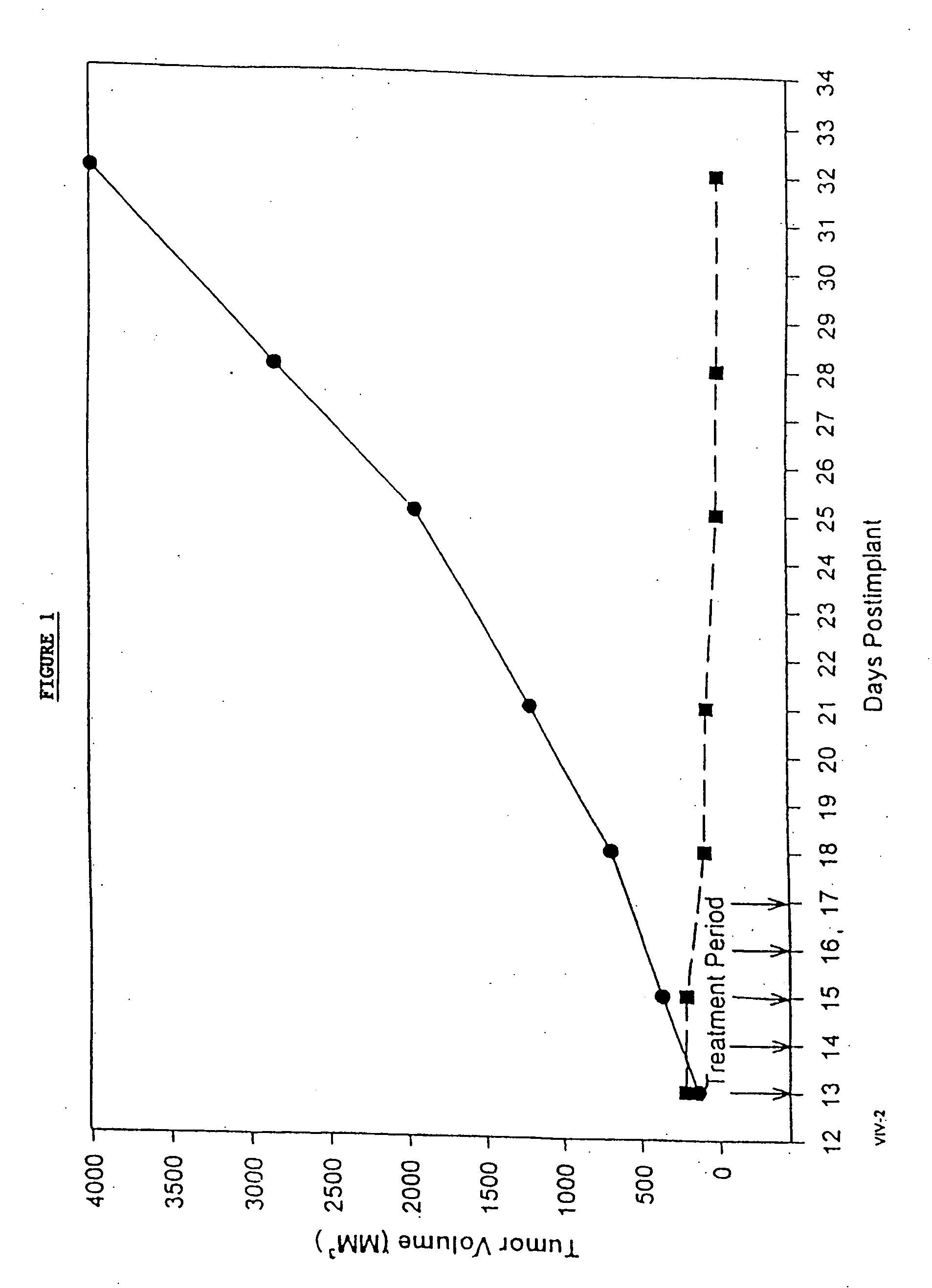
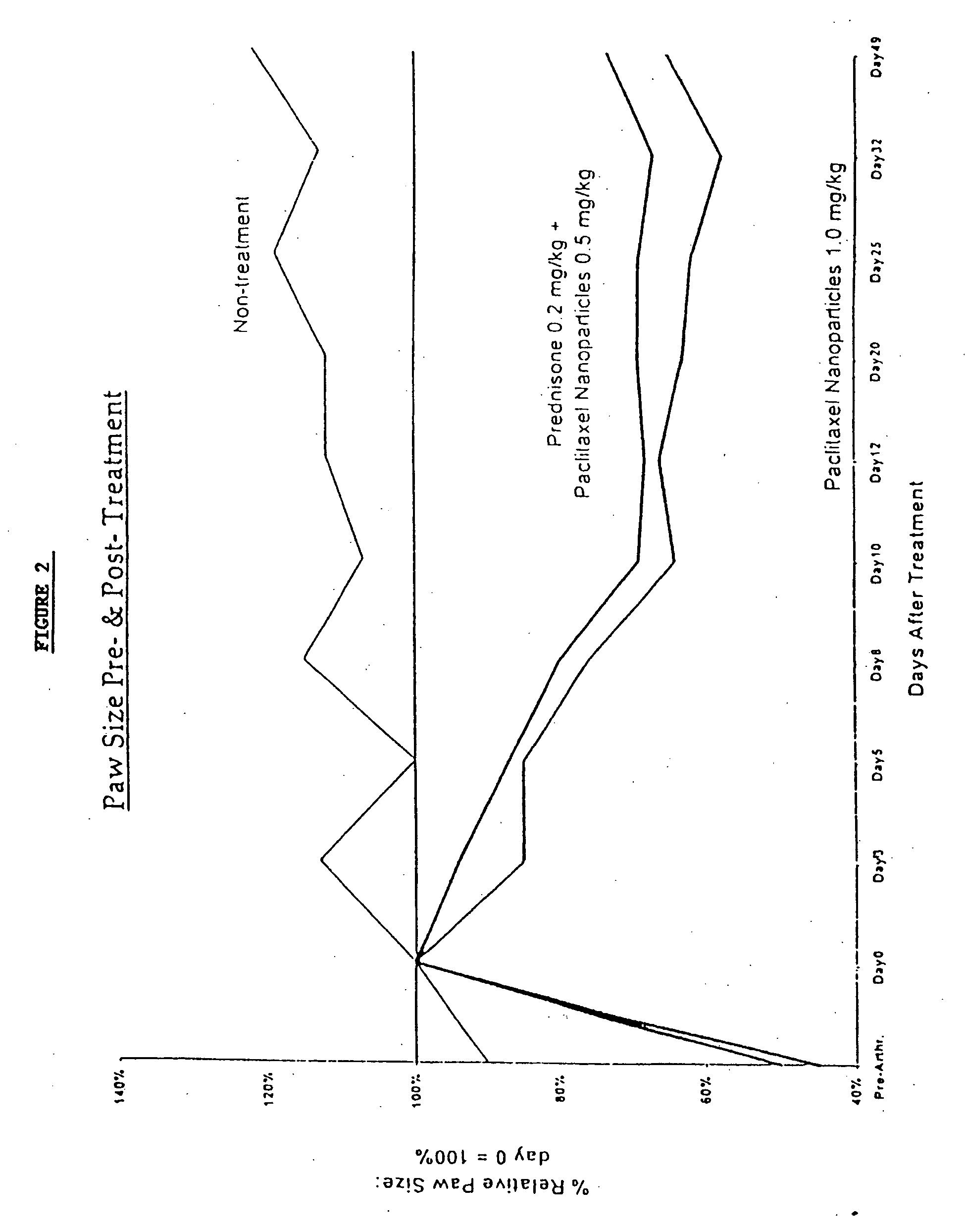
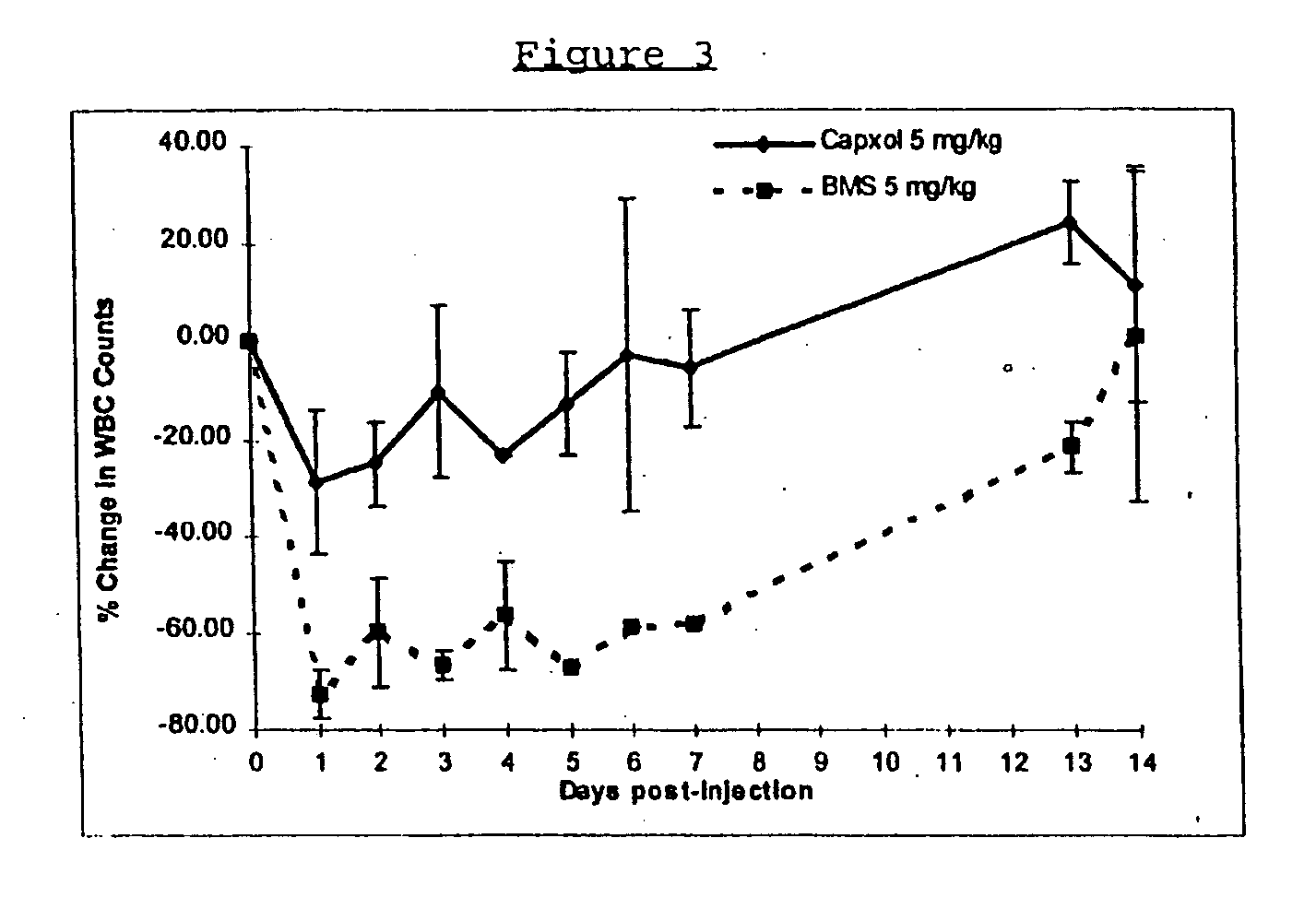
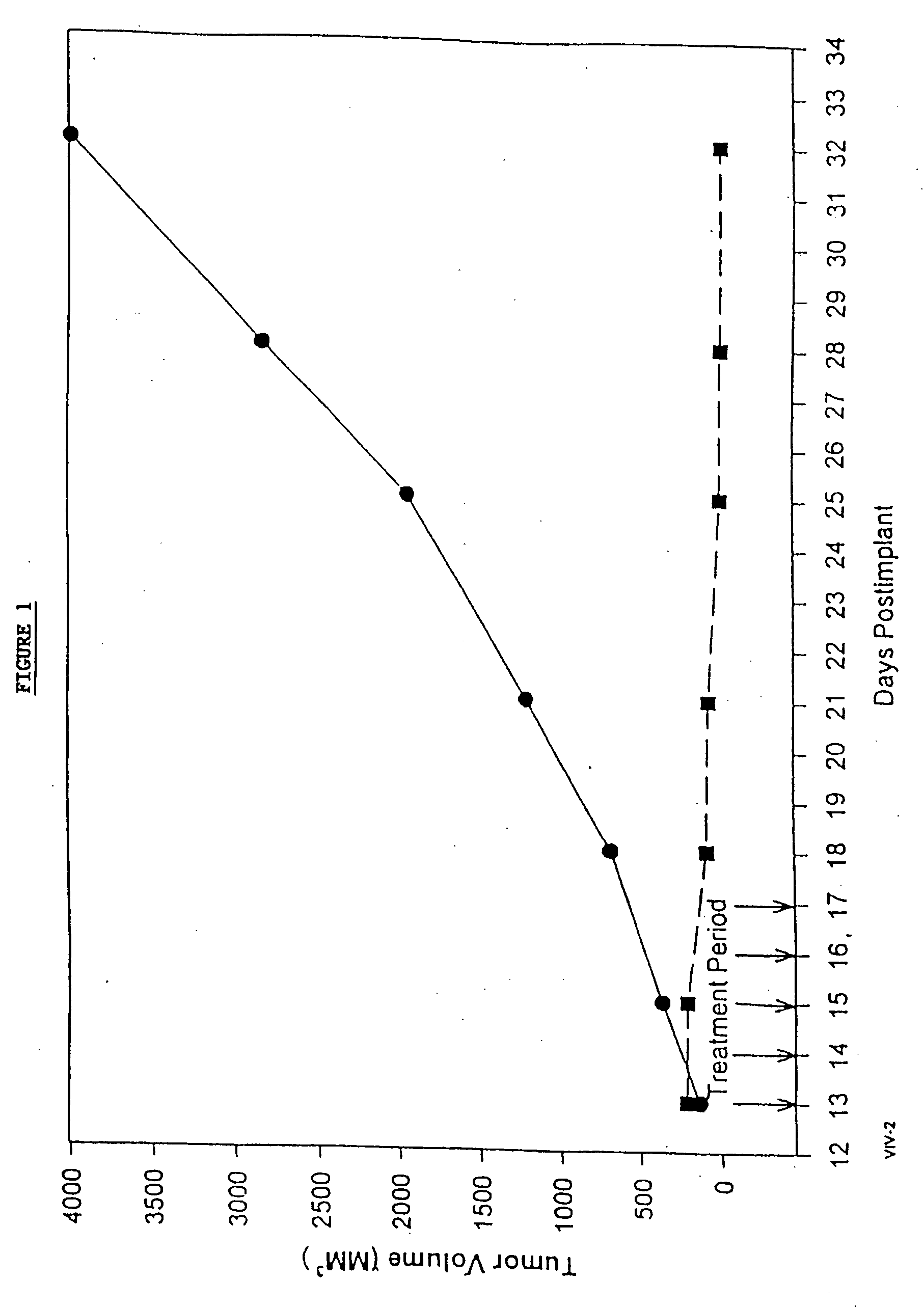
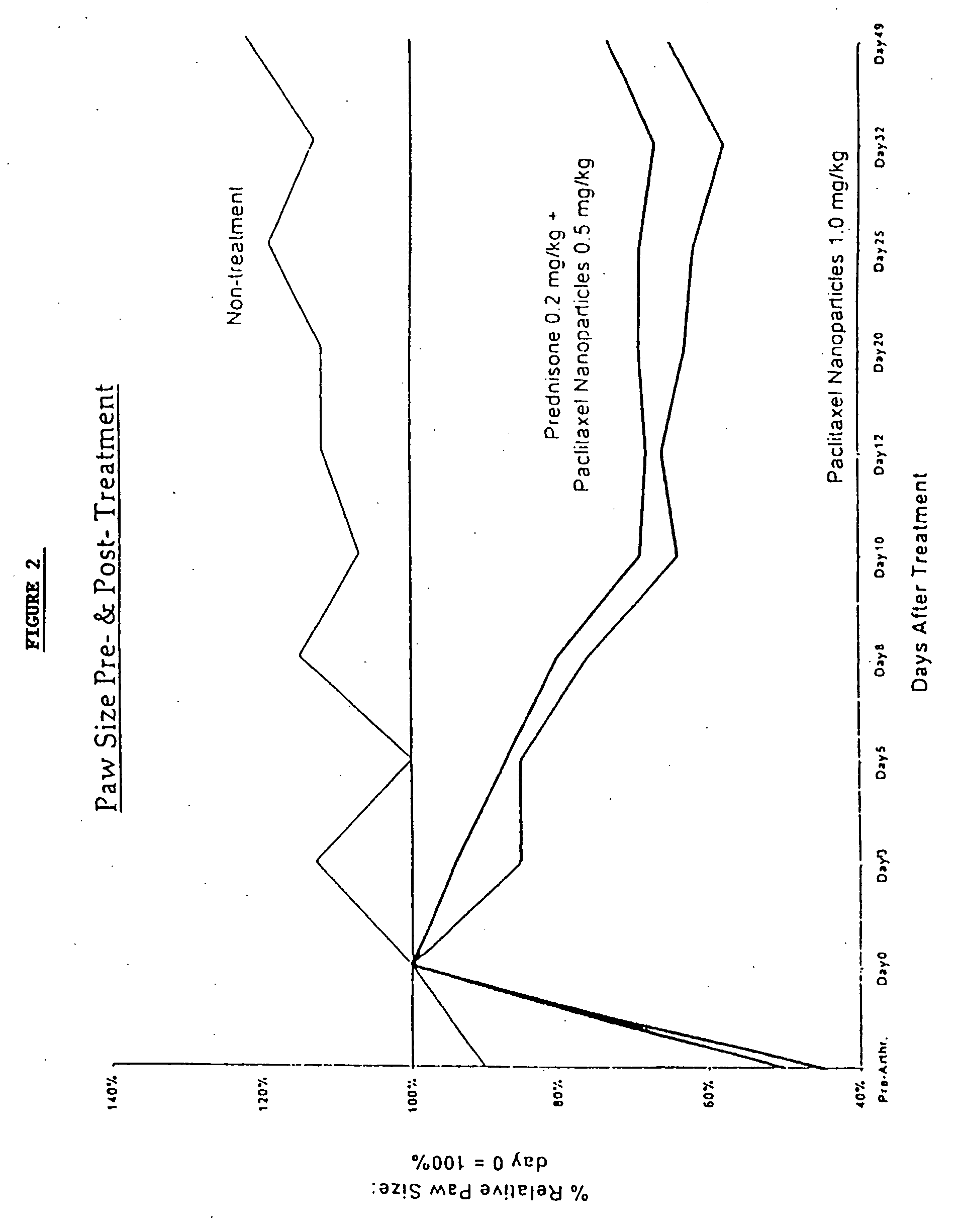
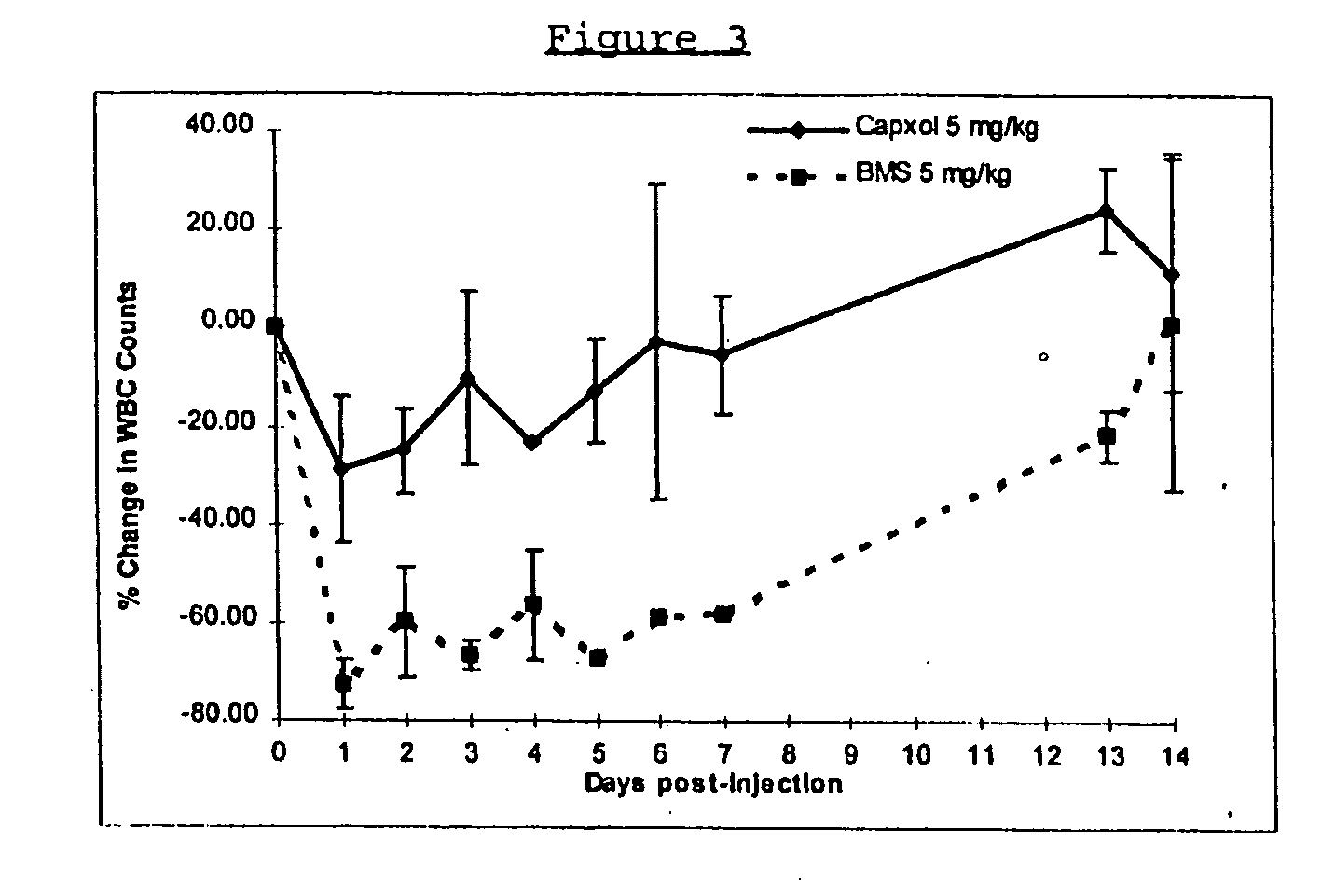
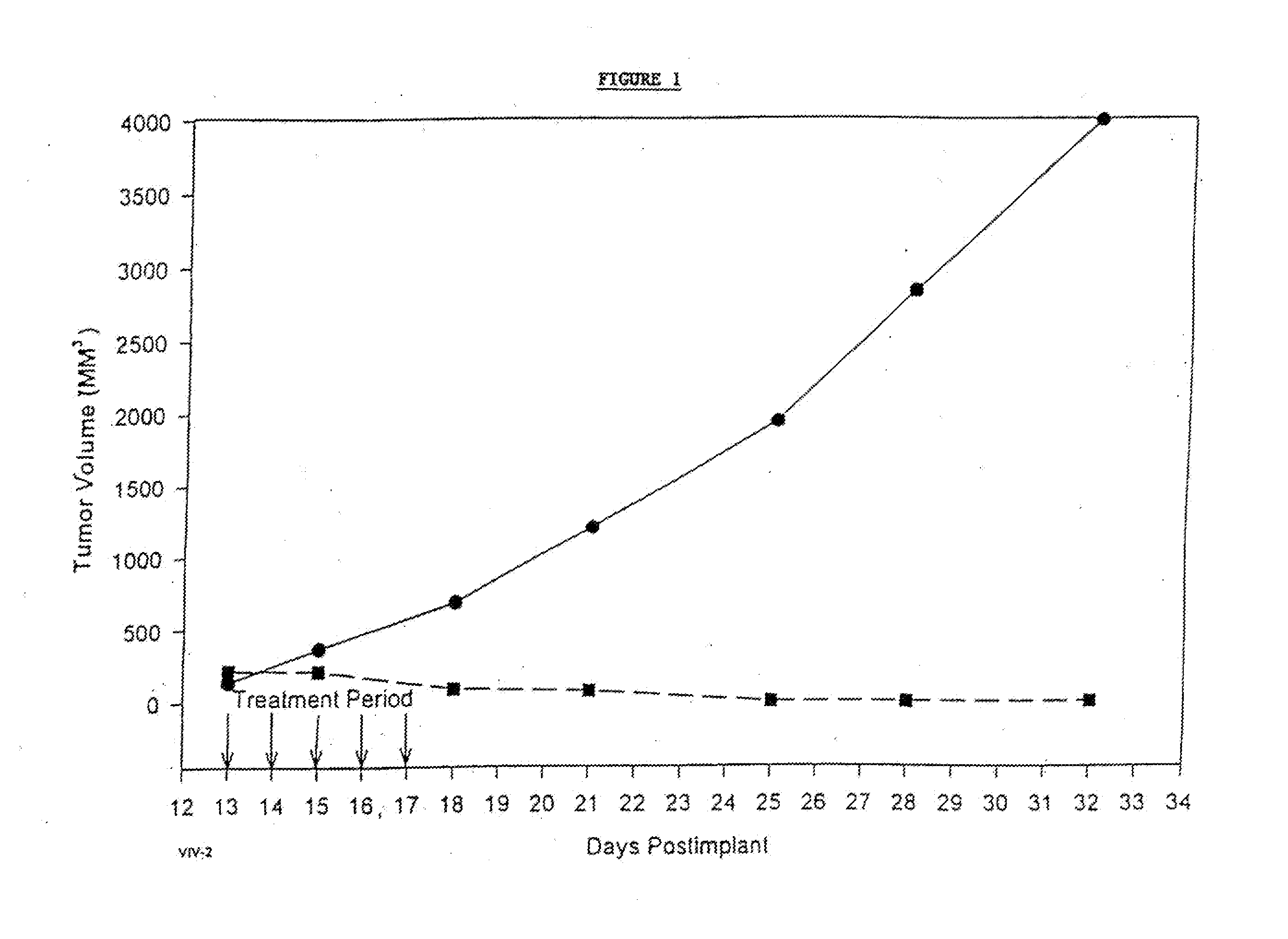
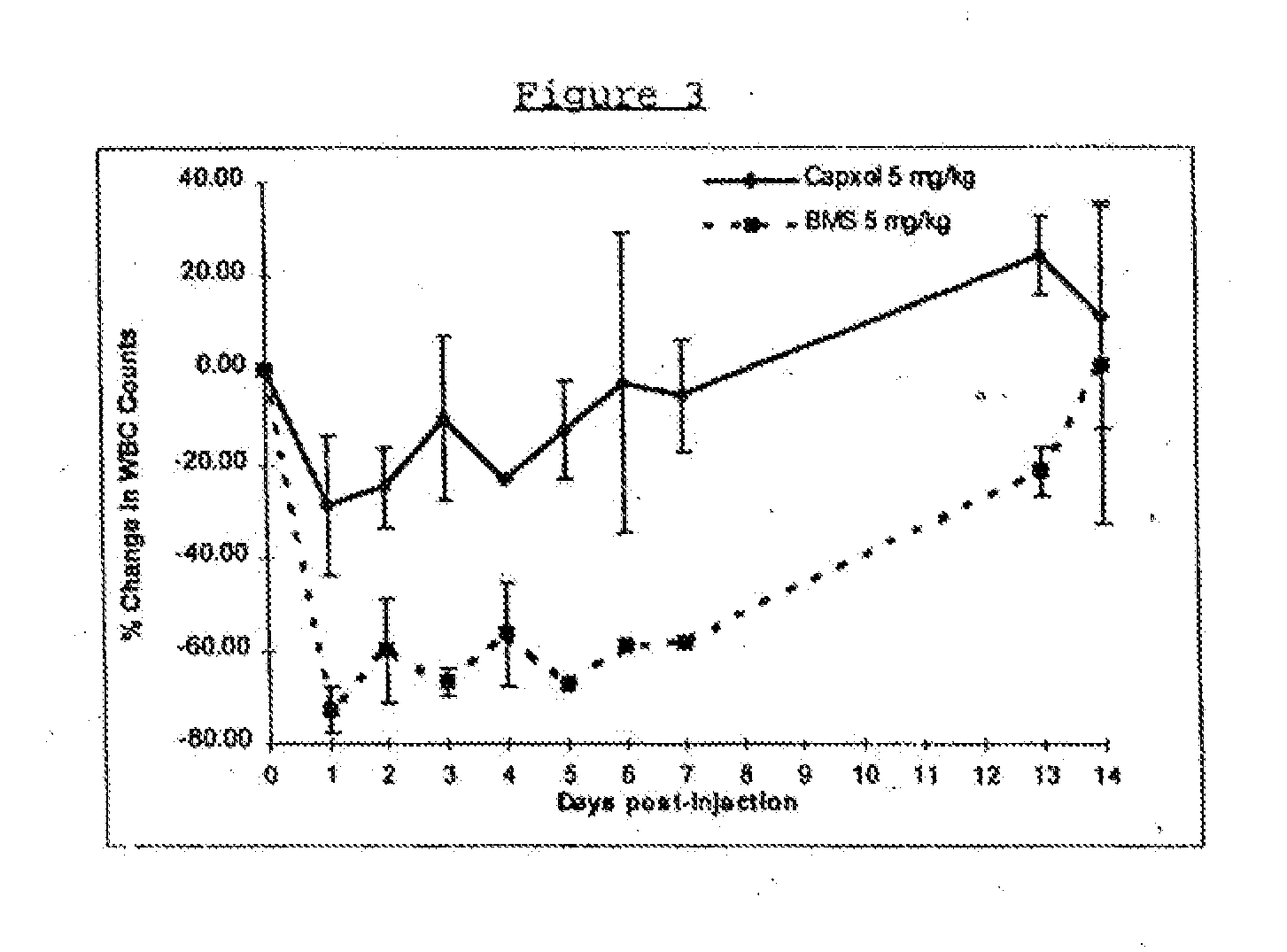
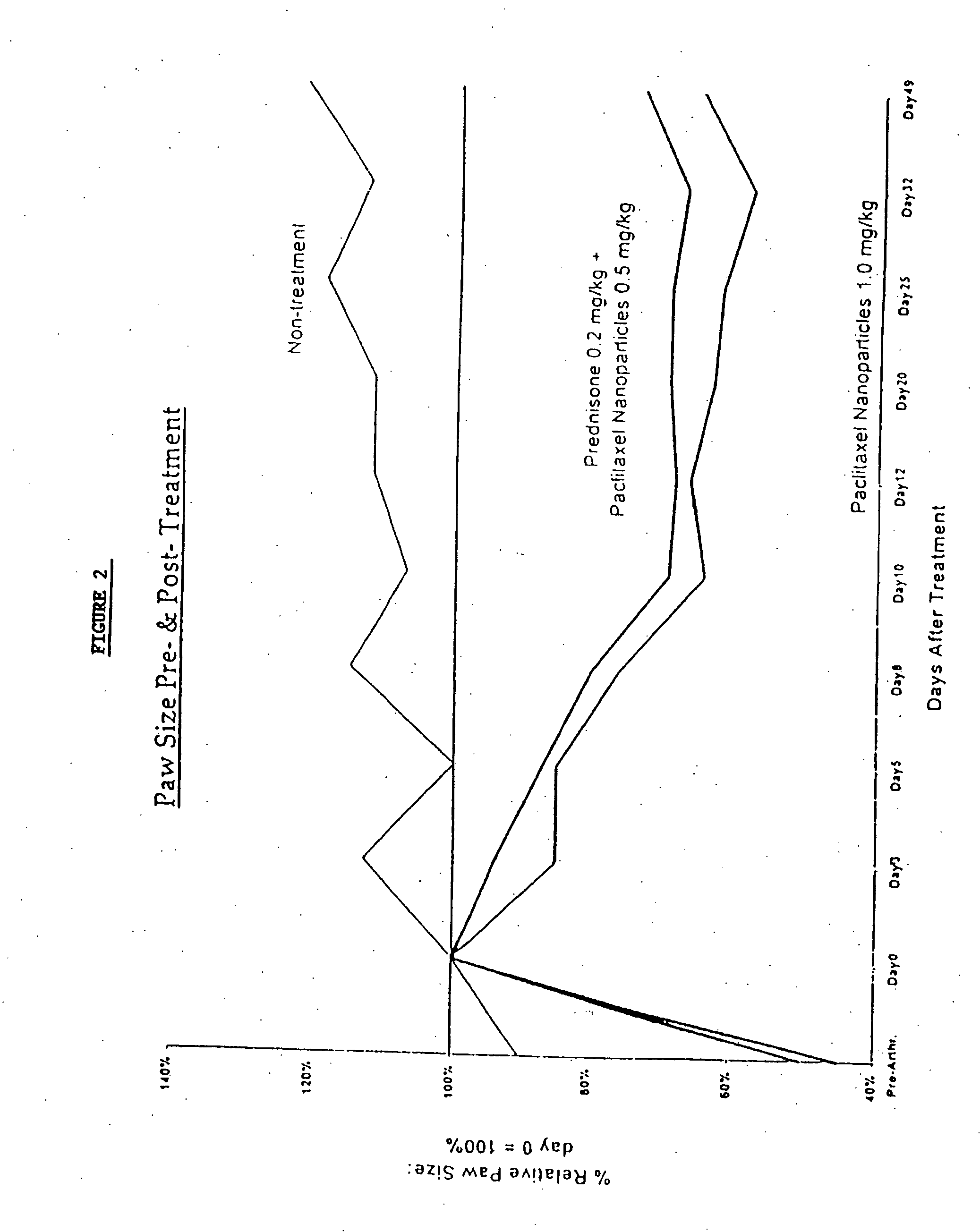
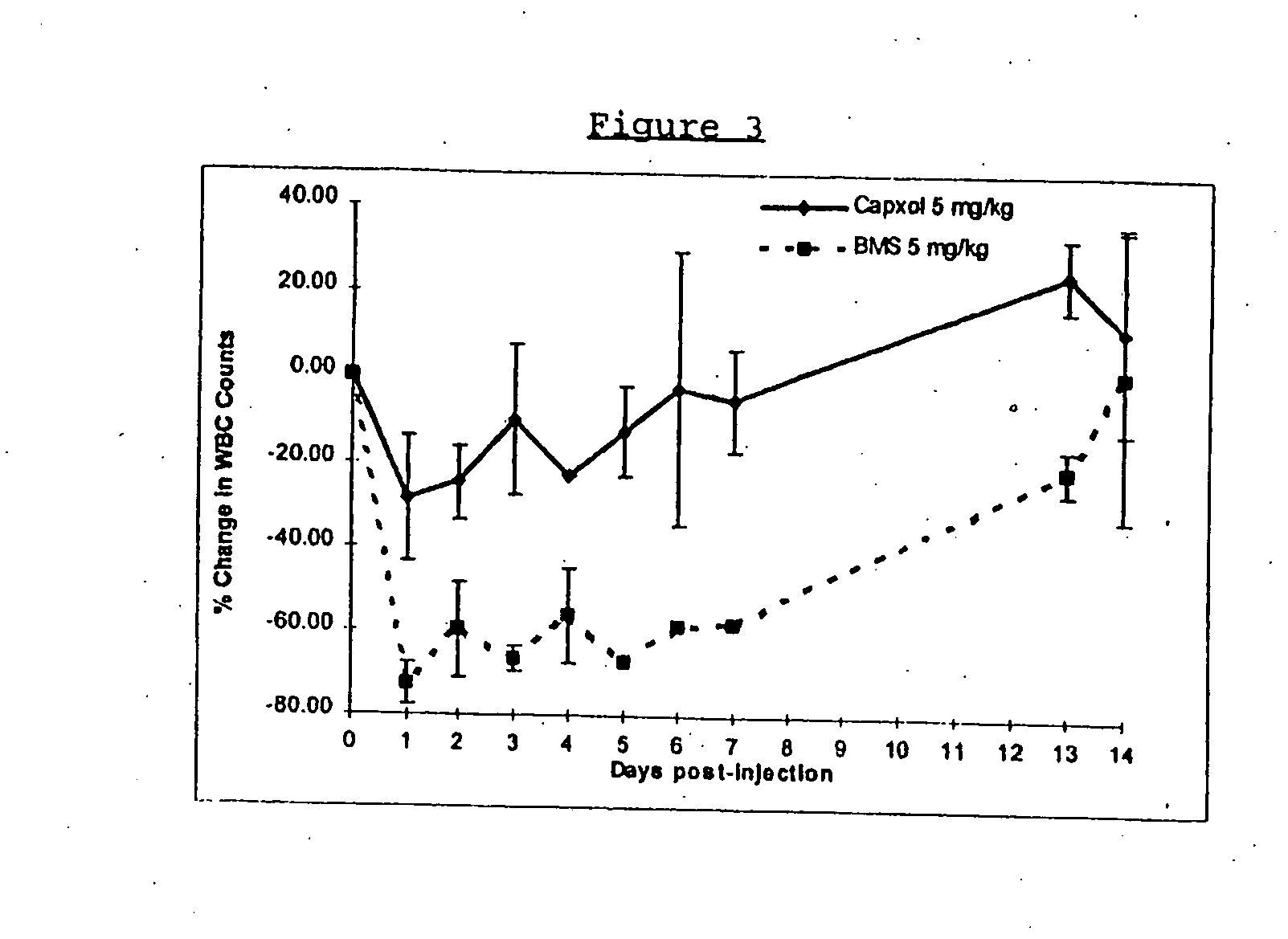
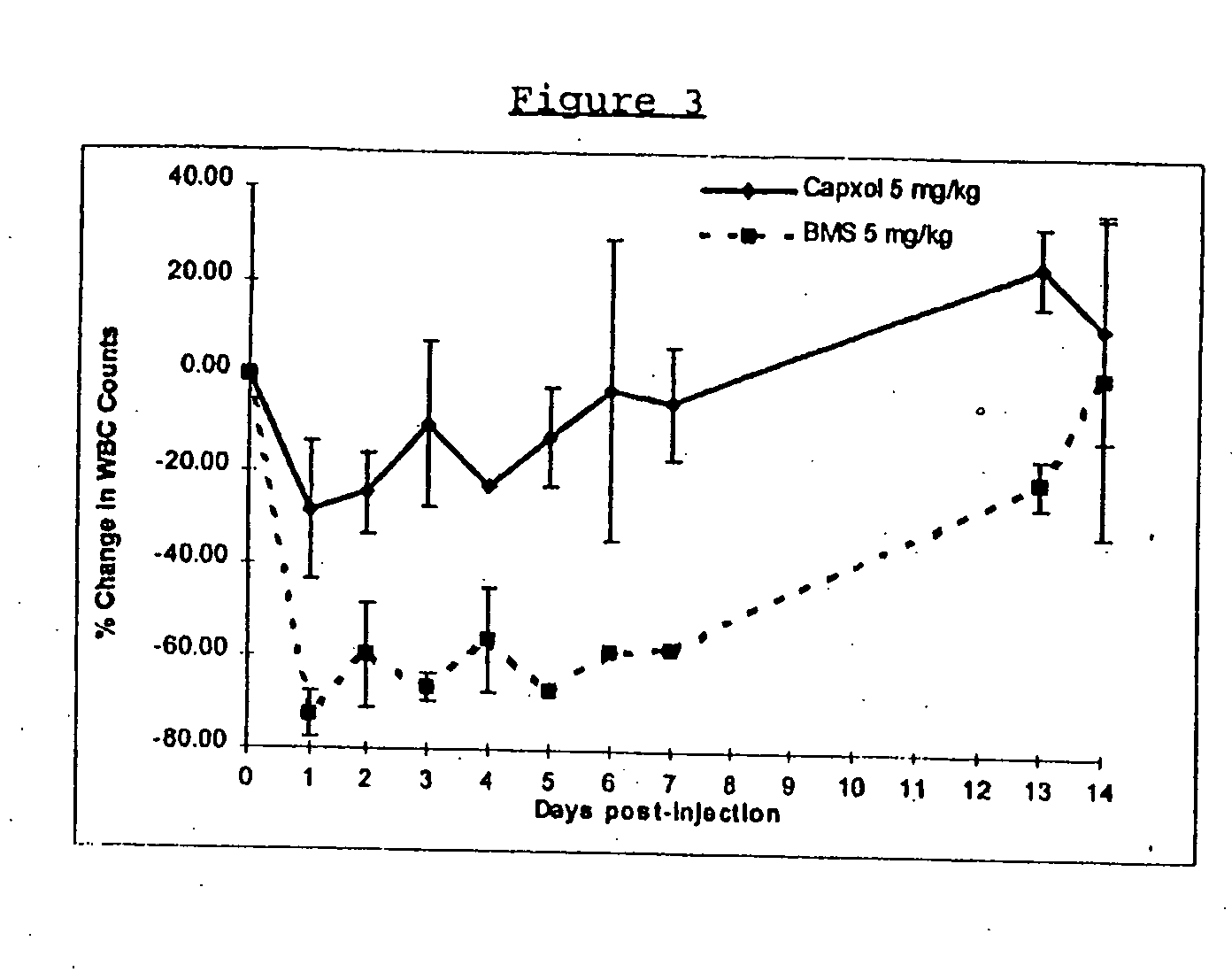
Protein stabilized pharmacologically active agents, methods for the preparation thereof and methods for the use thereof
InactiveUS6749868B1Low toxicityLong half-lifePowder deliveryEchographic/ultrasound-imaging preparationsSuspended particlesFree protein
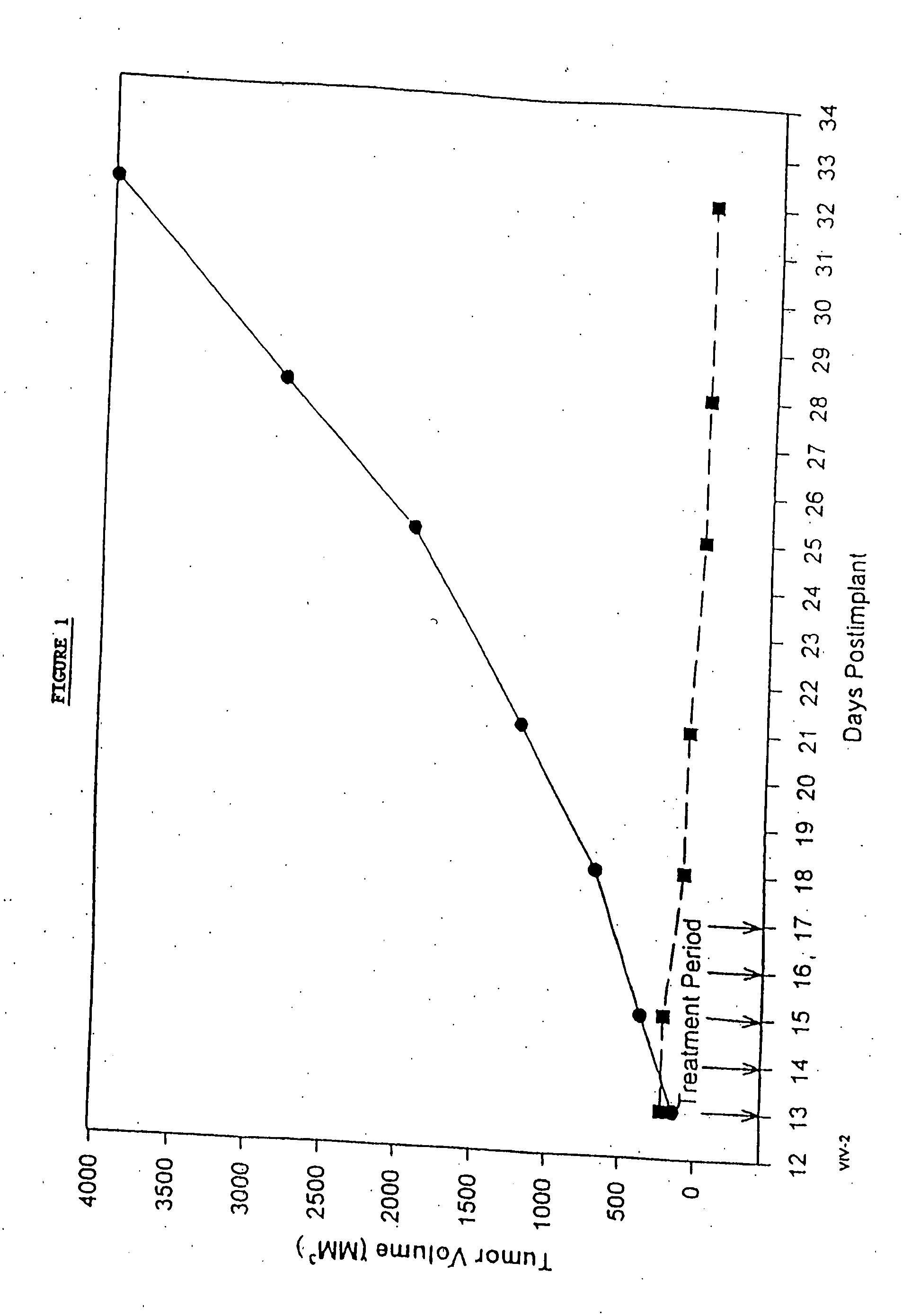
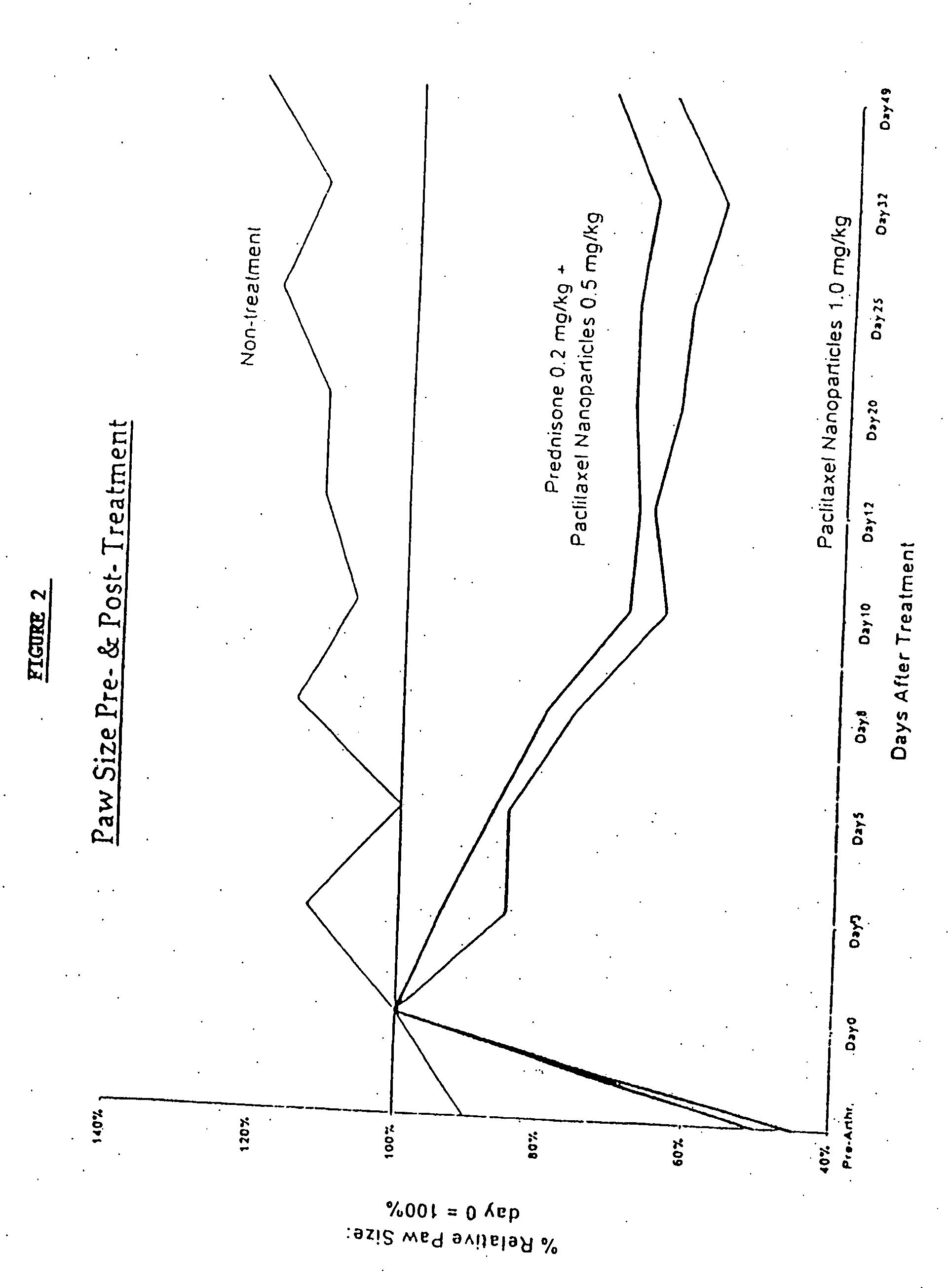
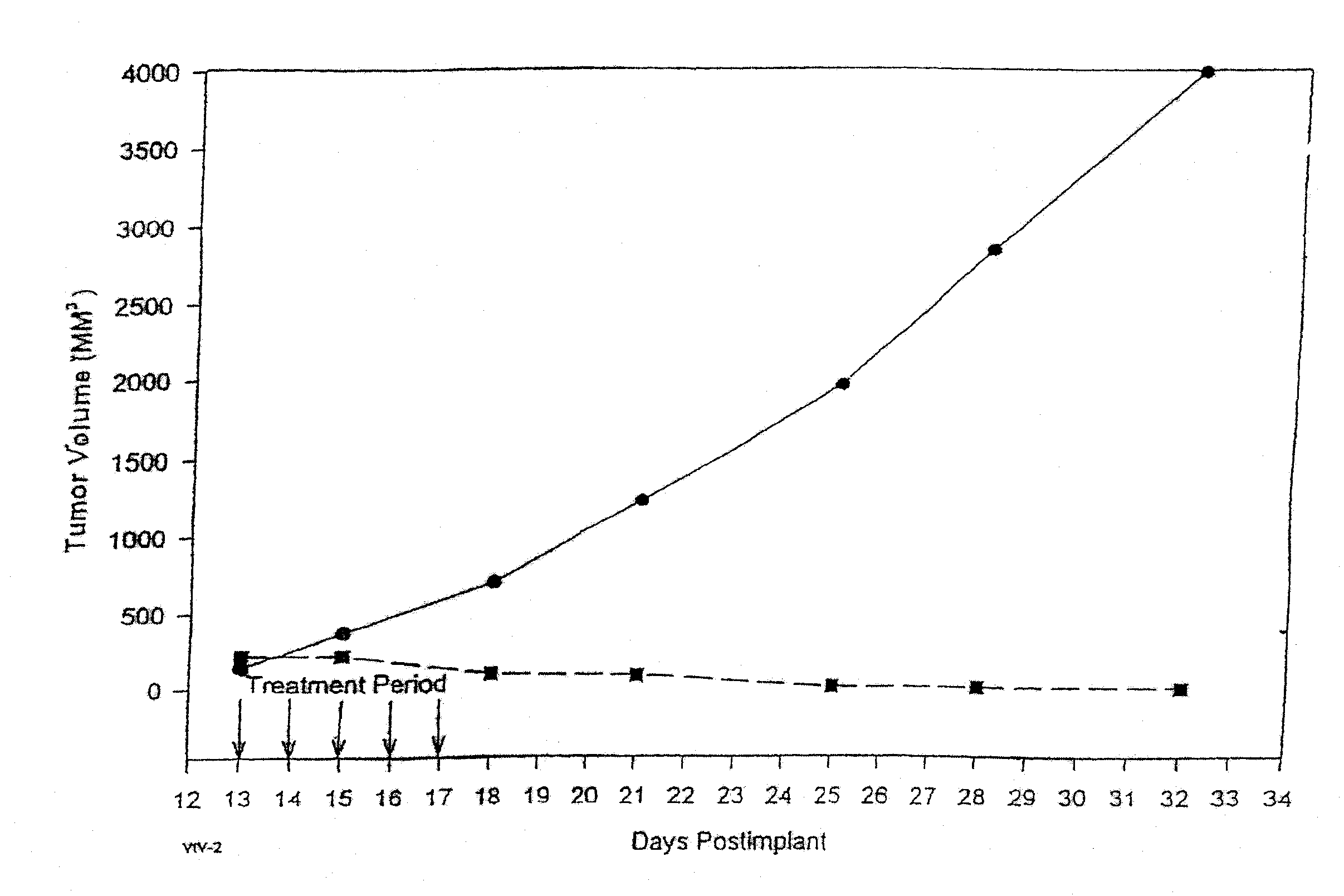
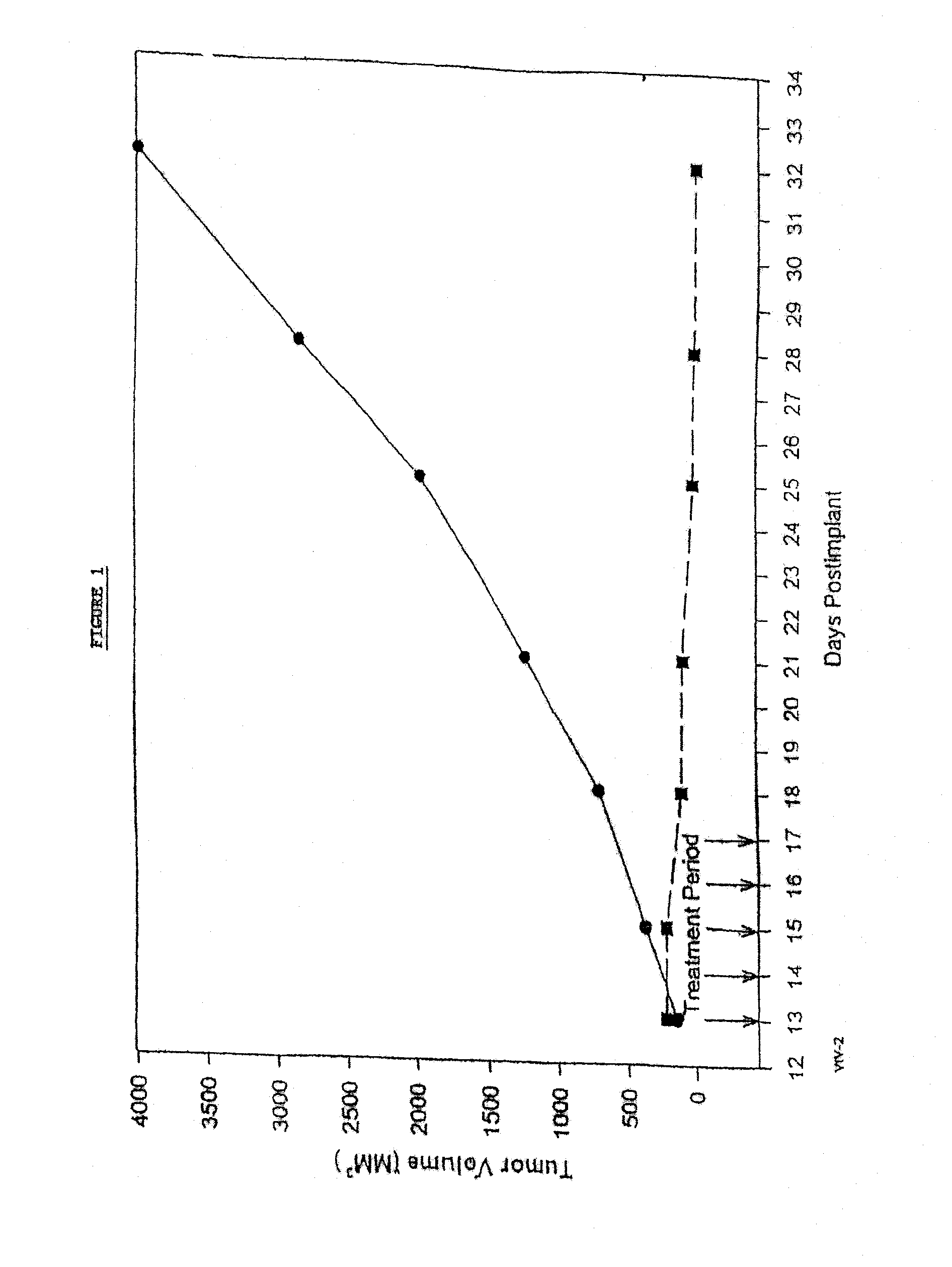
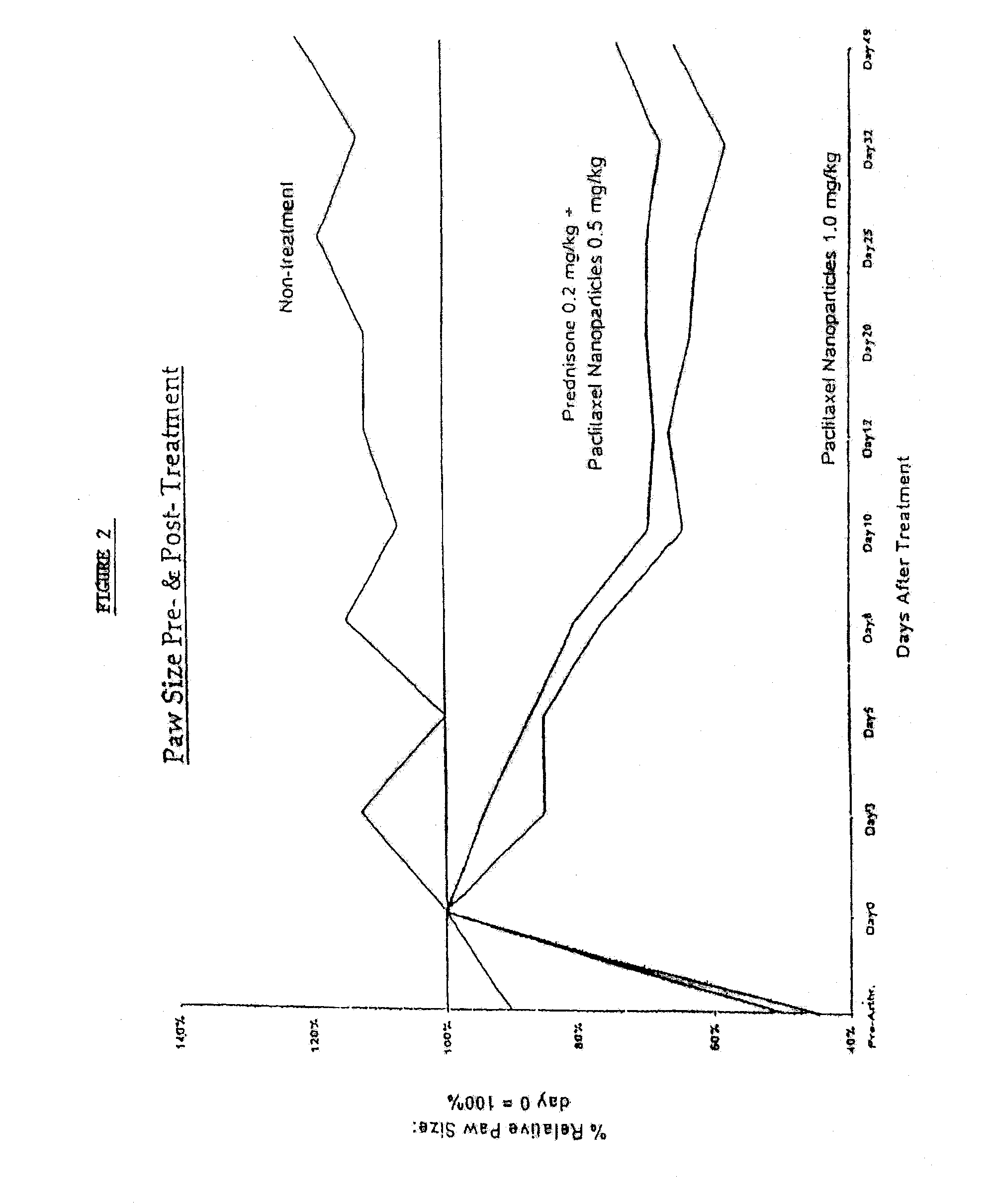
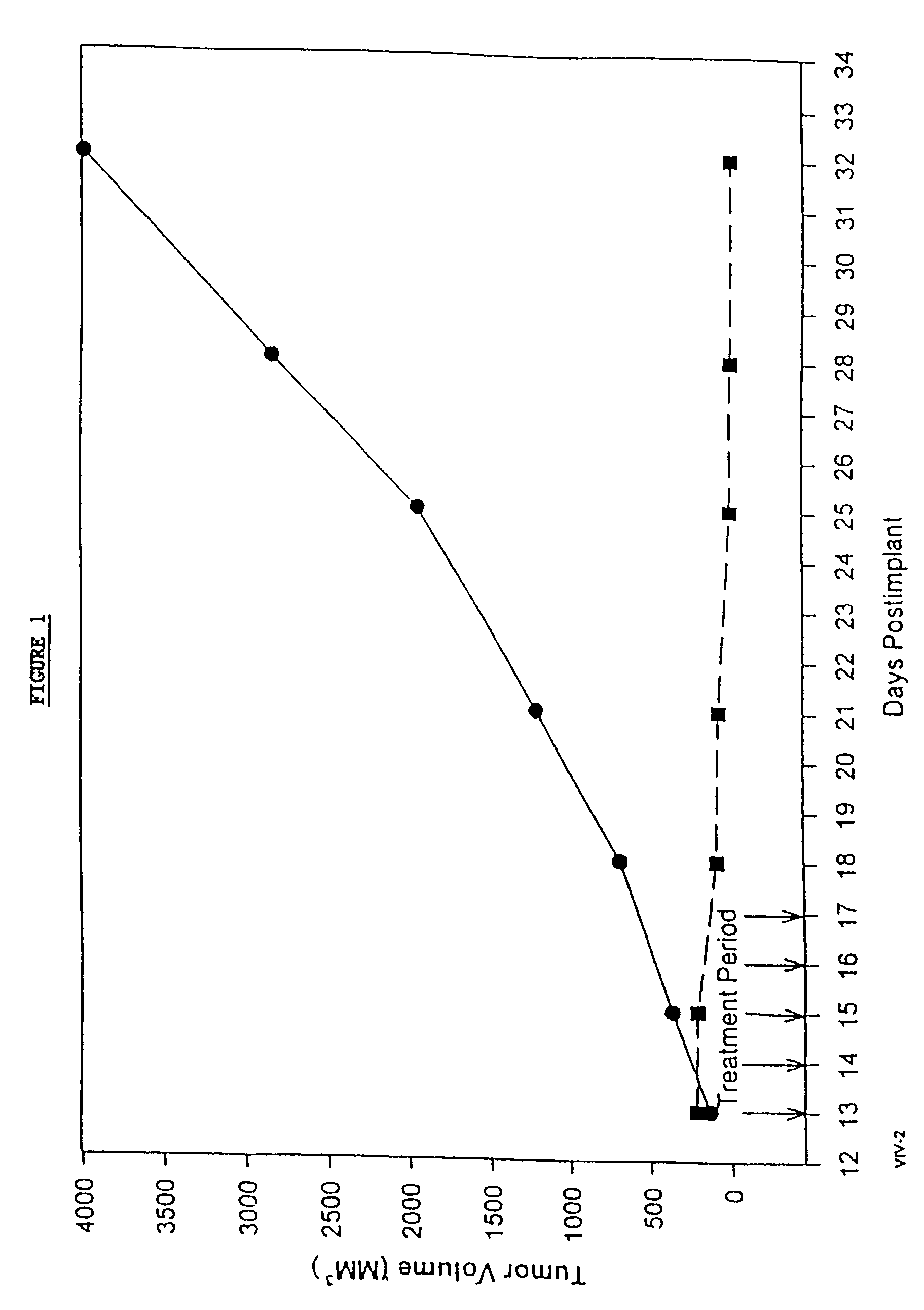
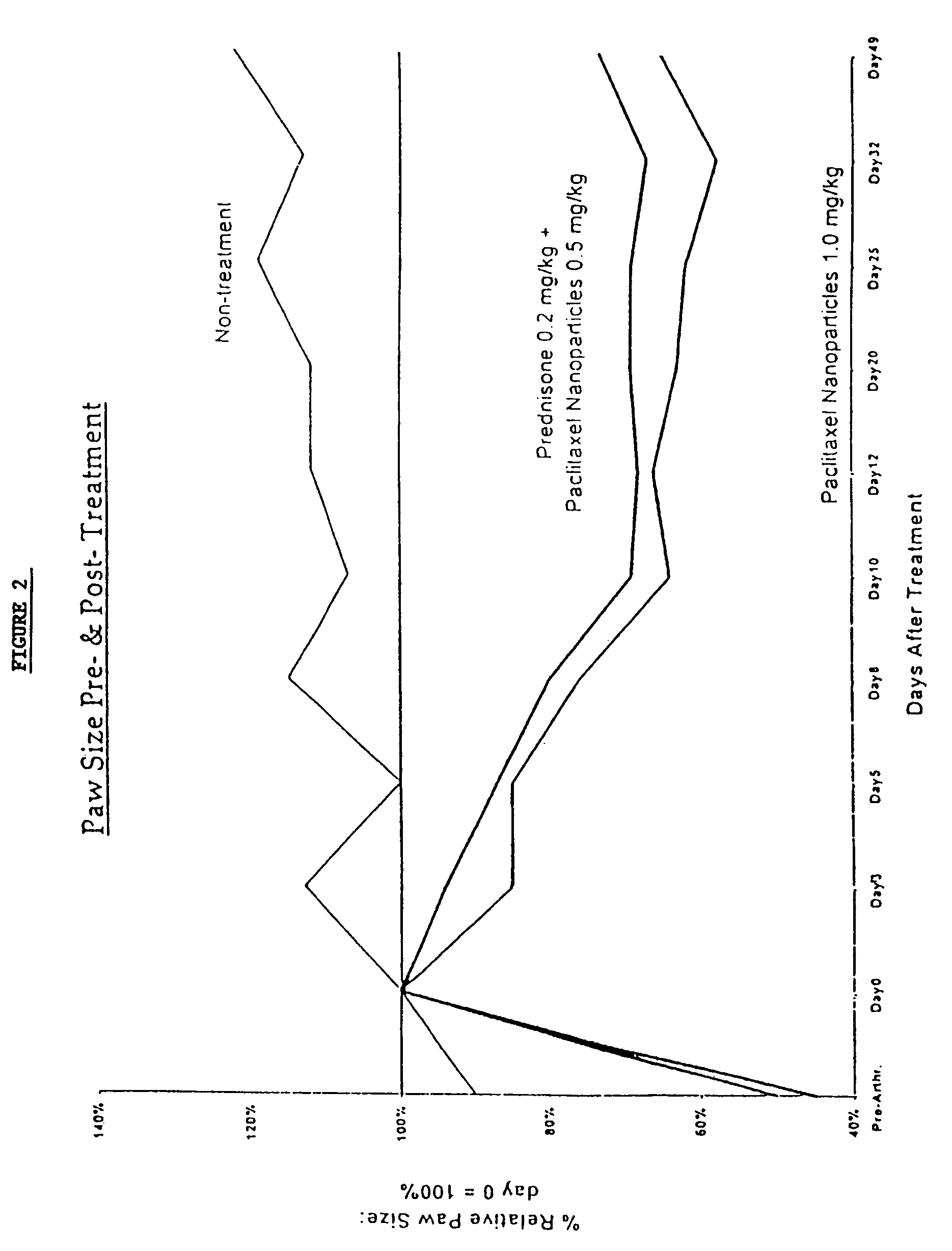
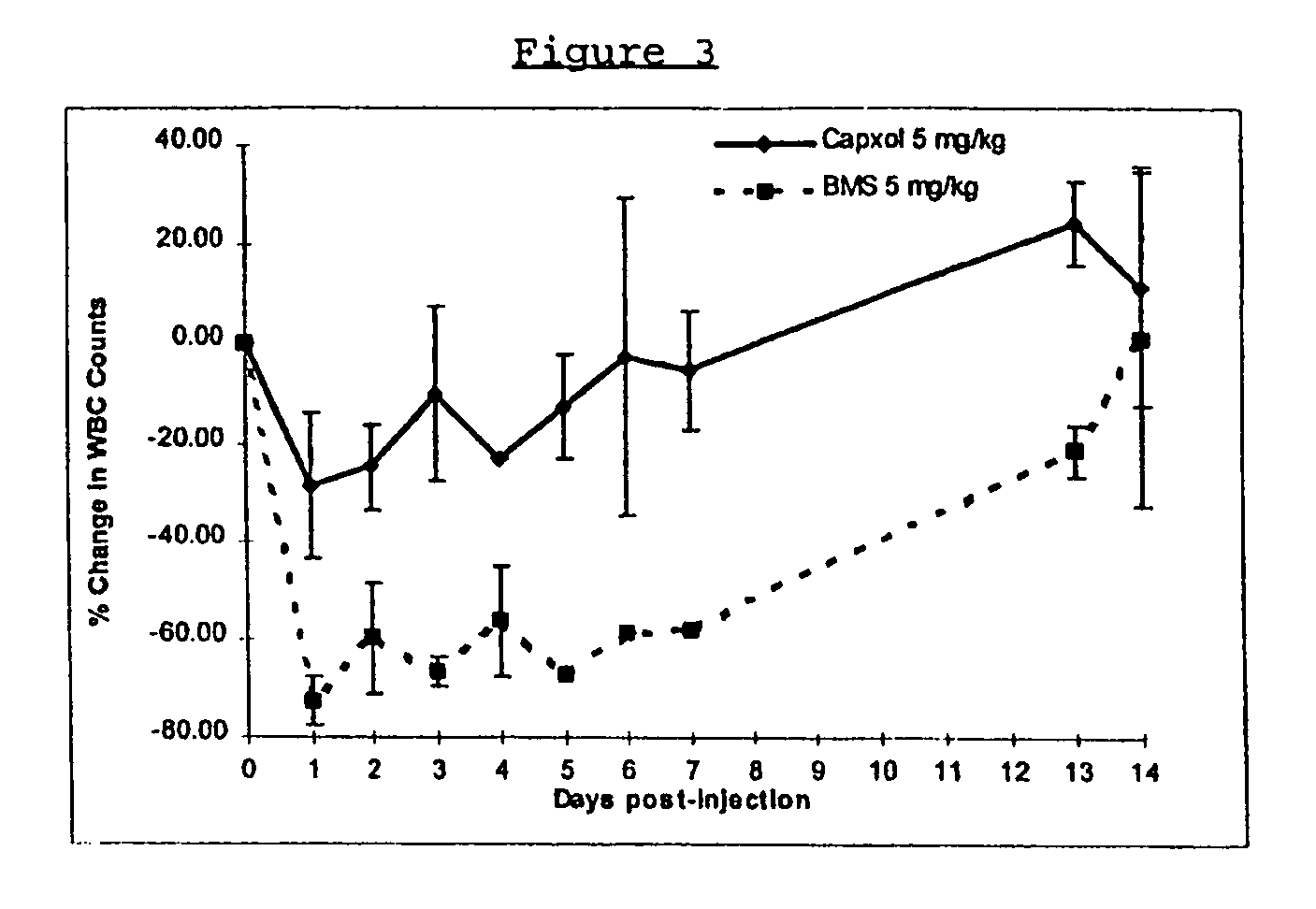
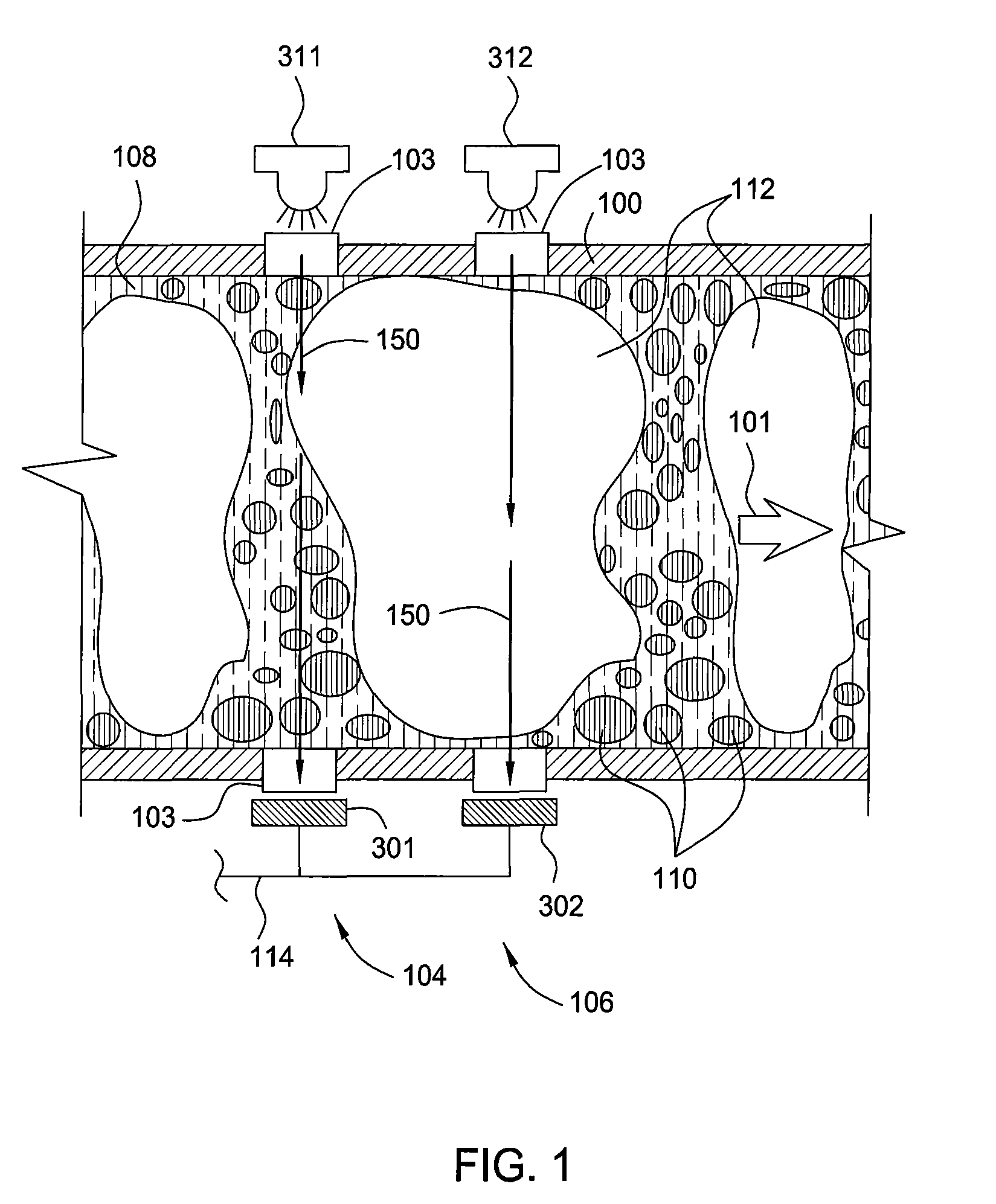
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful election of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
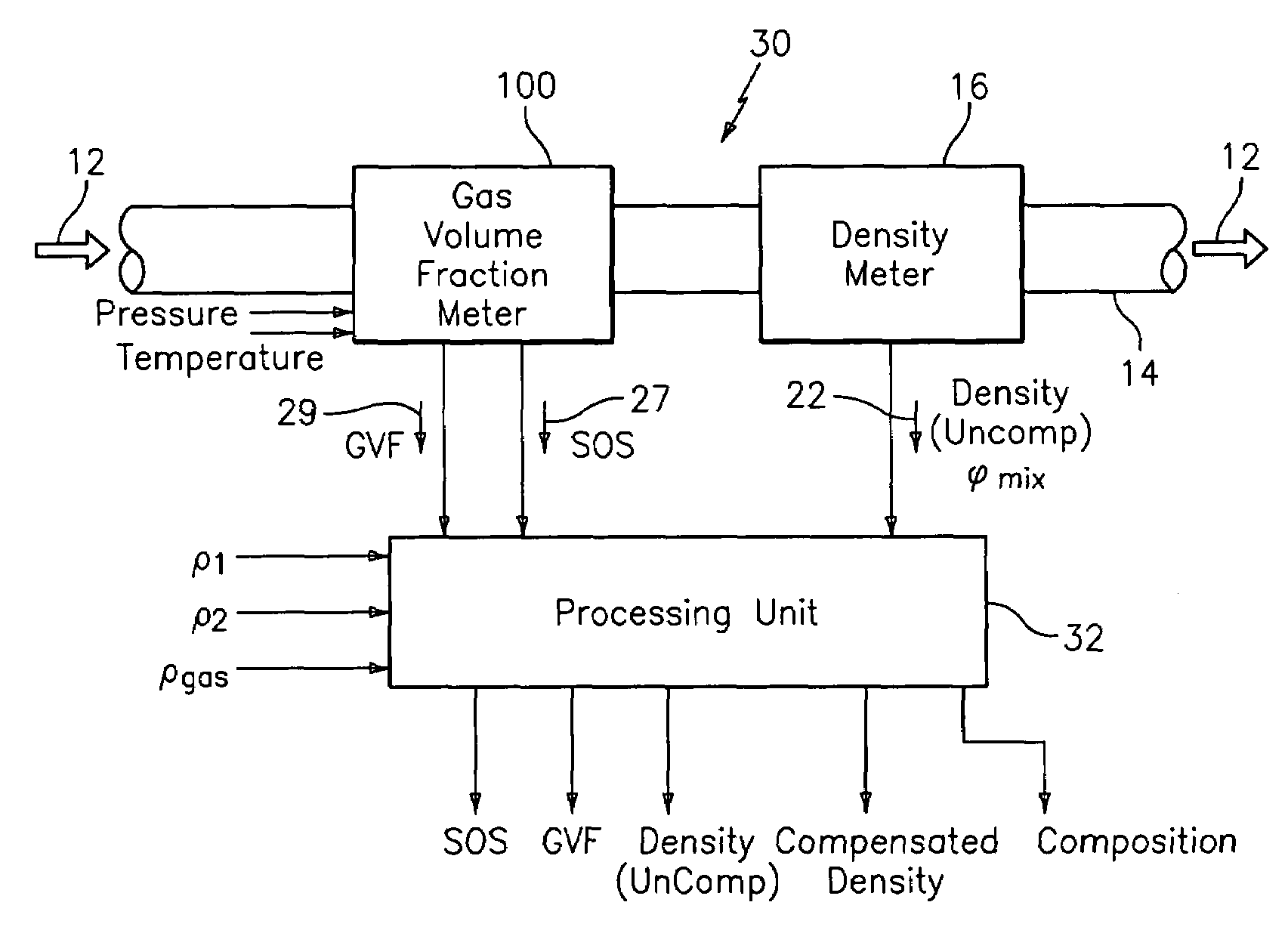
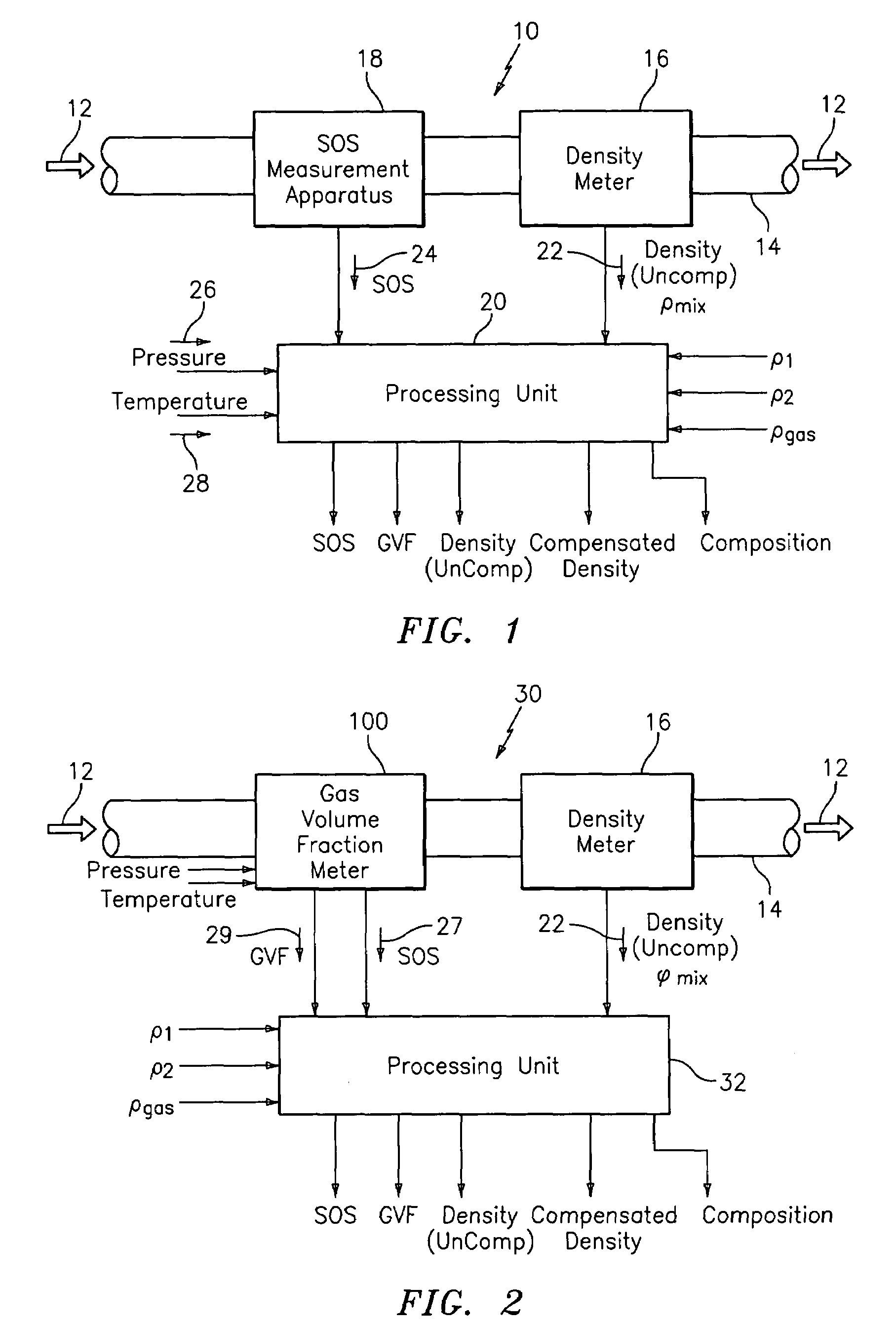
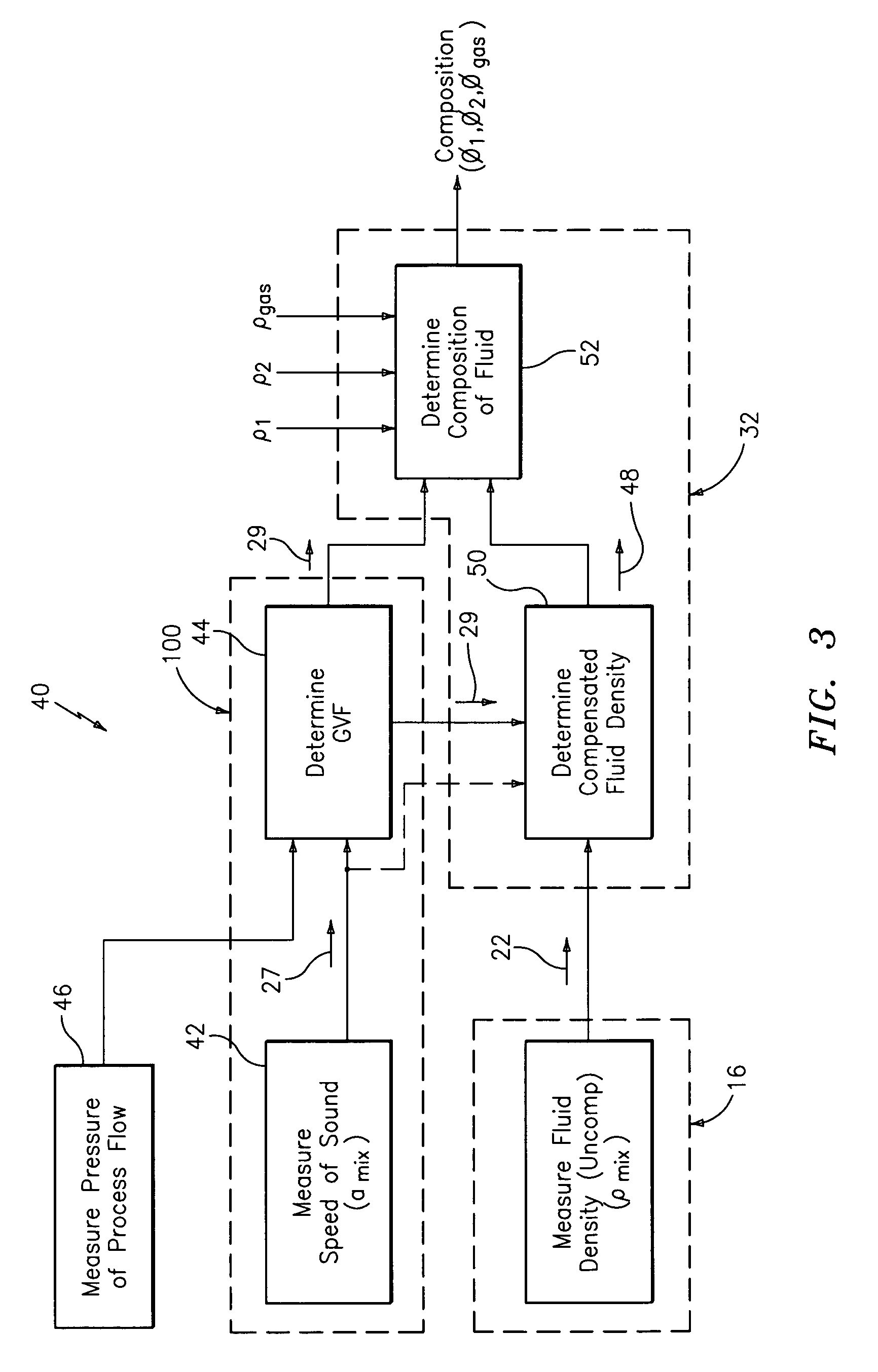
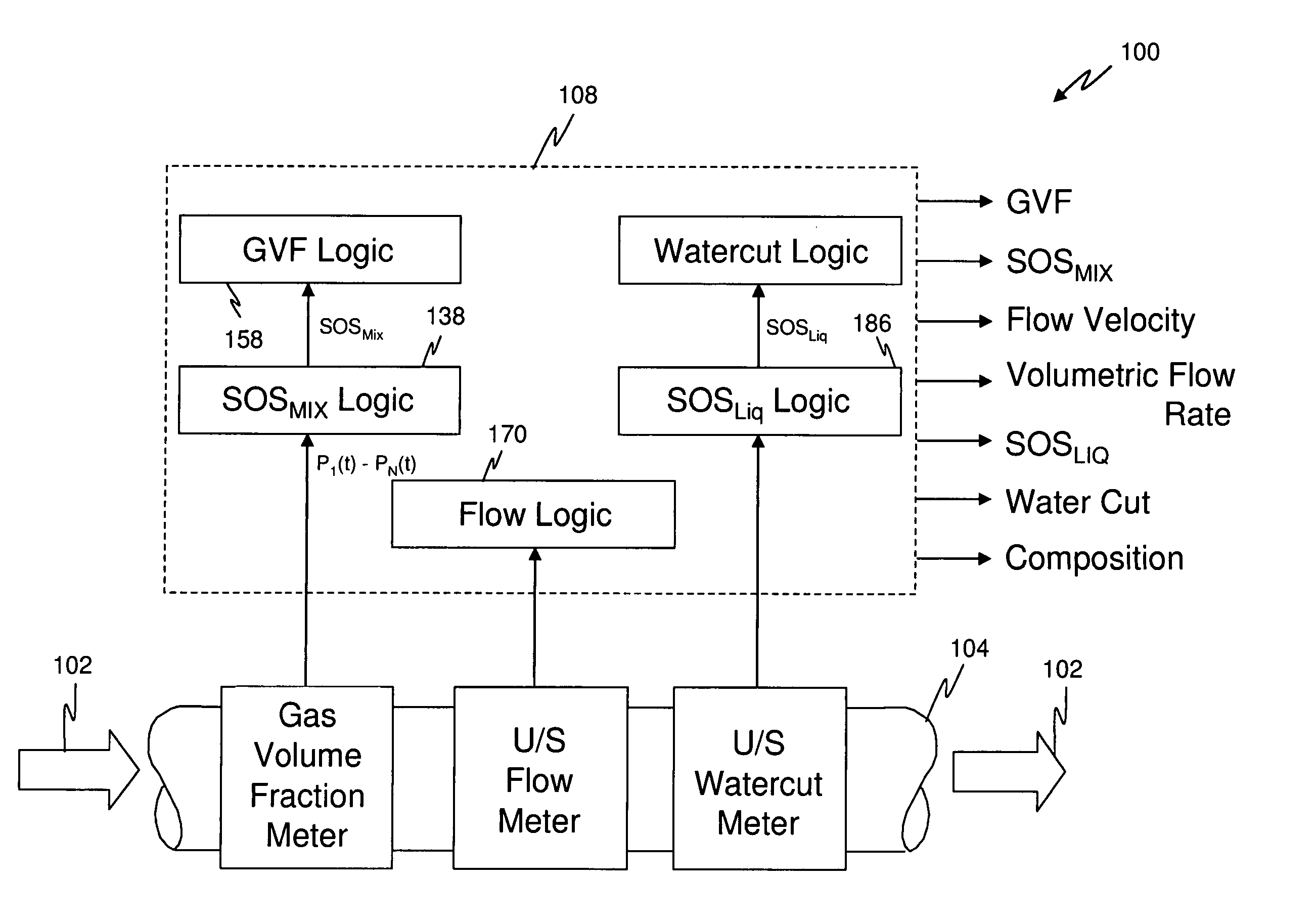
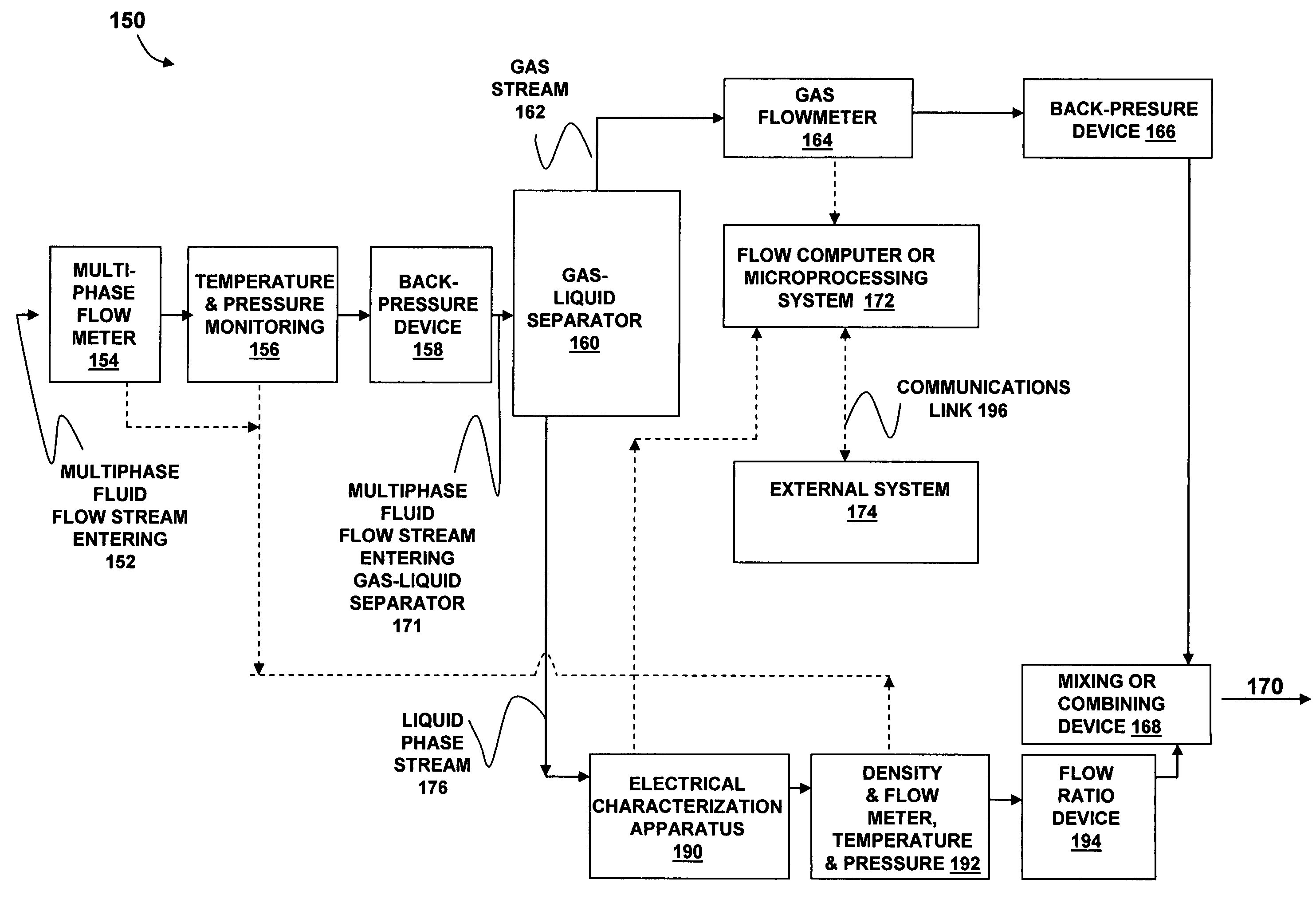
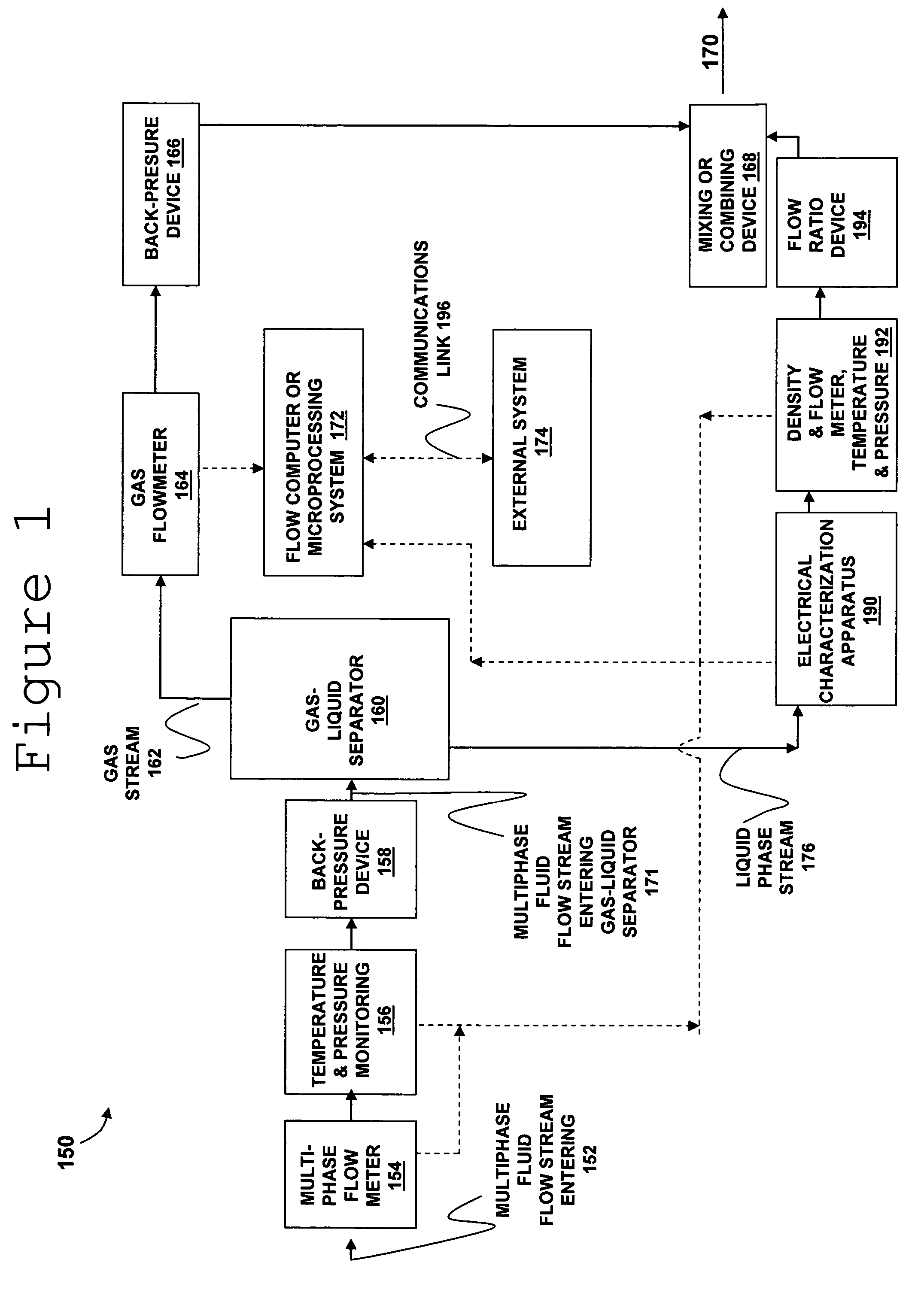
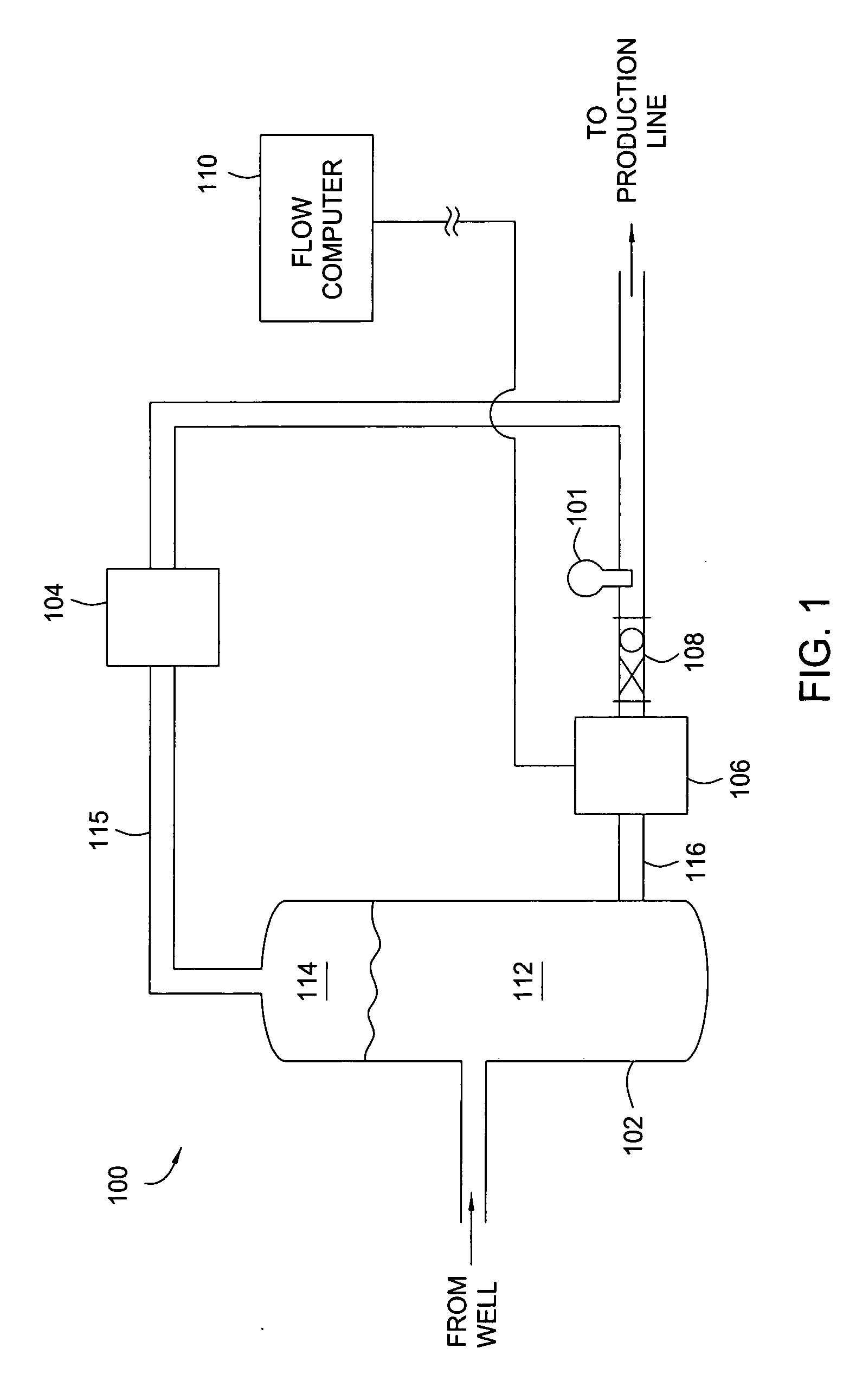
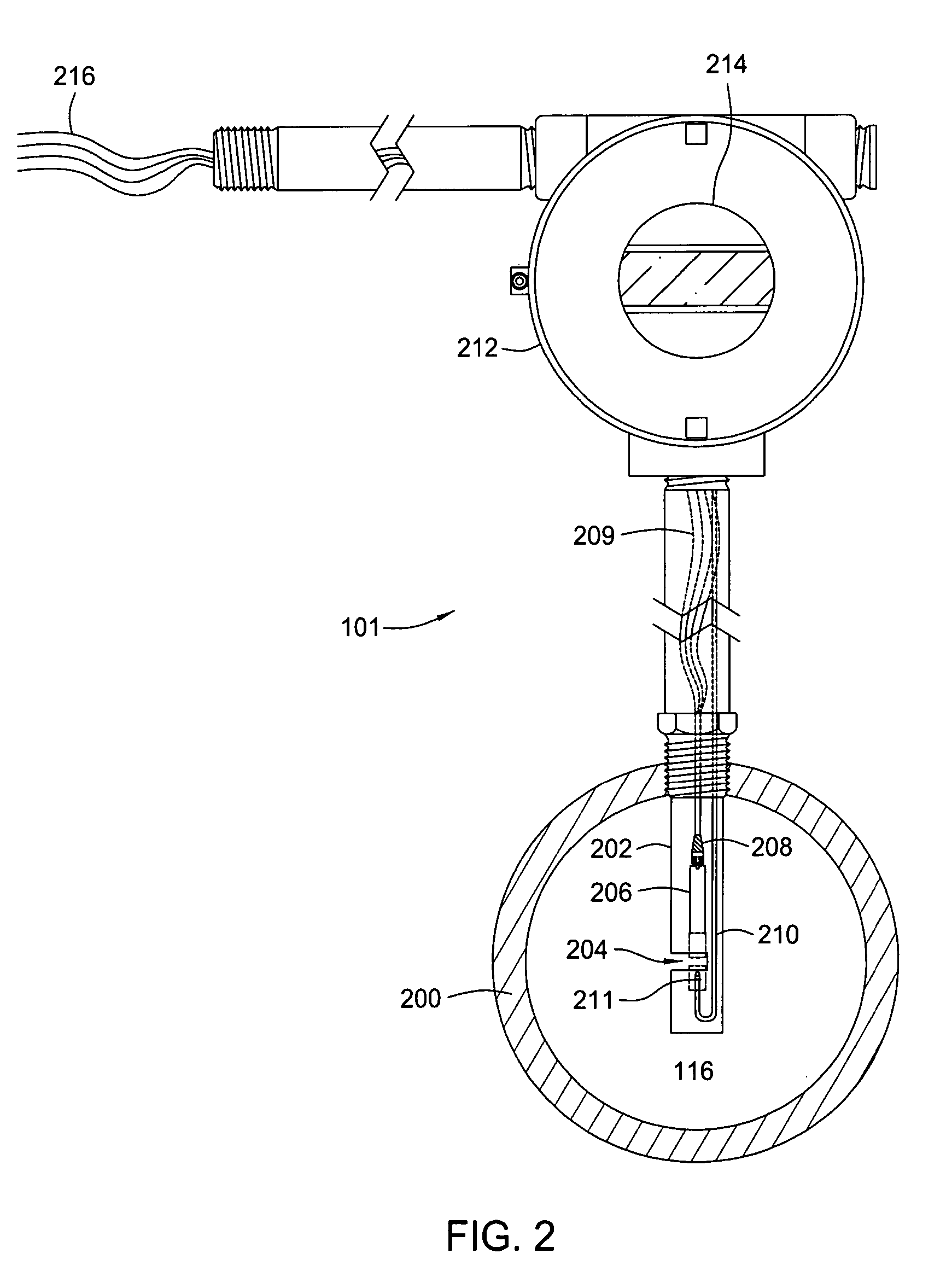
Apparatus and method for providing a density measurement augmented for entrained gas
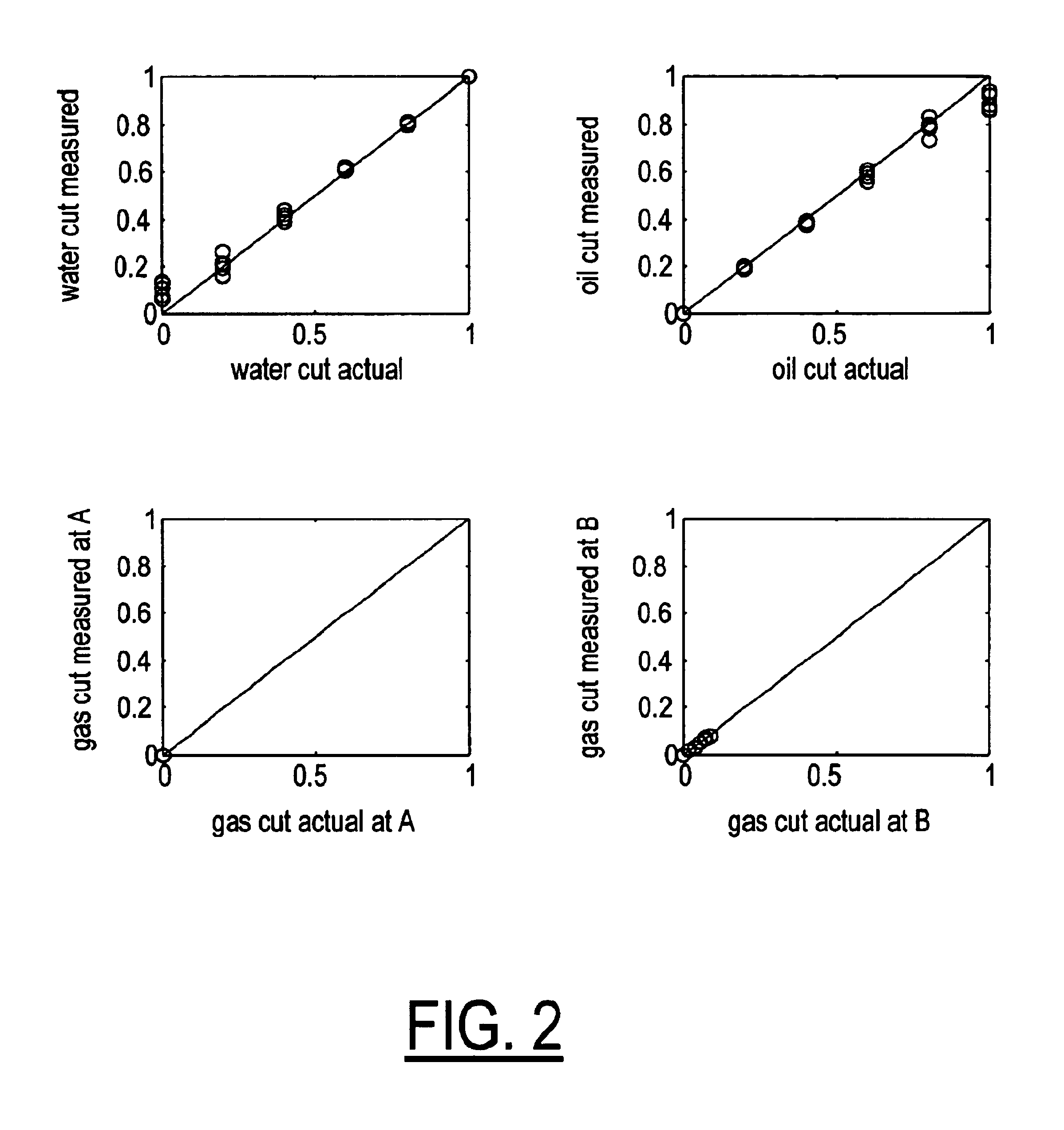
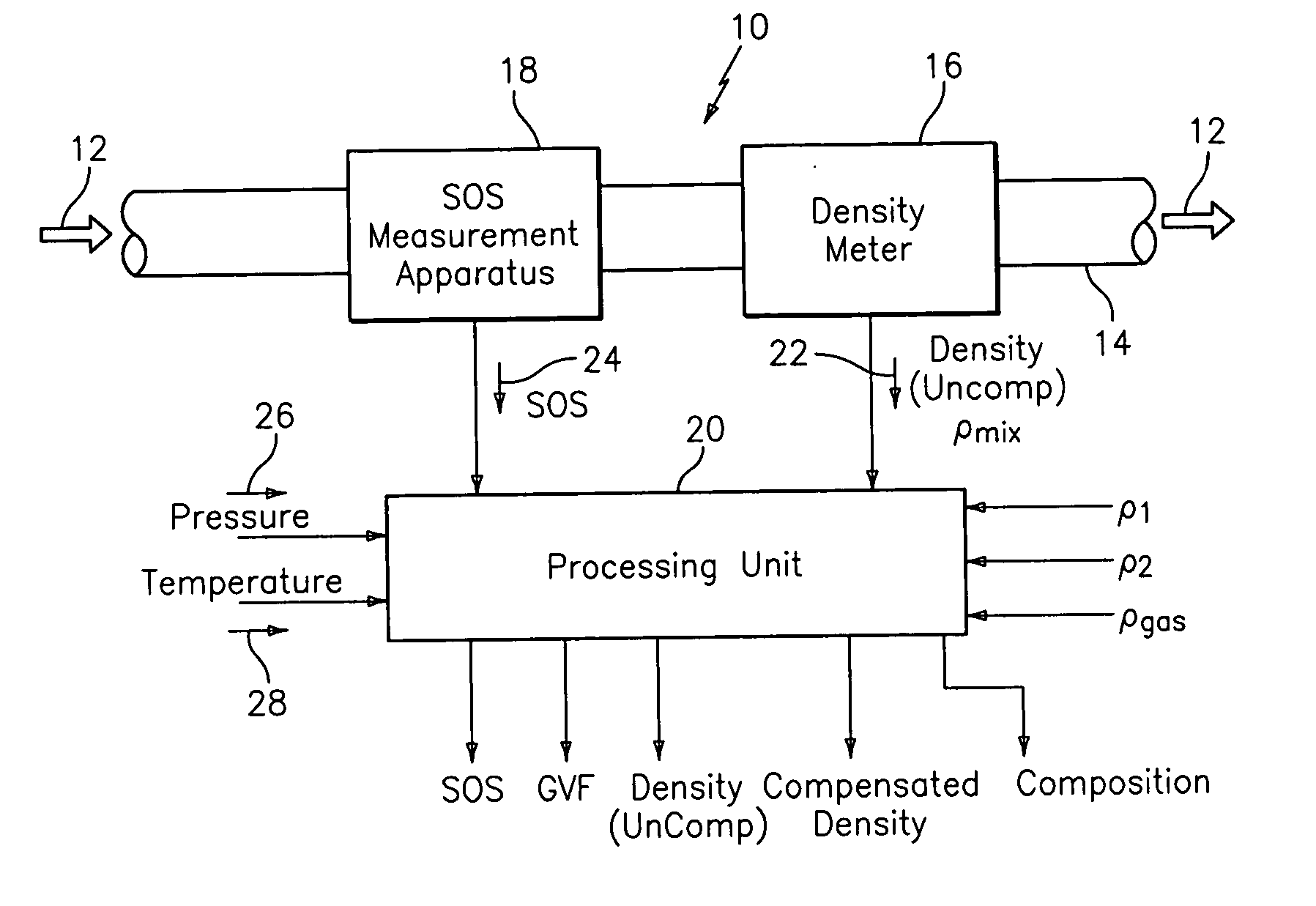
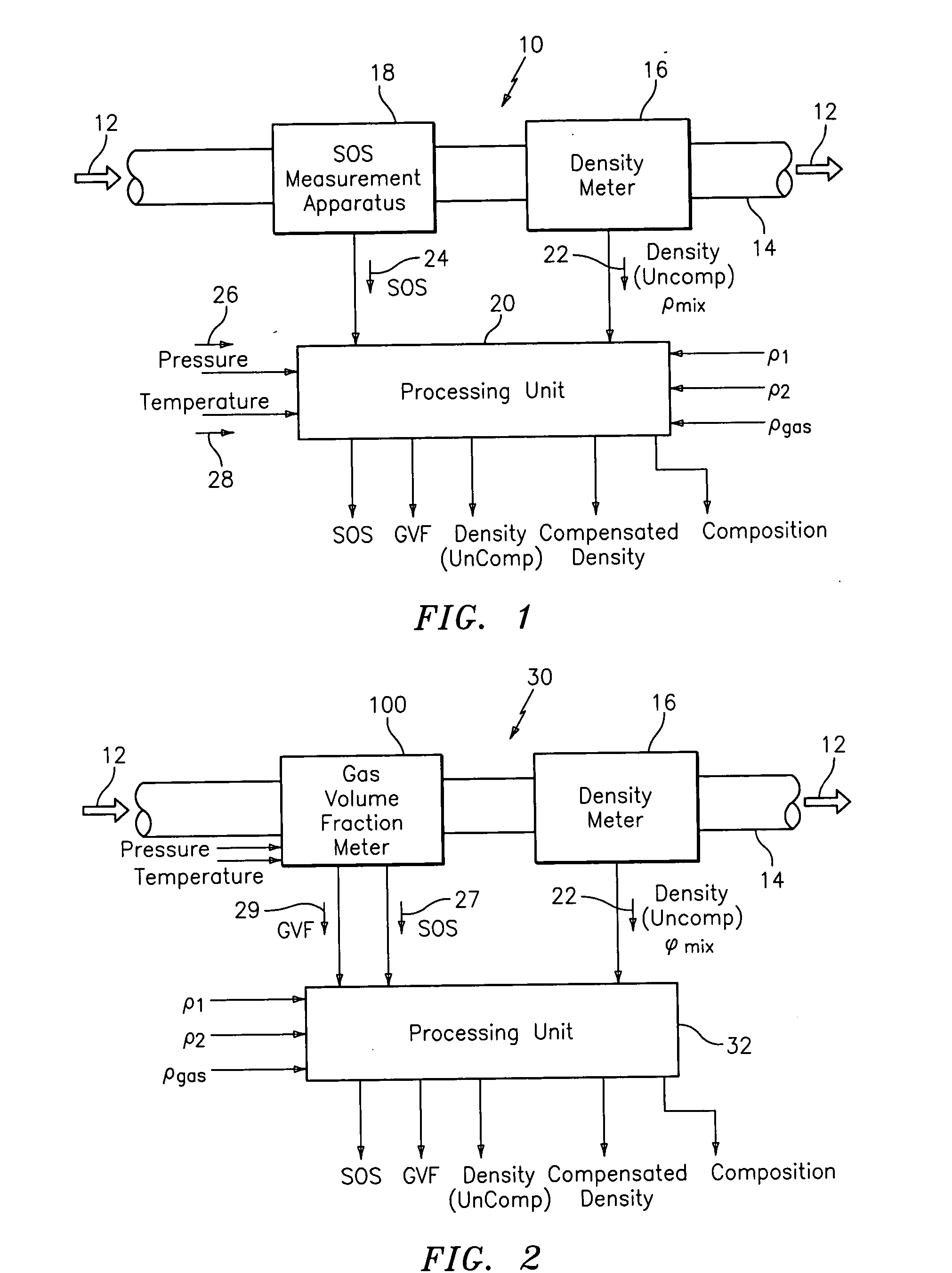
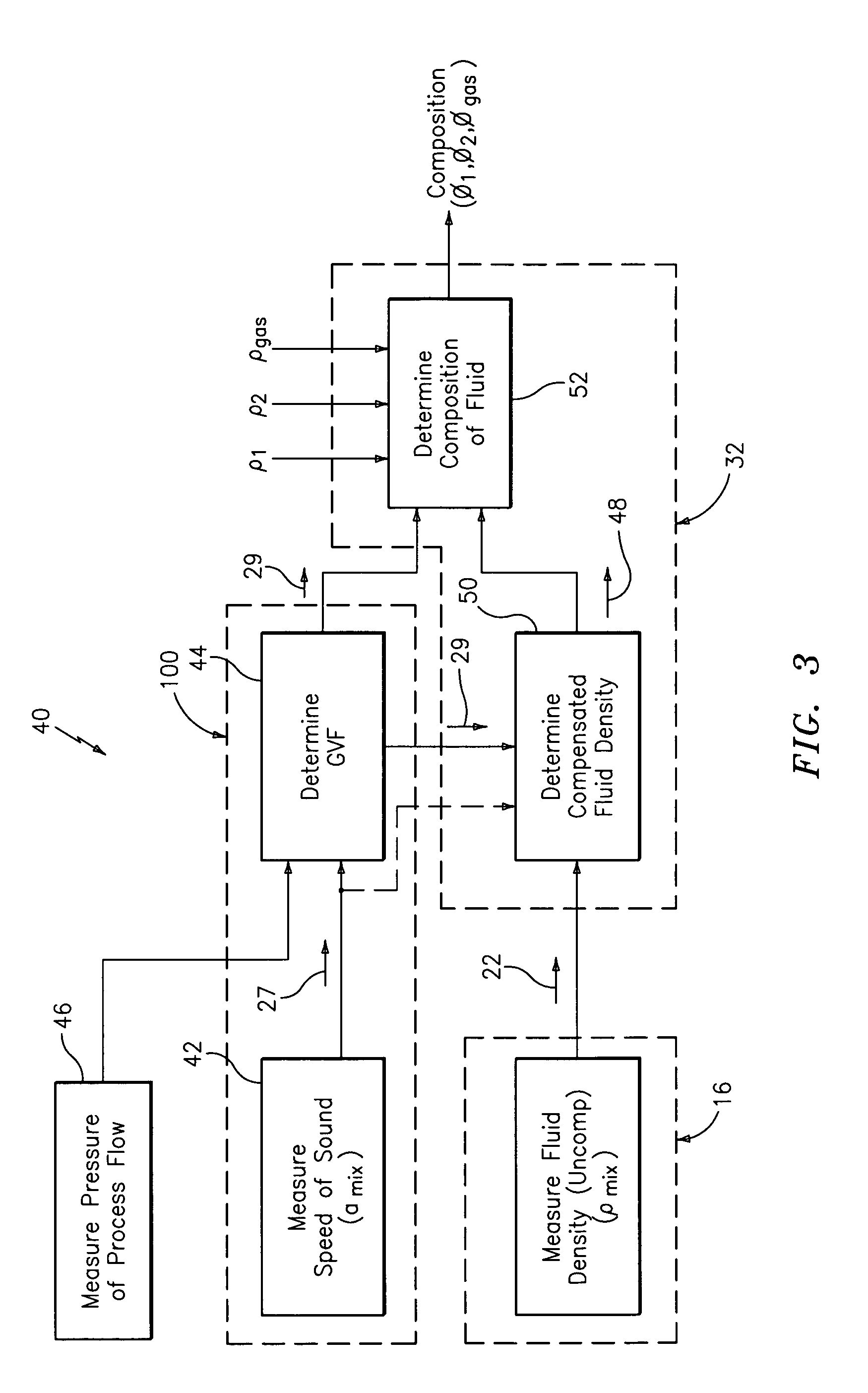
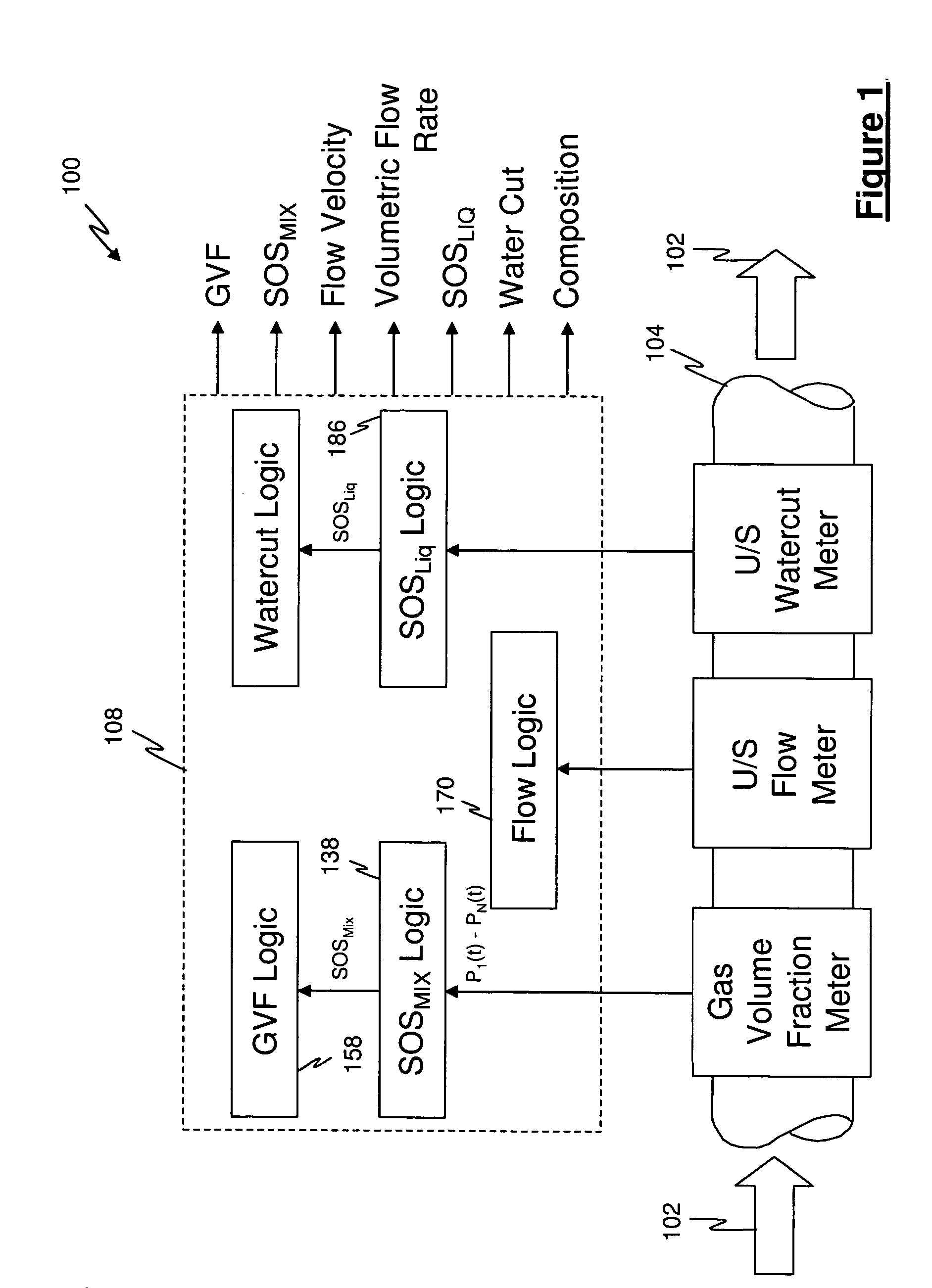
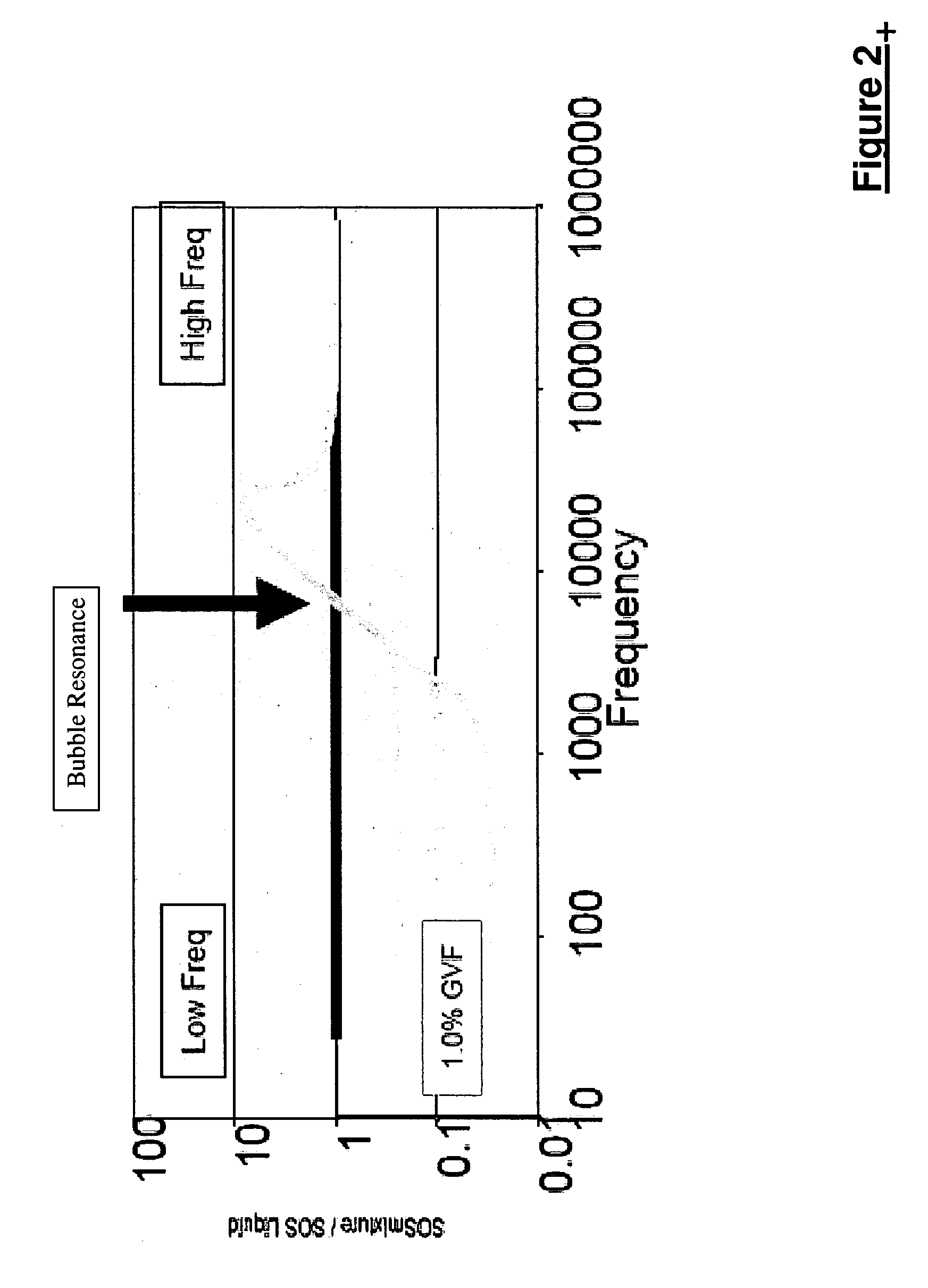
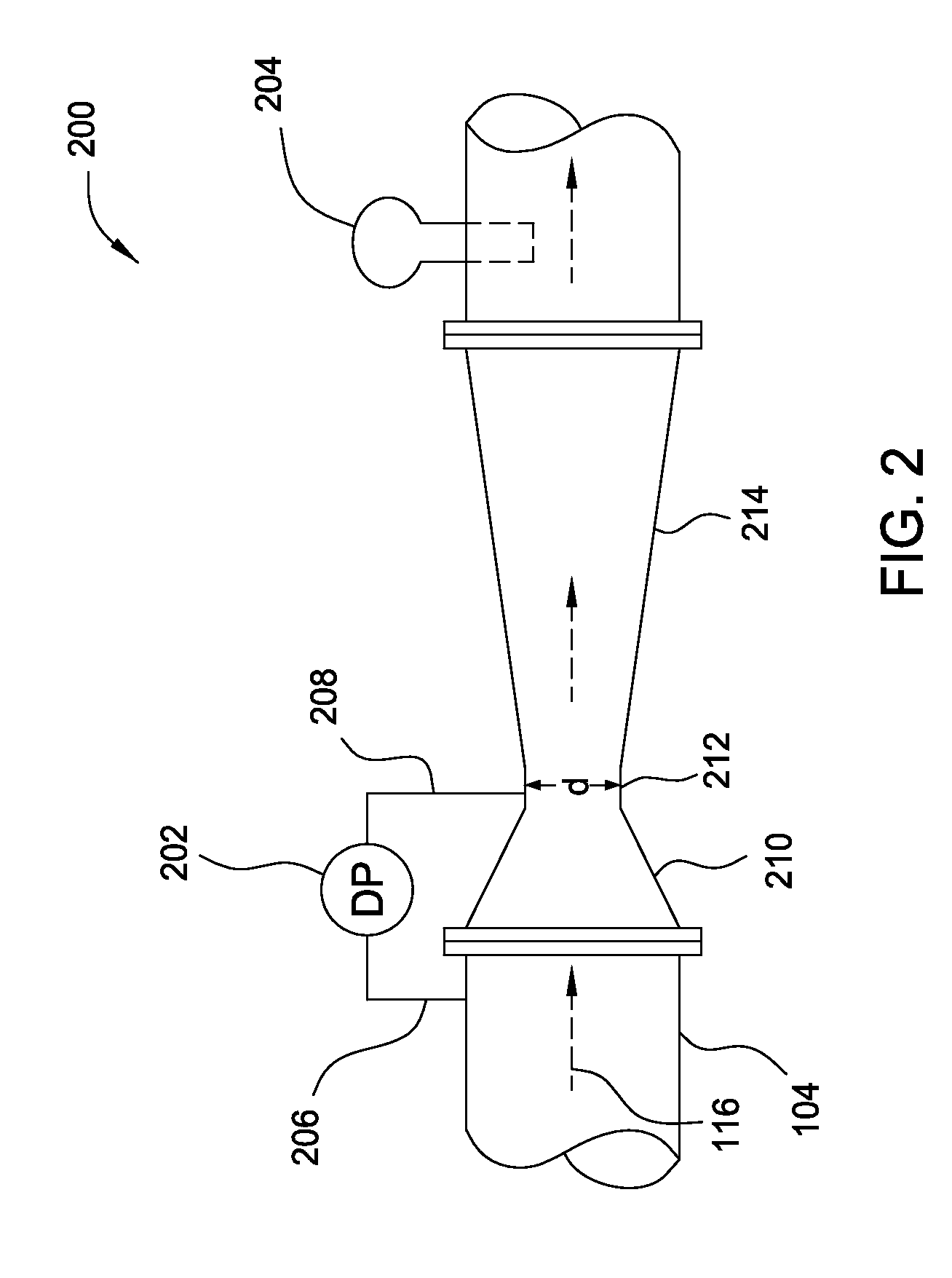
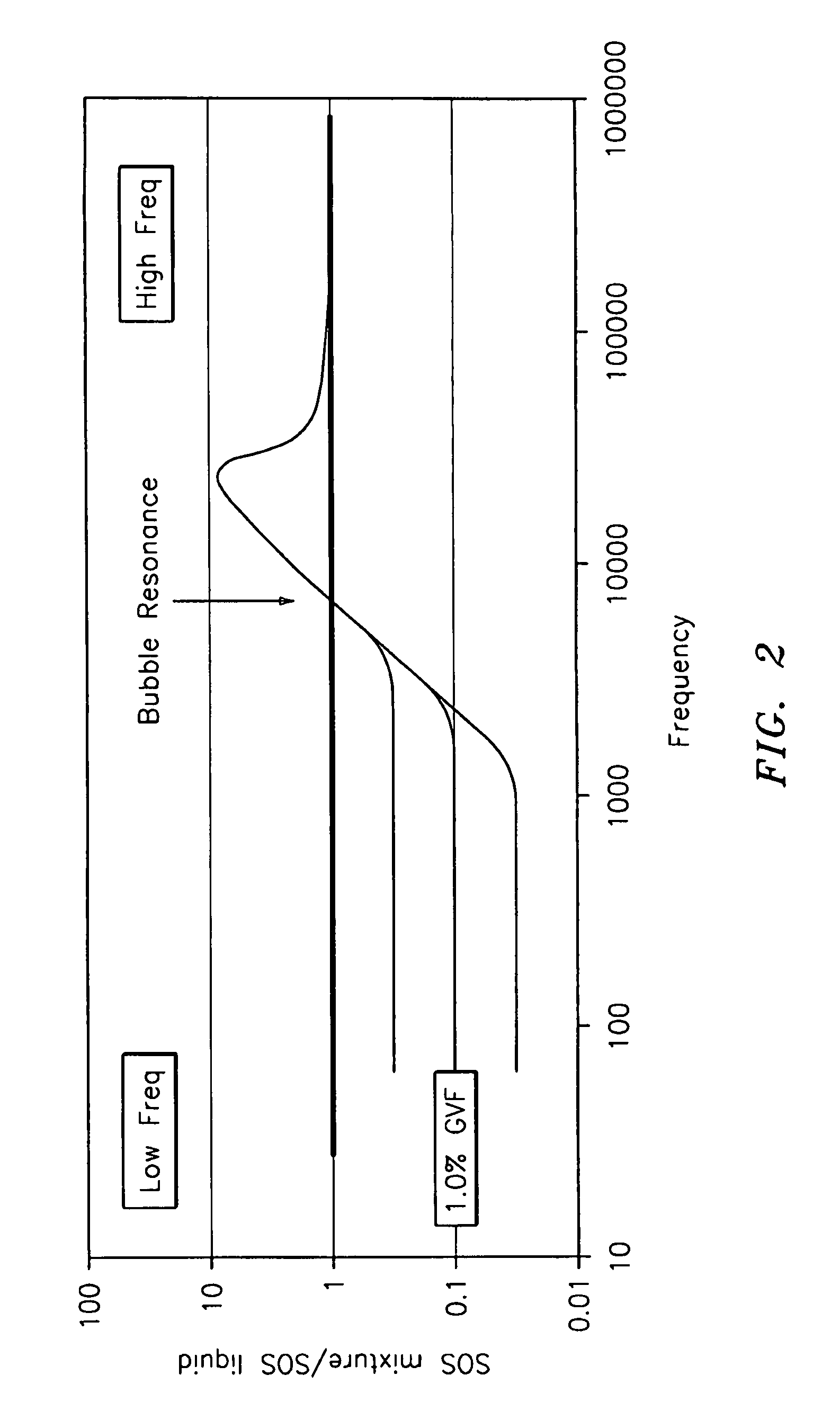
A flow measuring system combines a density measuring device and a device for measuring the speed of sound (SOS) propagating through the fluid flow and / or for determining the gas volume fraction (GVF) of the flow. The GVF meter measures acoustic pressures propagating through the fluids to measure the speed of sound αmix propagating through the fluid to calculate at least gas volume fraction of the fluid and / or SOS. In response to the measured density and gas volume fraction, a processing unit determines the density of non-gaseous component of an aerated fluid flow. For three phase fluid flows, the processing unit can determine the phase fraction of the non-gaseous components of the fluid flow. The gas volume fraction (GVF) meter may include a sensing device having a plurality of strain-based or pressure sensors spaced axially along the pipe for measuring the acoustic pressures propagating through the flow.
Owner:EXPRO METERS
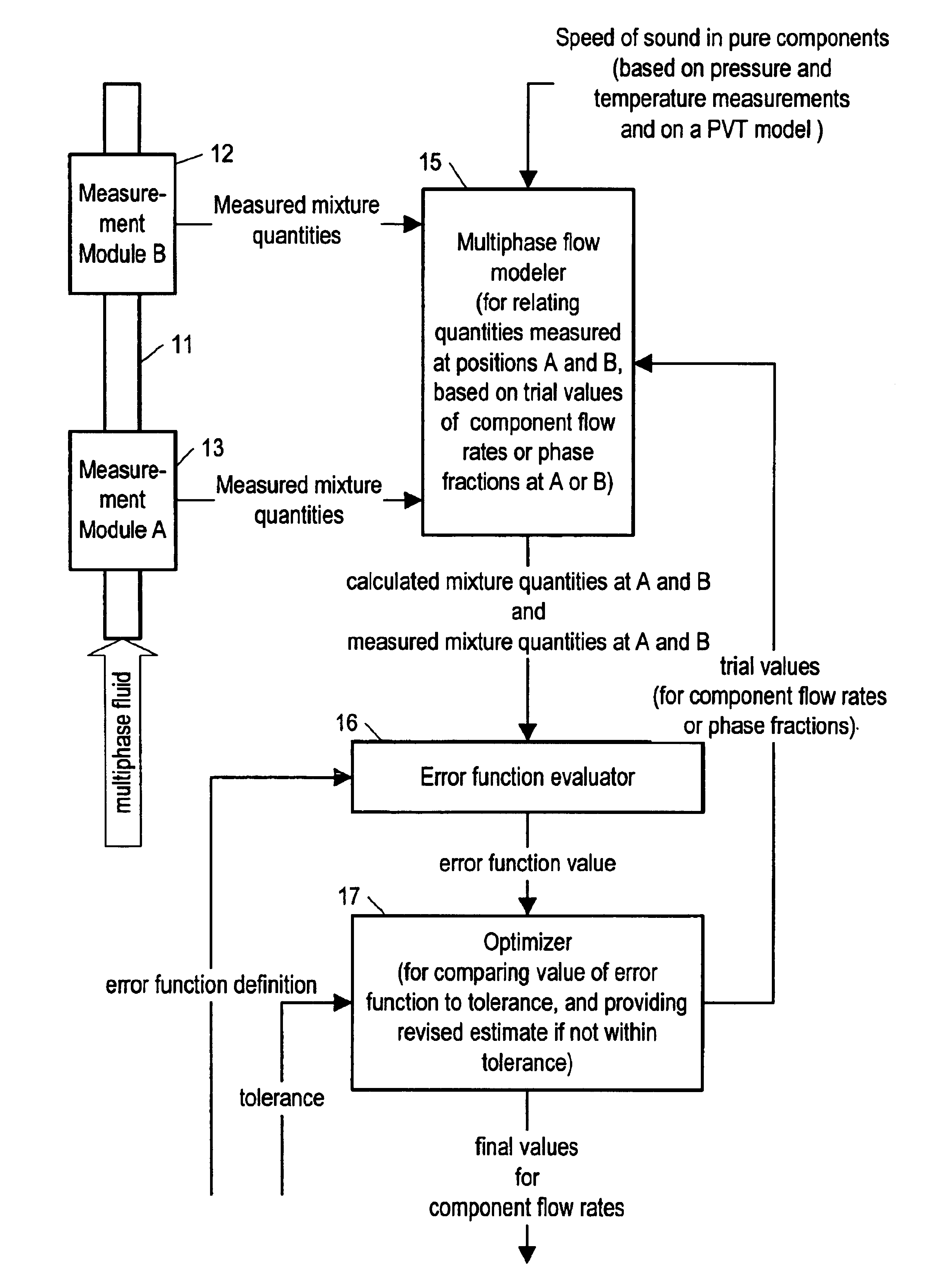
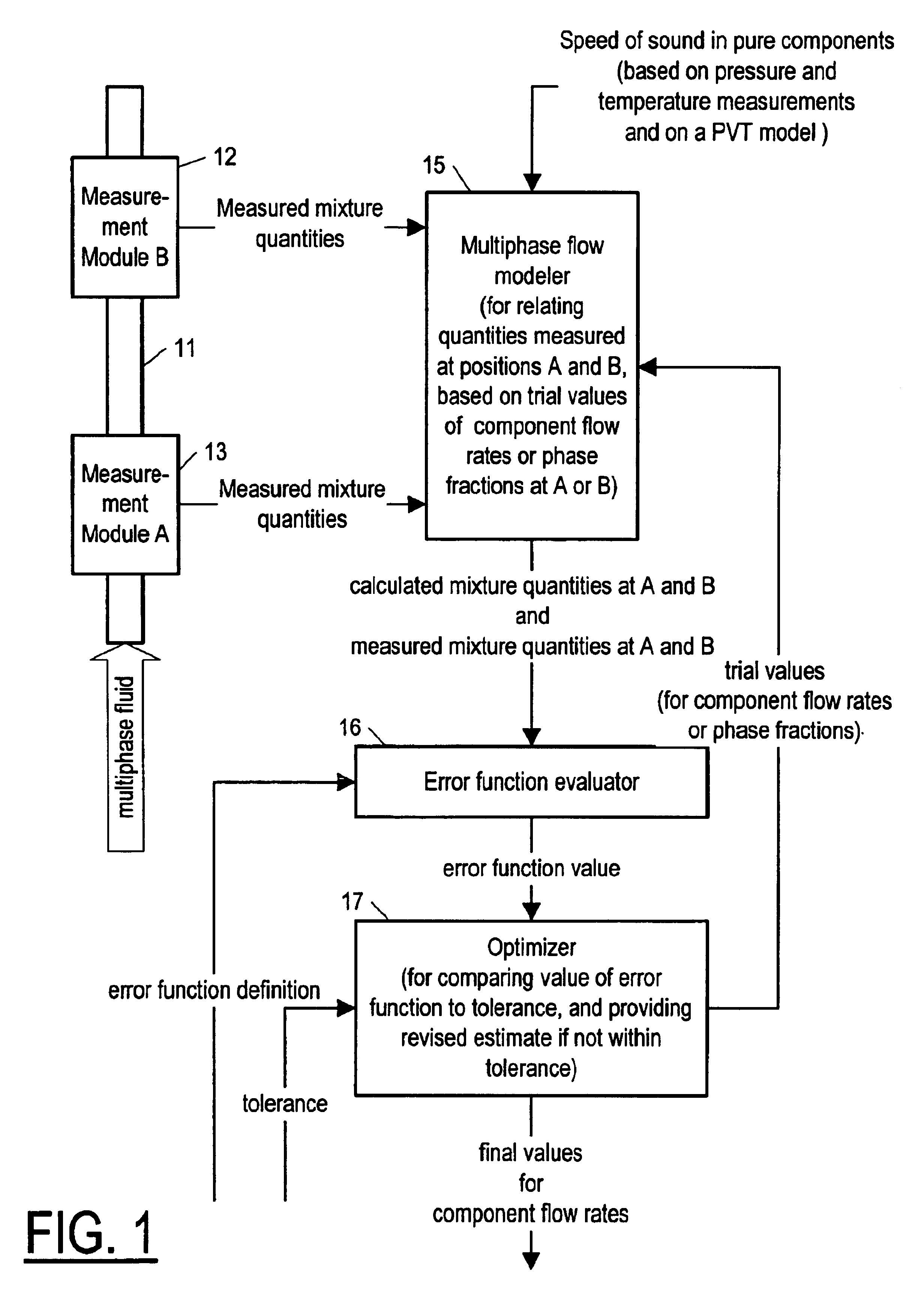
Method and apparatus for determining component flow rates for a multiphase flow
An apparatus and corresponding method for determining component flow rates of a multiphase fluid in a conduit, the fluid consisting of at least three known components, the method including the steps of: measuring at each of two different positions along the conduit at least four mixture quantities, typically the sound speed, the flow velocity of the multiphase fluid, the pressure and the temperature; providing a speed of sound in each of the components at the measured pressures and temperatures; providing a trial value for each of either the component flow rates or the phase fractions; using a predetermined model to calculate values for the measured mixture quantities based on the trial values for each of either the component flow rates or the phase fractions; using a predetermined error function to determine an error value; and using a predetermined optimizing algorithm to determine whether the calculated values are acceptable, and, if they are not, to provide a new trial value for each of either the component flow rates or the phase fractions. In some applications, the error function is the sum of the squares of the difference between the measured and calculated values at each point.
Owner:WEATHERFORD TECH HLDG LLC
Apparatus and method for providing a density measurement augmented for entrained gas
ActiveUS20050061060A1Improve accuracySpecific gravity using flow propertiesVolume/mass flow by dynamic fluid flow effectThree-phaseDischarge measurements
A flow measuring system combines a density measuring device and a device for measuring the speed of sound (SOS) propagating through the fluid flow and / or for determining the gas volume fraction (GVF) of the flow. The GVF meter measures acoustic pressures propagating through the fluids to measure the speed of sound αmix propagating through the fluid to calculate at least gas volume fraction of the fluid and / or SOS. In response to the measured density and gas volume fraction, a processing unit determines the density of non-gaseous component of an aerated fluid flow. For three phase fluid flows, the processing unit can determine the phase fraction of the non-gaseous components of the fluid flow. The gas volume fraction (GVF) meter may include a sensing device having a plurality of strain-based or pressure sensors spaced axially along the pipe for measuring the acoustic pressures propagating through the flow.
Owner:EXPRO METERS
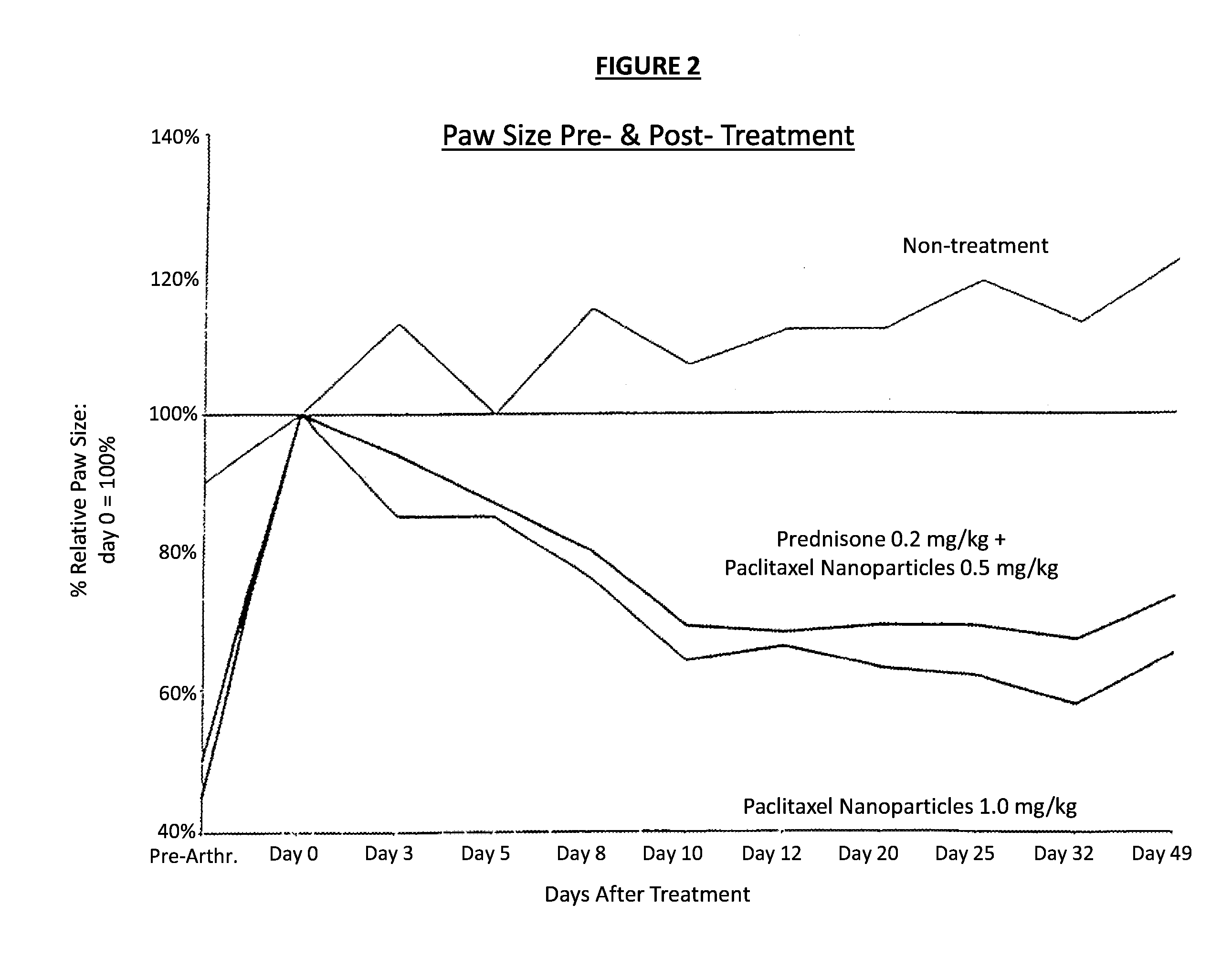
Formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS8853260B2Improve abilitiesPromote formationOrganic active ingredientsBiocideSuspended particlesWater insoluble
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
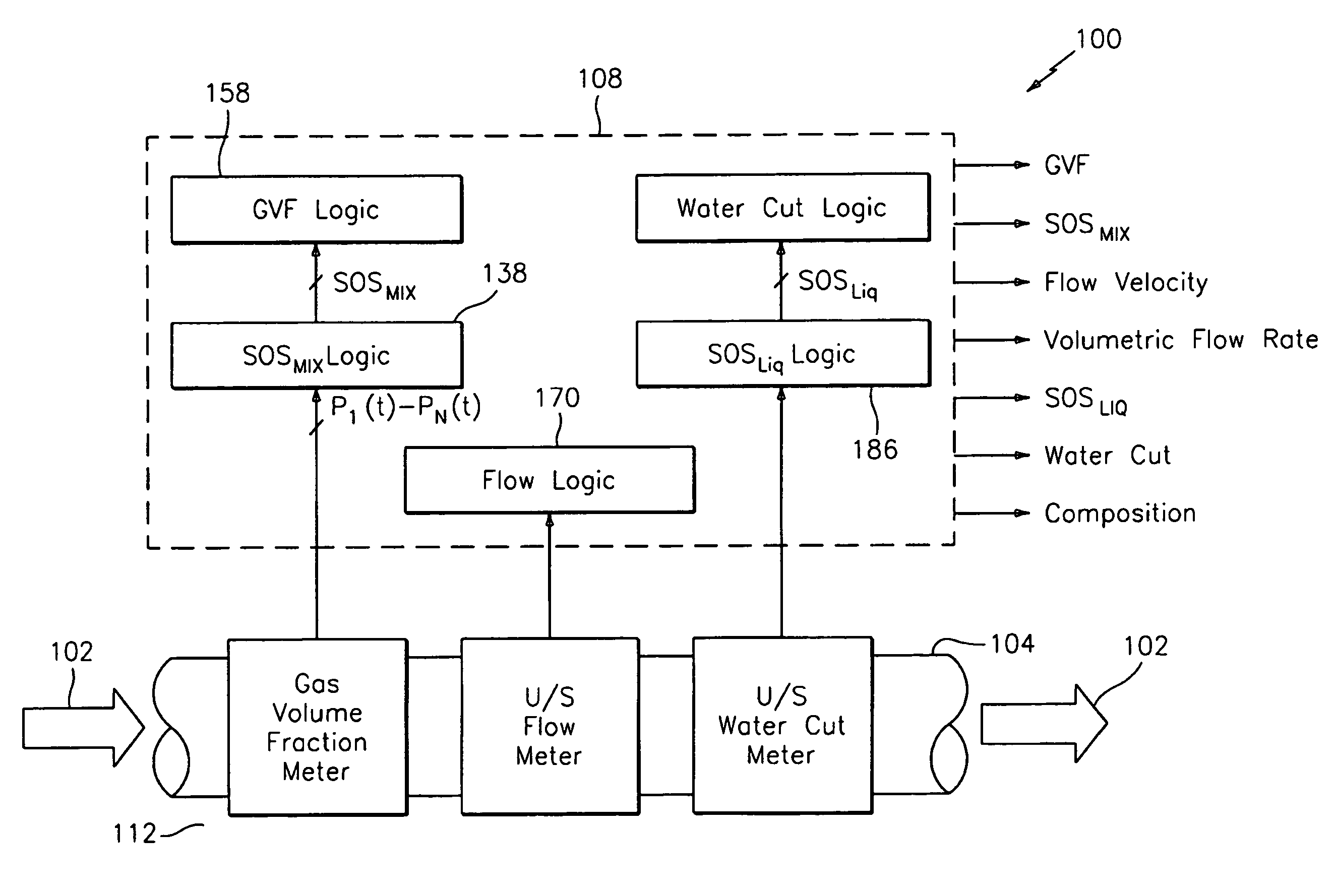
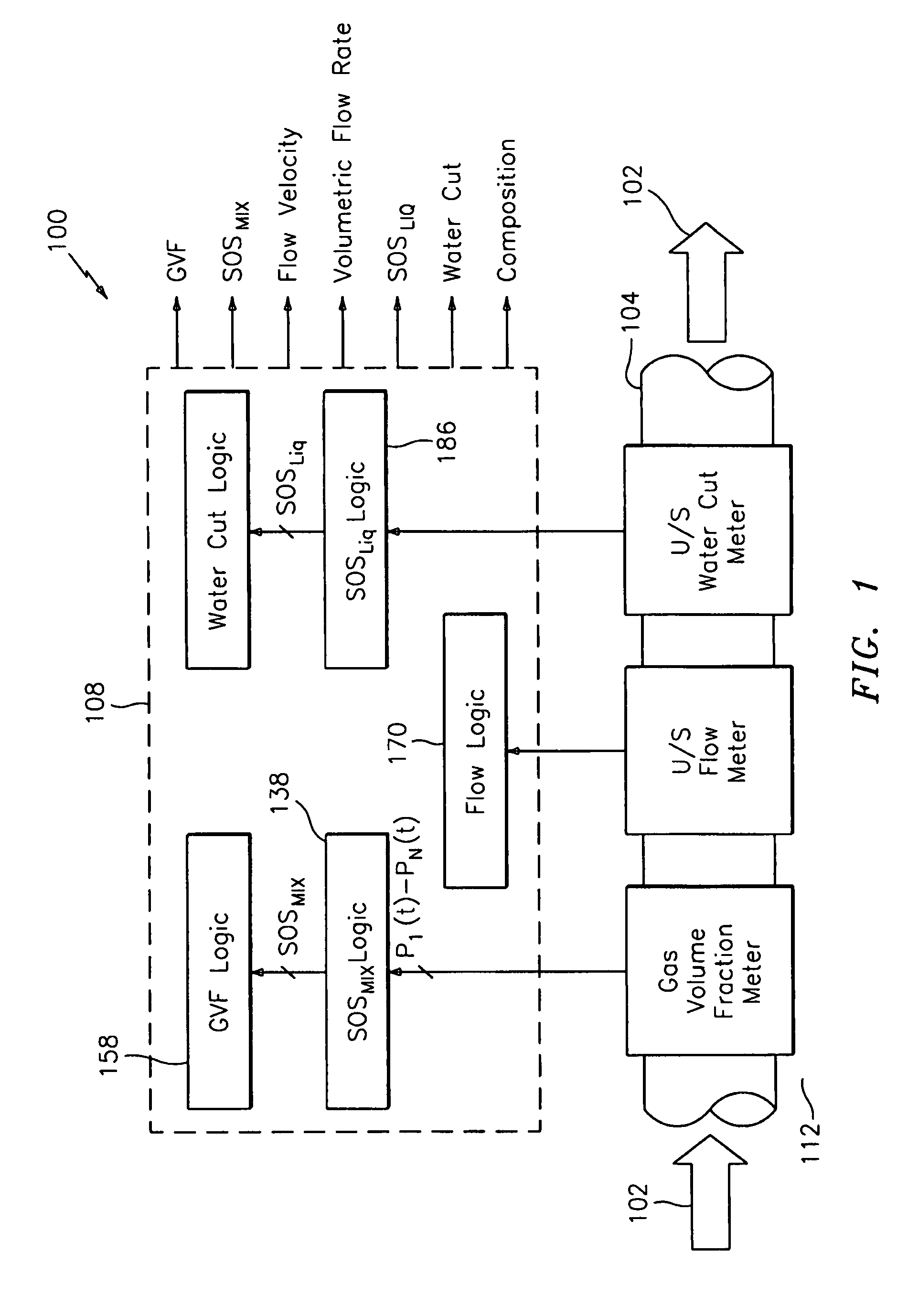
Apparatus and method for measuring a parameter of a multiphase flow
ActiveUS20070157737A1Volume meteringVolume/mass flow by dynamic fluid flow effectStream flowEngineering
An apparatus is provided that determines a characteristic of a multiphase fluid, such as an aerated oil and water fluid, flowing within a pipe. The apparatus includes a fluid flow meter, a water cut meter, and a density meter, wherein the density meter determines the density of the fluid flow to determine the gas volume (or void) fraction of the multiphase fluid flow. The output signal of each of the meters is provided to a multiphase flow model to provide a plurality of multiphase parameters, such as phase fraction, volumetric flow, mass flow of each of the phases of the multiphase mixture, optimized for various flow conditions. Each of the meters may be secured to the outer surface of the pipe using various means, such a clamping means.
Owner:EXPRO METERS
Novel formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS20070087022A1Eliminate side effectsHigh anticancer activityOrganic active ingredientsBiocideSuspended particlesActive agent
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Novel formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS20070093547A1Eliminate side effectsHigh anticancer activityBiocideOrganic active ingredientsSuspended particlesFree protein
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Water detection and 3-phase fraction measurement systems
ActiveUS20070114372A1Radiation pyrometryVolume/mass flow measurementWater detectorUltrasound attenuation
Methods and apparatus enable monitoring a hydrocarbon well for water within a flow stream of the well. A water detector includes a light source for emitting into a flow stream infrared light that includes a water absorbent wavelength band. A detector detects attenuation of the water absorbent wavelength band upon the infrared radiation passing through at least a portion of the flow stream. The water detector outputs a presence of water and / or a phase fraction or quantification of water as determined based on the attenuation. Detecting attenuation of a substantially transmissive wavelength band with respect to water simultaneously with detection of the attenuation of the water absorbent wavelength band can enable correction for non-wavelength dependent attenuation.
Owner:WEATHERFORD TECH HLDG LLC
Translucent or opaque colored glass-ceramic article providing a cooking surface and its use
InactiveUS20050252503A1Minimizing contentEasy resistanceCooking-vessel materialsStoves/ranges foundationsChemical reactionAdditive ingredient
The translucent or opaque colored glass-ceramic article provides a cooking surface and has an adjustable light transmission in a visible range under 15%, as measured for a 4 mm sample thickness; a flaw-free upper surface with an impact resistance of greater than 18 cm breaking height, as tested with a 200 g steel ball in a falling ball test; a temperature difference resistance of greater than 500° C.; a high crystallinity with keatite mixed crystals as principal crystal phase in an interior of the glass-ceramic article and with a residual glass phase fraction of less than 8% by weight; a glassy upper surface layer of from 0.5 to 2.5 μm thick, which is substantially free of high quartz mixed crystals and which inhibits chemical reactions, and a content of enriched ingredients in the residual glass phase in the interior of the glass-ceramic and in the glassy surface layer of ΣNa2O+K2O+CaO+SrO+BaO+F+refining agents of from 0.2 to 1.6% by weight.
Owner:SCHOTT AG
Novel formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS20080160095A1Reduce morbidityLow toxicityBiocideOrganic active ingredientsSuspended particlesWater insoluble
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Novel formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS20080161382A1Eliminate side effectsHigh anticancer activityBiocideOrganic active ingredientsSuspended particlesWater insoluble
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS8137684B2Improve abilitiesPromote formationBiocideOrganic active ingredientsSuspended particlesActive agent
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Novel formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS20070092563A1Improve abilitiesPromote formationOrganic active ingredientsBiocideSuspended particlesActive agent
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
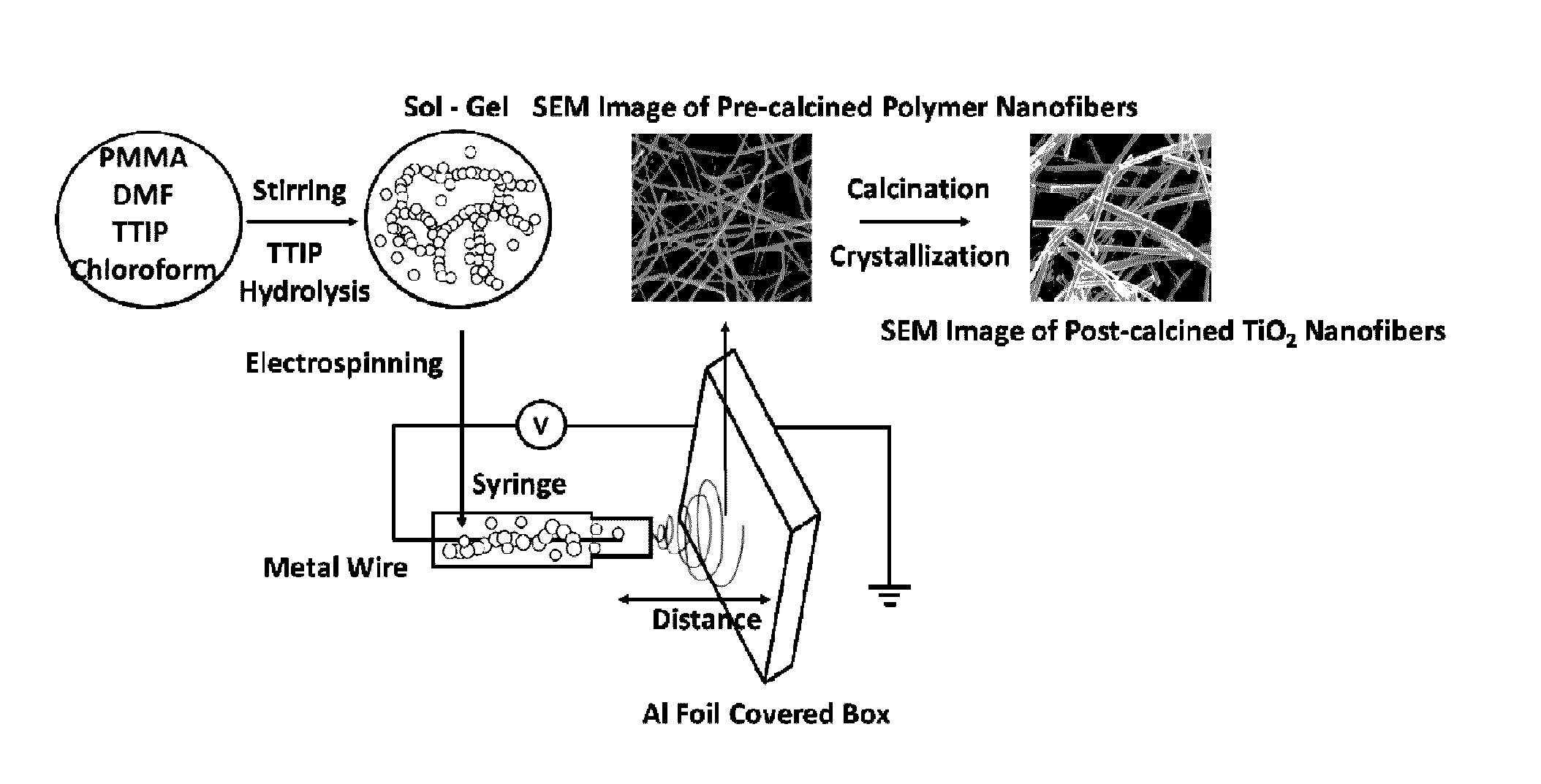
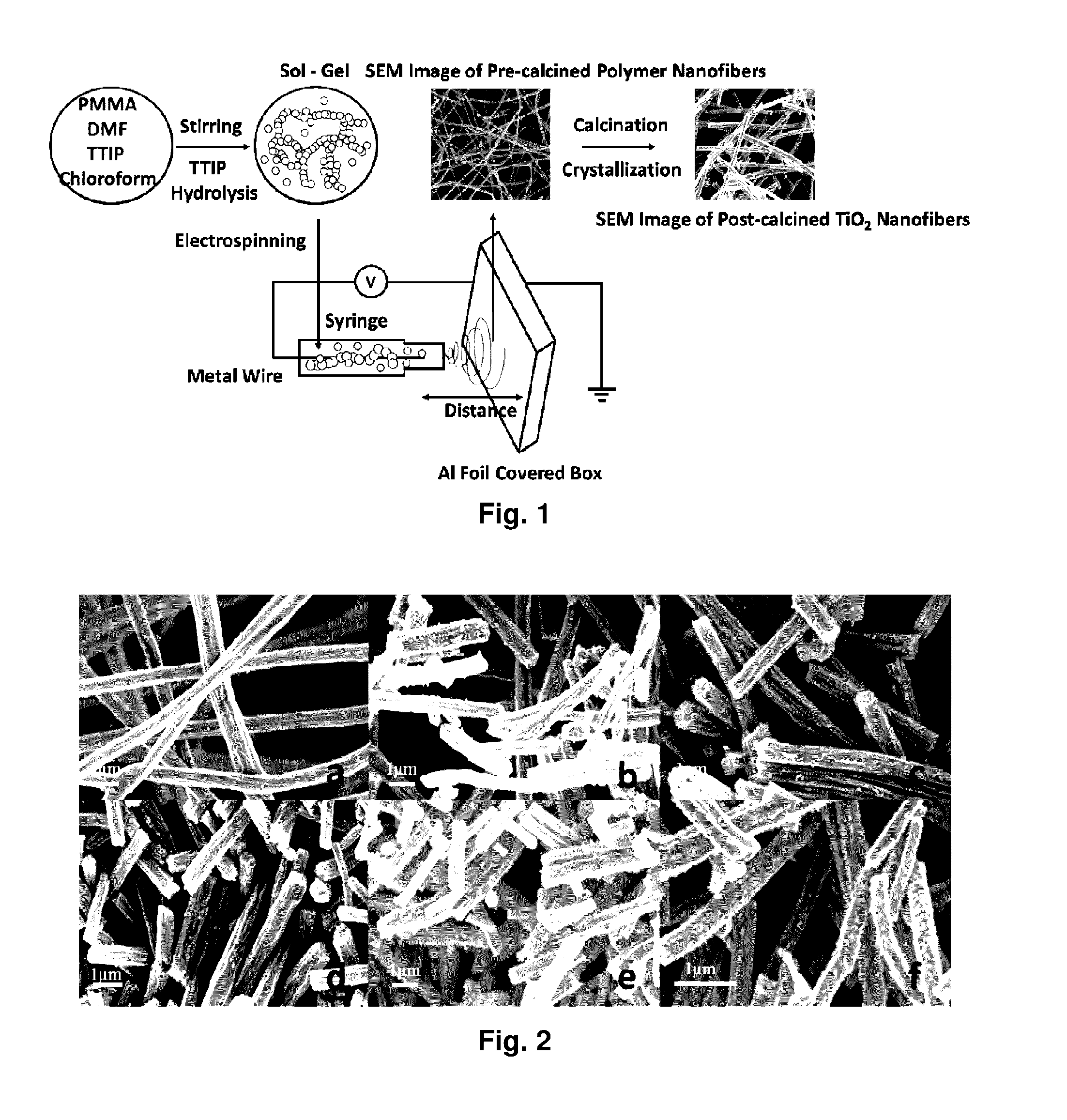
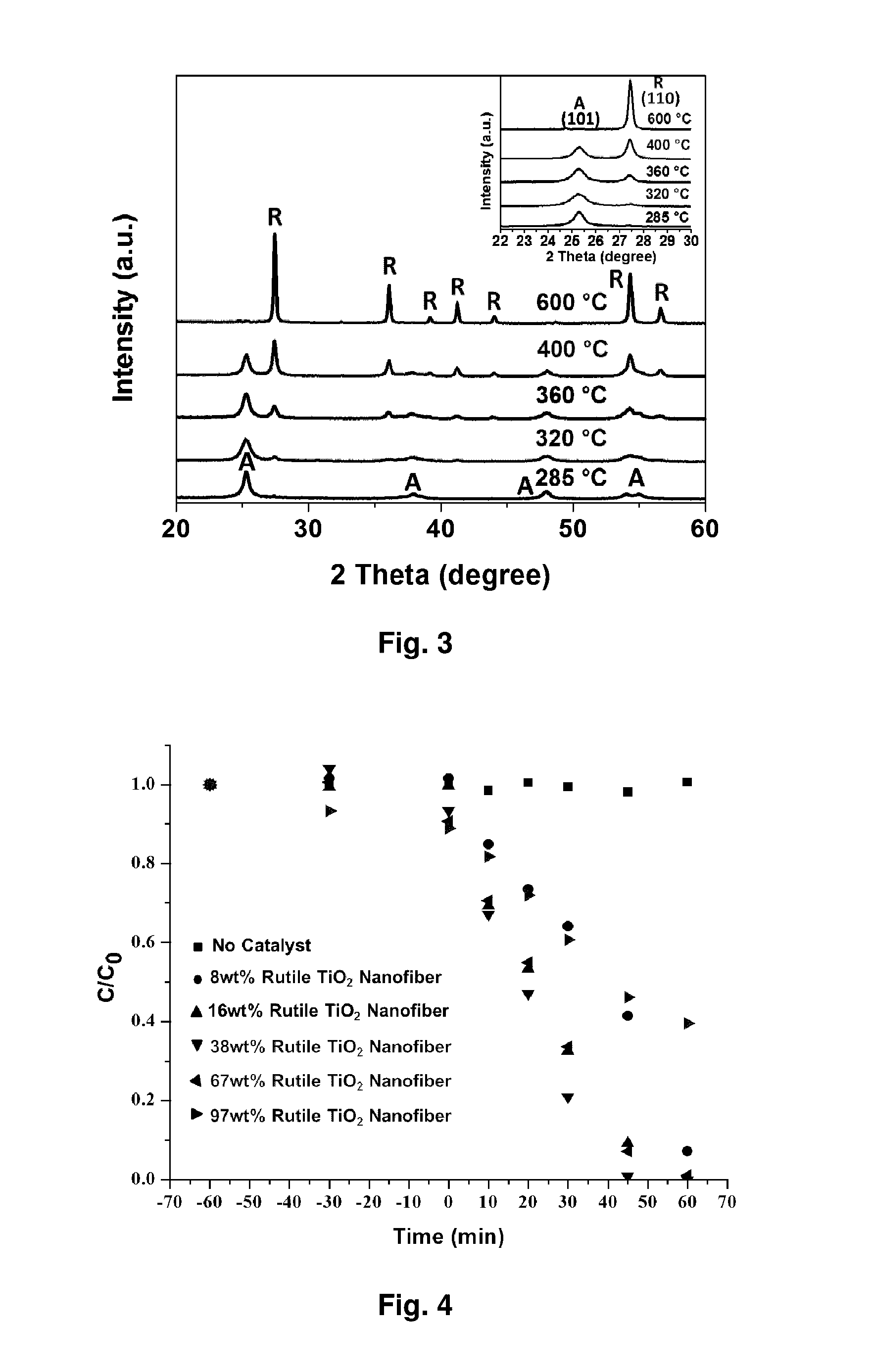
Metal oxide nanofibrous materials for photodegradation of environmental toxins
ActiveUS20170056873A1Rapid and inexpensiveAccelerated photodegradationWater/sewage treatment by irradiationWater treatment compoundsFiberRutile
Mixed-phase TiO2 nanofibers prepared via a sol-gel technique followed by electrospinning and calcination are provided as photocatalysts. The calcination temperature is adjusted to control the rutile phase fraction in TiO2 nanofibers relative to the anatase phase. Post-calcined TiO2 nanofibers composed of 38 wt % rutile and 62 wt % anatase exhibited the highest initial rate constant of UV photocatalysis. This can be attributed to the combined influences of the fibers' specific surface areas and their phase compositions.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
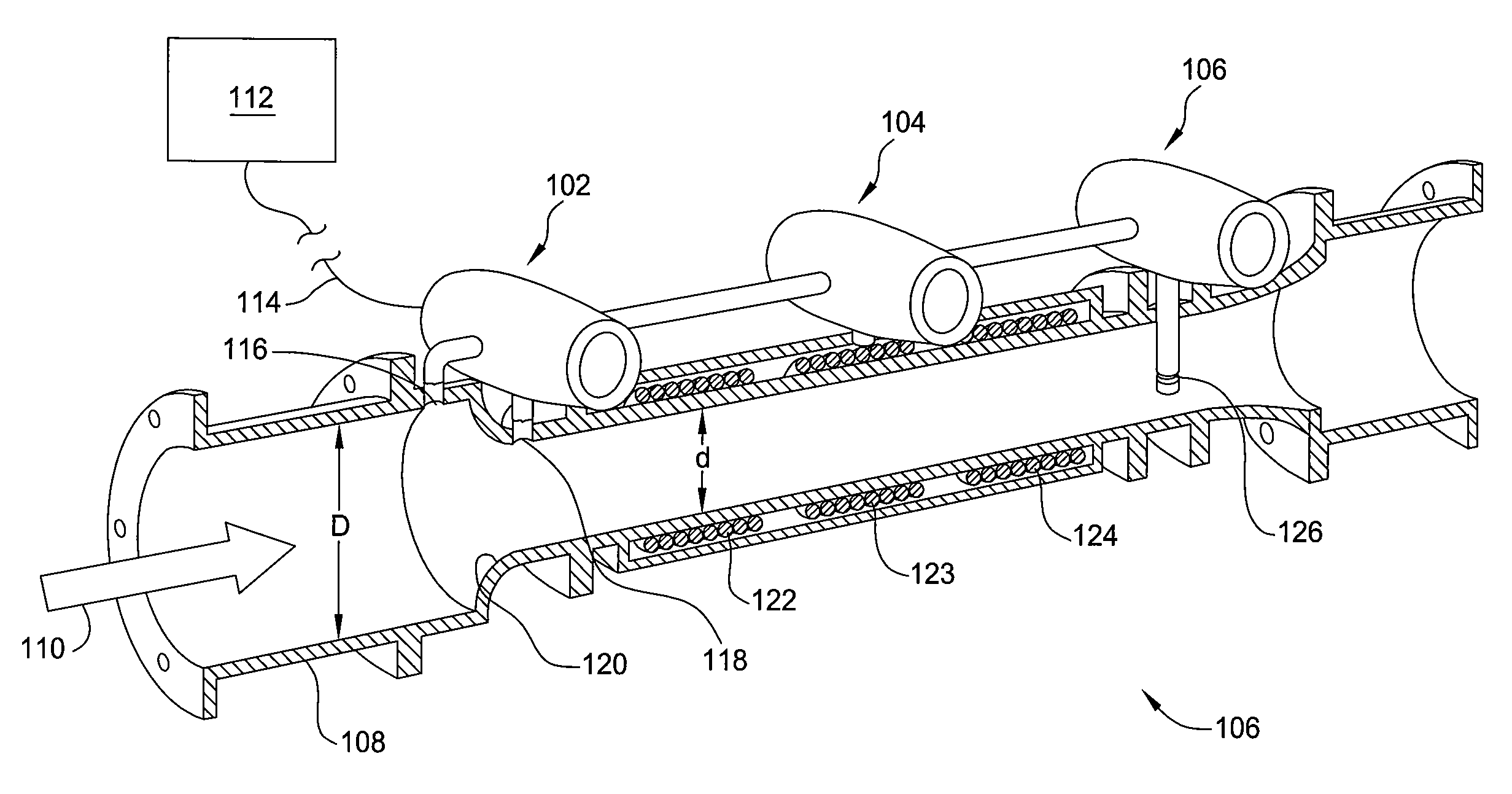
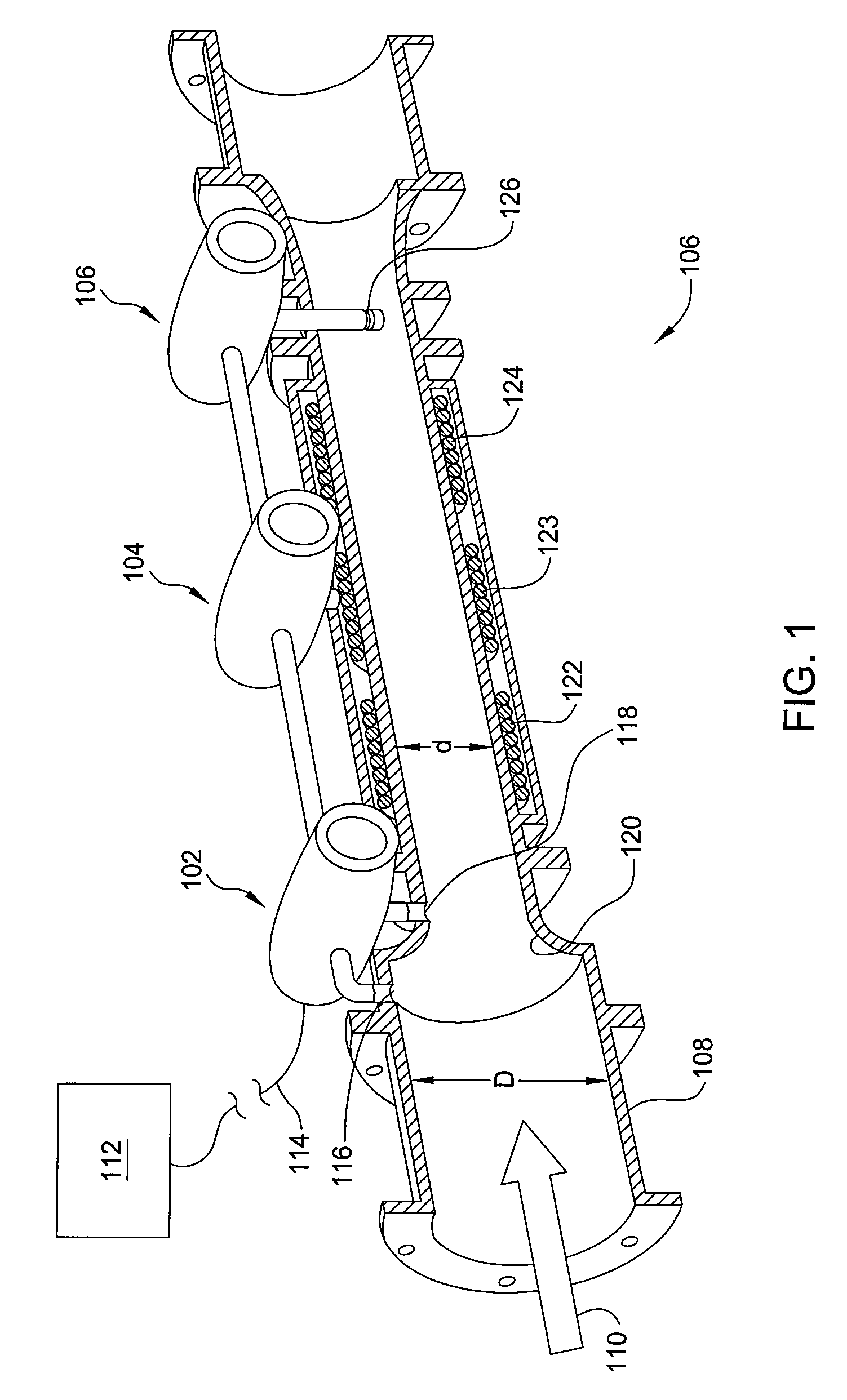
Multiphase flow meter for subsea applications using hydrate inhibitor measurement
ActiveUS20120046870A1Electric/magnetic detection for well-loggingSurveyDifferential pressureWater flow
Methods and apparatus for determining phase fractions (relative concentrations) within a multiphase fluid mixture, in the presence of an injected hydrate inhibitor. Combining this phase fraction information with a hydrate inhibitor injection rate (HIIR) enables resolving oil and water flow rates for the phase fractions. The liquid flow rates and a total combined flow rate of the fluid mixture—determined based on a differential pressure of the fluid mixture through a given area—enable resolving a gas flow rate.
Owner:WEATHERFORD TECH HLDG LLC
Novel formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS20070116761A1Reduce morbidityReduce decreaseBiocidePowder deliverySuspended particlesActive agent
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Novel formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS20150104521A1Improve abilitiesPromote formationBiocideOrganic active ingredientsSuspended particlesWater insoluble
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
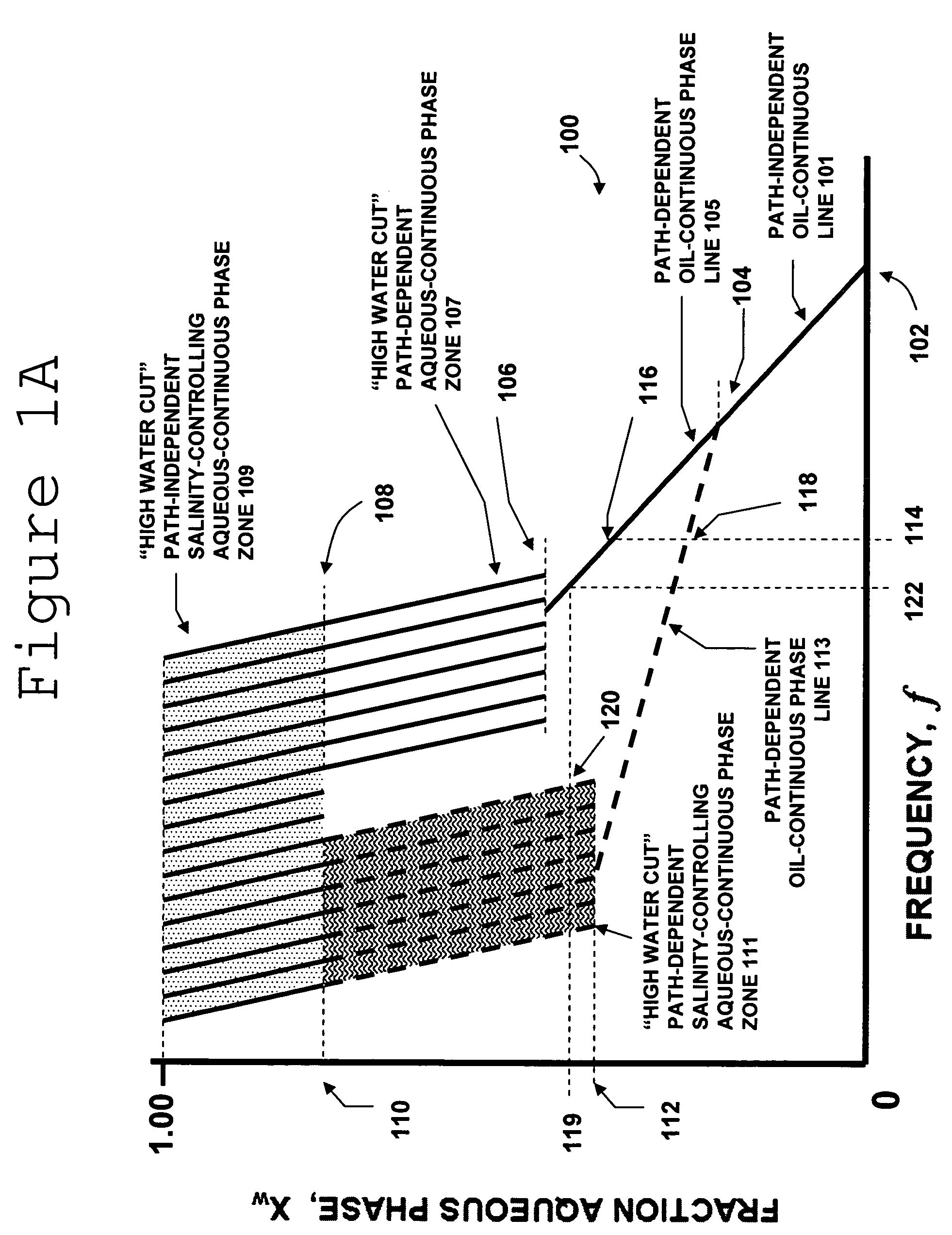
Multiphase fluid characterization
ActiveUS7523647B2Accurate measurementGreat advantageMaterial analysis by electric/magnetic meansSpecific gravity using flow propertiesPermittivityPetroleum oil
Methods for determining and validating a first phase fraction of a gravitationally-separated multiphase fluid by conducting physical and electrical property measurements on samples of the fluid. The water content of crude petroleum oil is determined after the oil and water have begun to separate into phase layers such as occurs during un-agitated holding of the crude petroleum oil. A series of measurements of electrical properties such as permittivity and physical properties such as density is collected. The density minima can be used to generate hindsight determinations of average properties, such as the dry oil phase density, which in turn can be used to increase the accuracy of the water percentage in the oil determined by the permittivity and density methods. Flow weighted averages of water percentages by each method can be used to determine and validate the water content of the crude petroleum oil.
Owner:PHASE DYNAMICS
Apparatus and method for measuring a parameter of a multiphase flow
An apparatus is provided that determines a characteristic of a multiphase fluid, such as an aerated oil and water fluid, flowing within a pipe. The apparatus includes a fluid flow meter, a water cut meter, and a density meter, wherein the density meter determines the density of the fluid flow to determine the gas volume (or void) fraction of the multiphase fluid flow. The output signal of each of the meters is provided to a multiphase flow model to provide a plurality of multiphase parameters, such as phase fraction, volumetric flow, mass flow of each of the phases of the multiphase mixture, optimized for various flow conditions. Each of the meters may be secured to the outer surface of the pipe using various means, such a clamping means.
Owner:EXPRO METERS
System for measuring phase fraction and phase interface in multiphase pipe flow by using monofilament capacitance probe
InactiveCN1865966AEliminate the effects ofRealize continuous real-time online measurementMaterial capacitanceEngineeringData treatment
The detection system for phase content rate and interface in multiphase tube flow comprises: a single-wire capacitor and probe device, and a connected C / V conversion circuit to convert the capacitor value of probe into dc voltage and send to following device, and a signal process device to control last circuit and process, display and store data. This invention eliminates effect of fluid electric feature to detection result, and realizes real-time and on-line detection with well precision.
Owner:XI AN JIAOTONG UNIV
Bodies coated with a hard material and method for the production thereof
ActiveUS20100233511A1High bonding strengthEasy to adaptNatural mineral layered productsChemical vapor deposition coatingMilling cutterCoating system
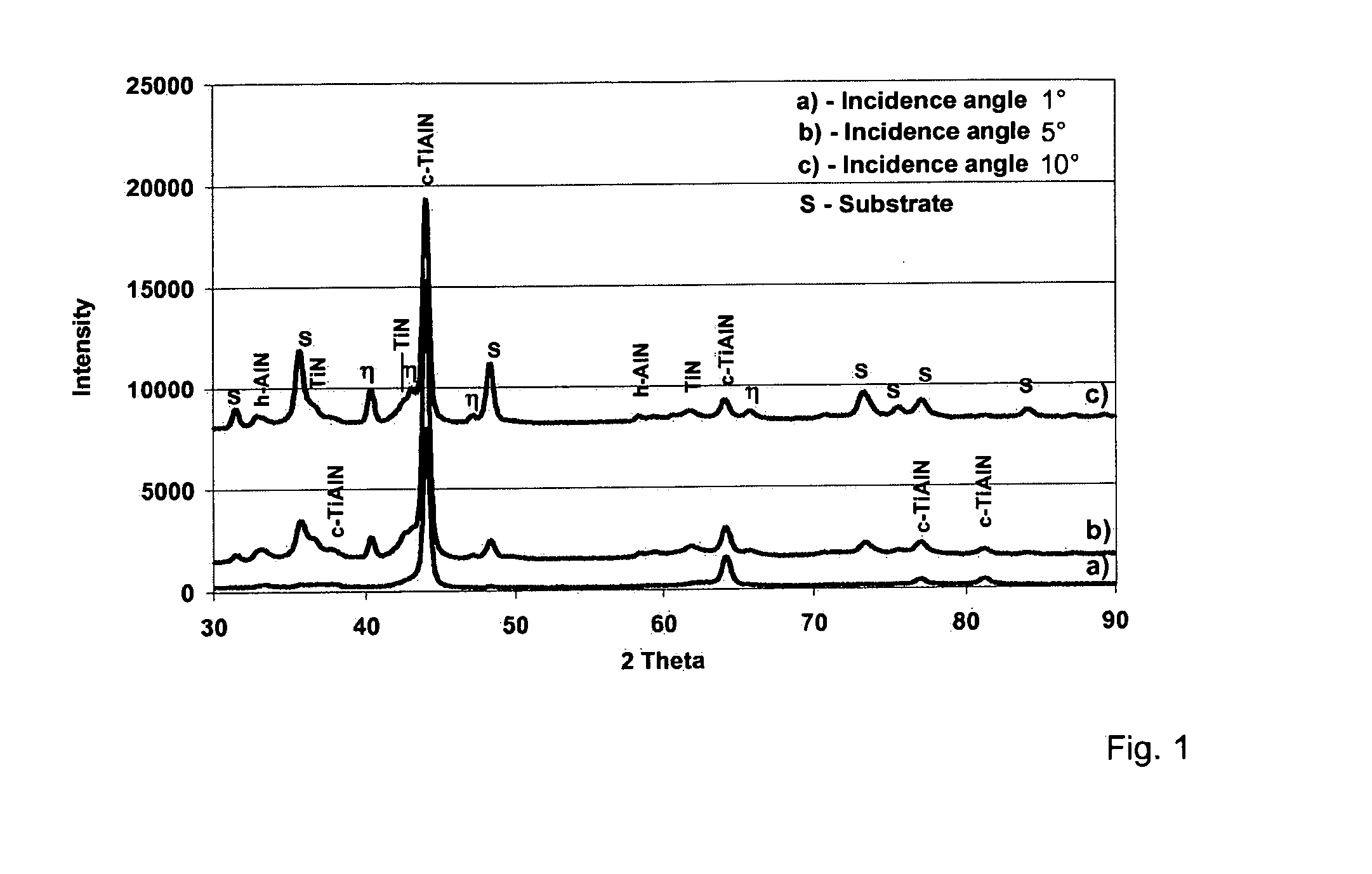
The invention relates to bodies coated with a hard material, comprising a multi-layer coating system containing at least one Ti1-xAlxN hard material coating and to a multi-stage CVD method for producing the bodies. The aim of the invention is to achieve excellent adhesion of the Ti1-xAlxN hard material coating in bodies coated with a hard material comprising a multi-layer coating system containing at least one Ti1-xAlxN hard material coating and a high degree of wear resistance. According to the invention, the bodies coated with a hard material comprising a multi-layer coating system containing at least one Ti1-xAlxN hard material coating are characterized by the following features: the coating system consists of a) a bonding coating applied to the body, consisting of TiN, Ti(C,N) or TiC; b) a phase gradient coating that is applied to the bonding coating; and c) the single or multi-phase Ti1-xAlxN hard material coating or coatings applied to the phase gradient coating. The phase gradient coating consists of a TiN / h-AlN phase mixture on the side facing the bonding coating and has an increasing phase fraction of fcc-TiAlN with an increasing coating thickness, (the fraction being >50%), towards the Ti1-xAlxN hard material coating(s) and a corresponding simultaneous decrease in the phase fractions of TiN and h-AlN. The coating according to the invention can be used in particular for tools and components consisting of steel, hard metals, cermets and ceramics, such as for example drills, milling cutters and indexable cutting inserts.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
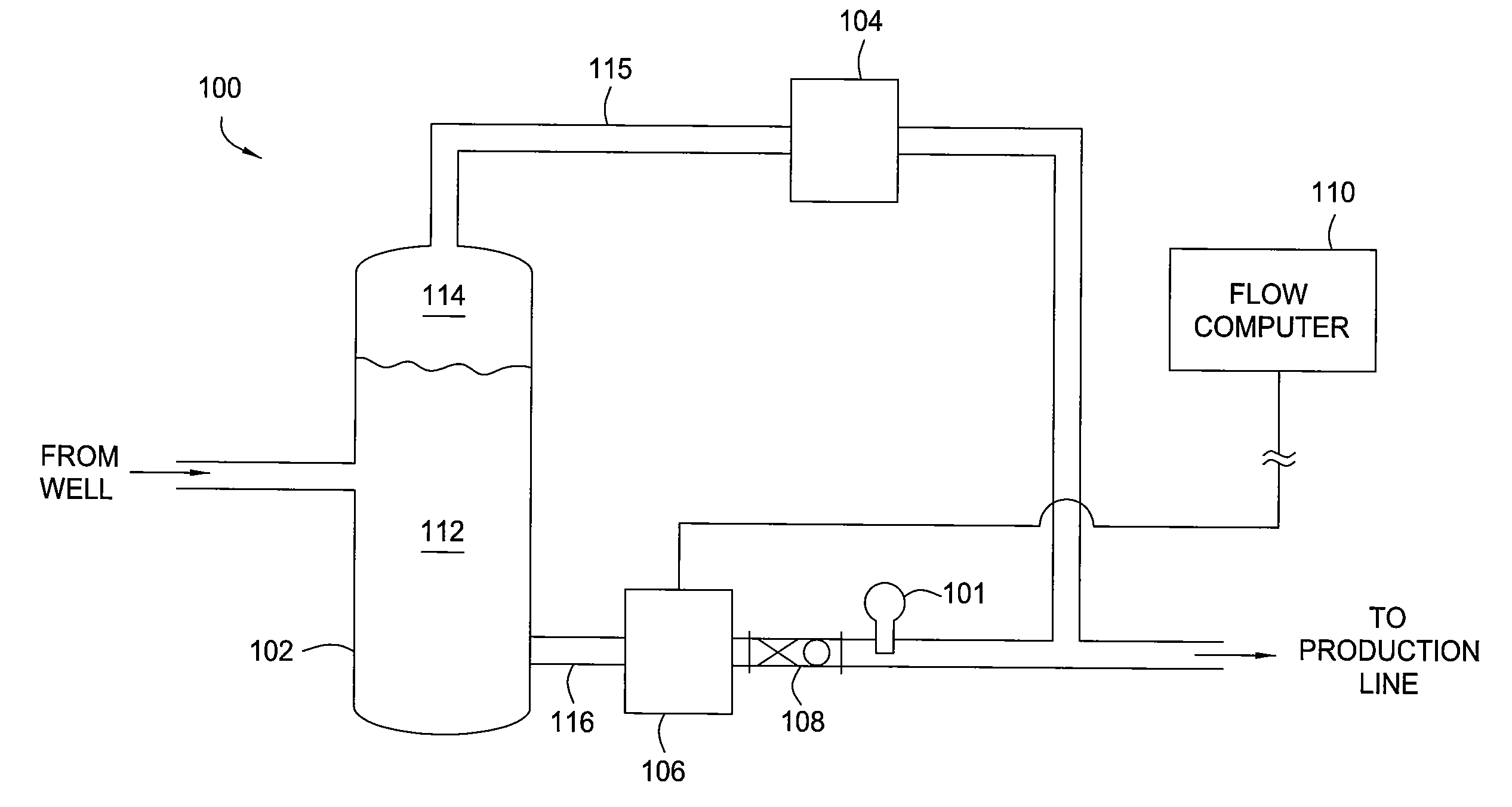
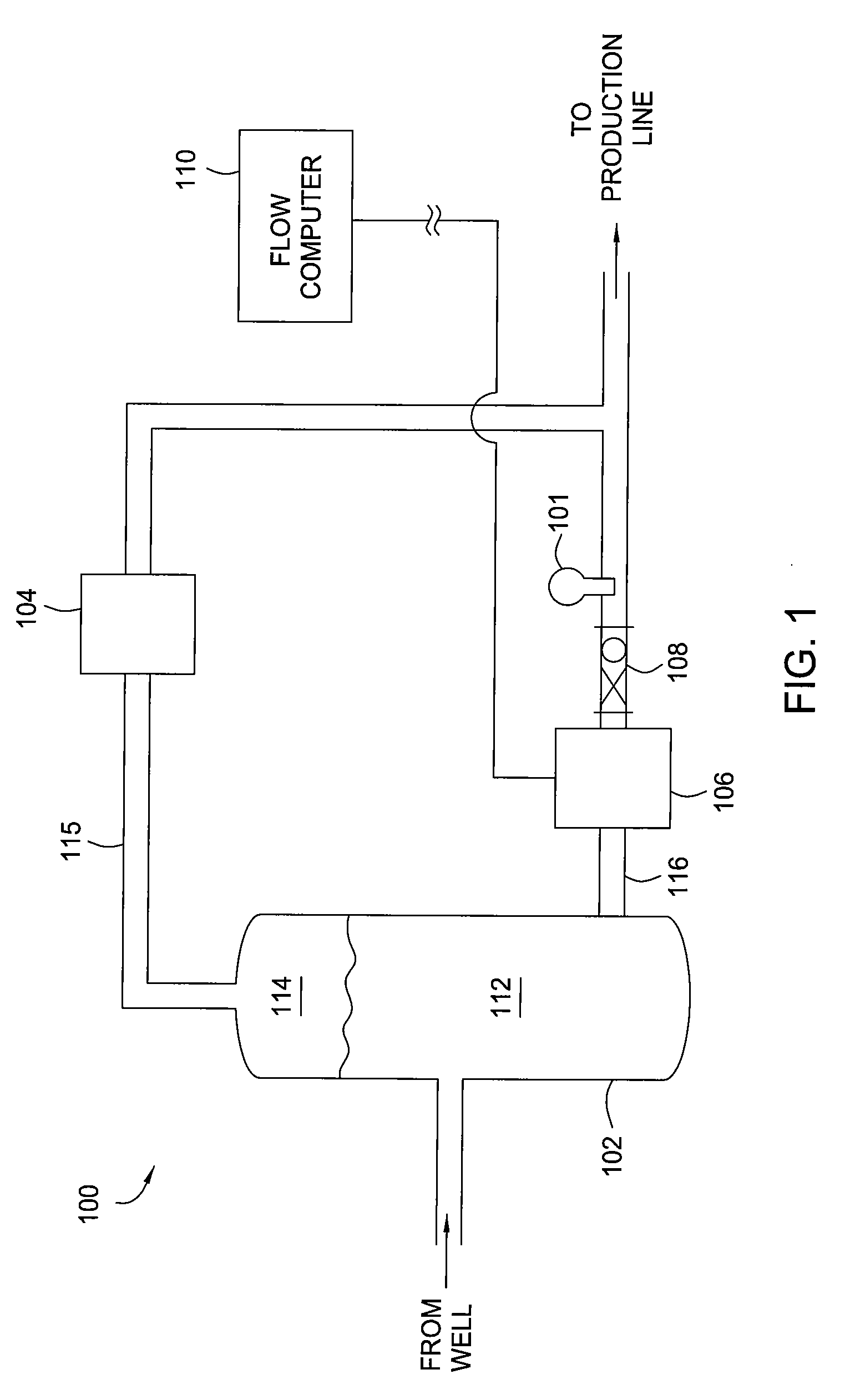
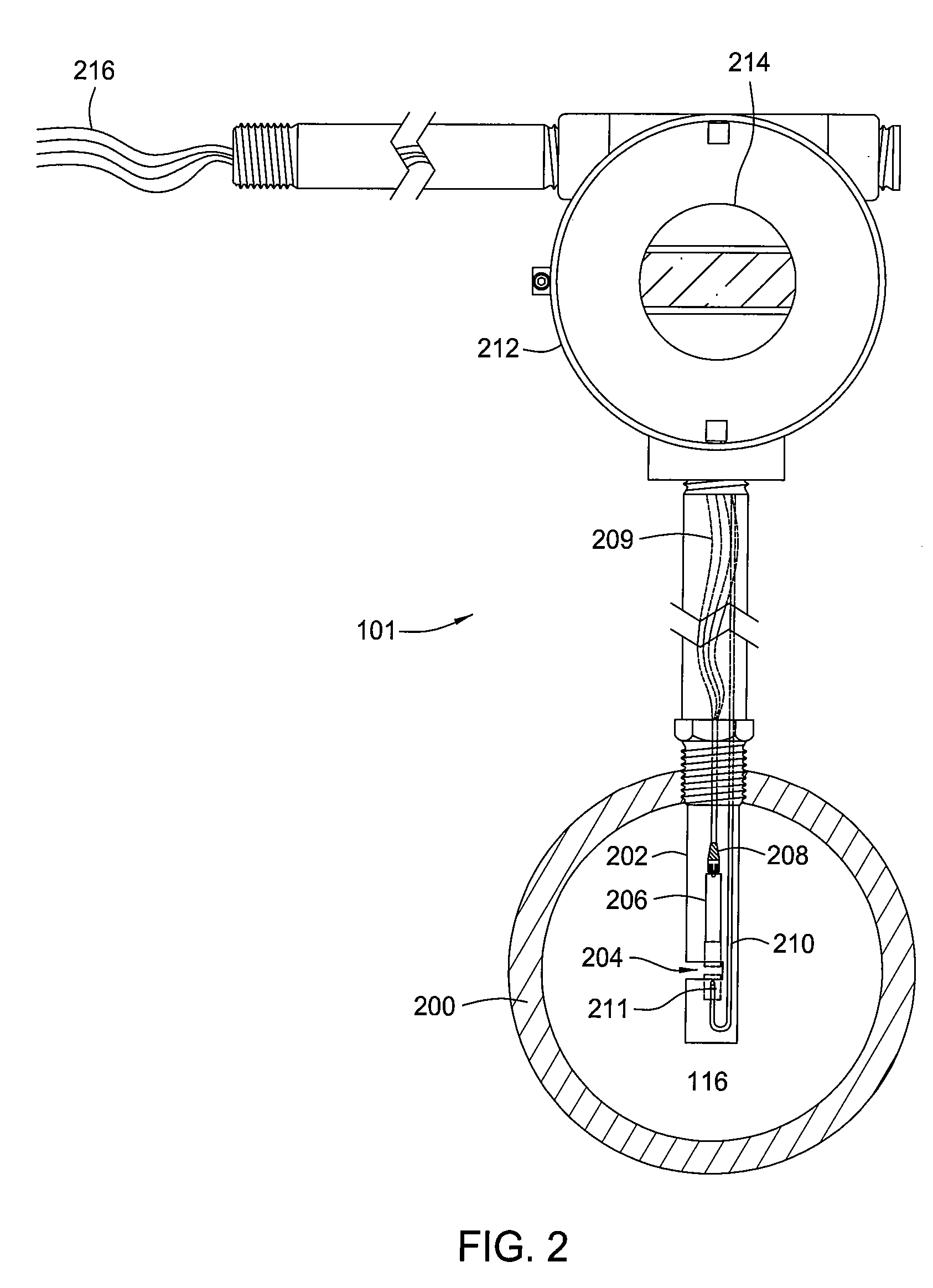
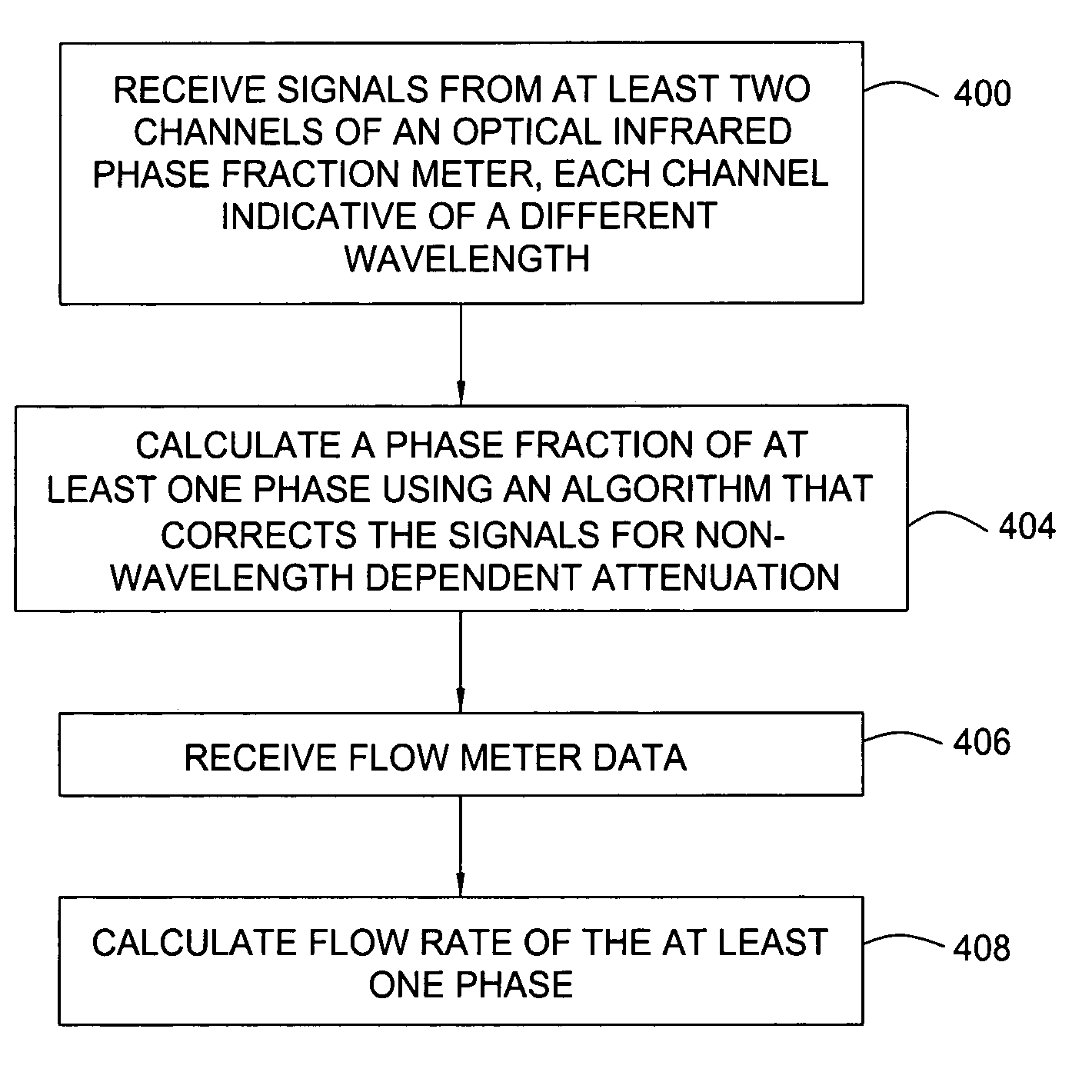
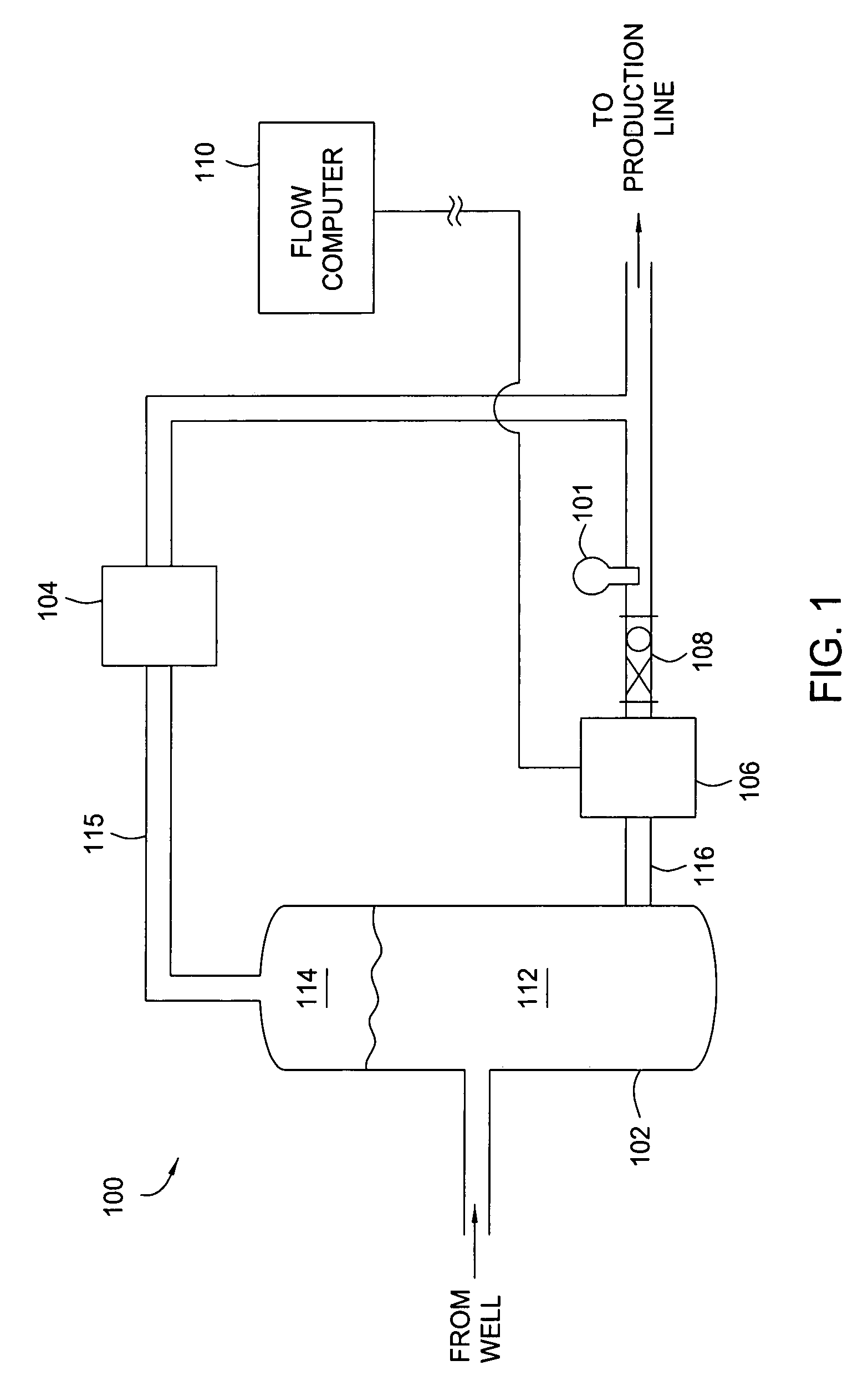
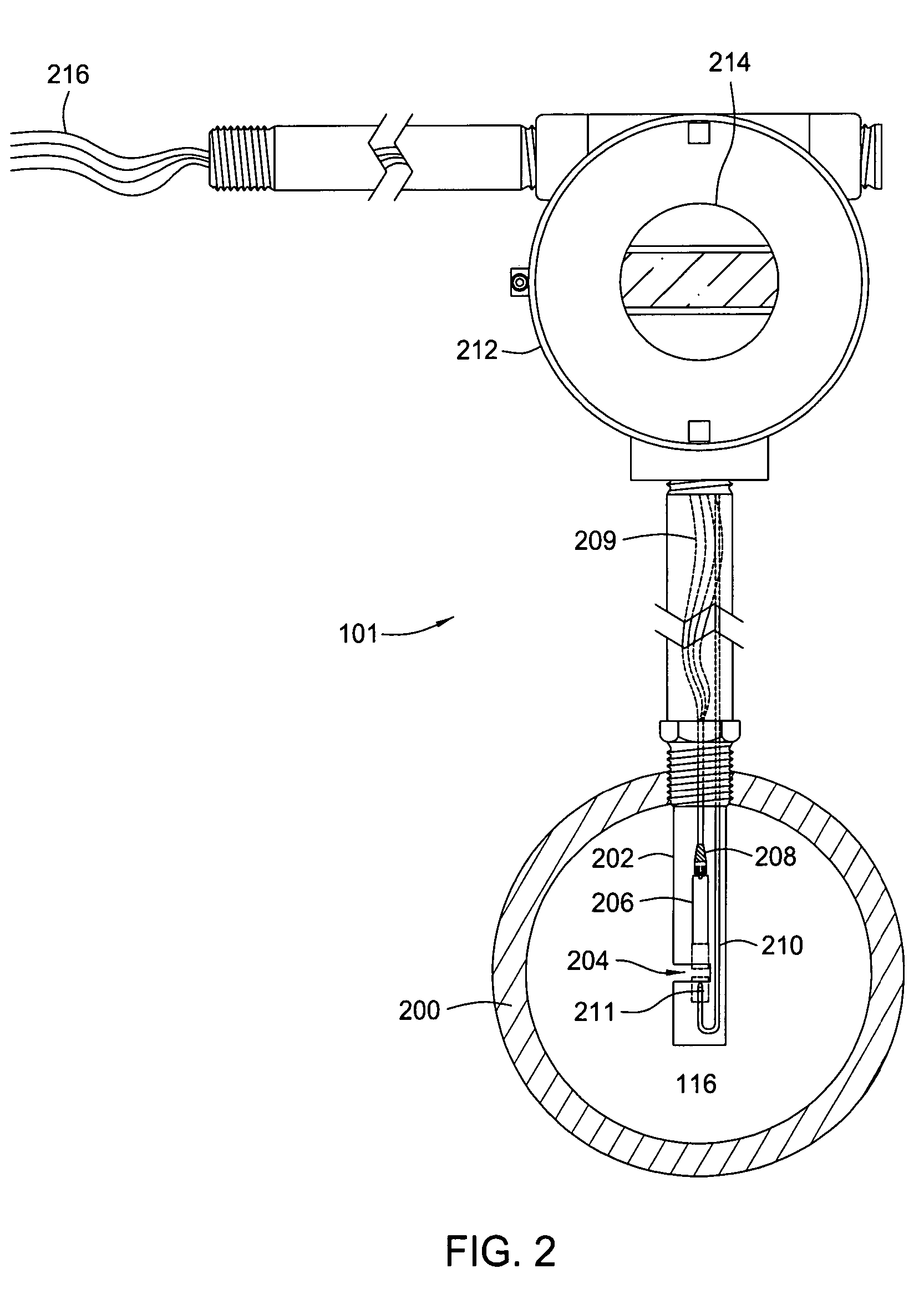
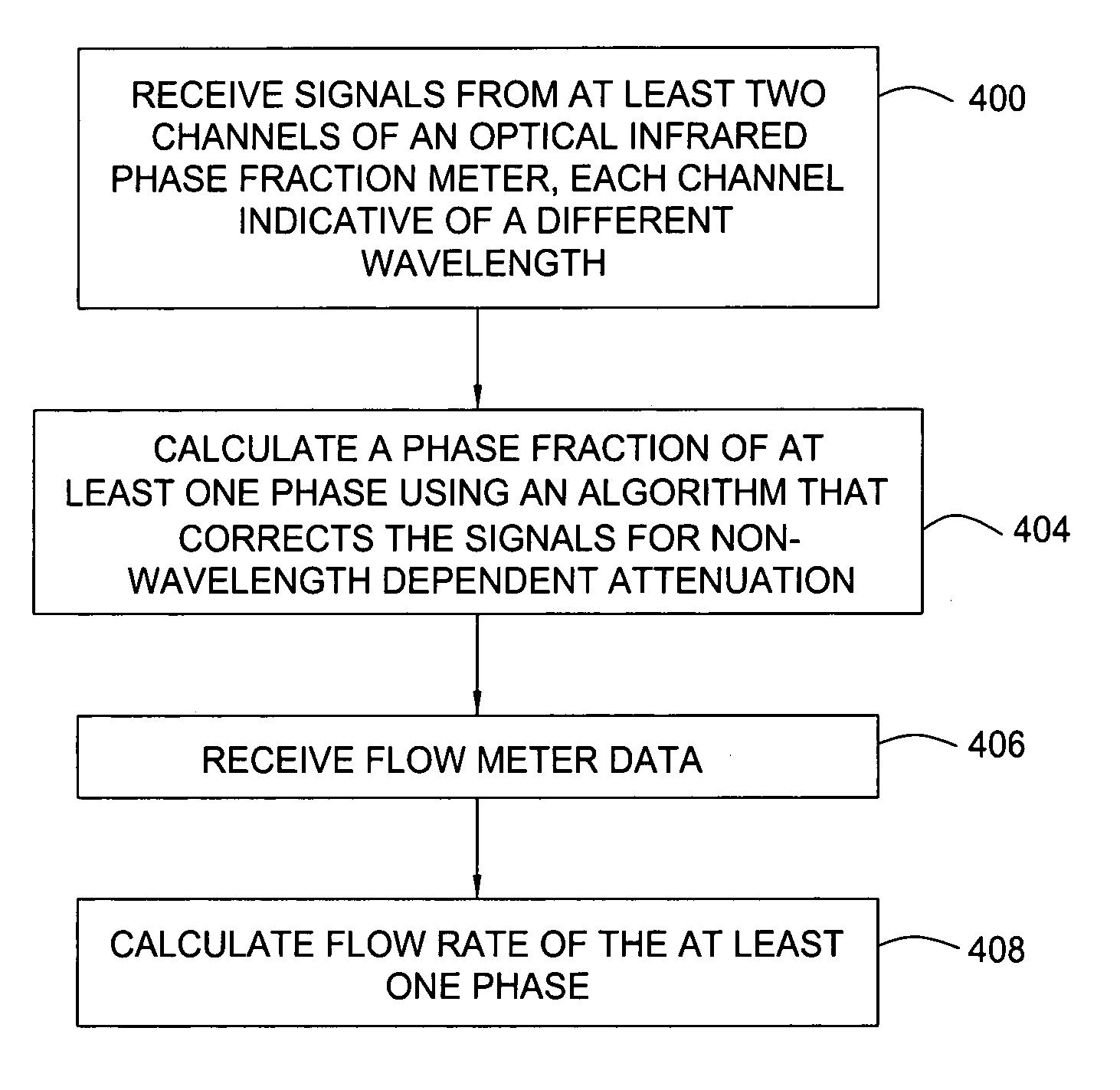
Multi-channel infrared optical phase fraction meter
ActiveUS7233001B2Improve accuracyIncrease rangeRadiation pyrometryVolume/mass flow measurementUltrasound attenuationLength wave
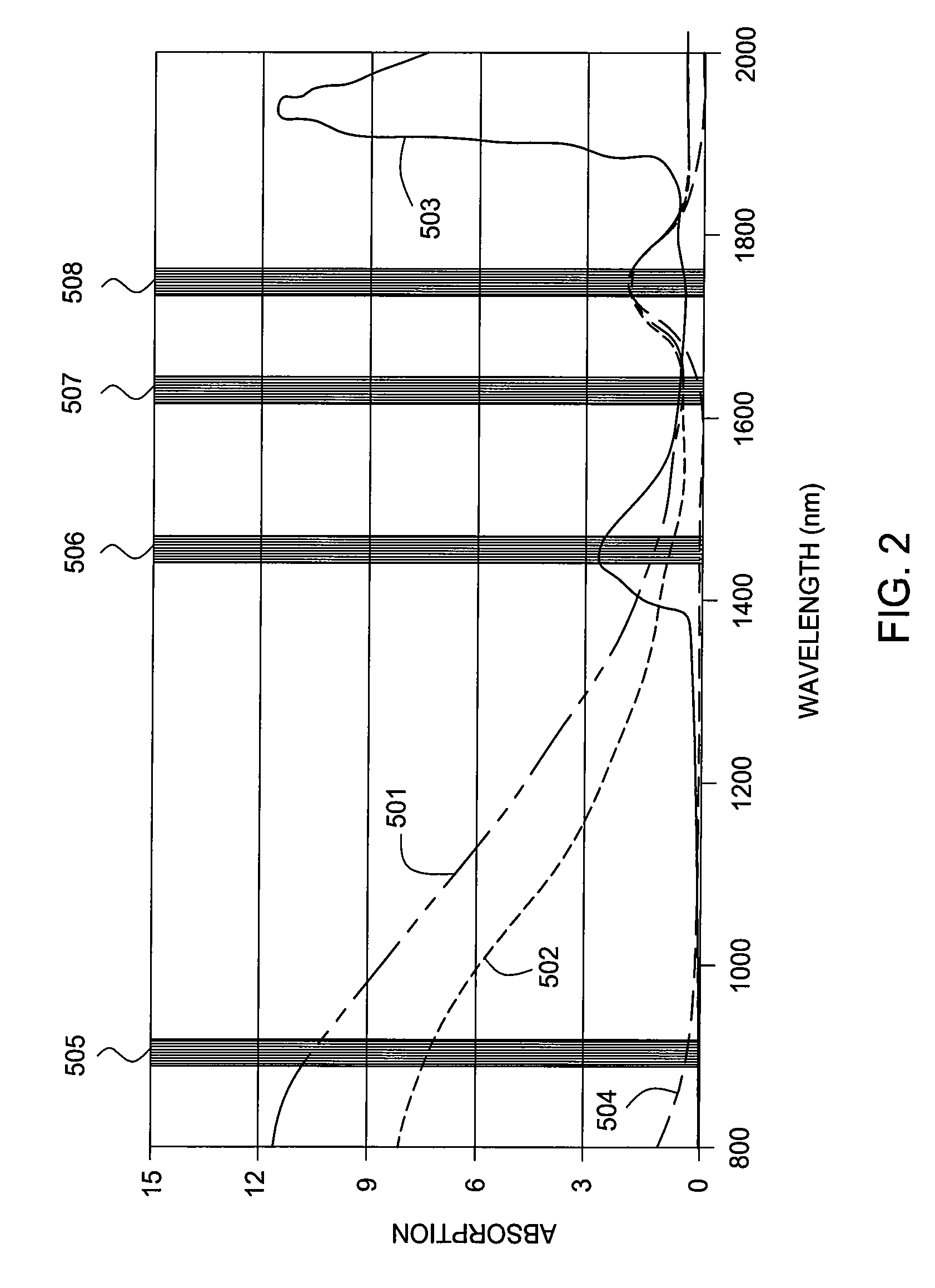
Methods and apparatus for measuring a phase fraction of a flow stream are disclosed. An infrared phase fraction meter includes a light source for emitting into a flow stream infrared radiation that includes first and second wavelength bands. The first wavelength band substantially transmits through first and second phases of the flow stream and is substantially absorbed by a third phase. In contrast, the second wavelength band is substantially absorbed by the second phase relative to the first and third phases. One or more detectors simultaneously detect attenuation of the first and second wavelength bands upon the infrared radiation passing through at least a portion of the flow stream, and a phase fraction of the second phase is determined based on the attenuation. As an example, the first, second and third phases are gas, water and oil, respectively, produced from a well.
Owner:WEATHERFORD TECH HLDG LLC
Novel formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS20070117862A1Eliminate side effectsHigh anticancer activityPowder deliveryBiocideSuspended particlesFree protein
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Multi-channel infrared optical phase fraction meter
ActiveUS20060186340A1Improve performanceLow costRadiation pyrometryVolume/mass flow measurementUltrasound attenuationLength wave
Methods and apparatus for measuring a phase fraction of a flow stream are disclosed. An infrared phase fraction meter includes a light source for emitting into a flow stream infrared radiation that includes first and second wavelength bands. The first wavelength band substantially transmits through first and second phases of the flow stream and is substantially absorbed by a third phase. In contrast, the second wavelength band is substantially absorbed by the second phase relative to the first and third phases. One or more detectors simultaneously detect attenuation of the first and second wavelength bands upon the infrared radiation passing through at least a portion of the flow stream, and a phase fraction of the second phase is determined based on the attenuation. As an example, the first, second and third phases are gas, water and oil, respectively, produced from a well.
Owner:WEATHERFORD TECH HLDG LLC
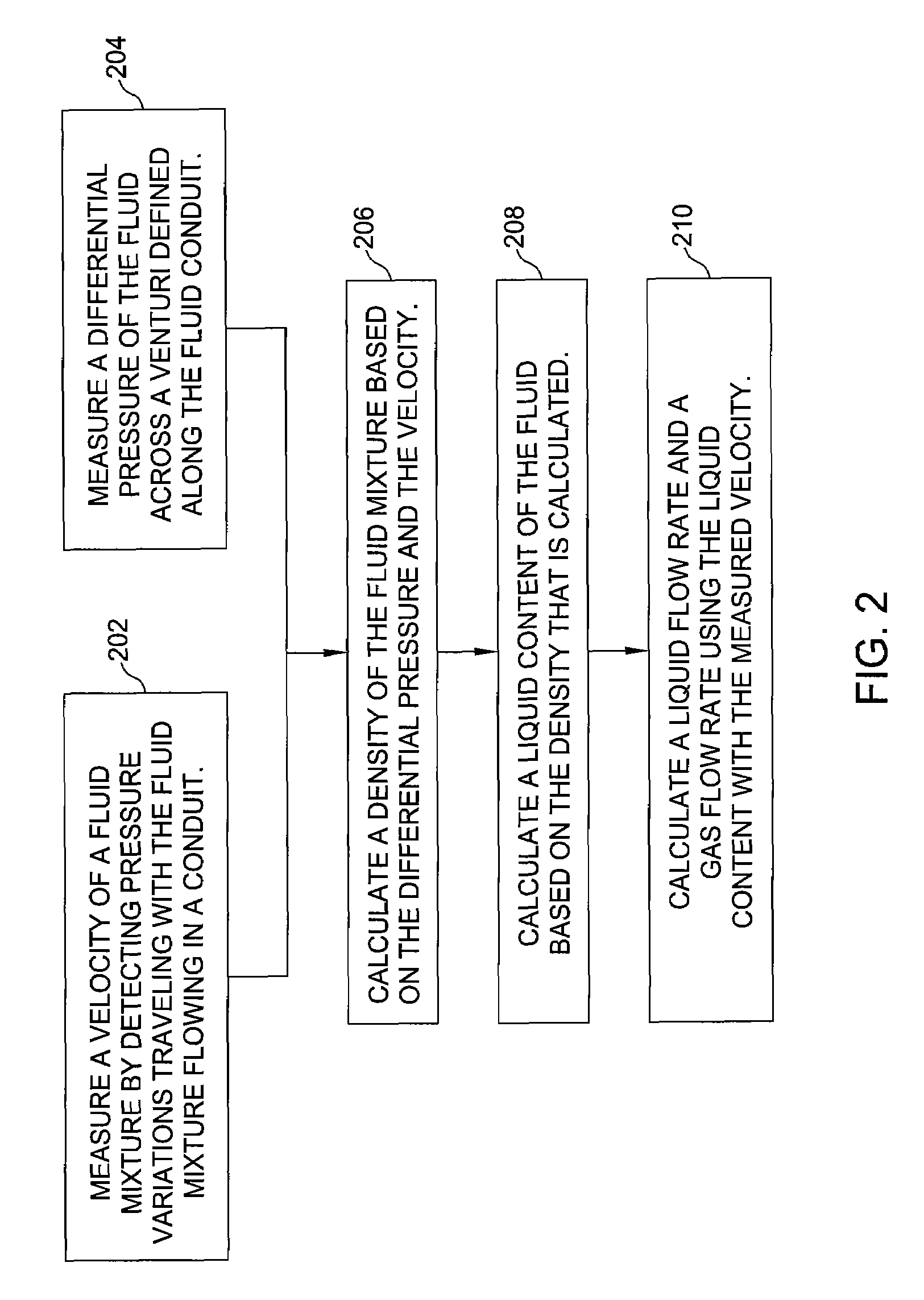
Wet-gas flowmeter
ActiveUS7654155B2Material analysis by optical meansVolume/mass flow by differential pressureLiquid ratioDifferential pressure
Methods and apparatus determine phase fractions for phases within a fluid mixture flow under a wide range of flow conditions including wet-gas flow. Appropriate flow algorithms can utilize this phase fraction information with a total flow rate of the mixture to find individual flow rates for phases, such as oil, water, and gas or gas and liquid, which can represent a combination of oil and water phases. For some embodiments, a multiphase flowmeter includes an array of spatially distributed pressure sensors configured to determine a velocity of the mixture flow and hence the total flow rate, which is applied with information from a differential pressure meter to calculate the bulk density of the fluid mixture. Further, additional speed of sound information or a water-in-liquid ratio as may be determined by spectral analysis can enable differentiation between the oil and water phases.
Owner:WEATHERFORD TECH HLDG LLC
In-well full-bore multiphase flowmeter for horizontal wellbores
ActiveUS20140012507A1Electric/magnetic detection for well-loggingMaterial analysis using sonic/ultrasonic/infrasonic wavesMomentumSurface roughness
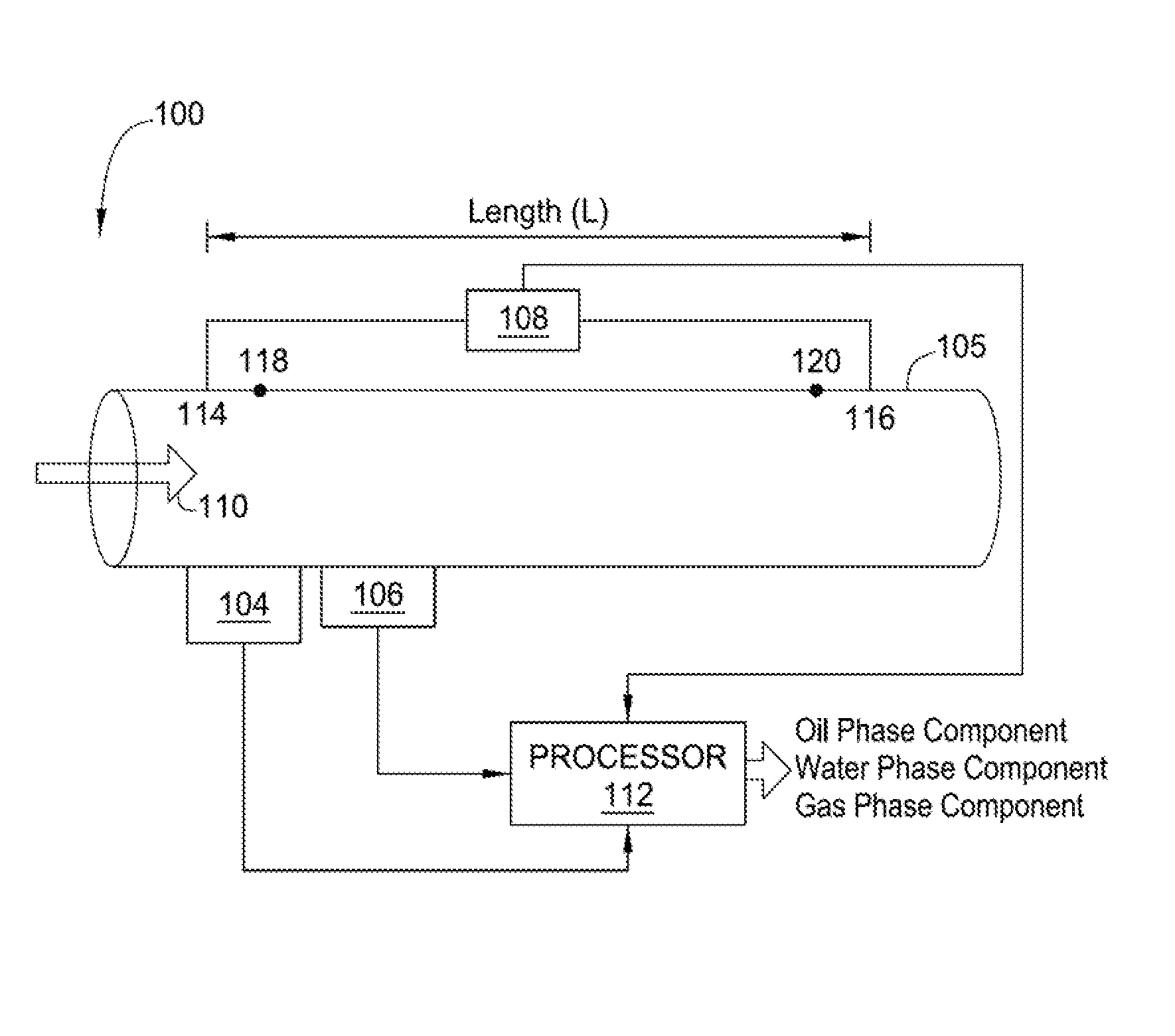
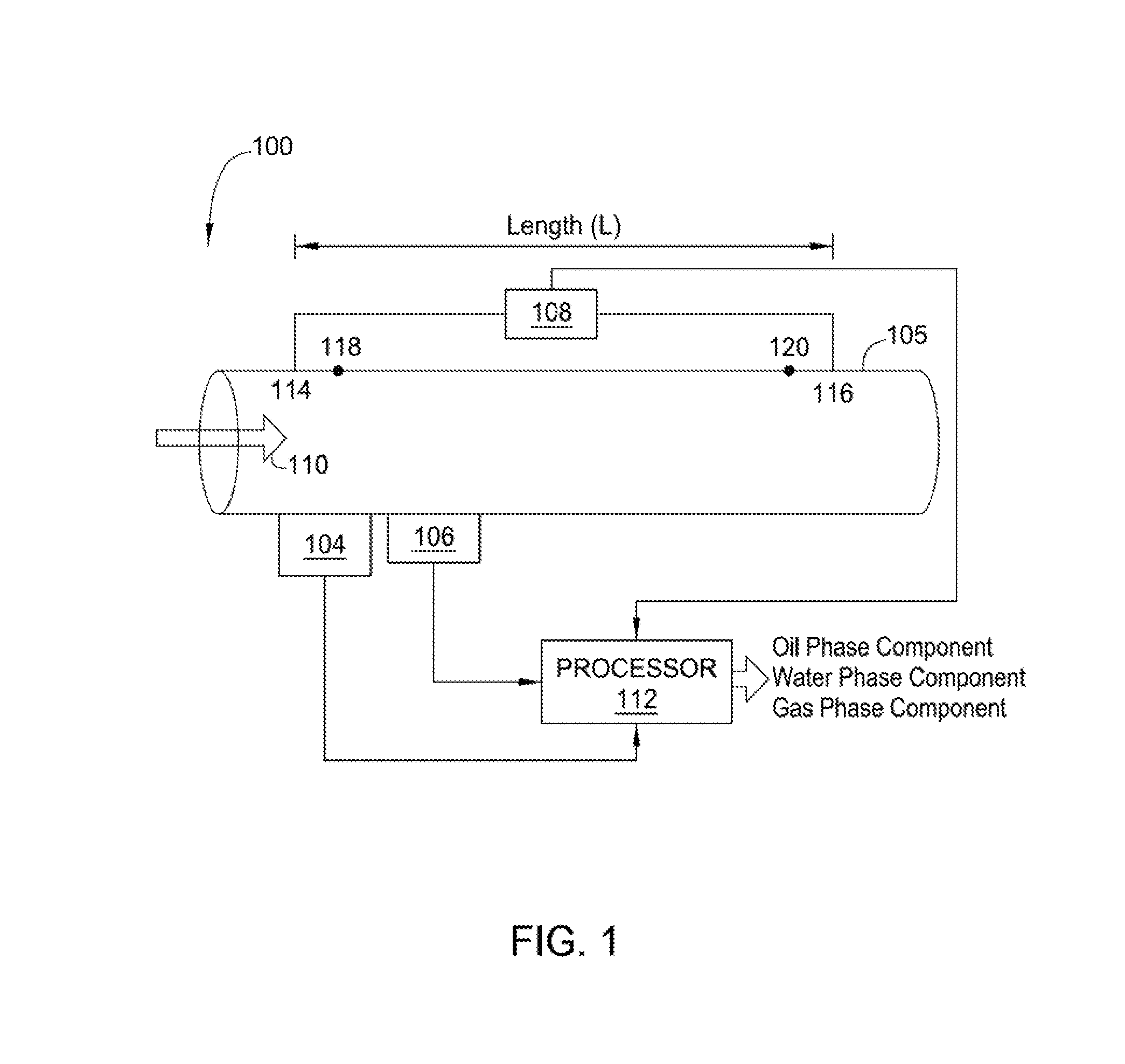
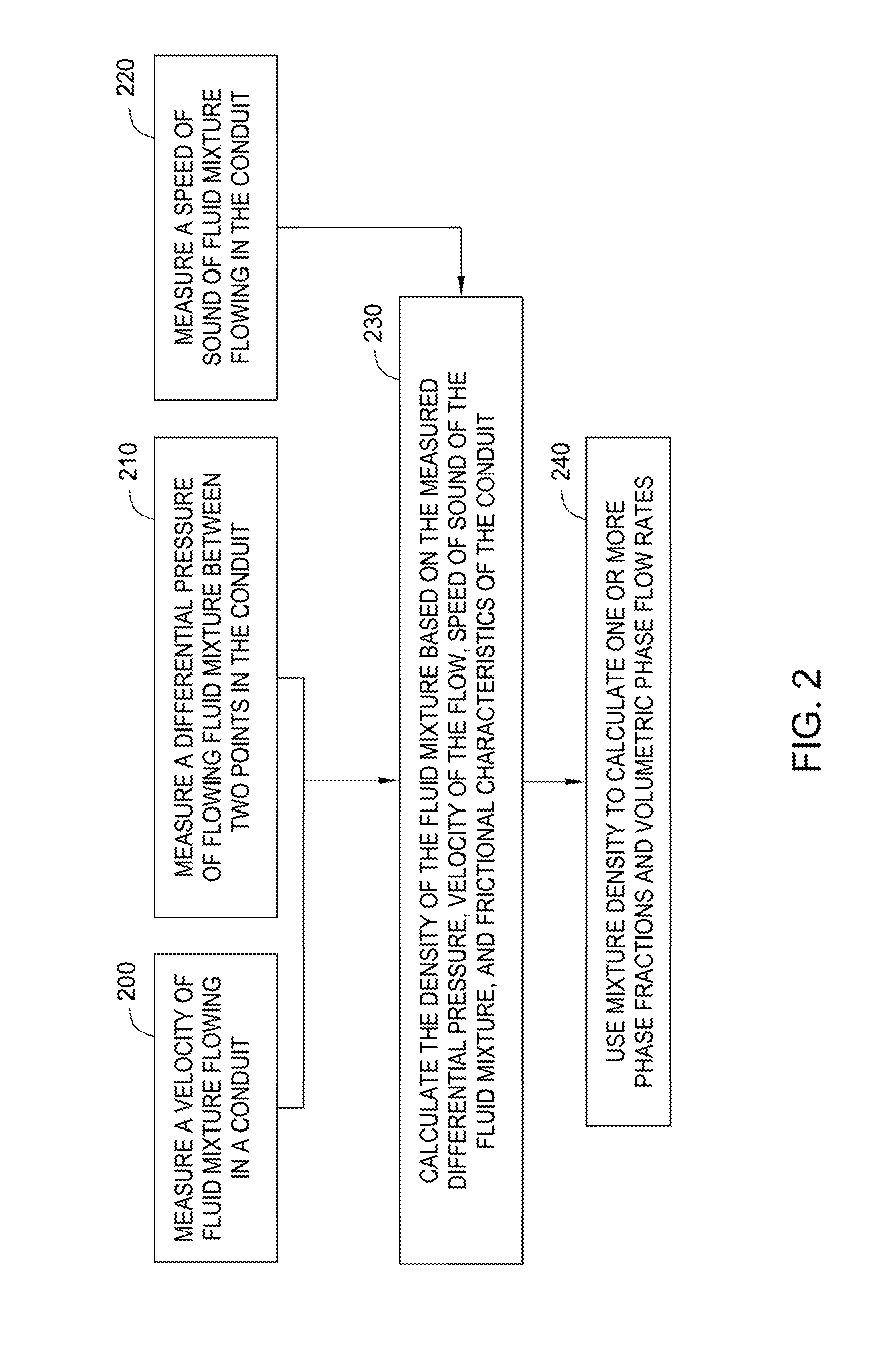
Methods and apparatus for measuring individual phase fractions and phase flow rates in a multiphase flow based on velocity of the flow, speed of sound through the fluid mixture, and the density of the fluid mixture. Techniques presented herein are based on measuring frictional pressure drop across a flowmeter conduit, determining a surface roughness term for the conduit during initial flow tests or through other mechanical means, implementing a correction method to balance the momentum equation, and calculating the fluid mixture density using the measured pressure drop. The techniques may be applicable to measuring flow parameters in horizontally oriented conduits and, more generally, conduits of any orientation.
Owner:WEATHERFORD TECH HLDG LLC
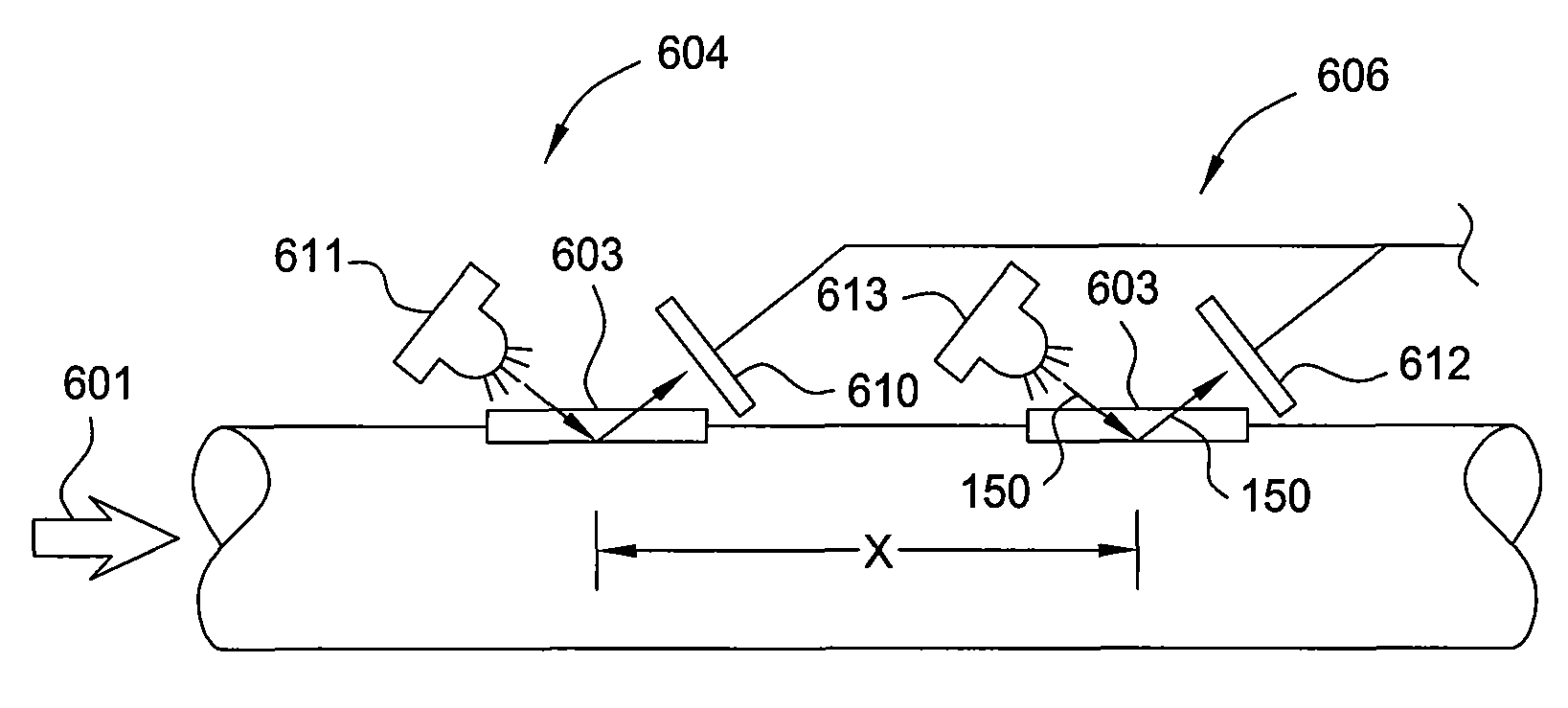
Optical multiphase flowmeter
Method and apparatus enable direct measurement of at least one flow velocity for one or more phases within a multiphase fluid mixture flowing in a conduit. Some embodiments provide determination of actual individual phase flow rates for three phases (e.g., oil, water and gas) that are distinct from one another within the fluid mixture. A multiphase flowmeter according to embodiments of the invention includes at least two optical sensors spatially distributed along a length of the conduit and designed to detect light interactions with the fluid mixture unique to the phases such that detected time-varying signals can be processed via cross-correlation or an array processing algorithm to provide desired individual phase flow velocity for oil, water and / or gas phases. This flow velocity can be applied to phase fraction measurements, which can be obtained utilizing the same flowmeter or another separate device, to calculate the flow rates for the phases.
Owner:WEATHERFORD TECH HLDG LLC
Novel formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS20070122465A1Improve abilitiesPromote formationPowder deliveryBiocideSuspended particlesActive agent
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Device and method for metering oil-gas-water three-phase flow in thickened oil in whole range
ActiveCN103759772AVolume/mass flow by differential pressureIndirect mass flowmetersDifferential pressureProduct gas
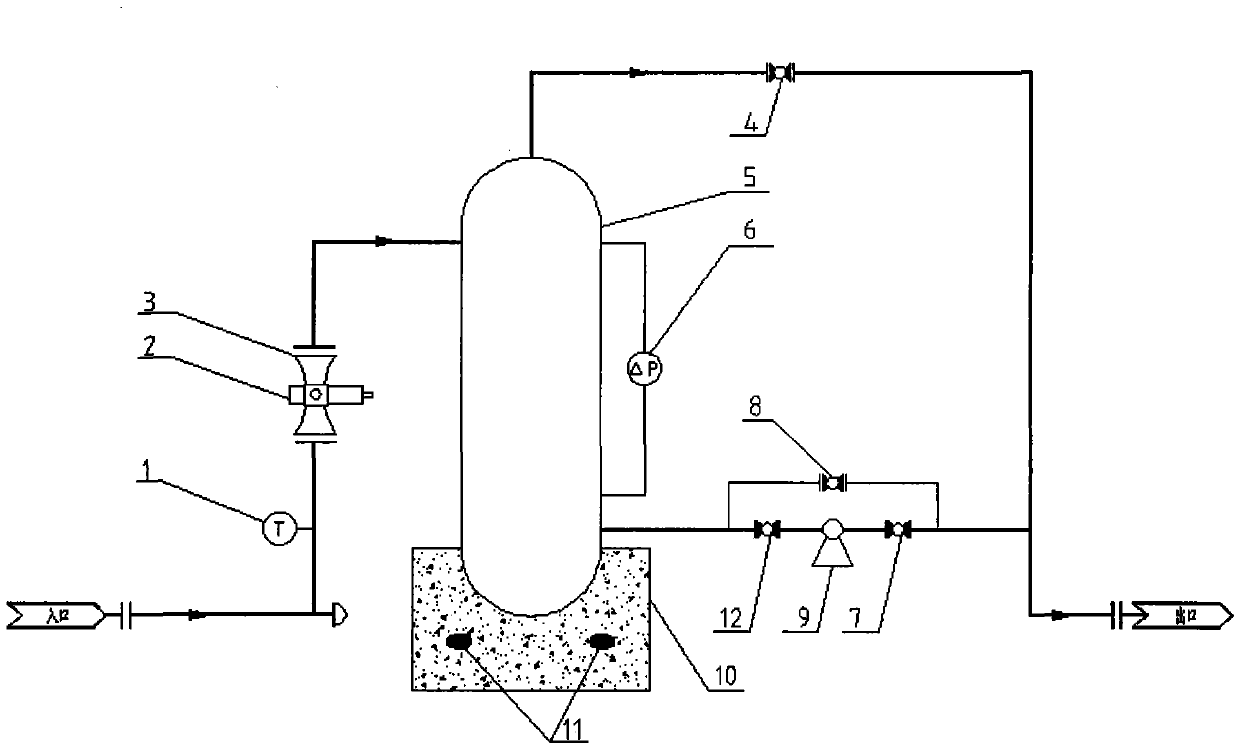
The invention provides a device for metering oil-gas-water three-phase flow in thickened oil in a whole range. The device comprises a Venturi flow meter 3, a double-energy gamma ray phase fraction instrument 2, a tank 5 and a differential pressure transmitter 6, wherein the double-energy gamma ray phase fraction instrument 2 is arranged on a vertical round tube at the upstream and the downstream of the Venturi flow meter or on a throat portion of a Venturi tube and comprises a double-energy gamma ray generator and a gamma ray detector, and due to the arrangement mode of the double-energy gamma ray generator and the gamma ray detector, gamma rays emitted by the double-energy gamma ray generator can penetrate through a tube cross section of the throat portion of the Venturi tube 3 in the radial direction and arrive at the gamma ray detector; the tank 5 is located at the downstream of the Venturi tube 3 and is used for accumulating or discharging the thickened oil, the top of the tank is provided with a gas outlet, and the bottom of the tank is provided with a liquid outlet; due to the arrangement mode of the differential pressure transmitter 6, the differential pressure transmitter 6 can measure differential pressure delta P between a lower pressure tapping and a liquid level caused by accumulation of the thickened oil in the tank 5. The invention further relates to a method for metering oil-gas-water three-phase flow in the thickened oil in the whole range.
Owner:HAIMO TECH GRP CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com