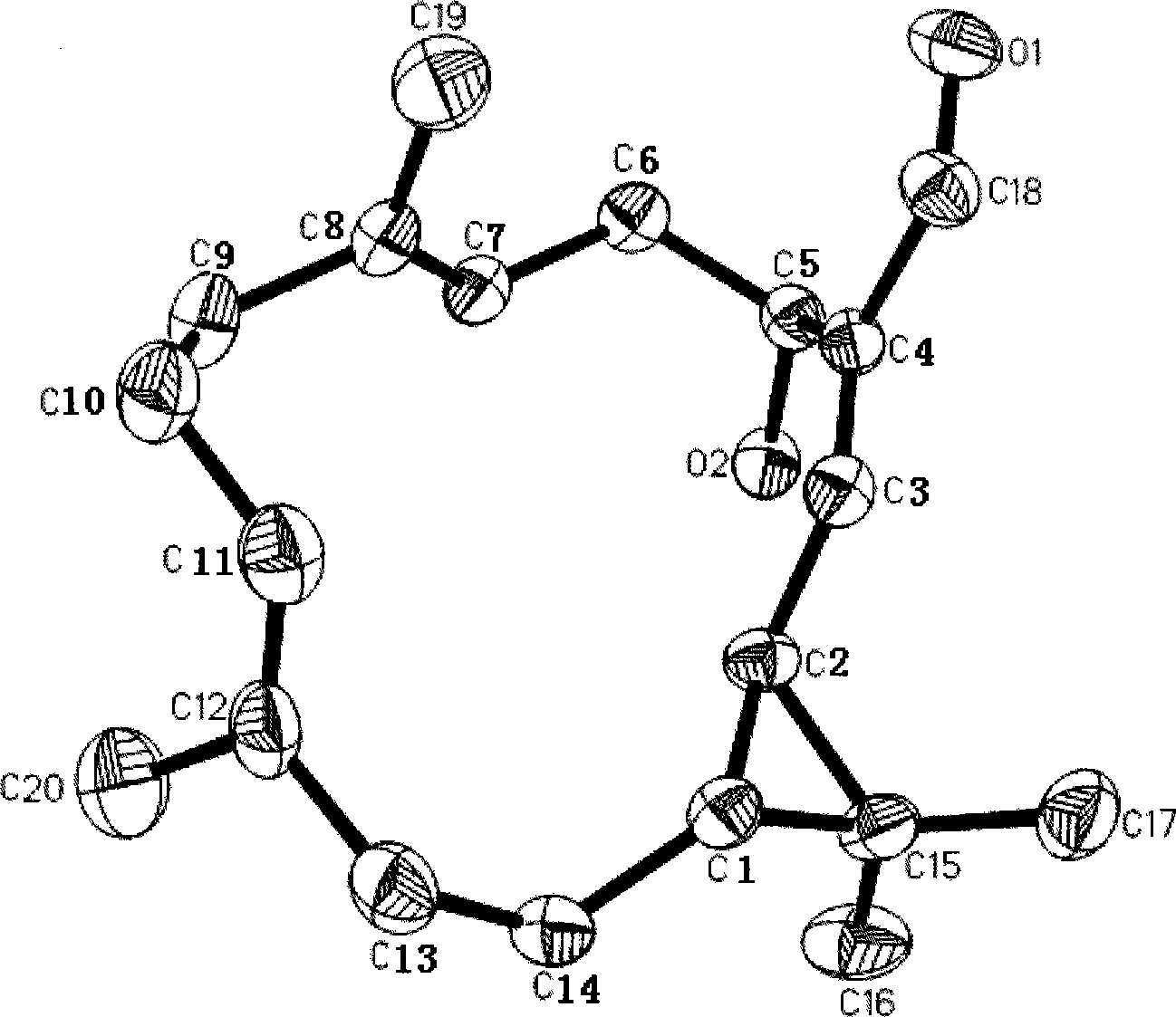
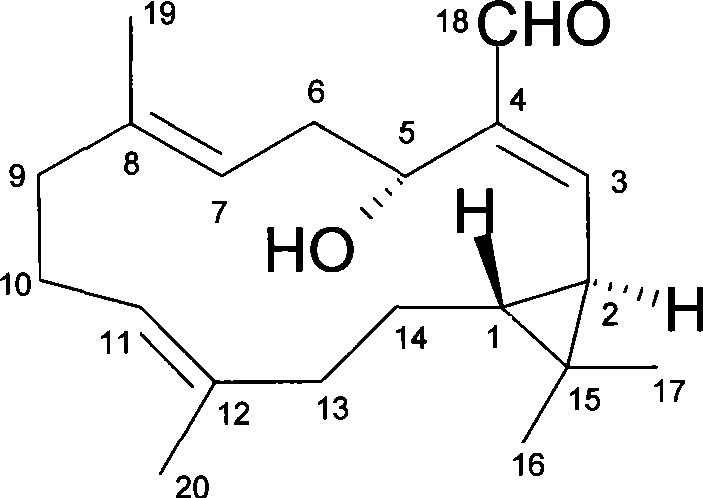
Anti-tumor diterpenoid isoeuphpekinensin in euphorbia perkinensis root and extraction method thereof
A compound and anti-tumor technology, applied in the separation/purification of anti-tumor drugs, carbonyl compounds, organic chemistry, etc., can solve the problems of few research reports on chemical composition and biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Extraction and separation method of diterpenoid isoeuphpekinensin
[0032] Select 1 kilogram of dried roots of Euphorbia sp. grown in the suburbs of Nanjing, Jiangsu, China, and extract 3 times with 2.5 times the amount (volume) of 95% ethanol under heating and refluxing, combine the 3 extractions, and recover ethanol under reduced pressure to obtain 68.3 grams of liquid extract; The stream extract was subjected to silica gel column (200-300 mesh) chromatography, eluted with petroleum ether and petroleum ether: ethyl acetate gradient (100:0, 99:1, 98:2, 96:4, 80:20) , detected by thin layer chromatography, collected the fractions containing isoeuphpekinensin, combined and concentrated, and then subjected to silica gel column chromatography, eluted with petroleum ether-ethyl acetate (100:0, 99:1, 98:2) gradient, thin layer Chromatographic detection, the diterpene compound isoeuphpekinensin pure product.
Embodiment 2
[0033] Example 2 In vitro anti-tumor test (Alamar blue method)-human lung cancer cell line NCI-H1299
[0034] Human lung cancer cell line NCI-H1299 was cultured in RPMI-1640 medium containing 10% calf serum, 100U / ml penicillin, and 0.1mg / ml streptomycin at 37°C, 5% CO. 2 Routine culture in an incubator. Digested with 0.25% trypsin plus 0.02% EDTA and passaged. Take cells in logarithmic growth phase, digest with trypsin and prepare cell suspension with RPMI-1640 medium containing 10% calf serum, the cell concentration is about 1×10 5 1 / ml, inoculated in a 96-well culture plate, 180 μL per well; 20 μL of solutions containing isoeuphpekinensin samples with different concentrations were added to each well of the experimental group (samples were dissolved in dimethyl sulfoxide, explained in the medium), and 20 μL of dimethyl sulfoxide was added to each well. Sulfone (final concentration 2 After culturing in the incubator for 48 hours, 20 μl of Alamarblue solution was added to eac...
Embodiment 3
[0035] Example 3 In vitro antitumor test (Alamar blue method) - human nasopharyngeal carcinoma cell line KB
[0036] The specific method is the same as that in Example 2, the sample concentrations are 2.5, 5, 10, 12.5, and 25 μg / ml, respectively, and the inhibition rates are 3.35%, 24.91%, 48.35%, 79.73%, and 93.54%, respectively. SPSS software was used to calculate the IC of isoeuphpekinensin inhibiting KB tumor cell line 50 was 8.52 μg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com