Perfluorination method for end group of fluorine-containing polymer
A fluoropolymer and perfluorinated technology, which is applied in the field of perfluorinated terminal groups of fluoropolymers, and can solve the problems of inability to apply terminal groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 900 units of water and 1 unit of ammonium perfluorooctanoate into a horizontal reactor with a volume of 1400 units of stirring (the material is new No. 3 steel), and add HFP / TFE blend monomer after evacuation treatment. The temperature is 80 / 20. When the temperature rises to 95°C, the pressure in the reactor rises to 3.5 MPa and 2.5 parts of ammonium persulfate solution is quickly added to the system, and the concentration of ammonium persulfate solution is 2.5%.
[0029] Stirring was continued during the reaction, and TFE monomer was supplemented to ensure that the reaction pressure was at 3.5 MPa. At the same time, ammonium persulfate was added at a rate of 0.2 parts / min. After continuing the reaction for 2 hours, the stirring was stopped, and the remaining monomers in the reactor were recovered. Take 1500ml of the resulting product dispersion, add 5ml of 0.3% nitric acid solution, and coagulate under the condition of 12000 rpm to obtain a white powder in the uppe...
Embodiment 2
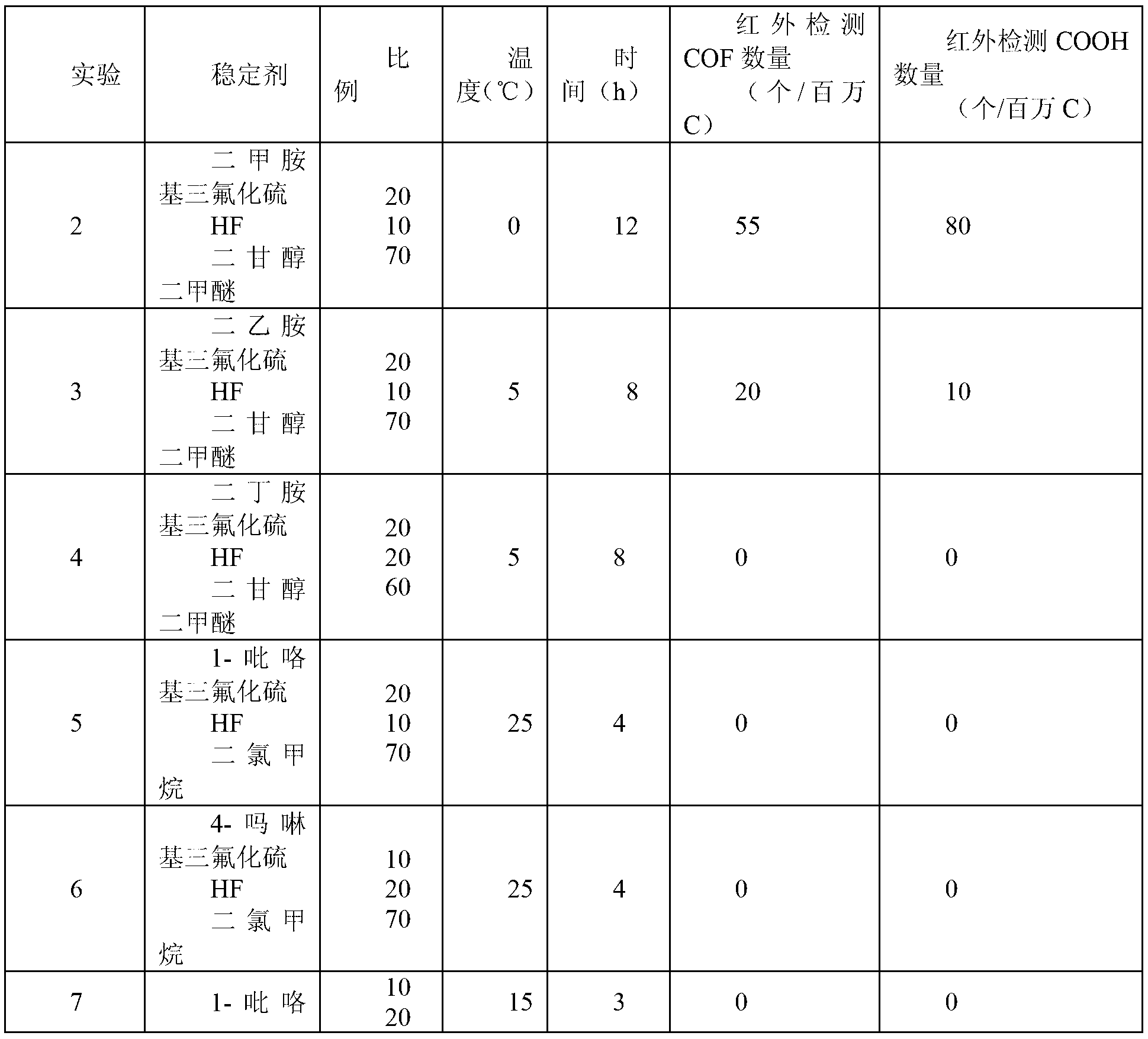
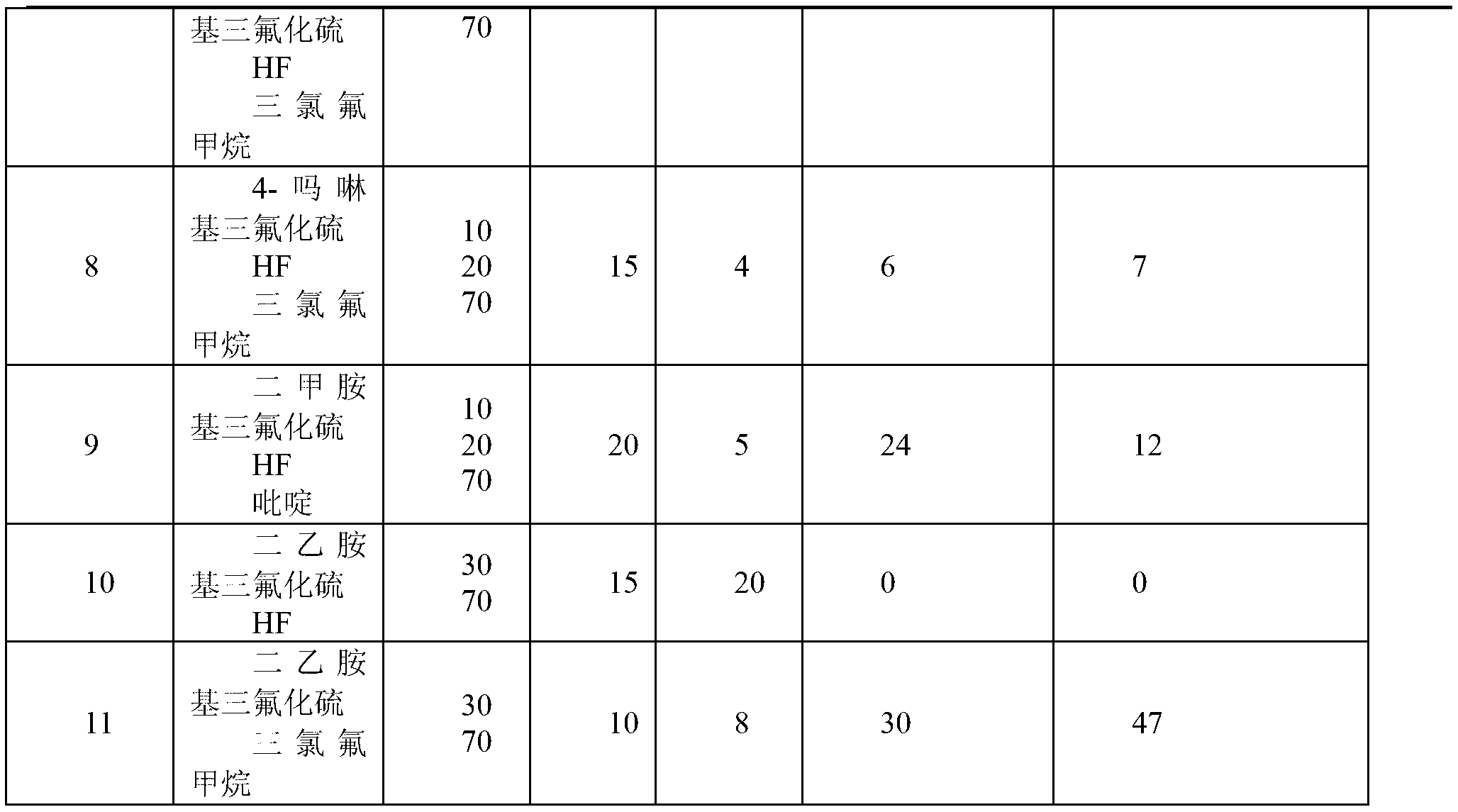
[0033]Take the FEP powder obtained by polymerization in Example 1, press it into tablets at 250°C, place it in a 500ml stainless steel tank, and at the same time evacuate the tank, inhale 100g of the prepared end group stabilizer, and treat it at the corresponding temperature After some time, drain the stabilizer, wash the flake FEP with 0.5% NaOH alkali and dry it, then dry it after wiping it with acetone, measure the thickness of the flake, and detect it with a Fourier transform infrared spectrometer. The obtained results are shown in Table 1:
[0034]
[0035]
Embodiment 3
[0037] Add 900 units of water and 1 unit of ammonium perfluorooctanoate to a horizontal reactor with a volume of 1400 units of stirring (the material is new No. 3 steel), and add HFP / VDF blend monomer after evacuation treatment. 80 / 20, when the temperature rises to 75°C, the pressure in the reactor rises to 1.0MPa, quickly add 3.0 parts of ammonium persulfate solution to the system, the concentration of ammonium persulfate solution is 2.5%, react for 1.5 hours, during which additional blending unit body to ensure that the pressure is maintained at not less than 1.0Mpa.
[0038] Take 1500ml of the resulting product dispersion, add 5ml of 3% magnesium chloride solution, and coagulate under the condition of 12000 rpm to obtain a white powder. Wash the white powder several times in an ion-free aqueous solution, and then dry it in an oven at 120°C for 24 hours to obtain a white flaky fluororubber. The Mooney viscosity ML is tested. 1+10 121℃ for 55.
[0039] The fluorine rubber ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melt index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com