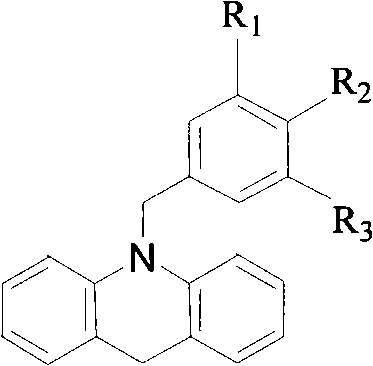
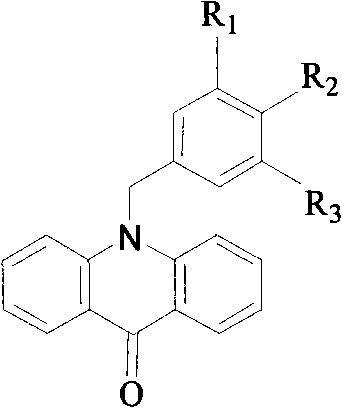
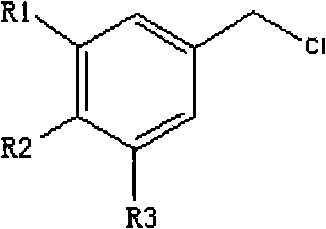
N-benzyl-9, 10- dihydro-acridine compound and preparation method thereof
A benzylacridone and compound technology, applied in the field of N-benzyl-9, can solve the problems of cumbersome reaction steps and harsh reaction conditions, and achieve the effect of simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Synthesis of N-benzylacridone
[0032] Under the protection of argon, in a three-necked round-bottomed flask containing 15 mL of dry N,N-dimethylformamide (DMF), add 585 mg (3 mmol) of acridone (purchased from Guangzhou Qihua Medical Equipment Co., Ltd., No. A0133), 160mg (4.0mmol) sodium hydride, stirred at room temperature for 1h, then added 6mmol benzyl chloride, 990mg (6mmol) potassium iodide, stirred overnight, added 30ml water, stirred rapidly under ice-water bath, yellow crystals were precipitated, filtered to obtain yellow Crystal: recrystallized with ethanol to obtain 770 mg of light yellow crystal N-benzylacridone with a yield of 90% and a melting point of 180-181° C. (determined by X-4 micro melting point apparatus). The characterization data of N-benzylacridone are as follows: 1 H NMR (300MHz, CDCl 3 ): δ5.61 (s, 2H), 7.20-7.37 (m, 9H), 7.62 (m, 2H), 8.60 (m, 2H).
[0033] 2) Synthesis of N-benzyl-9,10-dihydroacridine
[0034] Add N-benzylacridone (1m...
Embodiment 2
[0036] 1) Synthesis of N-(3,5-dimethoxy)benzylacridone
[0037] Under the protection of argon, in a three-necked round-bottomed flask containing 15 mL of dry N,N-dimethylformamide (DMF), add 585 mg (3 mmol) of acridone (purchased from Guangzhou Qihua Medical Equipment Co., Ltd., No. A0133), 160mg (4.0mmol) sodium hydride, stirred at room temperature for 1h, then added 6mmol 3,5-dimethoxyl-benzyl chloride (prepared according to the method in Gao Chunmei's 2007 doctoral dissertation of Guangzhou Institute of Chemistry, Chinese Academy of Sciences), 990mg ( 6mmol) potassium iodide, stirred overnight, added 30ml of water, stirred rapidly under an ice-water bath, yellow crystals were precipitated, filtered to obtain yellow crystals; recrystallized with ethanol to obtain yellow crystals, namely N-(3,5-dimethoxy) Benzylacridone 940mg, yield 91%, melting point: 229-230°C (determined by X-4 micro melting point apparatus). The characterization data of N-(3,5-dimethoxy)benzylacridone ar...
Embodiment 3
[0041] 1) Synthesis of N-(3,5-dibenzyloxy)benzylacridone
[0042] Under the protection of argon, in a three-necked round-bottomed flask containing 15 mL of dry N,N-dimethylformamide (DMF), add 585 mg (3 mmol) of acridone (purchased from Guangzhou Qihua Medical Equipment Co., Ltd., No. A0133), 160mg (4.0mmol) sodium hydride, stirred at room temperature for 1h, then added 6mmol3,5-dibenzyloxy-benzyl chloride (prepared according to the method in Gao Chunmei's doctoral dissertation of Guangzhou Institute of Chemistry, Chinese Academy of Sciences in 2007), 990mg (6mmol ) Potassium iodide, stirred overnight, added water, stirred rapidly under ice-water bath, yellow crystals were precipitated, filtered to obtain yellow crystals; recrystallized with ethanol to obtain light yellow crystals, namely N-(3,5-dibenzyloxy)benzyl Acridone 1.3g, yield 87%, melting point: 170-171°C. The characterization data of N-(3,5-dibenzyloxy)benzylacridone are as follows: 1 H NMR (500MHz, CDCl3): δ4.93(s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com