Ether electrolyte and lithium air battery
An electrolyte and ether technology, applied in the field of lithium-air batteries, can solve the problems of poor long-term cycle stability of lithium-air batteries, affecting the performance of lithium-air batteries, oxidation decomposition, etc., and achieve excellent chemical stability, excellent Coulombic efficiency and rate performance , the effect of high oxygen solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036]The present invention provides a kind of preparation method of ether electrolyte, preferably comprises the following steps:
[0037] The lithium salt and the organic solvent are mixed, stirred, and left to stand to obtain an ether electrolyte.
[0038] In the present invention, the types and sources of the lithium salt and the organic solvent are consistent with the types and sources of the lithium salt and the organic solvent described in the above technical solution, and will not be repeated here.
[0039] In the present invention, the mixing temperature of the lithium salt and the organic solvent is preferably 10°C to 50°C, more preferably 15°C to 45°C, most preferably 20°C to 30°C; the mixing time is preferably 1 min to 15min, more preferably 5min to 10min; the stirring temperature is preferably 10°C to 50°C, more preferably 15°C to 45°C, most preferably 20°C to 30°C; the stirring time is preferably 0.5h to 2h, more preferably 1h-1.5h; the stirring rate is preferabl...
Embodiment 1
[0059] In a glove box filled with high-purity argon, weigh 2.8709g LiN(SO 2 CF 3 ) 2 , take 10mL tetraethylene glycol dimethyl ether (TEGDME) with a pipette gun, make a mixed solution, stir evenly and let it stand for 2h to obtain an ether electrolyte;
[0060] Using Super P Li carbon as the positive electrode, metal lithium sheet as the negative electrode, glass fiber membrane as the diaphragm, and the ether electrolyte prepared above as the electrolyte, a lithium-air battery was assembled in a glove box filled with argon. The above-mentioned test battery was subjected to a constant current charge and discharge test in pure oxygen at a current density of 50 mA / g at room temperature, and the charge and discharge cut-off voltage was 2.0V-4.5V.
[0061] figure 1 It is the first charge and discharge curve diagram of the lithium-air battery obtained in Example 1 of the present invention, wherein, curve 1 is the charging process, and curve 2 is the discharging process. from fi...
Embodiment 2
[0065] In a glove box filled with high-purity argon, weigh 2.8709g LiN(SO 2 CF 3 ) 2 , take 10mL ethylene glycol dimethyl ether (DME) with a pipette gun, make a mixed solution, stir it evenly and let it stand for 2 hours to obtain an ether electrolyte;
[0066] Using Super P Li carbon as the positive electrode, metal lithium sheet as the negative electrode, glass fiber membrane as the diaphragm, and the ether electrolyte prepared above as the electrolyte, a lithium-air battery was assembled in a glove box filled with argon.
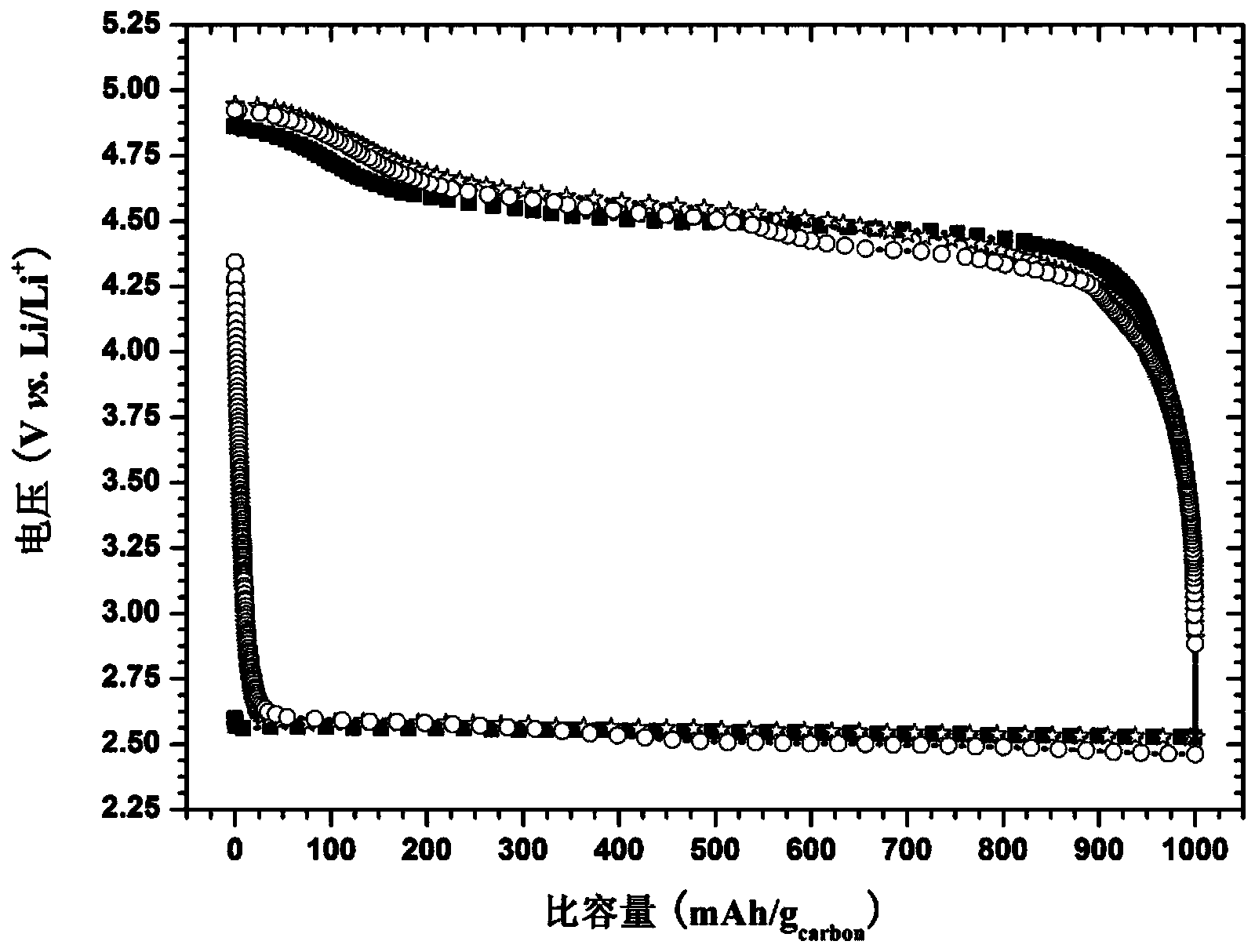
[0067] The above battery was subjected to a constant current charge and discharge test in pure oxygen at a current density of 100 mA / g at room temperature, and a cycle performance test was performed with a limited charge and discharge capacity of 1000 mAh / g. image 3 It is the lithium-air battery charge-discharge curve graph that the embodiment 2 of the present invention obtains, It is the charge and discharge curve of the first cycle; It is the cha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com