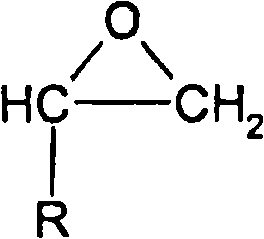
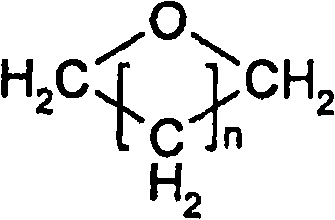
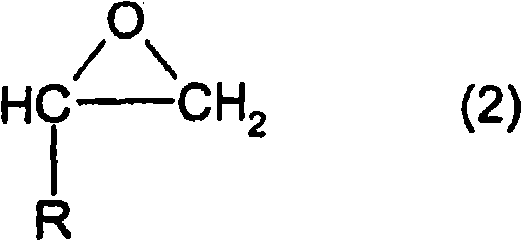
Method for producing alkylene glycol diethers
A technology of alkylene glycol diether and alkyl, which is applied in the field of preparing chain alkylene glycol diether, and can solve problems such as difficult sales
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 4
[0070] Example 4: HBF 4 / H 2 SO 4 catalytic
[0071] Similar to Comparative Example 1, 200 mg (2.28 mmol) of fluoroboric acid, 231 mg (2.28 mmol) of sulfuric acid and 157 g (3.41 mol) of dimethyl ether were initially charged in a 1-liter steel autoclave, and heated at 55° C. and 15 bar, 15.0 g (0.34 mol) of ethylene oxide were added. After an after-reaction time of 50 minutes and draining of the dimethyl ether, 30.7 g of residue were isolated. This residue has the following composition:
[0072] 59.9% Ethylene glycol dimethyl ether
[0073] 2.1% 1,4-dioxane
[0074] 6.8% Ethylene glycol monomethyl ether
[0075] 17.8% diglyme
[0076] 3.0% Diethylene glycol monomethyl ether
[0077] 4.3% Triglyme
[0078] 0.7% triethylene glycol monomethyl ether
[0079] 1.2% Tetraglyme
[0080] 0.3% Pentaethylene glycol dimethyl ether
[0081] 3.9% Unknown
Embodiment 5
[0082] Example 5: HBF 4 / H 3 PO 4 catalytic
[0083] Similar to Comparative Example 1, 200 mg (2.28 mmol) of fluoroboric acid, 223 mg (2.28 mmol) of phosphoric acid and 157 g (3.41 mol) of dimethyl ether were initially charged in a 1 liter steel autoclave, and heated at 55° C. and 15 bar, 15.0 g (0.34 mol) of ethylene oxide were added. After an after-reaction time of 50 minutes and draining of the dimethyl ether, 23.8 g of residue were isolated. This residue has the following composition:
[0084] 60.1% Ethylene glycol dimethyl ether
[0085] 1.7% 1,4-dioxane
[0086] 5.0% Ethylene glycol monomethyl ether
[0087] 16.9% diglyme
[0088] 4.6% Diethylene glycol monomethyl ether
[0089] 3.8% Triglyme
[0090] 1.6% triethylene glycol monomethyl ether
[0091] 0.9% Tetraglyme
[0092] 1.4% tetraethylene glycol monomethyl ether
[0093] 0.1% Pentaethylene glycol dimethyl ether
[0094] 3.9% Unknown
Embodiment 6
[0095] Example 6: HBF 4 / HNO 3 catalytic
[0096] Similar to Comparative Example 1, 200 mg (2.28 mmol) of fluoroboric acid, 288 mg (2.96 mmol) of nitric acid and 157 g (3.41 mol) of dimethyl ether were initially charged in a 1 liter steel autoclave, and heated at 55° C. and 15 bar, 15.0 g (0.34 mol) of ethylene oxide were added. After an after-reaction time of 50 minutes and draining of the dimethyl ether, 29.7 g of residue were isolated. This residue has the following composition:
[0097] 59.8% Ethylene glycol dimethyl ether
[0098] 1.8% 1,4-dioxane
[0099] 3.0% Ethylene glycol monomethyl ether
[0100] 16.3% diglyme
[0101] 4.3% Diethylene glycol monomethyl ether
[0102] 3.8% Triglyme
[0103] 1.9% triethylene glycol monomethyl ether
[0104] 3.2% tetraglyme
[0105] 1.1% tetraethylene glycol monomethyl ether
[0106] 0.3% Pentaethylene glycol dimethyl ether
[0107] 4.5% Unknown
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com