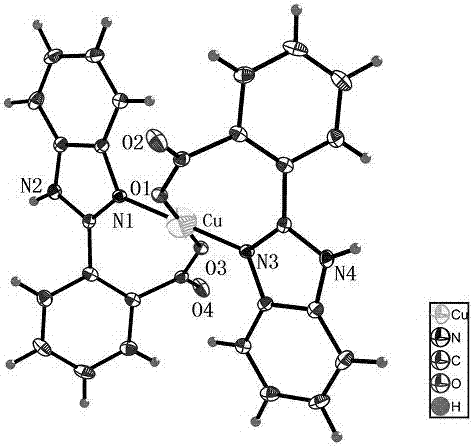
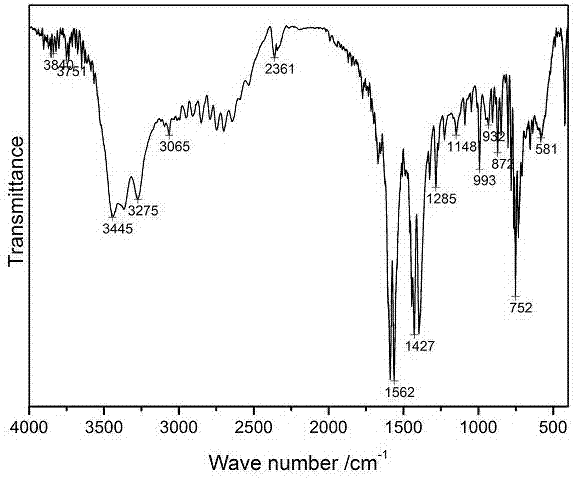
2-benzimidazolyl benzoic acid copper complex with anti-fungal activity and preparation method
A kind of technology of benzimidazolyl benzene and formyl benzoic acid, applied in the metal complex of benzimidazole heterocyclic compound and preparation field thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 0.24 g Cu(NO 3 ) 2 •3H 2 O (1 mmol) and 0.24 g 2-benzimidazolylbenzoic acid (1 mmol), then add 20 mL of absolute ethanol as a solvent, stir at room temperature for 15 min to mix the reactants evenly, seal the reaction vessel and place at 100 °C React for 48 h. A reddish-brown crystalline solid was formed with a yield of 38%.
Embodiment 2
[0029] Add 0.24 g Cu(NO 3 ) 2 •3H 2 O (1 mmol) and 0.24 g 2-benzimidazolylbenzoic acid (1 mmol), then add 20 mL of a mixed solvent of absolute ethanol and water (V 乙醇 : V 水 = 1:1), stirred at room temperature for 15 min to mix the reactants evenly, sealed the reactor and reacted at 100 °C for 48 h. A reddish-brown crystalline solid was formed with a yield of 68%.
[0030] As a comparative experiment;
Embodiment 3
[0032] Add 0.24 g Cu(NO 3 ) 2 •3H 2 O (1 mmol) and 0.24 g 2-benzimidazolylbenzoic acid (1 mmol), then add 20 mL of distilled water as solvent, stir at room temperature for 15 min to mix the reactants evenly, seal the reaction vessel and react at 100 °C for 48 h. Because 2-benzimidazole benzoic acid is insoluble in water, it cannot be mixed with Cu 2+ reaction, no target product was formed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com