Synthesis method of boron aza-naphthalene thiophthene hetero arene and derivative thereof
A synthesis method and a technology for heteroaromatic hydrocarbons are applied in the field of synthesis of boraza naphthothiophene heteroaromatic hydrocarbons and derivatives thereof, and can solve the problems that potential application characteristics are not widely developed and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
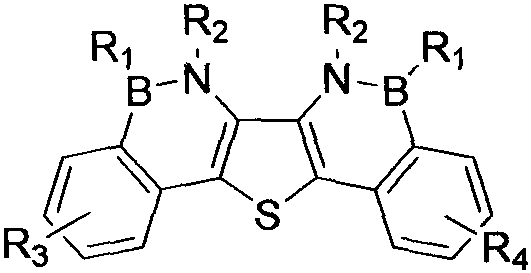
[0031] An overall synthesis method of borazabenzothiophene heteroaromatic derivatives of the present invention comprises the following synthesis route and steps:
[0032]
[0033] Some of the above-mentioned compounds are given examples, and the details are as follows:
Embodiment 1
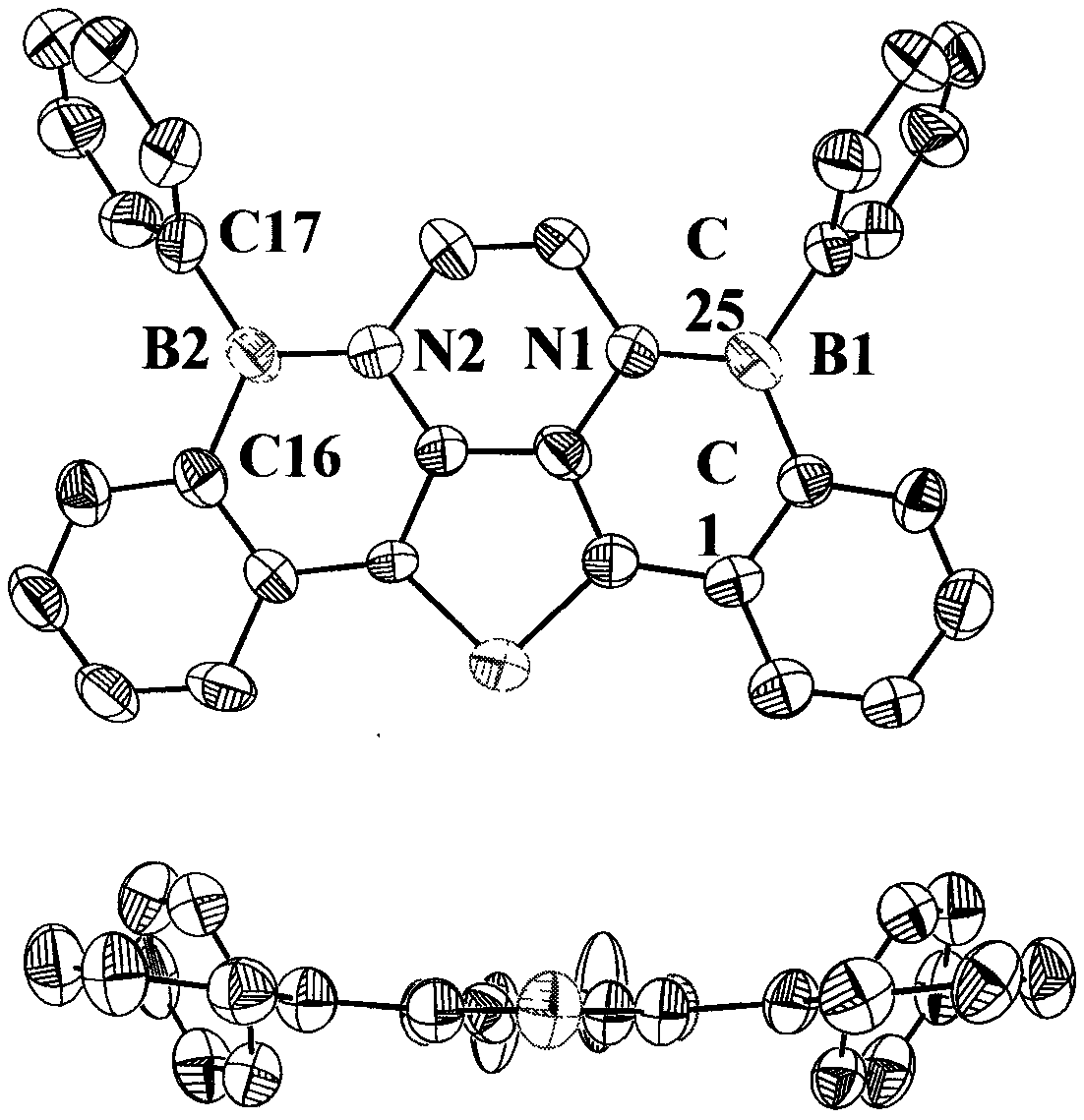
[0034] Embodiment 1: the synthesis of double 1,2-azaborinaphthothiophene compound 1
[0035] 1) Synthesis of Compound 4: Accurately weigh 2,5-dibromo-3,4-dinitrothiophene (1.00equiv, 5mmol, 1.66g), phenylboronic acid (2.10equiv, 11.5mmol, 1.48g), potassium carbonate (2.00equiv, 10.0mmol, 1.38g) and tetrakistriphenylphosphine palladium (0.05equiv, 0.25mmol, 0.30g) were added to a 100mL round-bottomed flask, and the system was evacuated using a vacuum oil pump, and the Schlenk Line was used to introduce High-purity nitrogen, repeated three times, added solvent tetrahydrofuran and water (volume ratio 4:1, 40mL), heated the reaction system to 100°C for reaction, and reacted overnight. The next day, TLC monitoring, rotary evaporator to remove solvent, dichloromethane and water extraction three times, combined organic phase, added anhydrous magnesium sulfate to dry for five minutes, then suction filtered, liquid phase was removed by rotary evaporator, and the residue was washed with...
Embodiment 2
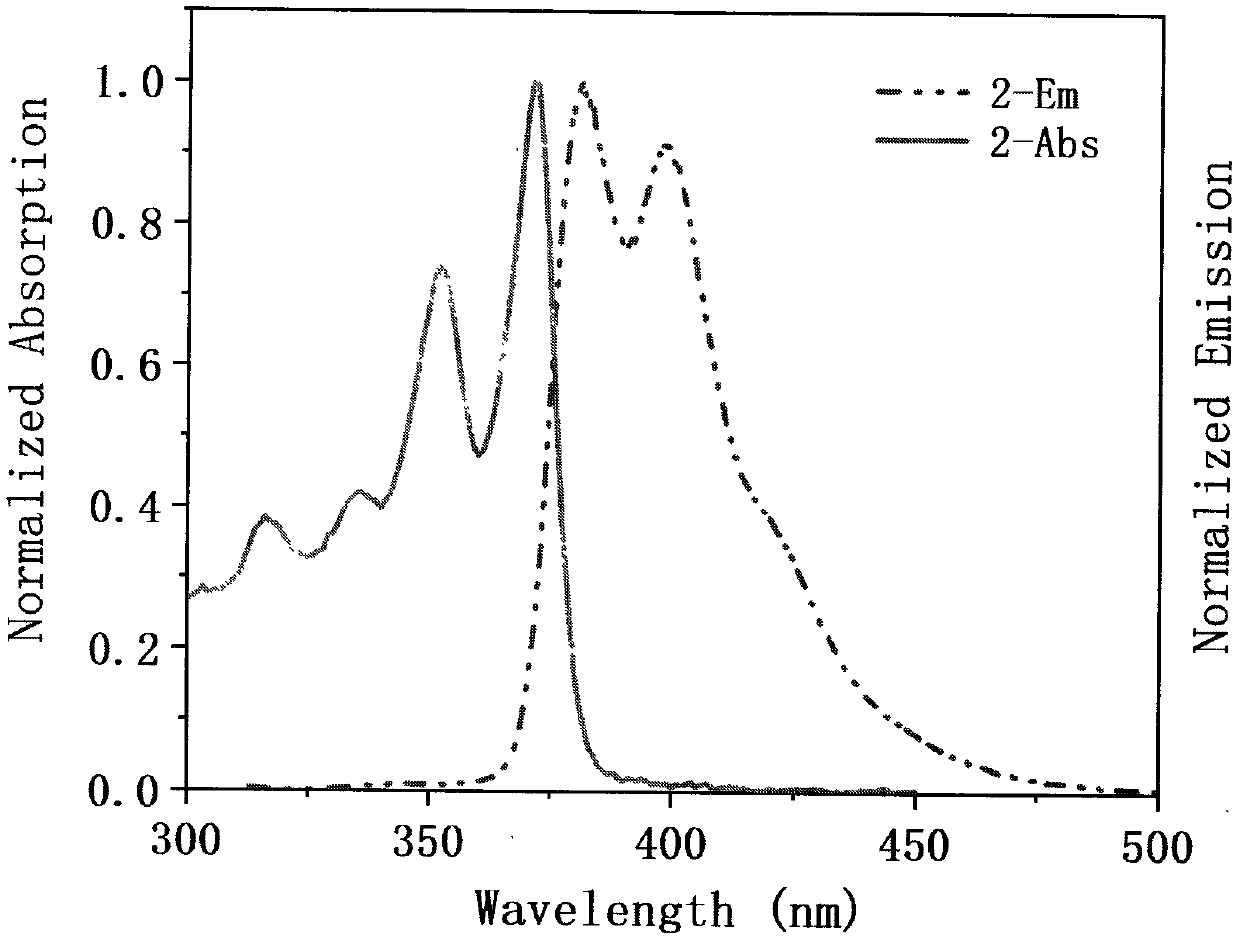
[0041] Example 2: Synthesis of two 1,2-azaborinaphthothiophene compound derivatives 2
[0042] 5) Synthesis of Compound 6: Weigh Compound 5 (1.00equiv, 0.63mmol, 170.00mg), add sodium carbonate (4.00equiv, 2.40mmol, 260.00mg) into a round bottom flask, add a stir bar, connect a spherical condenser, add Rubber stopper, vacuumize the system, use the Schlenk Line to feed high-purity nitrogen, repeat three times, connect a nitrogen balloon as a buffer, slowly add 40wt% glyoxal aqueous solution (1.10equiv, 0.71mmol, 0.13mL), Add solvent ethanol 10mL, react overnight at room temperature. TLC monitoring, rotary evaporation to remove solvent, dichloromethane and water extraction three times, combined organic phases, adding anhydrous magnesium sulfate to dry for five minutes, and suction filtration, liquid phase was removed by rotary evaporator, the residue was dissolved with a small amount of toluene, and used The silica gel chromatography column was subjected to column chromatograph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com