Bipyridine bisphenol-aluminum catalyst for preparing unsaturated polyester and preparation method
A bipyridyl bisphenol and catalyst technology, which is applied in the field of bipyridine bisphenol-aluminum catalyst for preparing unsaturated polyester and its preparation, can solve the problems of lack of high activity and low biological toxicity metal-organic complex catalytic system and the like, Achieving the effect of simple and efficient synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
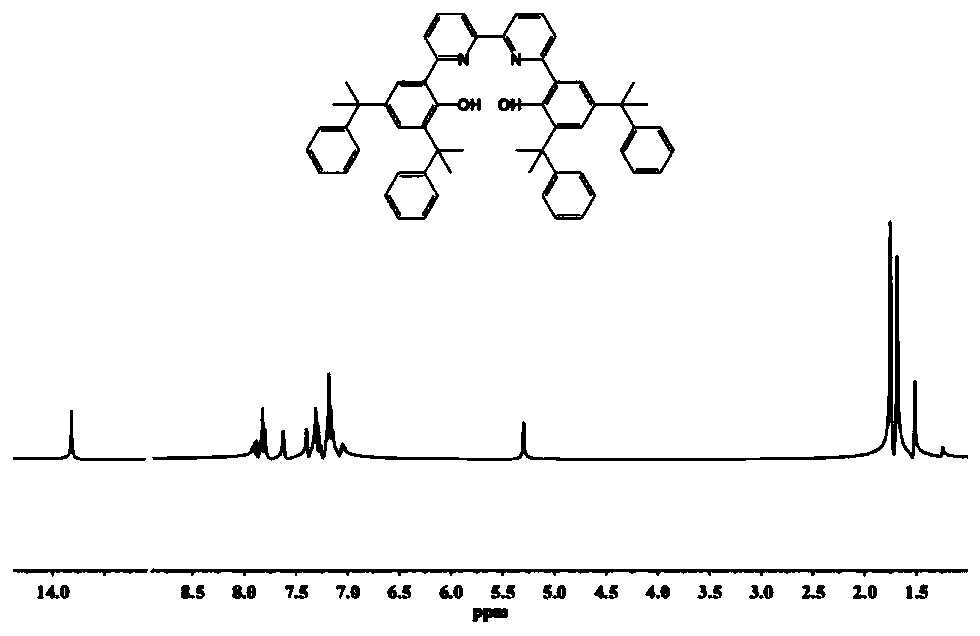
specific Embodiment 1-9
[0093] Specific examples 1-9 are the first steps for the synthesis of ligands LA to LI:
[0094]
Embodiment 1
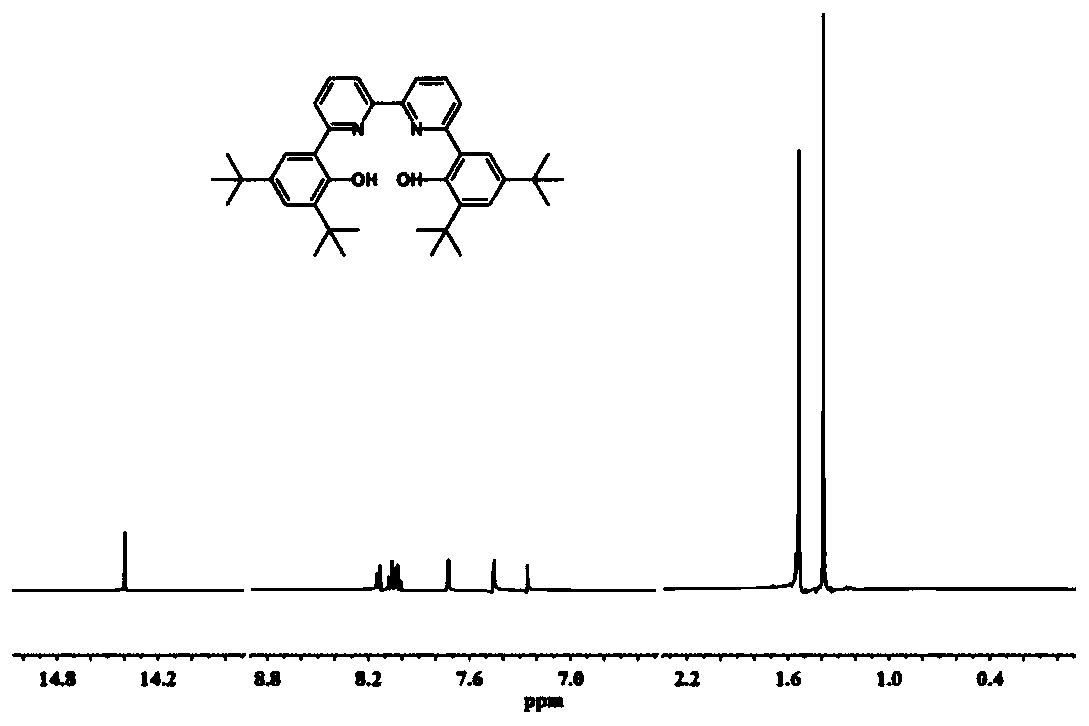
[0096] The synthesis steps of the compound LA: under the protection of nitrogen, dissolve 2,4-di-tert-butylphenol (10.3g, 50mmol) in 100mL dichloromethane, and slowly add 55mL liquid bromine dichloride under the condition of 0℃ Methane solution (1.0mol / L), gradually return to room temperature and react for 12h, use NaHSO after the reaction 3 Quench the reaction, extract the organic phase with dichloromethane, anhydrous NaSO 4 After drying, concentrating, and separating by column chromatography, 13.49 g of 2,4-di-tert-butyl-6-bromophenol was obtained with a yield of 95%. Under nitrogen, dissolve 2,4-di-tert-butyl-6-bromophenol in 1,4-dioxane and add [1,1'-bis(diphenylphosphino)ferrocene] 3.5g of palladium chloride, 18.0g of pinacol diboronic acid and 14g of potassium acetate, reacted at 80℃ for 16h. After the reaction, the reaction solution was directly concentrated and separated by column chromatography to obtain 2,4-di-tert-butyl as a white solid -6-Hydroxyphenylboronic acid p...
Embodiment 2
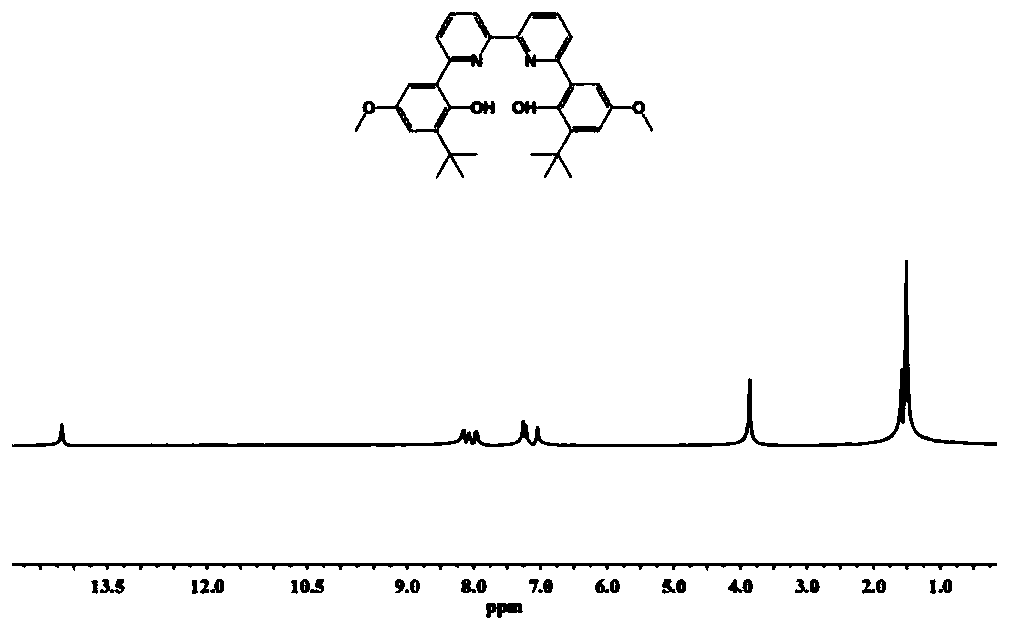
[0098] The synthesis steps of the compound LB: under the protection of nitrogen, dissolve 2-tert-butyl-4-methoxyphenol (9.0g, 50mmol) in 100mL dichloromethane, and slowly add 55mL liquid bromine under the condition of 0℃ Dichloromethane solution (1.0mol / L), gradually return to room temperature and react for 12h. After the reaction, use NaHSO 3 Quench the reaction, extract the organic phase with dichloromethane, anhydrous NaSO 4 After drying, concentrating, and separating by column chromatography, 11.6 g of 2-tert-butyl-4-methoxy-6-bromophenol was obtained with a yield of 90%. Under nitrogen, dissolve 2-tert-butyl-4-methoxy-6-bromophenol in 1,4-dioxane and add [1,1'-bis(diphenylphosphino) dicene Iron] palladium dichloride 3.0g, pinacol diborate 17.1g and potassium acetate 13.5g, reacted at 80℃ for 16h, after the reaction, the reaction solution is directly concentrated and separated by column chromatography to obtain white solid 2-tert-butyl 7.3 g of 4-methoxy-6-hydroxyphenylboro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com