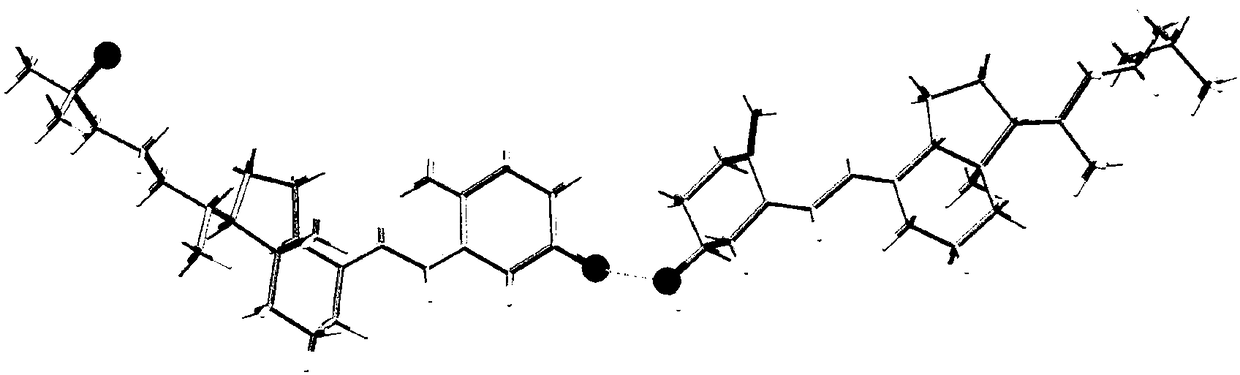
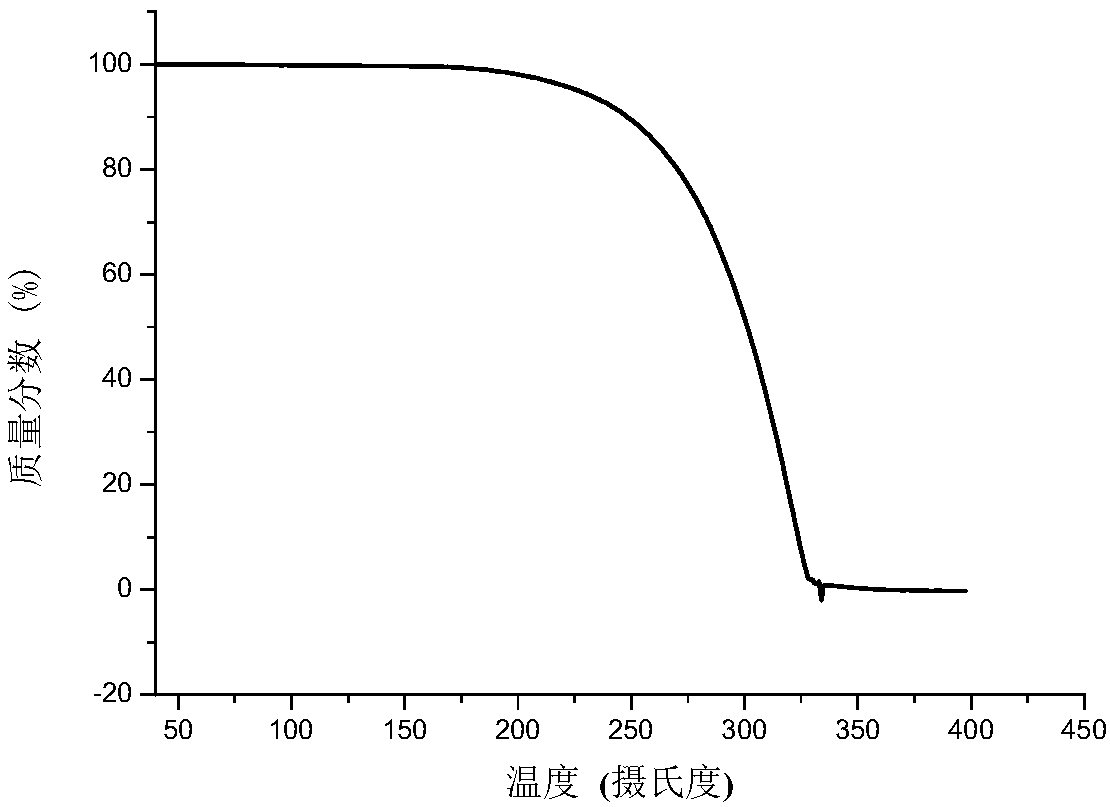
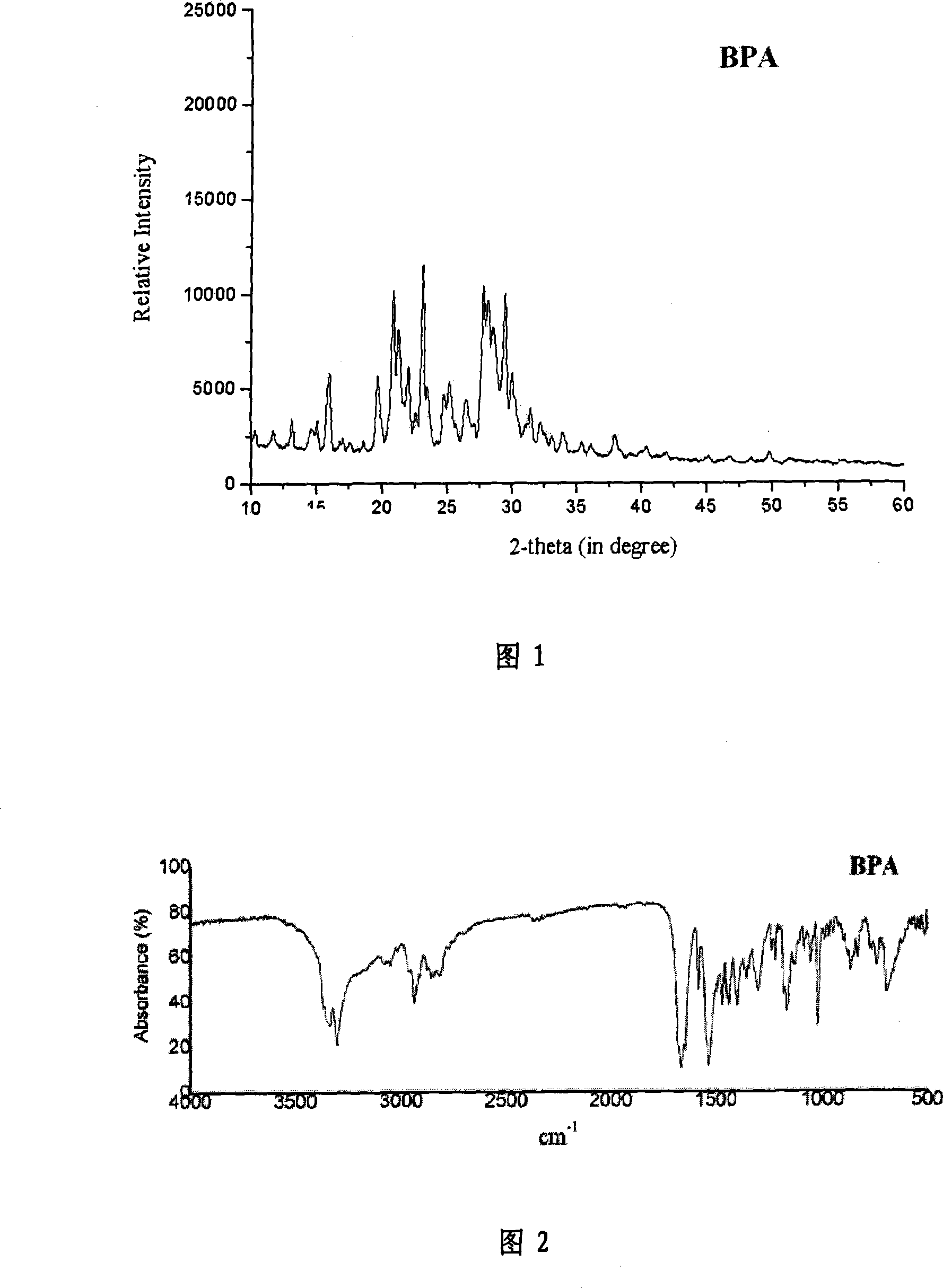
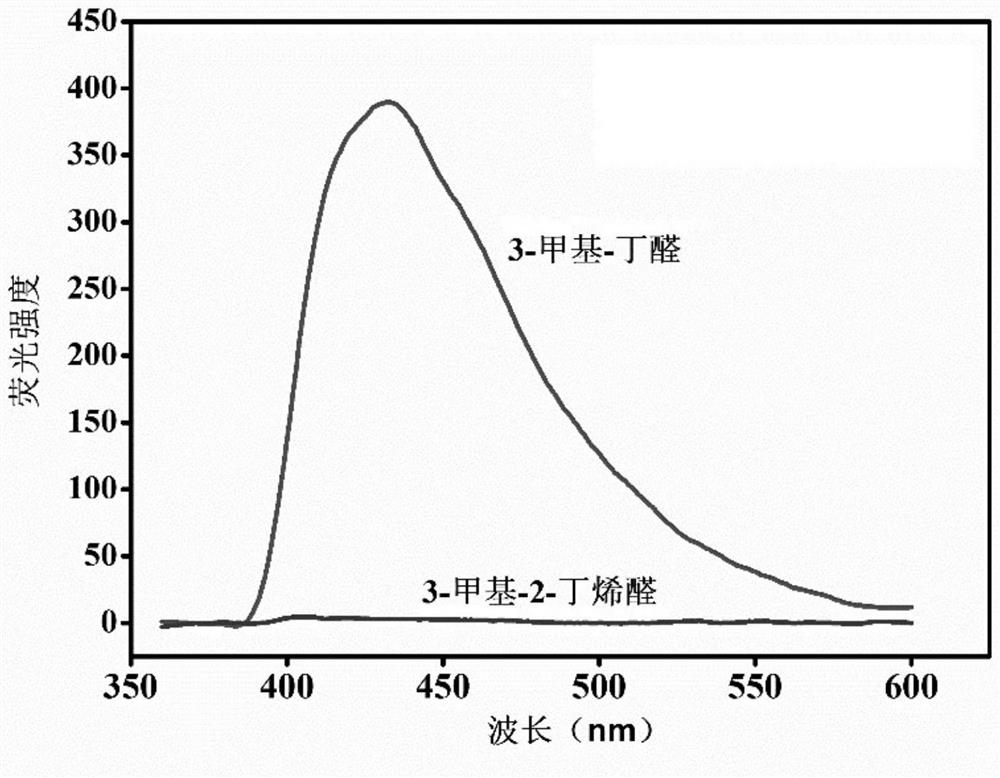
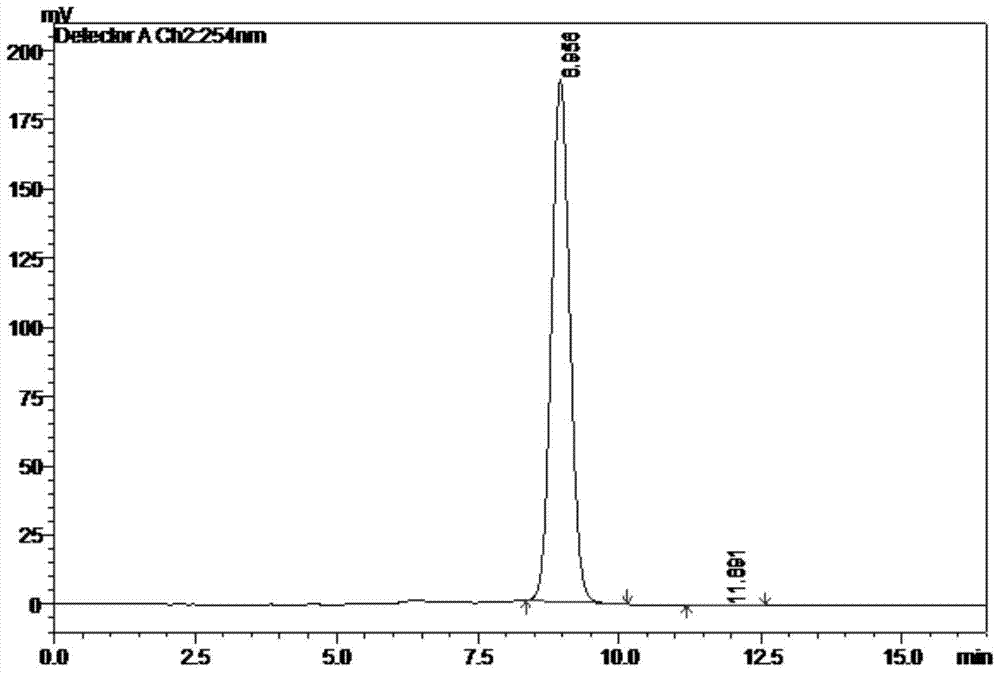
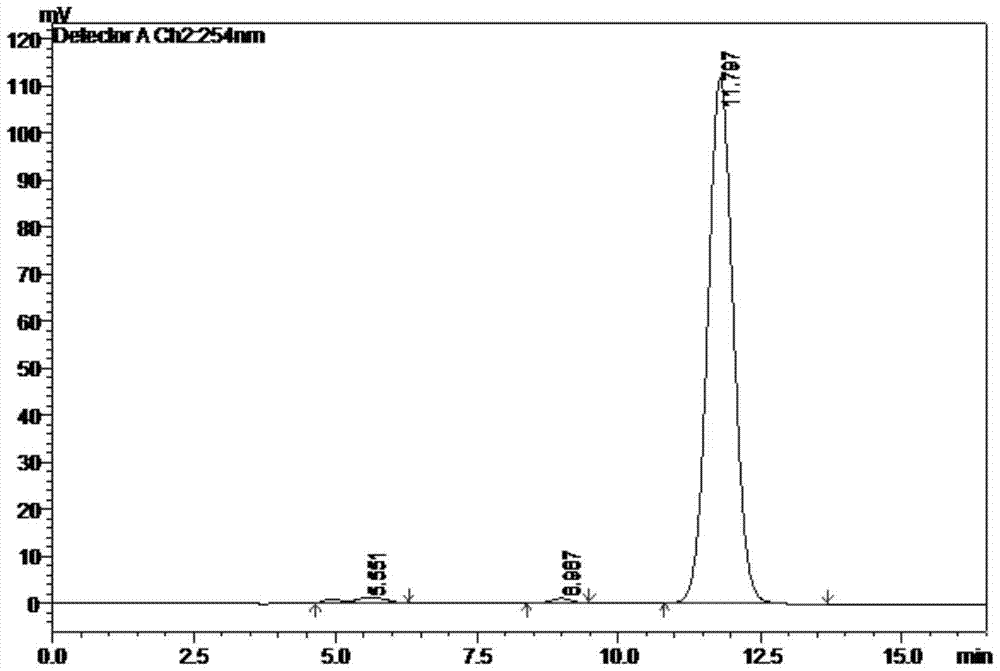
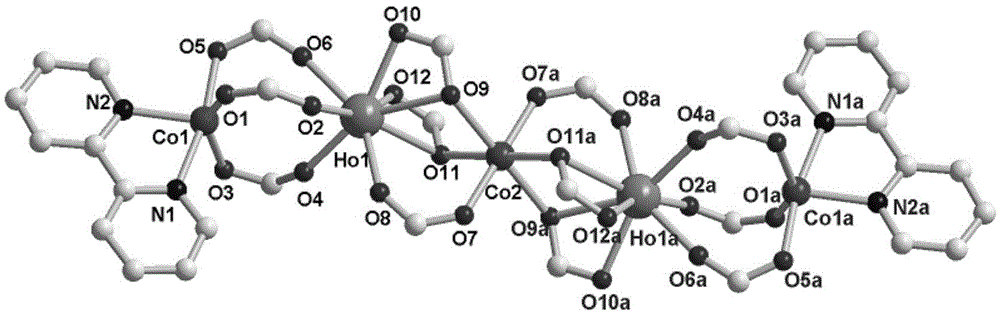
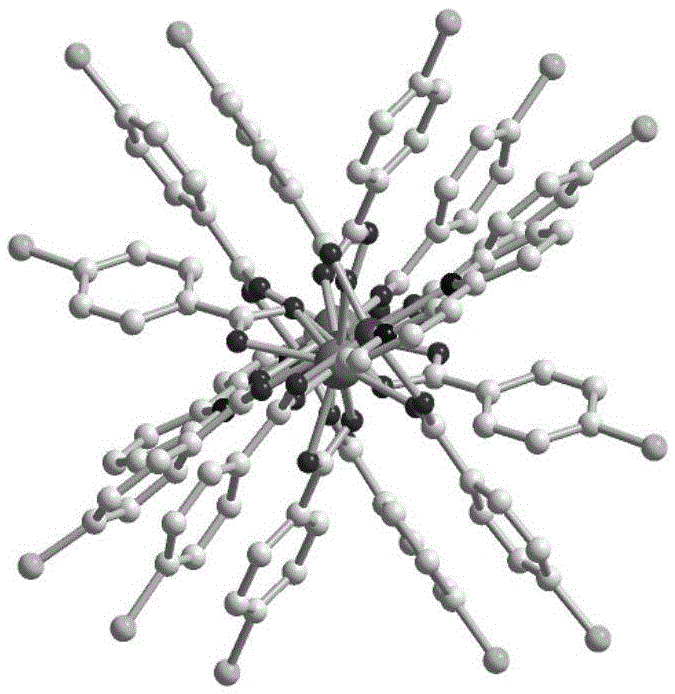
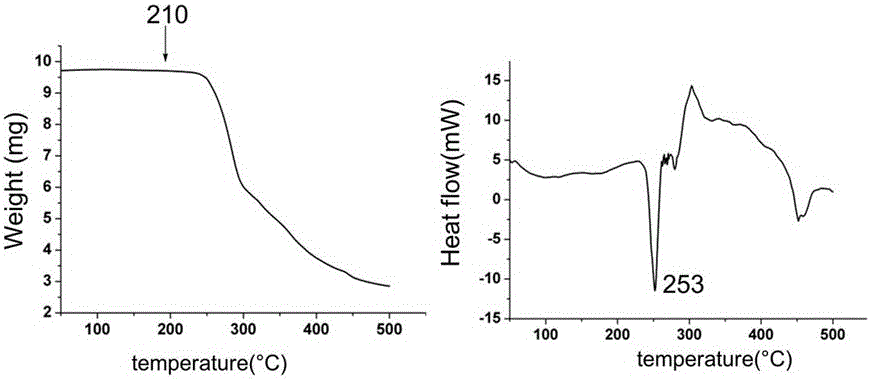
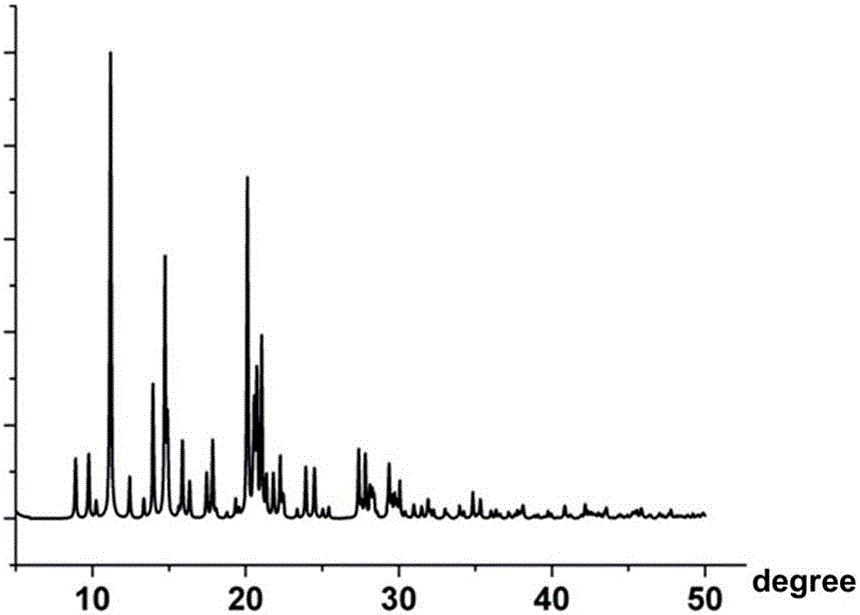
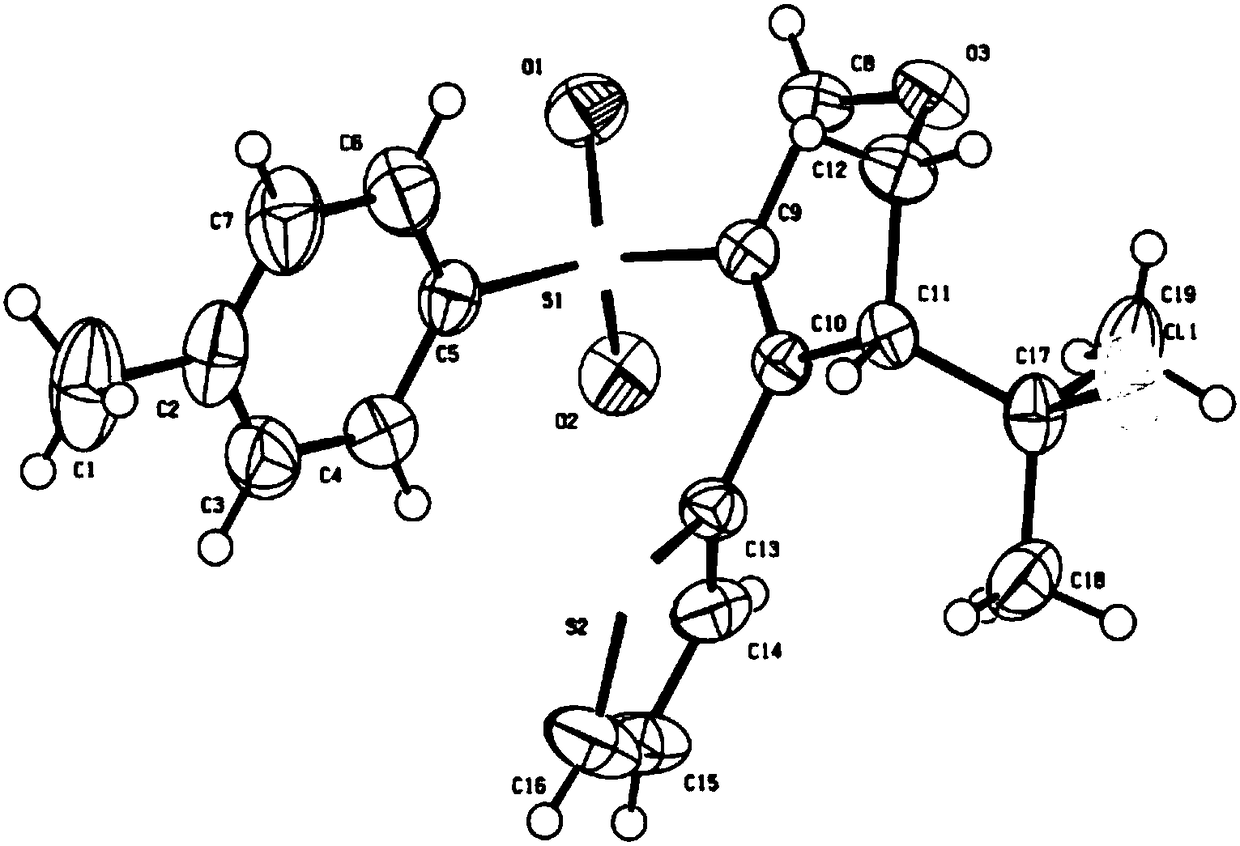
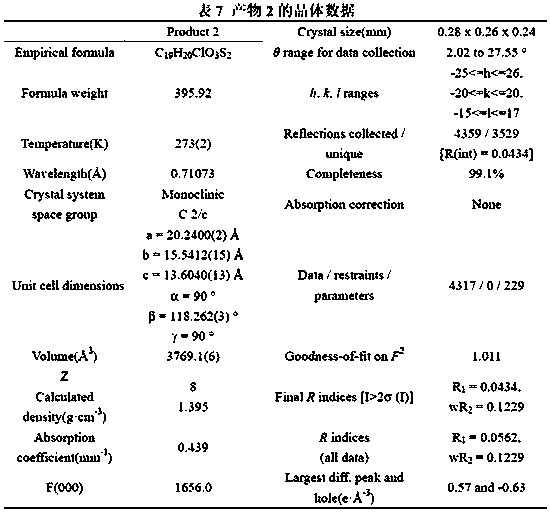
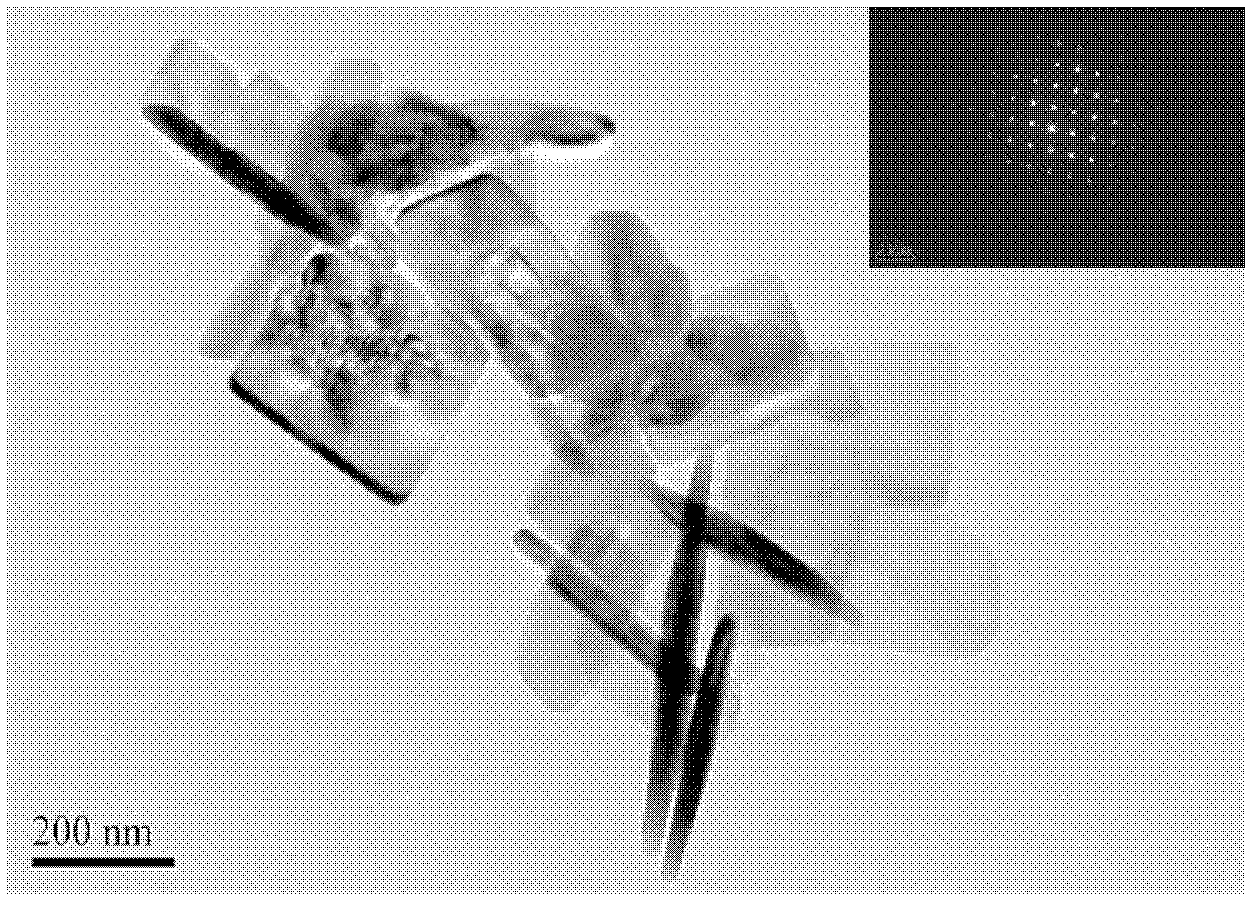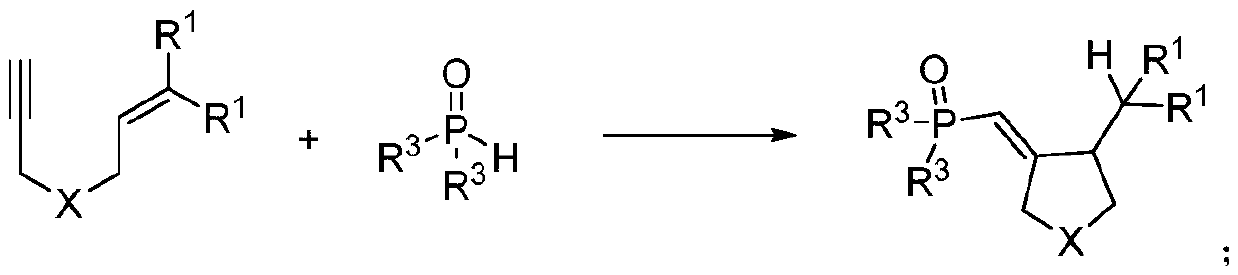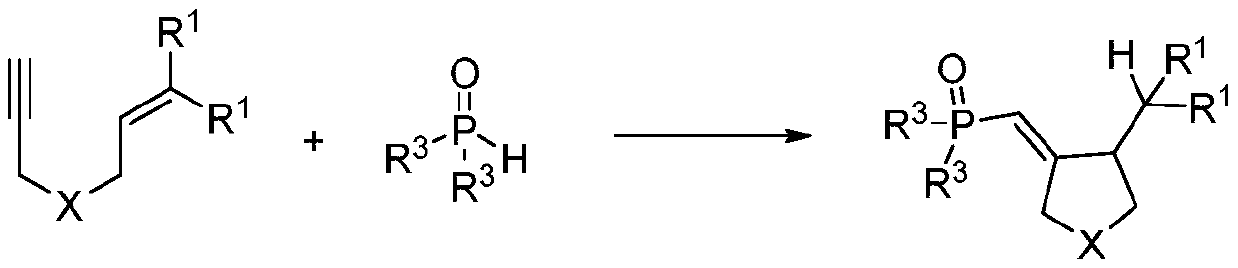Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
99 results about "Single Crystal Diffraction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Single-crystal diffraction is also used in the pharmaceutical industry, due to recent problems with polymorphs. The major factors affecting the quality of single-crystal structures are the crystal's size and regularity; recrystallization is a commonly used technique to
Synthesis technique of {001}-surface-exposed visible light titanium dioxide nanosheet with oxygen vacancy
InactiveCN102631907AUniform particlesEasy to manufacturePhysical/chemical process catalystsSingle Crystal DiffractionControllability
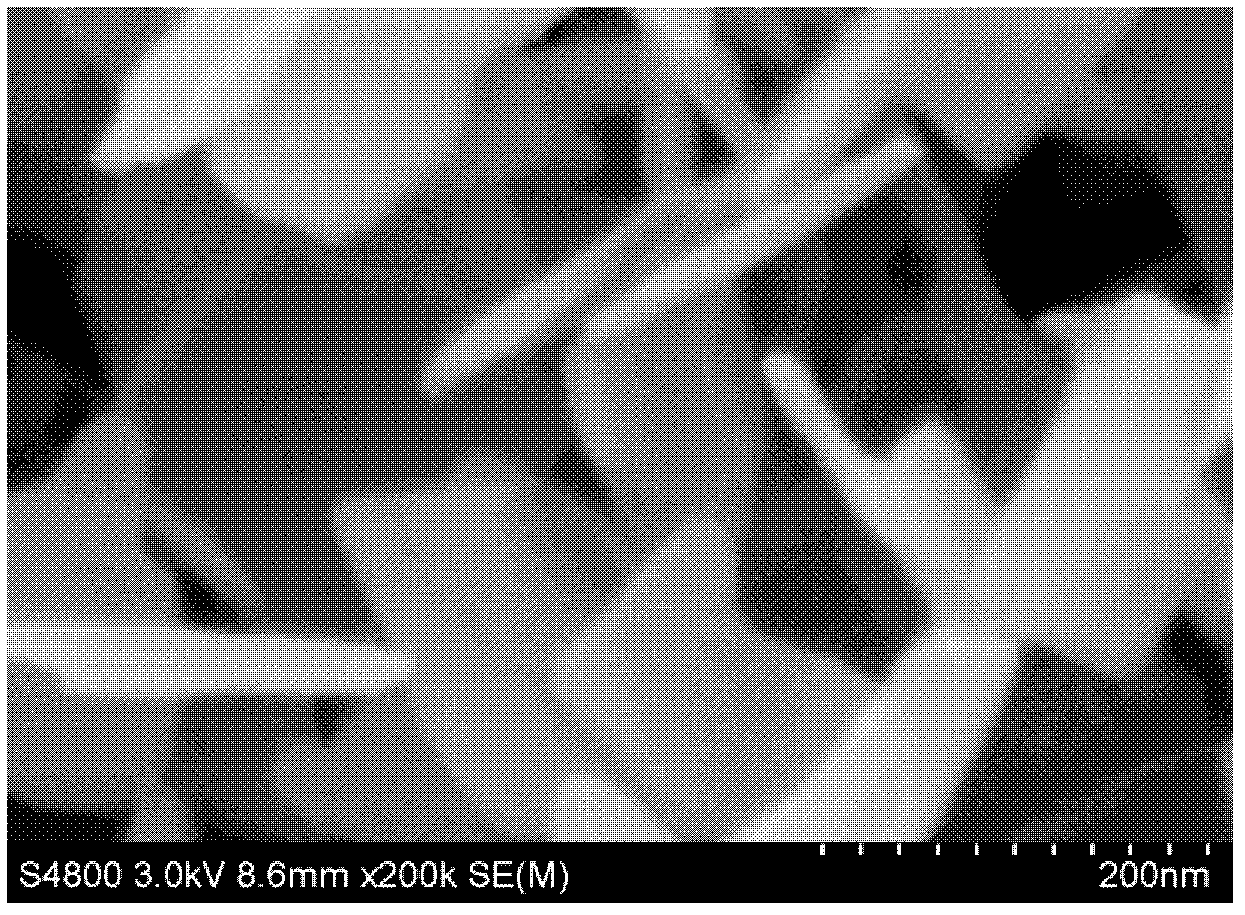
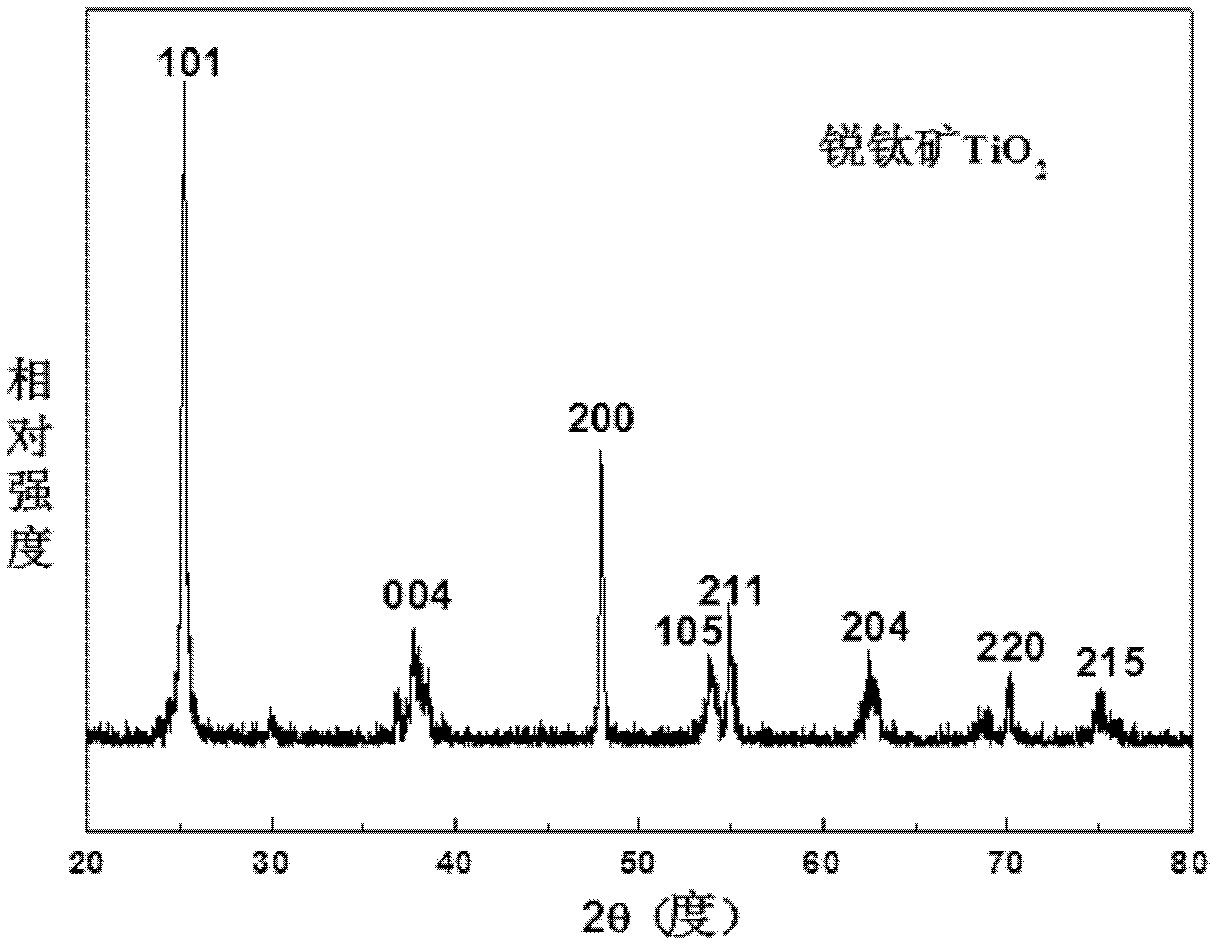
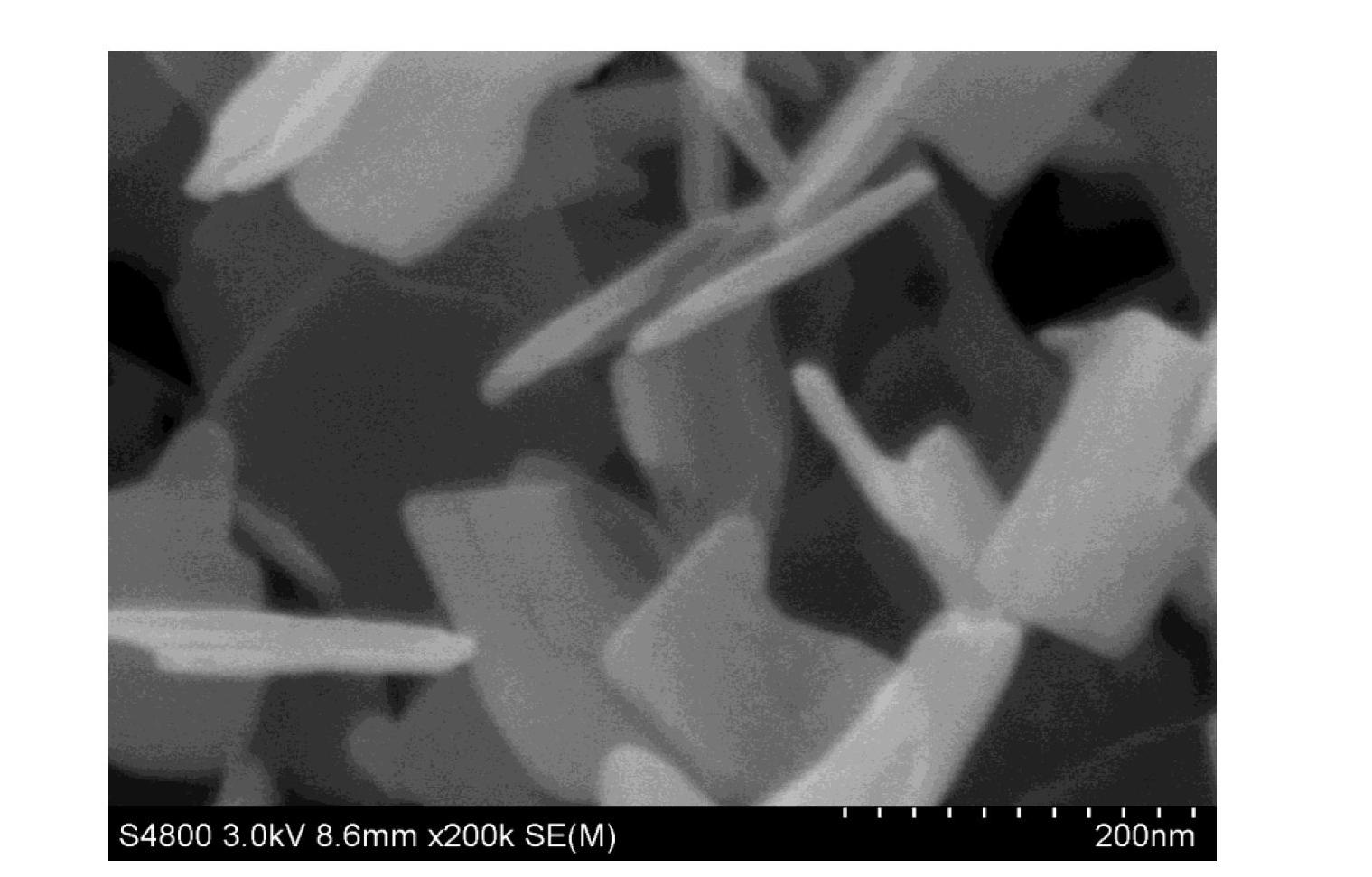
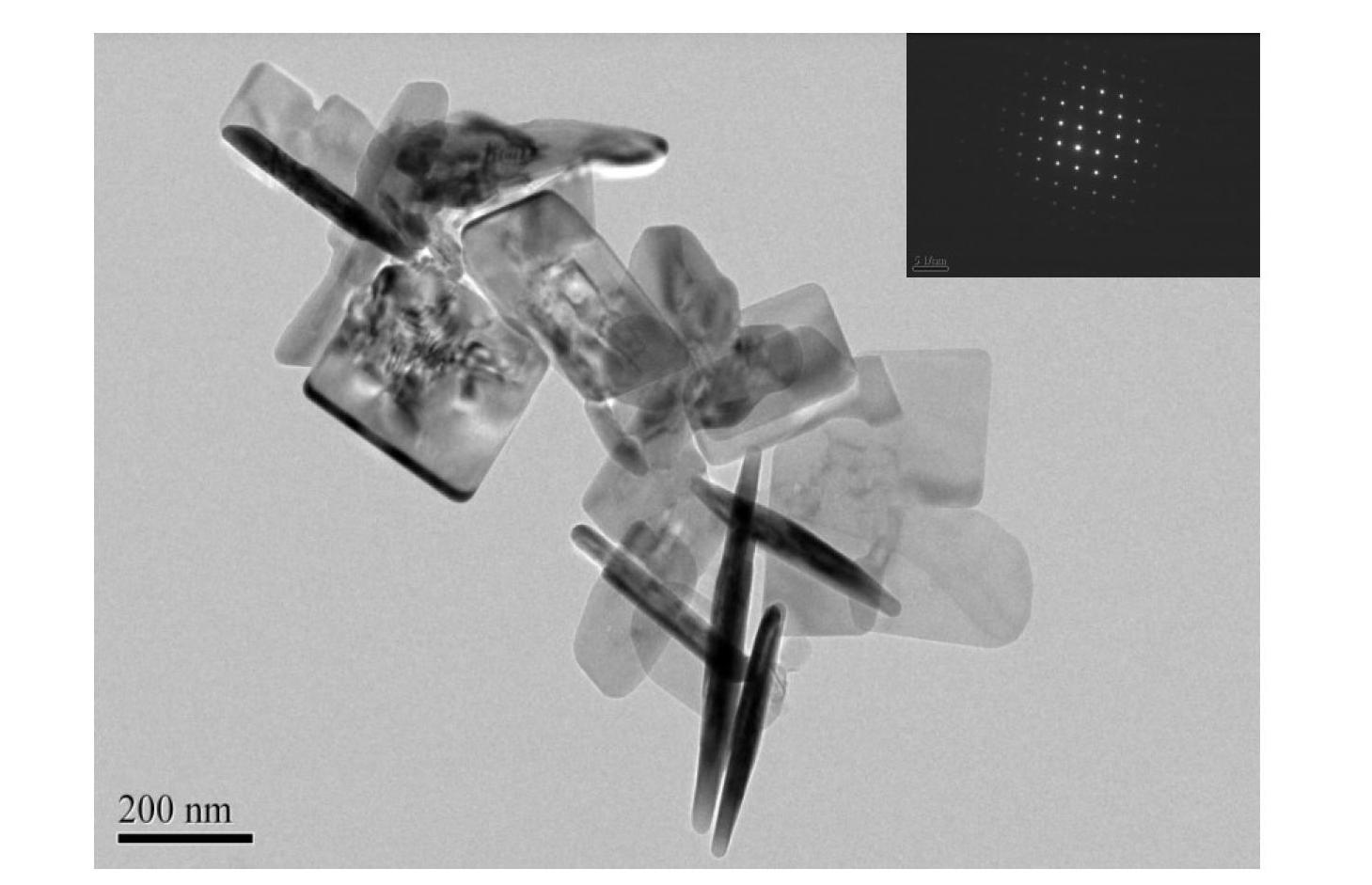
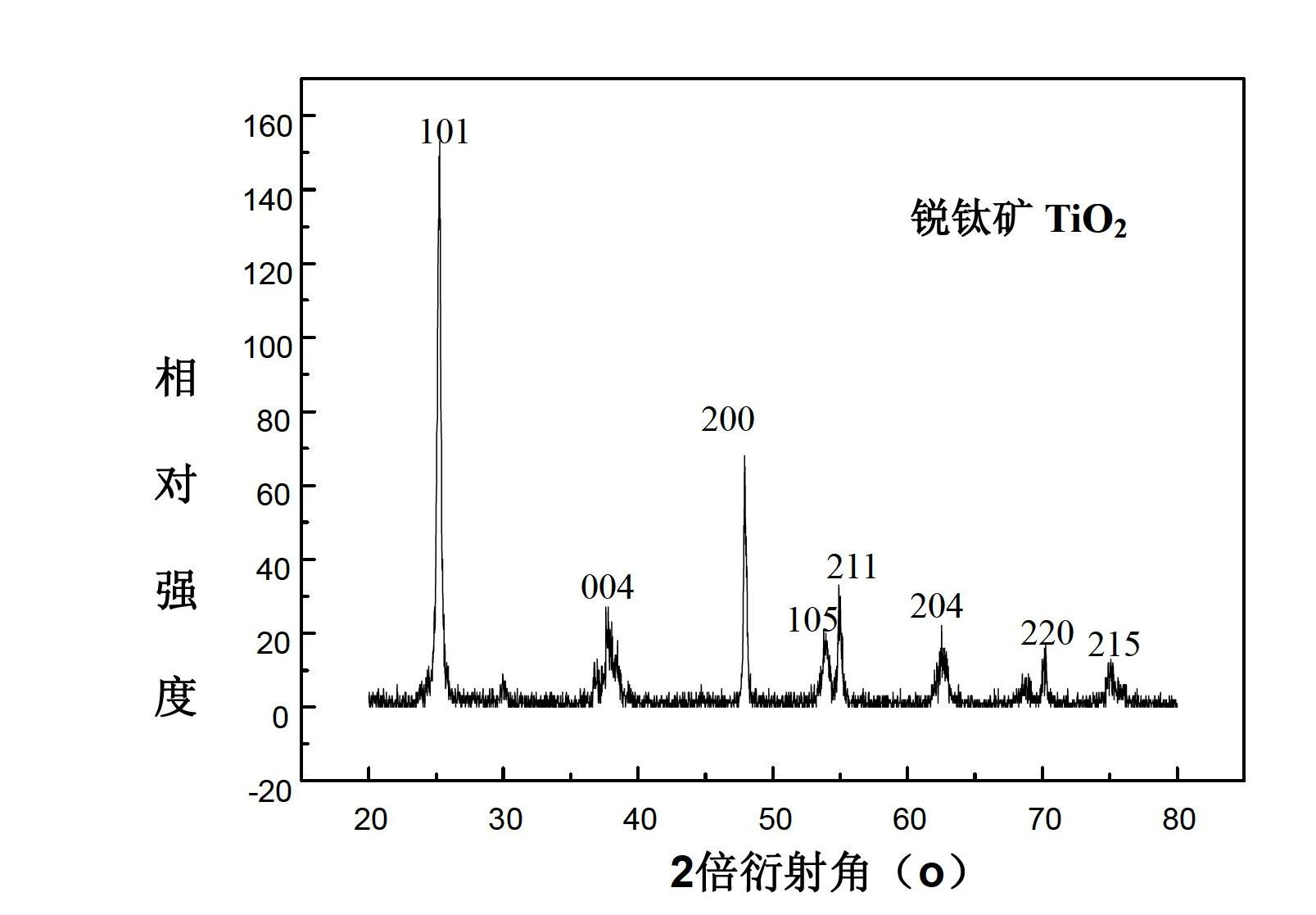
The invention discloses a synthesis technique of a {001}-surface-exposed visible light titanium dioxide nanosheet with oxygen vacancy. The raw material proportion, aging time and temperature and supercritical extraction conditions are controlled to obtain a titanium dioxide nanosheet with favorable degree of crystallization and obvious single-crystal diffraction, wherein the particle sizes are uniform (50-200nm), and the thickness of the nanosheet is 10-20nm. The invention has the advantages of simple preparation process, strong reaction condition controllability and short synthesis time, does not need to introduce other composite semiconductors or dope visible-light-responsive elements in the synthesis process, and does not need treatment after the reaction; and the prepared product has potential application value in the fields of environmental treatment, hydrogen generation by photolyzing water, dye-sensitized solar cells, photoelectric materials and the like.
Owner:SHANGHAI NORMAL UNIVERSITY
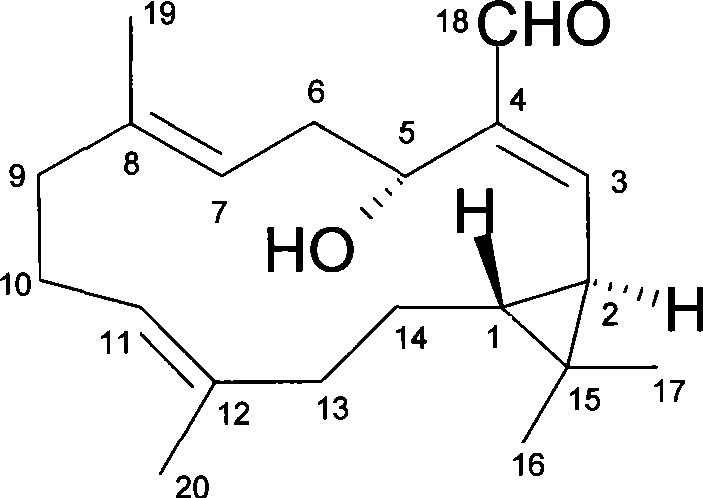
Anti-tumor diterpenoid isoeuphpekinensin in euphorbia perkinensis root and extraction method thereof
InactiveCN101397244ATumor suppressionAldehyde active ingredientsAntineoplastic agentsChemical structureChromatographic separation
The invention discloses an anti-tumor diterpenoid compound isoeuphpekinensin which is separated from euphorbia roots, the chemical structure is as the right formula, the chemical structure and the spatial configuration of the compound are determined via the spectral analysis, the integrated analysis of nuclear magnetic resonance spectroscopy and the X-ray single crystal diffraction technology particularly. The preparation method is as follows: the fresh euphorbia root is cut into slices and dried, then ethanol is used for reflux extraction, the ethanol is recycled under reduced pressure, a liquid extract is obtained, and then the chromatographic separation is carried out on the liquid extract by a silica gel column, thereby obtaining the compound. In vitro anti-tumor experiments show that the compound has significant inhibition effect on human nasopharyngeal carcinomas cells, KB cells and others which are six types of tumor cell lines in total. The invention provides the lead compound for developing new anti-tumor medicaments, thereby having important significance on developing and utilizing medicinal plant resources of China.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE +2
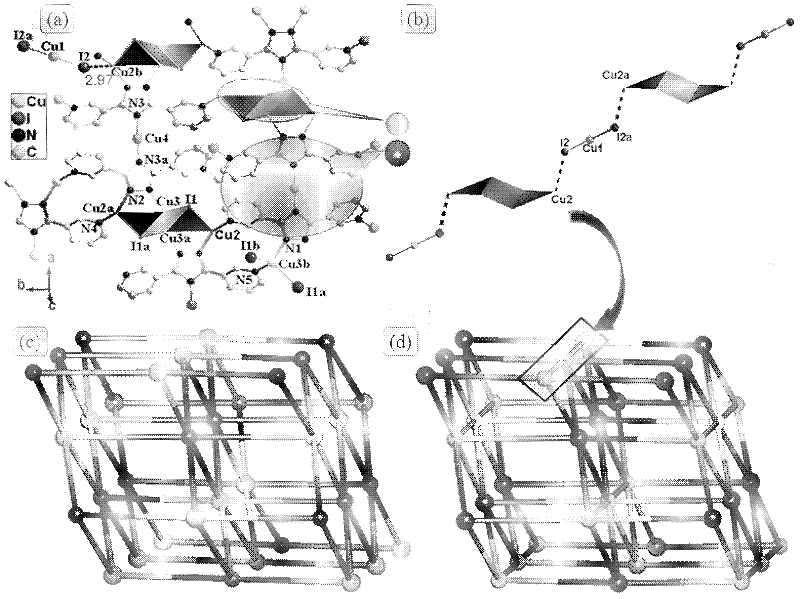
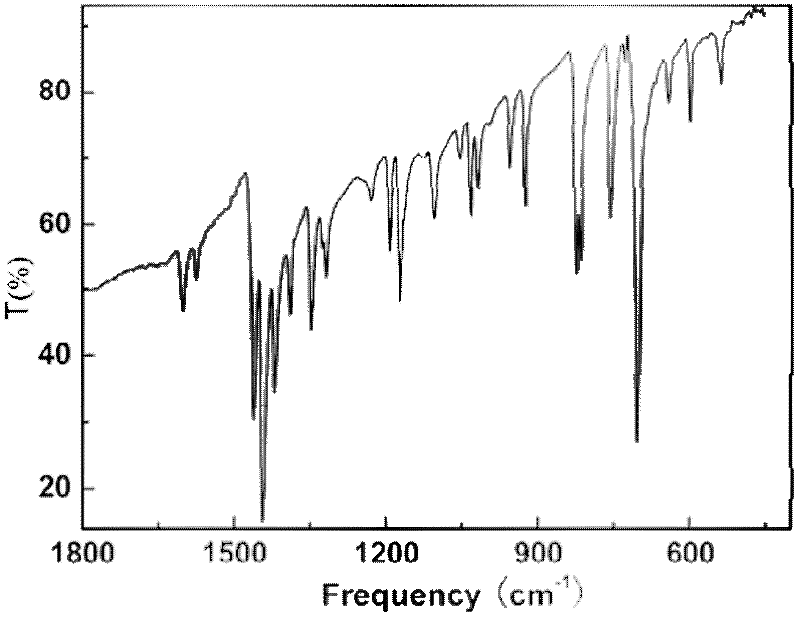
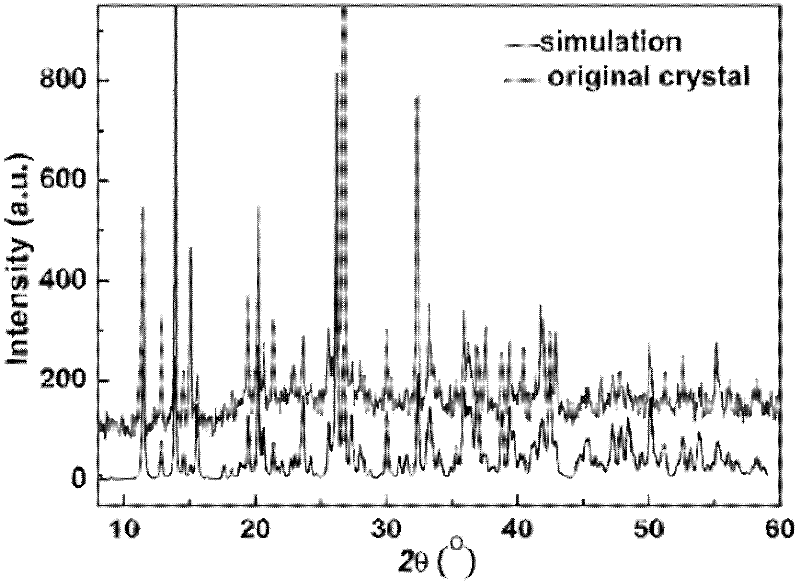
Novel tolyltriazole complex efficient fluorescent crystal material and preparation method thereof
ActiveCN102503960AImprove luminous efficiencyImprove thermal stabilityCopper organic compoundsLuminescent compositionsSingle Crystal DiffractionPhysical chemistry
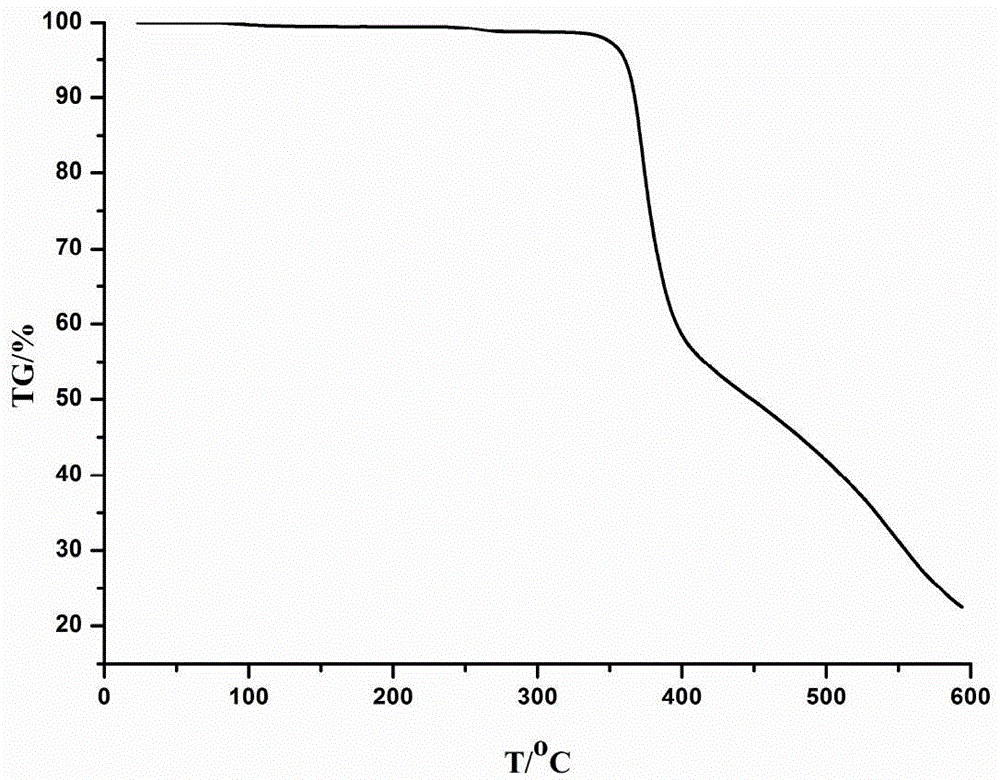
The invention discloses a novel tolyltriazole complex efficient fluorescent crystal material and a preparation method thereof, and relates to the preparation of efficient fluorescent crystal materials. The novel tolyltriazole complex efficient fluorescent crystal material comprises [Cu3(3,5-bi(3-pyridine)-1,2,4-tolyltriazole)I2]n. 3,5-bi(3-pyridine)-1,2,4-tolyltriazole generated by a ring conversion reaction of 2,5-bi(3-pyridine)-1,3,4-oxadiazole is used as a ligand, and a stable [Cu3(3,5-bi(3-pyridine)-1,2,4-tolyltriazole)I2]n crystal material with high luminous efficiency is prepared by a mild hydrothermal method. The single crystal property, the thermal stability and the efficient luminous property of a luminous crystal material are described by X-ray single crystal diffraction, X-ray powder diffraction, an infrared absorption spectrum, a thermogravimetric curve, an excitation spectrum, an emission spectrum, an absorption spectrum and the like. The obtained crystal material can emit bright yellow light under the irradiation action of a fluorescent lamp.
Owner:见喜纳米材料技术研发(河南)有限公司
Synthesis technology for visible light titanium dioxide nanosheet with exposed {001} face and oxygen defects
InactiveCN102659178AEasy to manufactureStrong controllability of reaction conditionsNanotechnologyTitanium dioxideOxygenSolvent
The invention discloses a synthesis technology for a visible light titanium dioxide nanosheet with an exposed {001} face and oxygen defects. The synthesis technology comprises the following steps of: 1-, dissolving a precursor of titanium in a solvent A to obtain a solution B; 2-, ageing the solution B at the temperature of 40 DEG C for two days; and 3-, treating a product after ageing is ended through the supercritical extraction technique to obtain the visible light titanium dioxide nanosheet with the exposed {001} face and the oxygen defects. The synthesis technology for the visible light titanium dioxide nanosheet with the exposed {001} face and the oxygen defects has the advantages that 1-, the preparation process is simple and convenient; the reaction condition is strong in controllability; the synthesis time is short; other compound semiconductors or corresponding elements doped with visible light do not need to be introduced in the synthetic process; and retreatment is not needed after the reaction is ended; 2-, a prepared catalyst is very good in crystalline and has obvious single crystal diffraction. The synthesis technology for the visible light titanium dioxide nanosheet with the exposed {001} face and the oxygen defects has a potential application value in the aspects of environmental control, producing hydrogen by water photolysis, dye sensitized solar cells, photoelectric materials and the like.
Owner:SHANGHAI NORMAL UNIVERSITY
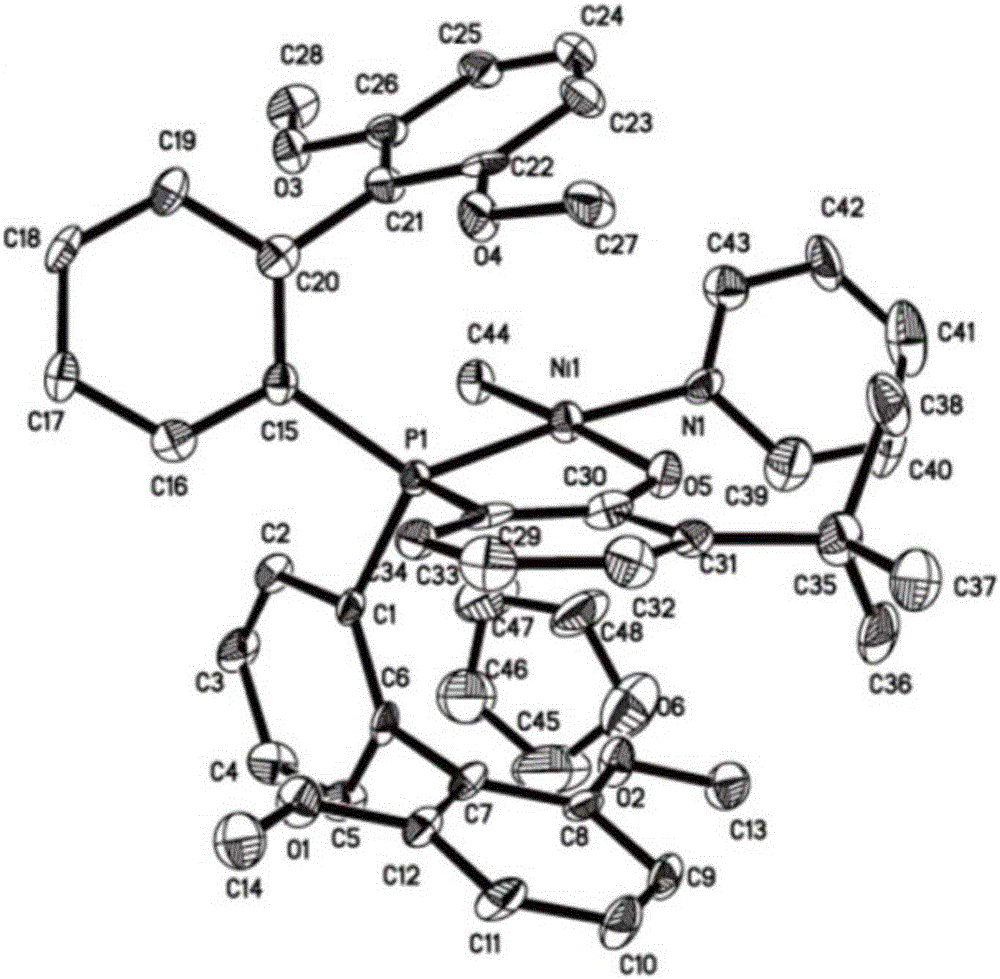
Large-steric-hindrance neutral nickel catalyst, preparation method and application in preparation of ethylene/polar monomer copolymers
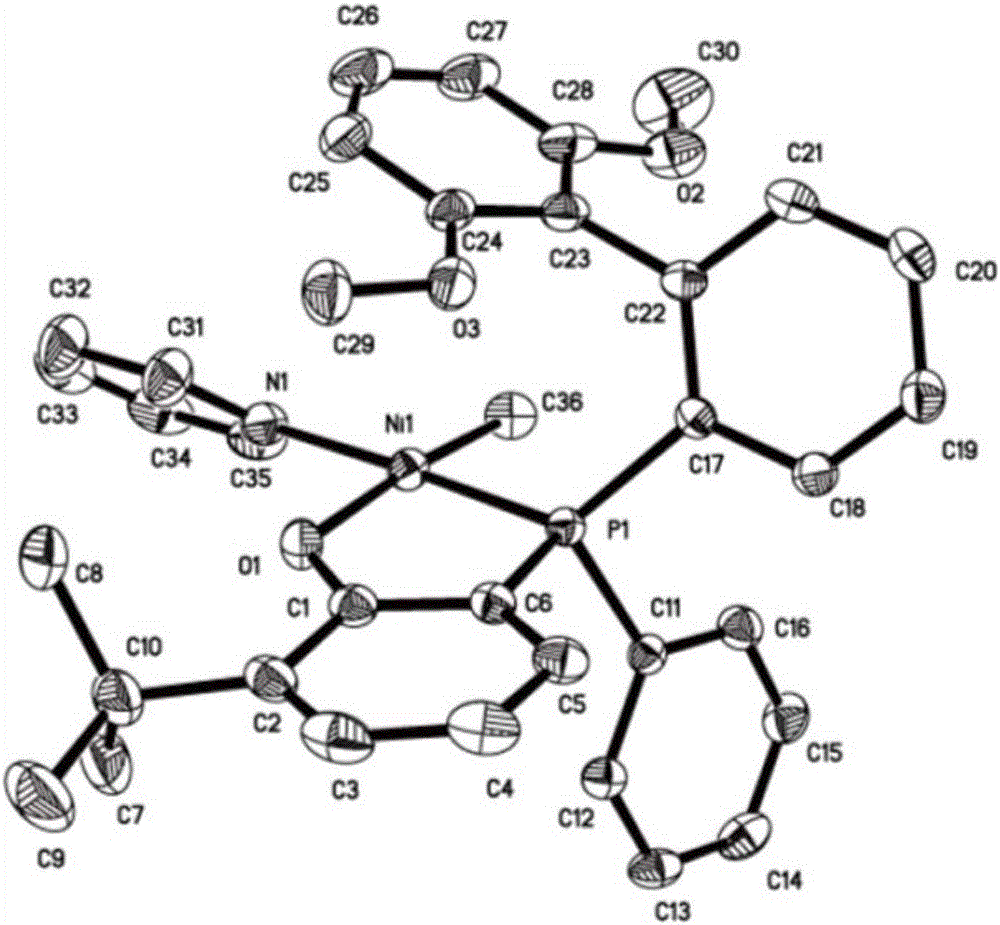
The invention relates to a large-steric-hindrance neutral nickel catalyst, a preparation method and an application in preparation of an ethylene / polar monomer copolymer. A phenol-phosphorous mono-anionic ligand with different substituents of R1, R2 and R3 is dissolved in a good solvent and added to a good solvent of a metal nickel source for a reaction, and the catalyst is obtained, and X-ray single crystal diffraction indicates that an obtained complex adopts a plane quadrangle structure. The synthesized catalyst single component is used for triggering ethylene polymerization. An obtained polymer is a high-molecular-weight linear polymer with the molecular weight up to 770 kg / mol. Copolymerization of polar monomers such as ethylene, methyl acrylate and the like is realized, the copolymer with different polar monomer insertion rates is obtained, and the polar monomer insertion rate is up to 8mol%. Experimental results indicate that the polar monomers are inserted in polymer chain tails and molecular chains, which is the major breakthrough of the neutral nickel catalyst in the olefin polymerization history.
Owner:TIANJIN UNIV
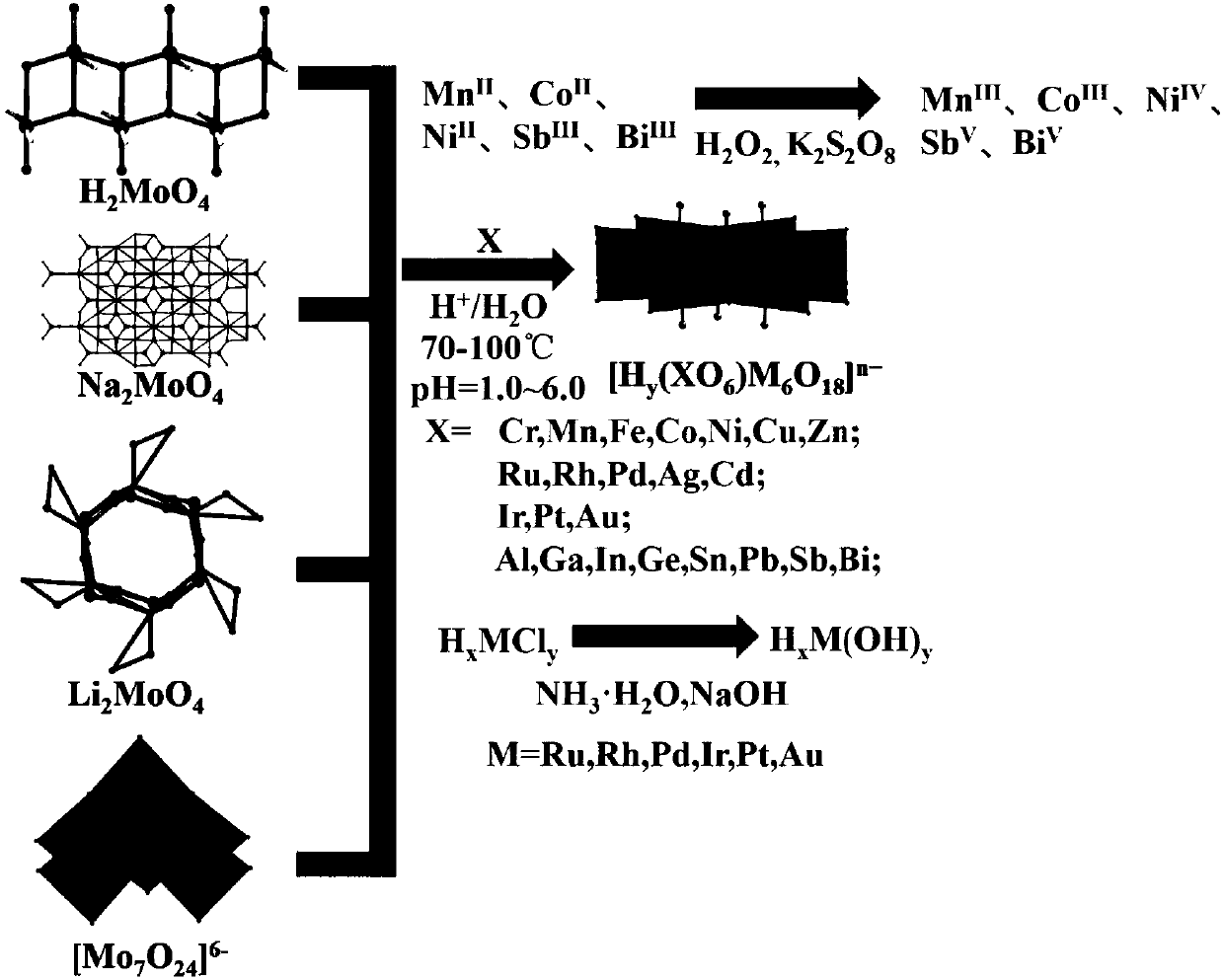
Method for preparing protonated B-type Anderson-type heteropoly acid by aqueous phase method
InactiveCN109847775AHigh yieldHigh purityPhysical/chemical process catalystsHeteropoly acidSolid acid
The invention provides a universal method for preparing a protonated B-type Anderson-type heteropoly acid by an aqueous phase method. A commercialized water-soluble molybdate is used as the reaction raw material, the heteropoly acid is prepared by adjusting pH in a system through an inorganic acid and adding a corresponding central heteroatom metal salt, and the central heteroatom includes most oftransition metals and main group metals. The method is highly controllable, and simple and easy to operate, the yield is up to 95%, the purity is high, and the large-scale production in a kilogram scale can be achieved. The topological structure of the heteropoly acid is determined by single crystal diffraction. The B-type Anderson-type heteropoly acid (NH4)n[Hy(XO6)Mo6O18] can be used as a special solid acid catalyst, and has broad application prospects in the fields of chemistry, life, environmental science and the like. Different from traditional methods that organic solvents are used in the production process, the method uses water as the solvent, and the preparation is carried out under mild conditions, so that the method is environmentally friendly, has economic benefits, and is inline with the development direction of green production advocated by the state.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
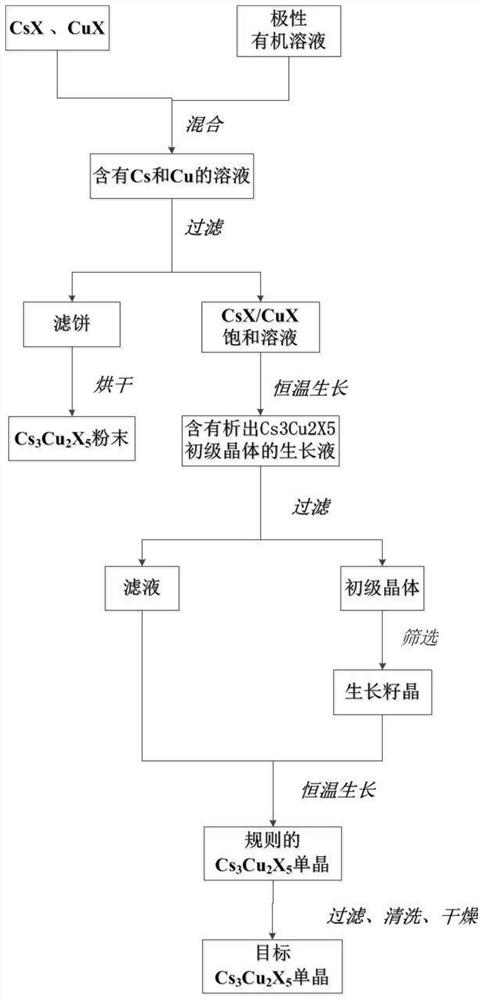
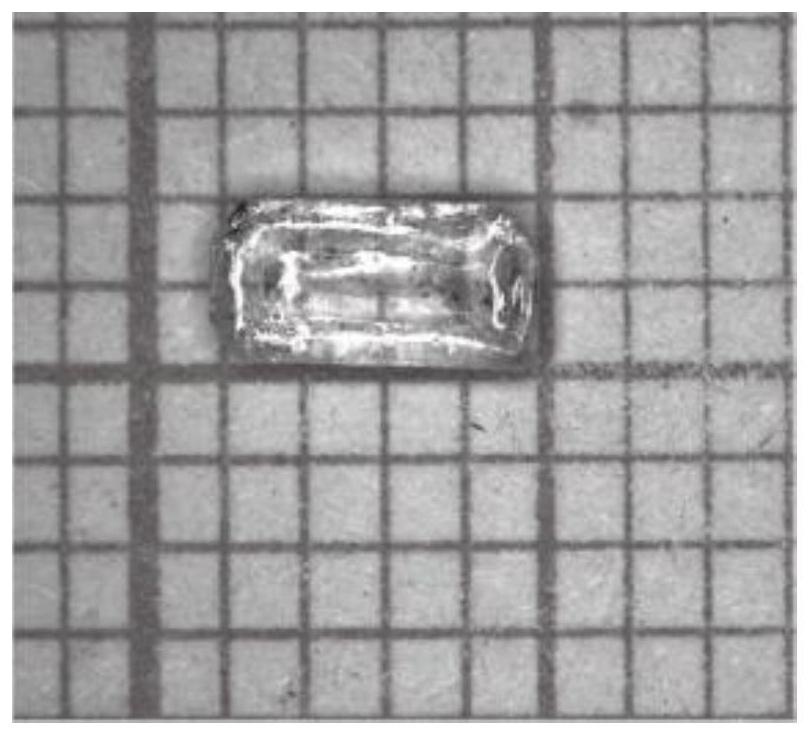
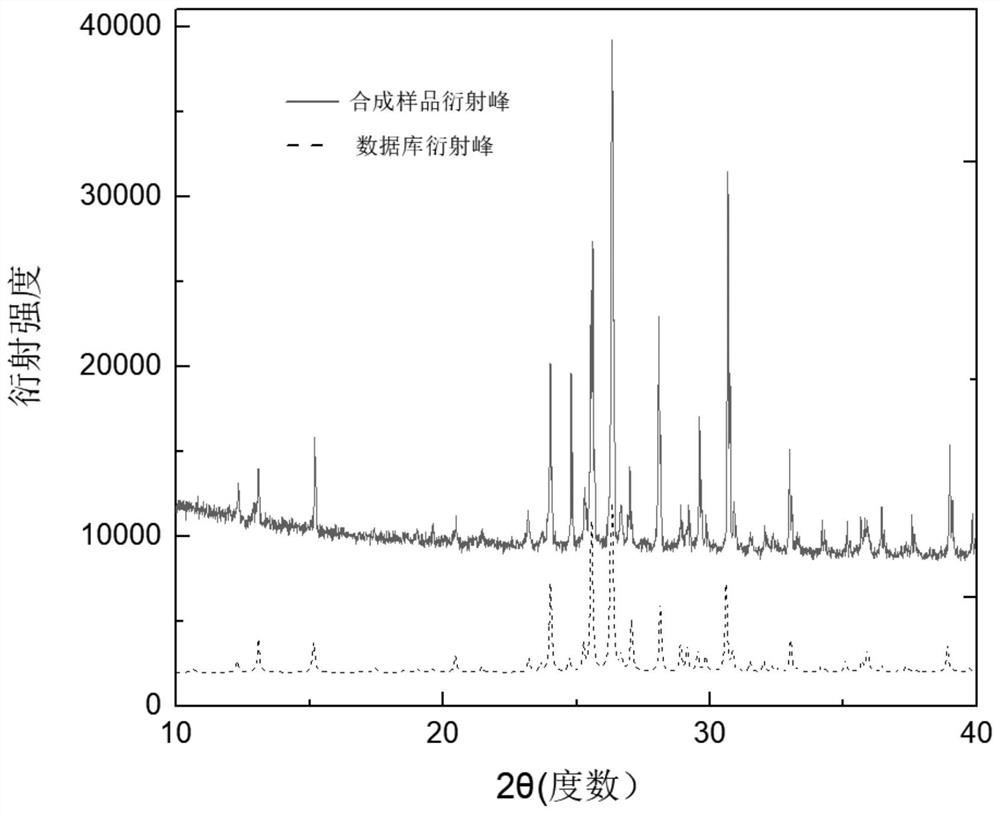
Method for growing copper-based lead-free perovskite single crystal by low-temperature solvent method
ActiveCN112853466AGrow fastStable growthPolycrystalline material growthFrom normal temperature solutionsSingle Crystal DiffractionPhysical chemistry
According to the method for growing the copper-based lead-free perovskite single crystal through the low-temperature solvent method, an organic mixed solution is used as a crystal growth solvent, and the high-quality and large-size Cs3Cu2X5 single crystal can be rapidly grown through the reaction of CsX and CuX under the condition that the temperature is lower than 100 DEG C. The method disclosed by the invention has the characteristics of short manufacturing period, low cost, easiness in operation, environmental friendliness and the like, the size of the synthesized crystal can reach a super-centimeter magnitude, and the diffraction angle of the grown single crystal is less than 0.03 degree.
Owner:江苏第二师范学院
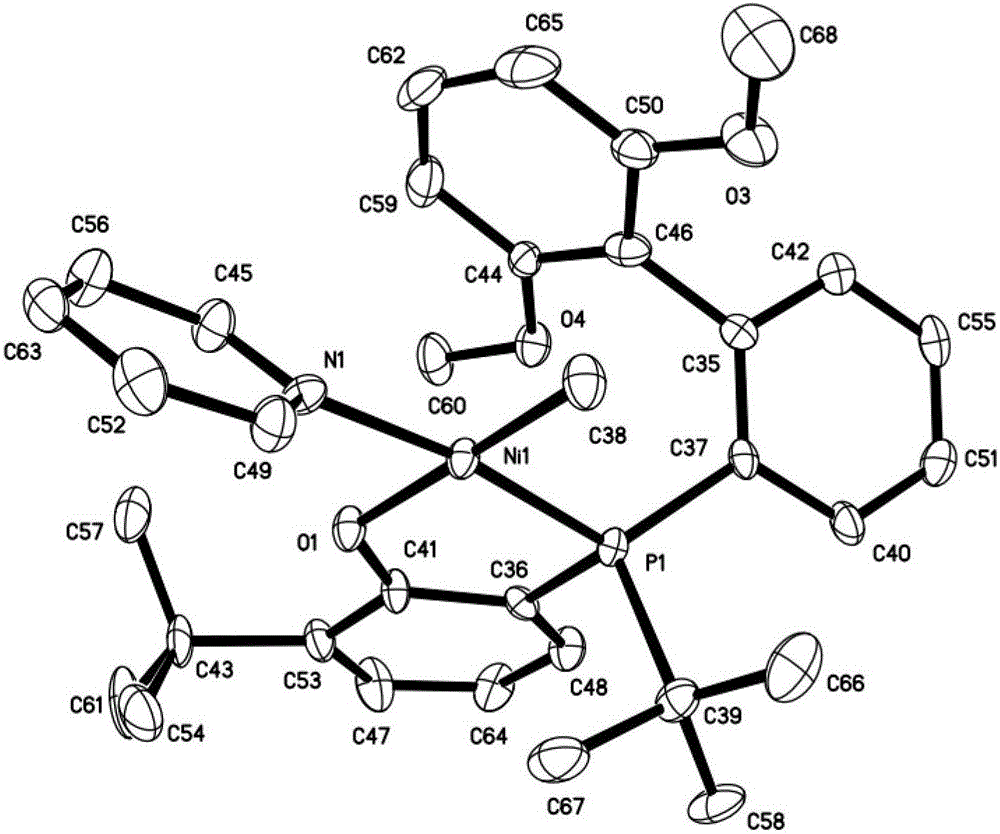
Bipyridine bisphenol-aluminum catalyst for preparing unsaturated polyester and preparation method
The invention relates to a bipyridine bisphenol-aluminum catalyst for preparing unsaturated polyester and a preparation method. The structural formula of the bipyridine bisphenol-aluminum complex is shown as I; wherein the X group is Cl, CH3COO, CF3COO or NO3, and the R1 group, the R2 group, the R3 group and the R4 group are simultaneously or independently H, a halogen atom, an alkoxy group, an alkane group or a substituted alkane group, aromatic hydrocarbon groups or substituted aromatic hydrocarbon groups. The preparation method comprises the following steps: preparing a bipyridine bisphenolligand with R1, R2, R3 and R4 through reaction of 2, 4-disubstituted phenol; and dissolving in a good solvent, adding into a good solvent of a metal aluminum source, and reacting to obtain the catalyst, and X-ray single crystal diffraction shows that the coordination compound has a distorted tetragonal pyramid structure. Unsaturated polyester is prepared by using the synthesized catalyst. The polyester segment content of the obtained unsaturated polyester is greater than 99%, and the catalyst can be further copolymerized with a vinyl monomer to prepare unsaturated polyester resin.
Owner:TIANJIN UNIV
Preparation method of multicolor luminous fluorescent crystal material for imidazole and silver complex
ActiveCN105885829ASimple processLow costGroup 1/11 organic compounds without C-metal linkagesOrganic chemistry methodsFluorescenceX-ray
The invention relates to efficient multicolor luminous fluorescent crystal material for preparing novel imidazole and silver complex and preparation method of the material. [Ag2(1,4-bis(2-methyl-imidazole-1yl)butane)Br2]n or [Ag2(1,4-bis(2-methyl-imidazole-1yl)butane)I2]n crystal material that is stable and high in luminous efficiency is prepared by means of coordination between 1,4-bis(2-methyl-imidazole-1-yl)butane and silver halide through a mild hydrothermal process. Through X-ray single crystal diffraction, X-ray powder diffraction, infrared absorption spectrum, thermogravimetric curve, excitation spectrum, emission spectrum and the like, it is proved that the luminous crystal material has single crystal characteristics, thermal stability and efficient multicolor luminous characteristic. Two isostructural multicolor luminous fluorescent crystal materials obtained can emit bright light yellow and deep yellow beams when exposed to a handheld ultraviolet lamp at room temperature.
Owner:NANJING UNIV OF TECH
Method for preparing high-purity topological insulator YbB6 single crystal
InactiveCN105350075AHigh purityQuality improvementPolycrystalline material growthBy zone-melting liquidsSingle Crystal DiffractionDiffractometer
The invention discloses a method for preparing a high-purity topological insulator YbB6 single crystal, and belongs to the technical field of topological insulator materials. At present, preparation and study on the high-purity topological insulator YbB6 single crystal are rarely carried out, the preparation process is complex, and the single crystal is poor in quality, low in purity and small in size, and can hardly be actually applied. The method of combining plasma activated sintering and suspension zone melting is adopted, and the high-quality, high-purity and large-size YbB6 single crystal is prepared in the high vacuum environment. High-purity YbB6 powder is adopted as initial raw materials, the prepared YbB6 single crystal is a cylinder with phi of 4.5 mm*20 mm, and it is shown through the testing result of a single-crystal diffractometer that the quality of the single crystal is good.
Owner:BEIJING UNIV OF TECH
Eutectic crystal of calcifediol and vitamin D3 as well as preparation method and application thereof
InactiveCN108129371AGood chemical stabilityImprove complianceOrganic active ingredientsMetabolism disorderSingle Crystal DiffractionX-ray
The invention relates to a eutectic crystal of calcifediol and vitamin D3 as well as a preparation method and application thereof. In the eutectic crystal, the mole ratio of the calcifediol to the vitamin D3 is 1 to 1. The eutectic crystal of the calcifediol and the vitamin D3 is subjected to comprehensive characterization by applying measures such as X-ray single crystal diffraction, X-ray powderdiffraction, thermogravimetric analysis, differential scanning calorimetry and infrared spectroscopic analysis, thereby finding that the chemical stability of the vitamin D3 of the eutectic crystal can be obviously improved under an illumination condition. The preparation method of the eutectic crystal of the calcifediol and the vitamin D3 is simple, and the eutectic crystal can be repeatedly prepared.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Pyrazinamide derivatives as well as preparation method and uses thereof
InactiveCN101182312AEasy to makeHigh yieldOrganic chemistrySurface/boundary effectAtomic force microscopyInfrared
The present invention relates to a novel pyrazinamide derivative and more particularly a hydrated crystal of N, N'-(4-nitrogen heterocyclic-1, 7-heptyl) double-pyrazinamide compound, the structure and characteristic of which are mensurated by X-ray single-crystal diffraction, X-ray powder diffraction, thermogravimetric analysis, infrared spectrum, nuclear magnetism and element analysis; a molecular formula of the hydrated crystal is C8H18.50N3.50O5, the crystal belongs to the orthorhombic system, a space group is P2<1>2<1>2, unit cell parameters are that a is 11.164, b is 26.05, c is 4.582, and alpha, beta and gamma are equal to 90 degrees; unit cell size is that V is 1332.4 <3>. The preparation process of the hydrated crystal is simple; the crystal has high yield and purity and good stability. The pyrazinamide derivative can form a two-component crystal with the water absorption quantity of 29.56 percent, and the absorption of organic compound towards water molecule has the reversibility, which ensures that the compound can be applied to the recycling technology of worn atomic force microscopy silicon tips.
Owner:TIANJIN NORMAL UNIVERSITY
Preparation method and application of cadmium-based metal organic framework Cd-MOF material
InactiveCN112300400AImprove flexibilityLarge conjugated structureFluorescence/phosphorescenceLuminescent compositionsCarboxyl radicalMetal-organic framework
The invention provides a preparation method and application of a cadmium-based metal organic framework Cd-MOF material. The preparation method is characterized by comprising the following steps: step1, dissolving cadmium nitrate in water, dissolving a ligand 5-(bis(4-carboxylbenzyl)amino isophthalic acid in N,N-diethylformamide, and mixing the ligand 5-(bis(4-carboxylbenzyl)amino isophthalic acidand N,N-diethylformamide with ethanol; step 2, pouring the mixed solution into a polytetrafluoroethylene reaction kettle lining, and carrying out ultrasonic treatment; step 3, putting the reaction kettle into a blast drying oven for heating; and step 4, cooling the reaction kettle to room temperature, and filtering the reaction solution to obtain the yellow petal-shaped Cd-MOF crystal. The synthesis method of the Cd-MOF fluorescent material is simple, the yield and the purity are high, the accurate structure of MOF can be analyzed through single crystal diffraction, and the Cd-MOF fluorescentmaterial synthesized by the method has the characteristics of simple operation, less time consumption and low cost when alpha,beta-unsaturated aldehyde and saturated aldehyde are distinguished and detected.
Owner:XUCHANG UNIV
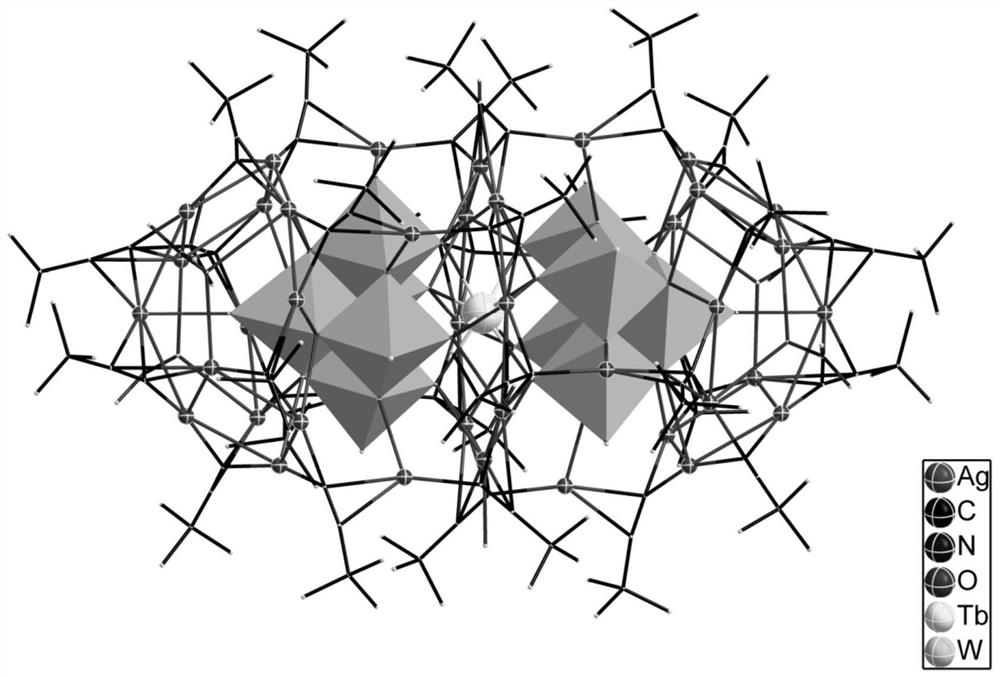
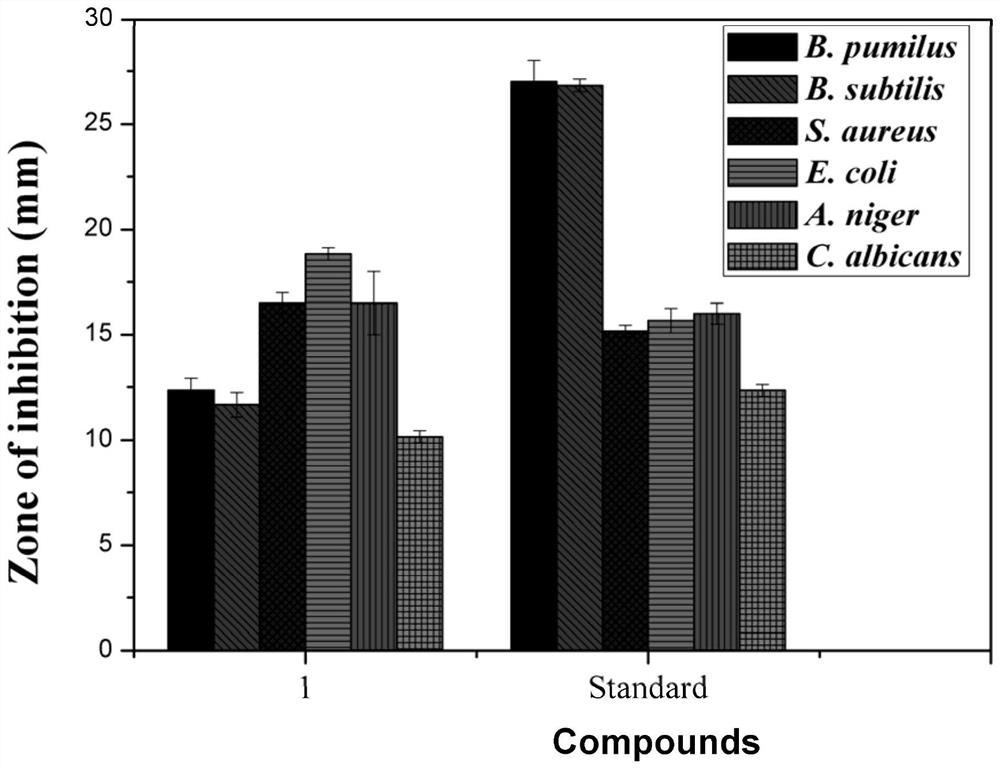
Rare earth-silver nanocluster antibacterial agent and preparation method thereof
PendingCN112690286AOvercome the lightOvercoming heat sensitivityBiocideTransportation and packagingInhibition zoneSingle crystal
The invention discloses a novel rare earth-silver nanocluster antibacterial agent and a preparation method thereof. According to the invention, rare earth and silver elements with antibacterial properties are combined together to form the rare earth-containing silver nanocluster. A novel rare earth-silver nanocluster antibacterial agent is developed by utilizing the synergistic effect of the rare earth and silver elements. The antibacterial agent is a microcrystalline product, the microstructure of the antibacterial agent is analyzed by an X single crystal diffractometer, and a certain contribution can be made to the research of the structure-activity relationship of the rare earth element antibacterial mechanism. The inhibition zone method test shows that the antibacterial property of the nanocluster on common bacteria and fungi is equivalent to that of a common antibacterial agent gentamycin / amphotericin. The prepared rare earth-silver nanocluster antibacterial agent can be applied to various fields of antibacterial macromolecules, antibacterial coatings, antibacterial fabrics and the like, and then is used for manufacturing ceramic bathroom accessories, fresh air systems, indoor decoration coatings, clothes and other products.
Owner:HUAQIAO UNIVERSITY +1
Synthetic method of phosphonyl methylene substituted five-membered cyclic compound
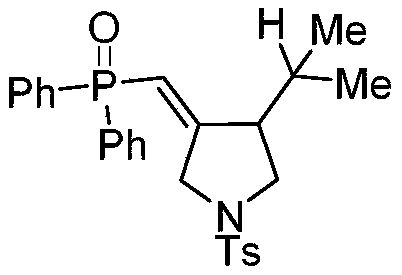
ActiveCN111072720ARaw materials are cheap and easy to getIn line with the concept of green environmental protectionGroup 5/15 element organic compoundsOrganic dyeAcyl group
The invention discloses a synthetic method of a phosphonyl methylene substituted five-membered cyclic compound. According to the method, 1,6-enyne and diaryloxy phosphorus are taken as reaction raw materials, an organic dye is taken as a photocatalyst, a reaction solvent is added, an illumination reaction is carried out under a nitrogen protection condition, a target product is synthesized, and the structure of the target product is represented and analyzed through IR, 1H NMR, 13C NMR, HRMS and X-ray single crystal diffraction. The method does not need any additive, high temperature and otherharsh and complex reaction conditions, and has the advantages of mild reaction conditions, simple operation, convenient subsequent treatment, easily available raw materials, easy derivatization to obtain different types of organic compounds, and the like.
Owner:YANGZHOU UNIV
Sesquiterpene and preparation method and use of sesquiterpene
The invention discloses a sesquiterpene and a preparation method and use of the sesquiterpene as an antitumor drug. The preparation method comprises the following steps: by taking dicotyledoneae asterales compositae eupatorium crofton weed all grass as a raw material, extracting by ethyl acetate; after concentration, carrying out silica gel column chromatography; determining the compound which is a novel sesquiterpene Eupatorium adenophorum B through detection by hydrogen spectrum and carbon spectrum of nuclear magnetic resonance as well as X-single crystal diffraction. Cell experiments show that the novel compound has a certain inhibiting effect to human lung cancer cells A549 and H1299 and human cervical carcinoma cell HeLa.
Owner:成都格纯生物医药有限公司
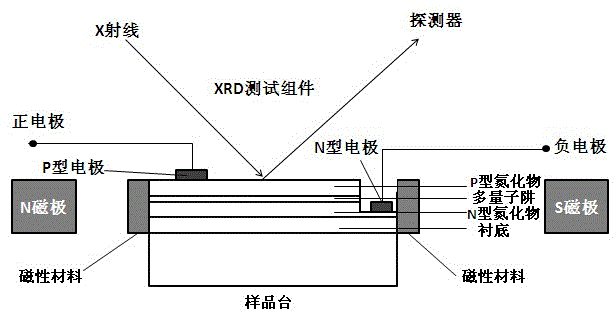
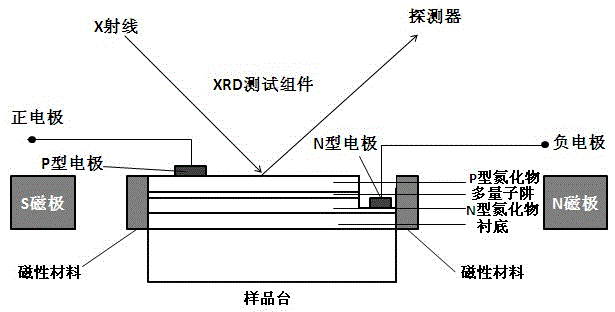
In-situ test system of nitride light emitting diode magnetoelectricity stress coupling
ActiveCN105606982AImprove luminous efficiencyIncrease stressIndividual semiconductor device testingSingle crystalParticle physics
The invention discloses an in-situ test system of nitride light emitting diode magnetoelectricity stress coupling. The system comprises a nitride light emitting diode, an additional current source, an XRD test component and a magnetic field generation apparatus. The system is characterized in that a magnetic material is formed on a side wall of the nitride light emitting diode; a current is added to two ends of a P-type electrode and an N-type electrode of the nitride light emitting diode and the nitride light emitting diode is placed on a sample stage of the XRD test component; the magnetic field generation apparatus generates a magnetic field around the nitride light emitting diode so that the nitride light emitting diode generates a magnetic field-electric field-stress coupling effect; XRD single crystal diffraction is used to test a lattice constant of the light emitting diode in a magnetic field and electric field change process in an in-situ test mode so as to carry out in-situ control on the magnetic field and the electric field and regulate and control a stress change of a nitride so that the light emitting diode acquires highest luminescence efficiency.
Owner:XIAMEN SANAN OPTOELECTRONICS TECH CO LTD
Two enantiomers of 2,6-diamido triptycene and detection and separation method thereof
The invention provides a detection and separation method of two enantiomers of 2,6-diamido triptycene with three-dimensional rigid structures and confirmation of absolute configurations thereof. Bridgehead carbons at the ninth sites and the tenth sites of the structures of the two enantiomers of the 2,6-diamido triptycene are chiral carbon atoms. According to the detection and separation method of the two enantiomers of the 2,6-diamido triptycene with the three-dimensional rigid structures and the confirmation of the absolute configurations thereof provided by the invention, high performance liquid chromatography (HPLC) is adopted, a chiral column is utilized for separating the two enantiomers, two optical voidness isomer components with ee percent being larger than 98 percent are obtained, and through X-ray single crystal diffraction analysis, the absolute configurations are confirmed as an (S,S) type and an (R, R) type, i.e., the two enantiomers are sequentially (9S, 10S)-9,10-dihydro-9,10-[1,2]benzanthracene-2,6-diamine and (9R, 10R)-9,10-dihydro-9,10-[1,2]benzanthracene-2,6-diamine. The prepared enantiomers can be used as key constructing units for synthesizing a chiral catalyst.
Owner:HUAZHONG UNIV OF SCI & TECH
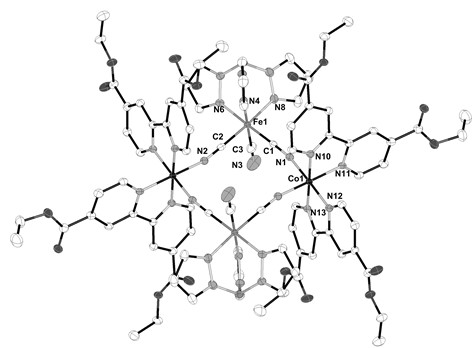
Zero-dimension linear five-core Ln2Co3 magnetic material and preparation method thereof
ActiveCN105669774ALess prone to magnetic interactionsImprove isolationCobalt organic compoundsOrganic/organic-metallic materials magnetismSingle Crystal DiffractionSynthesis methods
The invention provides a zero-dimension linear five-core Ln2Co3 magnetic material and a preparation method thereof. The magnetic material is a compound; the chemical formula of the compound is [Ho2Co3(C7H4ClO2)<12>(C<10>H8N2)2]; in the compound, five metal irons are in Co1-Ho1-Co2-Ho1a-Co1a linear arrangement. According to the preparation method, Ho(NO3)3.6H2O, CoCl2.(H2O)6, 4-chlorobenzoic acid (L) and bipy are added into a mixed solvent of H2O and C2H5OH; the pH is regulated to 5.0; after the materials are sufficiently and uniformly stirred, the materials are heated to 160 DEG C; the constant temperature is maintained for three days; then, program temperature reduction is performed to the room temperature; filtering and drying are carried out, and mauve blocky crystals suitable for single crystal diffraction can be obtained. The magnetic material provided by the invention has the advantages that the synthesis method is simple; the yield is high; the product is stable; the method is suitable for mass production. The magnetic test shows that the compound is a ferromagnetic material, and has great potential application values in the field of magnetic materials.
Owner:HUAIBEI NORMAL UNIVERSITY
Methanol indicator and preparation method thereof
InactiveCN102675373AExtensive inspectionGas chromatography is simple and fastMaterial analysis by observing effect on chemical indicatorCobalt organic compoundsX-raySingle crystal
The invention relates to a methanol indicator and a preparation method thereof. The methanol indicator disclosed by the invention is Fe2Co2 four-core compound bridged by cyano group and has a chemical formula of [Co2Fe2(CN)6(Tp)2(4,4'-DCEB)4](ClO4)2, wherein Tp is triparazole borate; and DCEB is 2,2-bipyridyl-4,4-ethylene terephthalate. The methanol indicator is displayed to be red in a general state and is immediately turned into green only when the methanol indicator is in contact with a methanol solvent. The structure of a compound which is displayed to be green after the methanol indicator is in contact with the methanol solvent is determined by X-ray single crystal diffraction and is verified to a square planar Fe2Co2 four-core compound bridged by the cyano group. The square planar Fe2Co2 four-core compound has a molecular formula of [Co2Fe2(CN)6(Tp)2(4,4'-DCEB)4](ClO4)2.2MeOH, wherein MeOH is methanol. The methanol indicator disclosed by the invention shows highly-selective response to methanol molecules and has no response to other various alcohol solvents and other various non-alcohol solvents. The methanol indicator disclosed by the invention has the advantages of simpleness in synthesis, easy operation, quickness in response, distinct color change and indication, capability of recycling and the like.
Owner:HUAZHONG NORMAL UNIV
Crystal form of salt formed from vinpocetine and phosphoric acid, and preparation method
InactiveCN105153147AImprove solubilityImprove stabilityOrganic chemistry methodsSolubilityO-Phosphoric Acid
The invention discloses a crystal form of a salt formed from vinpocetine and phosphoric acid, and a preparation method, and relates to the technical field of discovery and preparation of a crystal form of a pharmaceutical salt. The prepared salt formed from vinpocetine and phosphoric acid is detected through analytical methods, such as thermal gravimetric analysis (TGA), X-ray powder diffraction (P-XRD), X-ray single crystal diffraction (S-XRD), differential scanning calorimetry (DSC), and infrared spectroscopy (IR). Vinpocetine is a indissolvable medicine (the solubility of which is about 5 [mu]g / mL and pKa of which is 7.31), while it is proved through detection that the salt prepared from vinpocetine and phosphoric acid has excellent solubility and stability. Development of the novel salt form establishes foundation for development and application of new formulation.
Owner:JIANGSU QINGJIANG PHARMA
Method for synthesizing sulfonyl-substituted pyran type compound
The invention discloses a method for synthesizing a paratoluensulfonyl chloride substituted pyran type compound. The method comprises the following steps: adding paratoluensulfonyl chloride, 3-methyl-1-(propyl-2-alkynyl-1-oxy)-2-butene with different substituted groups, water and a photocatalyst to a solvent, performing reaction at room temperature under an illumination condition, performing a TLCmonitoring reaction process, and after the reaction is completed, performing column chromatography separation and purification to obtain the product. The method adopts novel light-catalyzed reactionand has the advantages of mild reaction conditions, simple operation, convenient subsequent treatment and the like, and the structure of the prepared product is characterized and analyzed through IR (Infrared Ray), 1H NMR (Nuclear Magnetic Resonance), 13C NMR, X-Ray single crystal diffraction and high resolution mass spectrum.
Owner:YANGZHOU UNIV
Crystal form and preparation method of salt prepared from vinpocetine and perchloric acid
ActiveCN105061419AImprove solubilityImprove stabilityOrganic chemistry methodsSingle Crystal DiffractionVinpocetine
The invention relates to a crystal form and a preparation method of a salt prepared from vinpocetine and perchloric acid, and belongs to the technical field of crystal form finding and preparation of medicinal salts. A result of detection through thermogravimetic analysis (TGA), X-ray powder diffraction (P-XRD), X-ray single crystal diffraction (S-XRD), differential scanning calorimetry (DSC) and infrared spectroscopy (IR) shows that the salt is the salt prepared from vinpocetine and perchloric acid. Vinpocetine is an insoluble drug (with the solubility is approximately equal to 5[mu]g / mL and pKa is 7.31), and a result of test of the salt prepared from vinpocetine and perchloric acid shows that the salt has good solubility and stability. The new dosage form of the salt is exploited, and the method lays a foundation for exploitation and application of new dosage forms.
Owner:JIANGSU QINGJIANG PHARMA
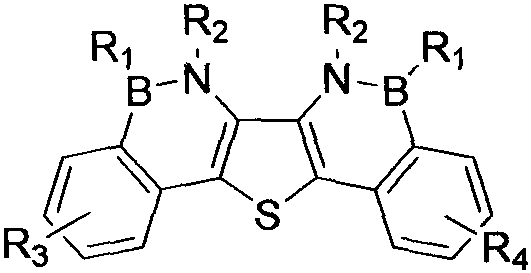
Synthesis method of boron aza-naphthalene thiophthene hetero arene and derivative thereof
InactiveCN109796480ASolid-state devicesSemiconductor/solid-state device manufacturingFuranSynthesis methods
The invention relates to a synthesis method of boron aza-naphthalene thiophthene hetero arene and a derivative thereof. The photophysical properties and single crystal diffraction structures of the compounds are tested, and the potential application value of the organic material in organic electrochemistry is further researched. According to the structural formula of the compound, R1, R2, R3 and R4 are independent substituted or non-substituted groups which comprise alkyl and aryl (benzene ring, thiophene ring, furan ring, pyrrole, pyridine, benzothiophene, benzofuran, benzopyrrole, quinoline,naphthalene ring, anthracene ring, phenalene, naphthacene, pyrene, a compound shown in the description, linear or angled pentacene, hexacene, indene, fluorene and the like). R3 and R4 can also be used as a single substituted halogen atom X : F, Cl, Br and I. The formula is shown in the description.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
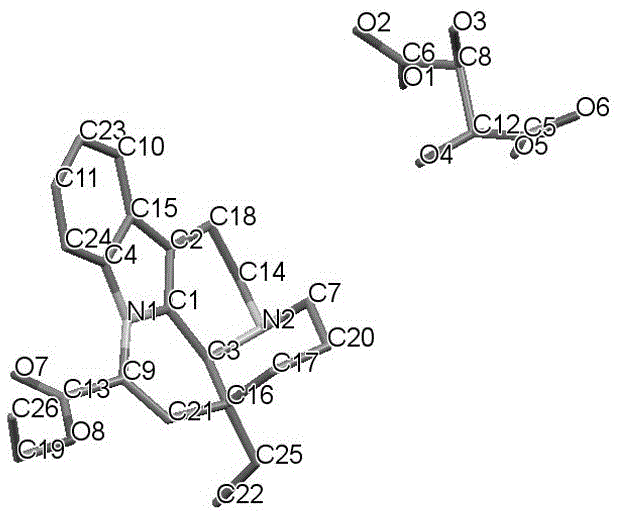
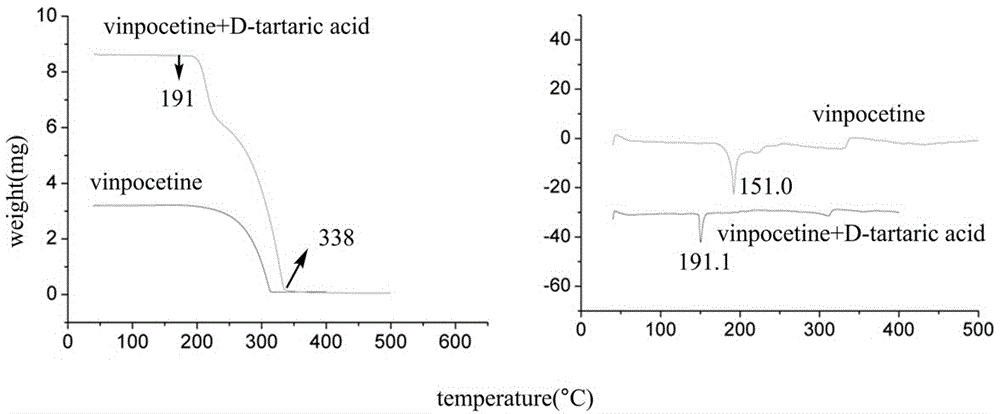
Crystal form of salt formed by vinpocetine and D-tartaric acid and preparation method of crystal form
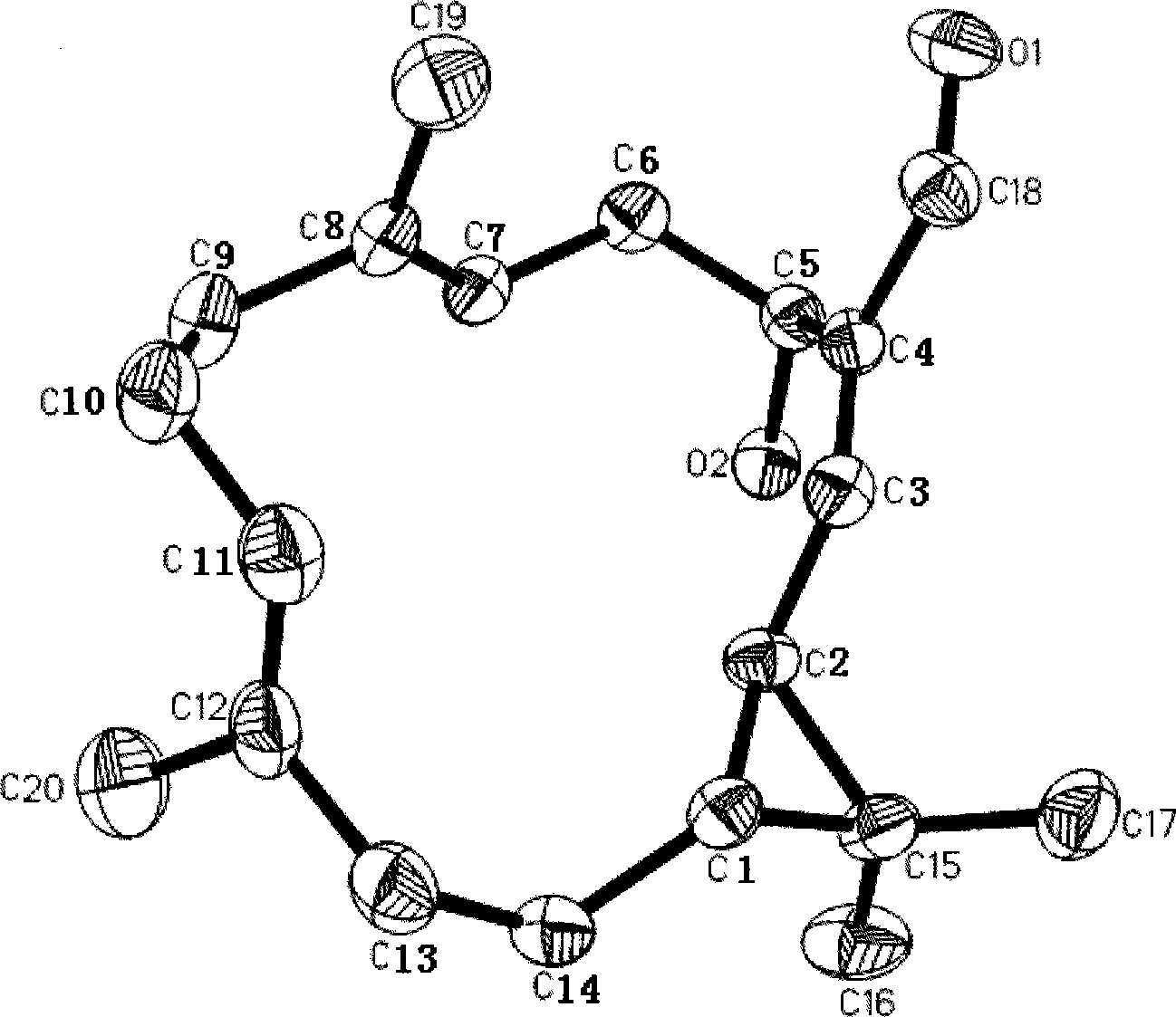
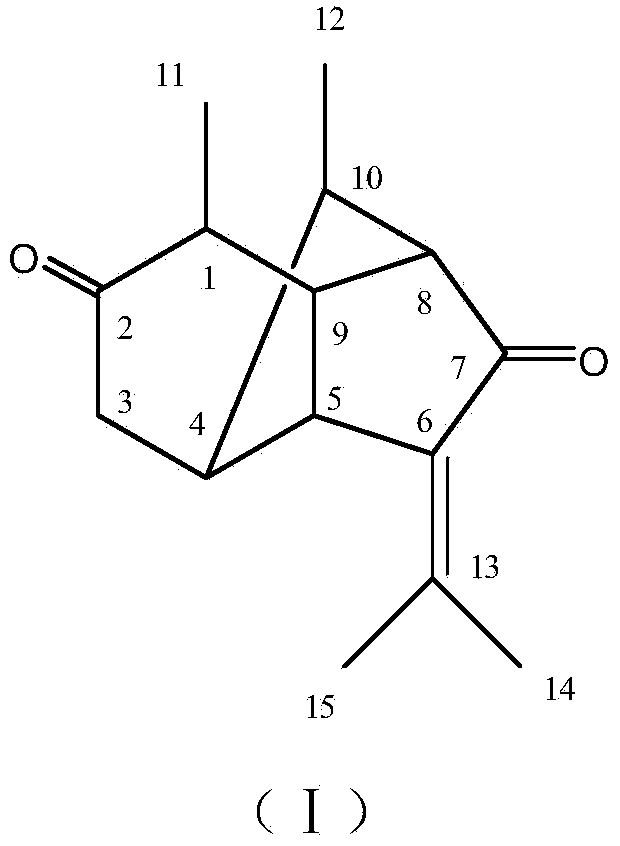
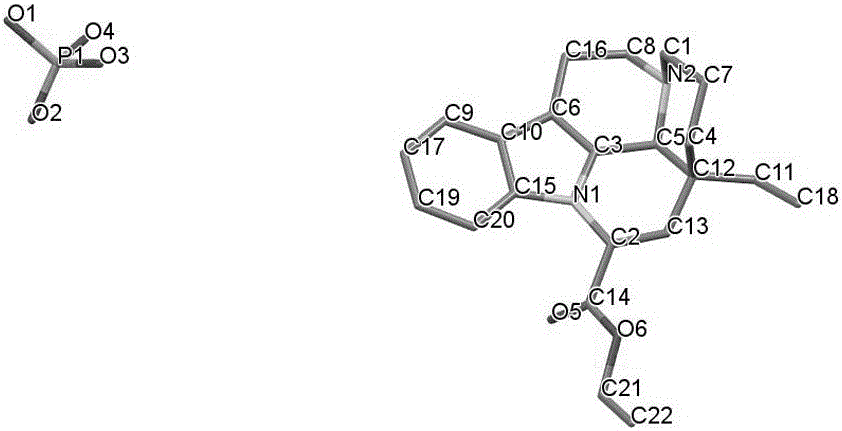
The invention relates to a crystal form of salt formed by vinpocetine and D-tartaric acid and a preparation method of the crystal form and belongs to the technical field of the discovering and preparation of the crystal form of medicine salt. The salt prepared by the method is detected as the salt formed by the vinpocetine and the D-tartaric acid through analyzing methods such as thermal gravimetric analysis (TGA), X-ray powder diffraction (P-XRD), X-ray single-crystal diffraction (S-XRD), differential scanning calorimetry (DSC) and infrared spectroscopy (IR). The vinpocetine is an insoluble drug (the solubility is about 5 microgram / mL and pKa=7.31), tests show that the salt formed by the vinpocetine and the D-tartaric acid is good in solubility and stability in the preparing process of solid preparations, and the development of new salt crystals lays a foundation for the development and application of the new dosage form of the salt. The spatial structure of the salt formed by the vinpocetine and D-tartaric acid is as shown in picture 1.
Owner:JIANGSU QINGJIANG PHARMA
Copper (1) Complex
InactiveUS20090105207A1Group 1/11 organic compounds without C-metal linkagesBiocideDiseaseIncurable diseases
Copper (I) chloride complex of pyrazinic acid is prepared, characterized by elemental analysis, IR, UV-visible spectra and its crystal structure determined by single crystal diffraction methods. This compound is found to exhibit a very positive influence as a drug, in a pharmaceutical acceptable composition for different incurable diseases, e.g. myopathy or weakness of muscles in general, infertility, and certain genetically causes disorders in mammals. These products were found to improve symptoms of myopathy, male infertility, fatigue and weakness of muscles for different reasons, and certain genetically causes disorders in mammals. It also enhanced regeneration of tissue affected by skin burns, scars, improved alopecia, decreased blood lipids and helped in loss of weight in obese patients. The observed wide scope of activities probably due to stem cell stimulation, enhancement the immune system and role of copper dependent enzyme activity.
Owner:HUSSEIN SALAH FATHI +2
A method for directional docking of cylindrical single crystals
ActiveCN110835782BOrientation means intuitiveImprove work efficiencyGeometric CADAfter-treatment detailsSingle Crystal DiffractionDiffractometer
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
2-benzimidazolyl benzoic acid copper complex with anti-fungal activity and preparation method
InactiveCN107043354ARaw materials are convenientEnhanced inhibitory effectBiocideOrganic chemistryBenzoic acidCopper benzoate
The invention discloses a 2-benzimidazolyl benzoic acid copper complex with anti-fungal activity and a preparation method. The complex is prepared by the following steps: taking 2-benzimidazolyl benzoic acid and Cu(NO3)2.3H2O to react in a mixed solvent of ethanol and water; controlling heating temprature to 100 DEG C and reacting for 48h. The molecular structure of the copper complex is confirmed through an X-ray single-crystal diffraction and infrared spectrum. An anti-fungal activity experiment shows that the copper complex has good inhibition activity on fungus wheat take-all and rice disease. The molecular structure of the copper complex is shown as follows: a formula is shown in the description.
Owner:SOUTHWEST FORESTRY UNIVERSITY
Wide-angle scattering test method and device based on single-crystal diffractometer
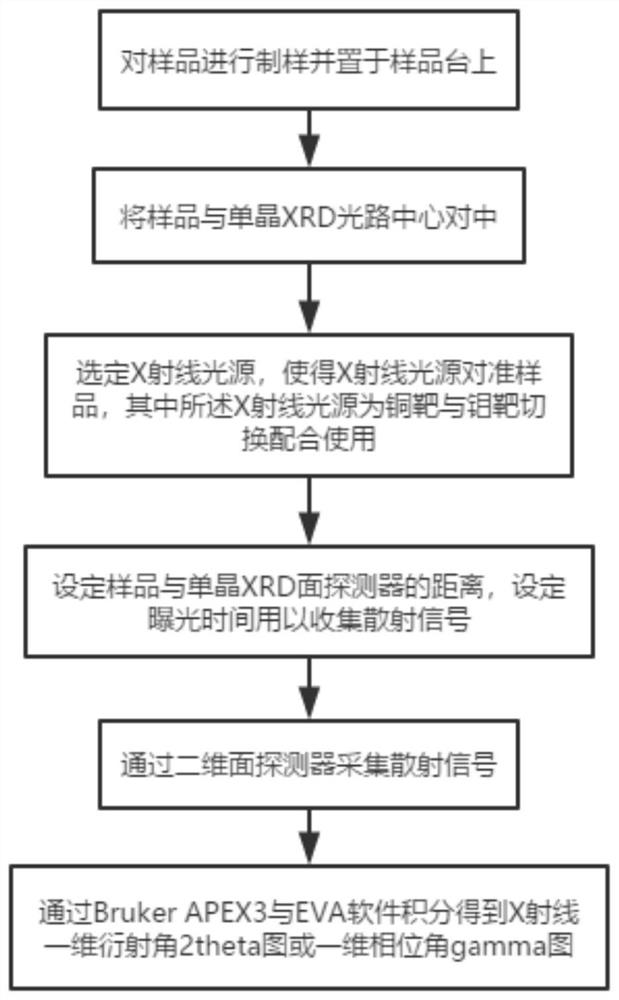
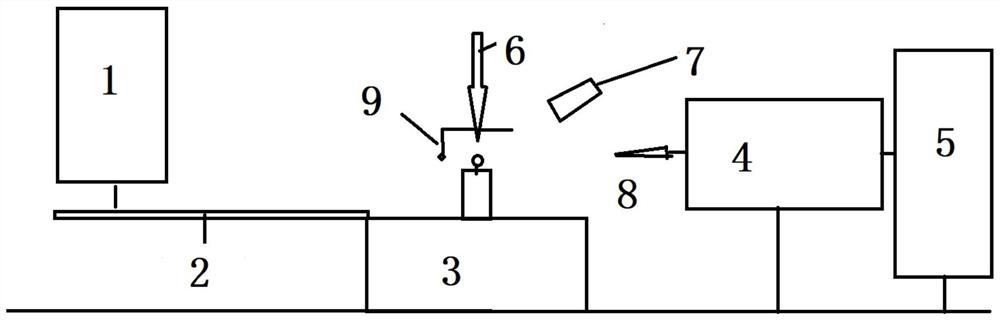
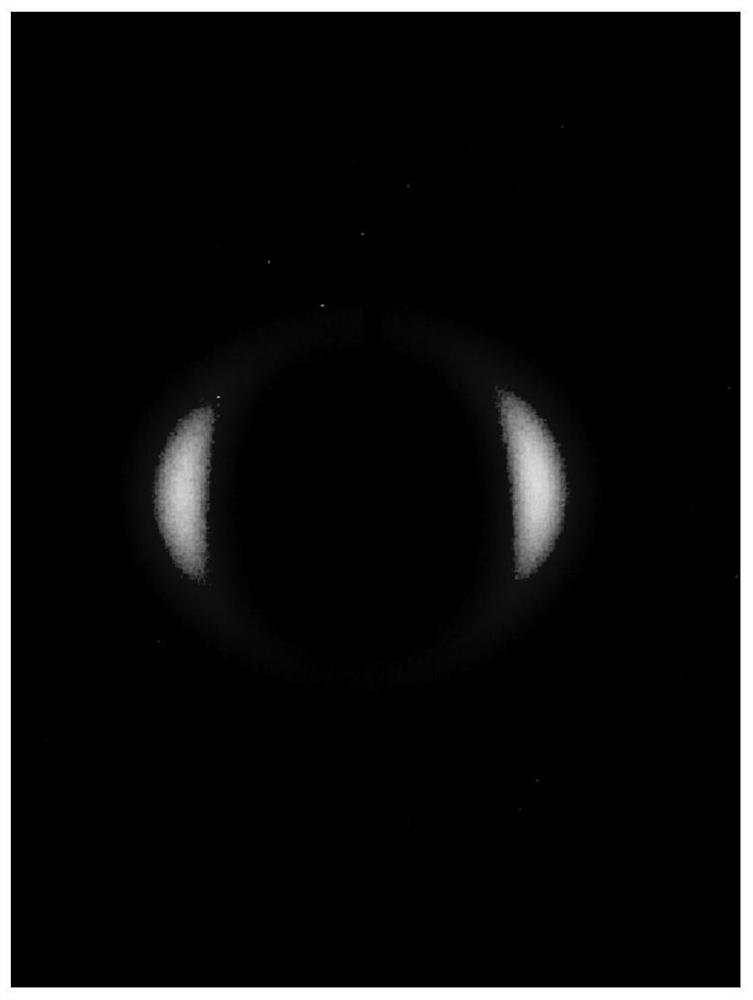
PendingCN113049617AReduce noiseReduce signal to noise ratioMaterial analysis using wave/particle radiationSingle Crystal DiffractionDiffractometer
The invention relates to a wide-angle scattering testing method and device based on a single-crystal diffractometer. According to the scheme, the wide-angle scattering testing method comprises the following steps: preparing and placing a sampleon a sample table; centering the sample with the single crystal XRD center; selecting an X-ray light source, so that the X-ray light source is aligned with the sample, and the X-ray light source is a copper target and a molybdenum target which are switched and matched; setting the distance between the sample and the single crystal XRD surface detector, and setting exposure time to collect scattered signals; collecting the scattered X-rays through a two-dimensional surface detector; and integrating the two-dimensional scattering spectrogram to obtain an X-ray one-dimensional diffraction angle 2theta diagram or a phase angle gamma diagram. The scheme combines the characteristics of spot focusing and two-dimensional surface detection of the single crystal XRD, expands the application field of the single crystal XRD, is compatible with the characterization of solid and liquid materials, has extremely high tolerance to the materials, gives consideration to wide-angle scattering of transmission and grazing incidence modes, and is rapid, efficient and economical.
Owner:WESTLAKE UNIV
A bipyridine bisphenol-aluminum catalyst for preparing unsaturated polyester and its preparation method
The invention relates to a bipyridine bisphenol-aluminum catalyst for preparing unsaturated polyester and a preparation method; the bipyridine bisphenol-aluminum complex has a structural formula such as I; the X group is Cl, CH 3 COO, CF 3 COO or NO 3 , R 1 , R 2 , R 3 and R 4 The groups are simultaneously or independently H, a halogen atom, an alkoxy group, an alkane group or a substituted alkane group; an aromatic hydrocarbon group or a substituted aromatic hydrocarbon group. Prepared by reaction of 2,4‑disubstituted phenols with R 1 , R 2 , R 3 and R 4 The bipyridine bisphenol ligand; then dissolved in a good solvent, and added to the good solvent of the metal aluminum source, and reacted to obtain a catalyst, X-ray single crystal diffraction showed that the complex distorted the structure of the square pyramid. The synthesized catalyst was used to prepare unsaturated polyester. The obtained unsaturated polyester has a polyester segment content greater than 99%, and can be used for further copolymerization with vinyl monomers to prepare unsaturated polyester resins.
Owner:TIANJIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com