Eutectic crystal of calcifediol and vitamin D3 as well as preparation method and application thereof
A technology of calcifediol and vitamin, which is applied in the field of co-crystal and preparation of calcifediol and vitamin D3, can solve the problems of poor patient compliance, difficulty in rationally matching use of vitamin D calcifediol, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Calcidiol and Vitamin D 3 Eutectic
[0057] At room temperature, weigh calcidiol (20.32g) and vitamin D 3 (19.23g) powder, completely dissolved in 500mL acetonitrile solution to form calcidiol and vitamin D 3 The unsaturated solution. Cool this unsaturated solution from room temperature to -20 degrees Celsius. After standing for 24 hours, there will be calcifediol and vitamin D. 3 The eutectic powder precipitated. Centrifuge and filter the suspension to obtain calcidiol and vitamin D 3 The eutectic (31.35g).
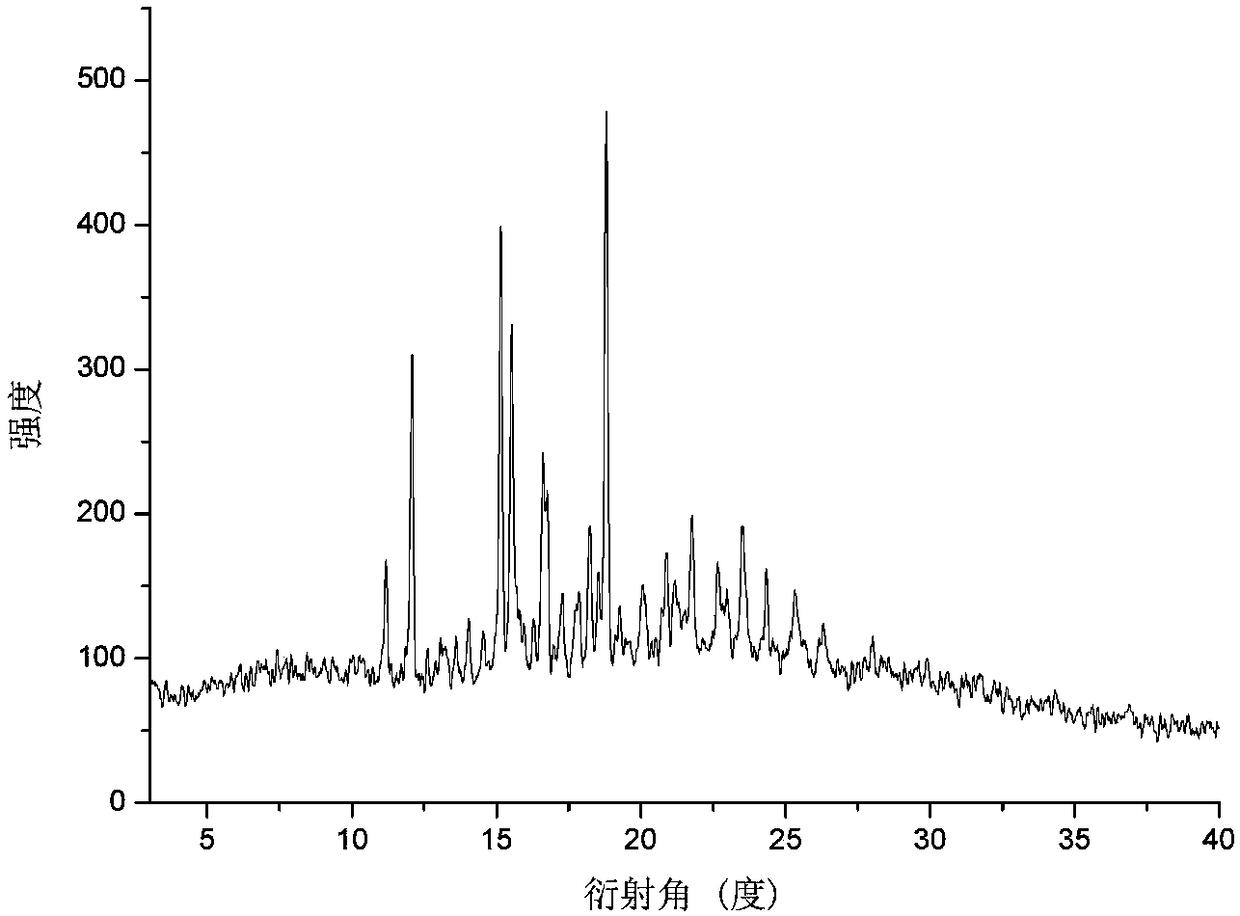
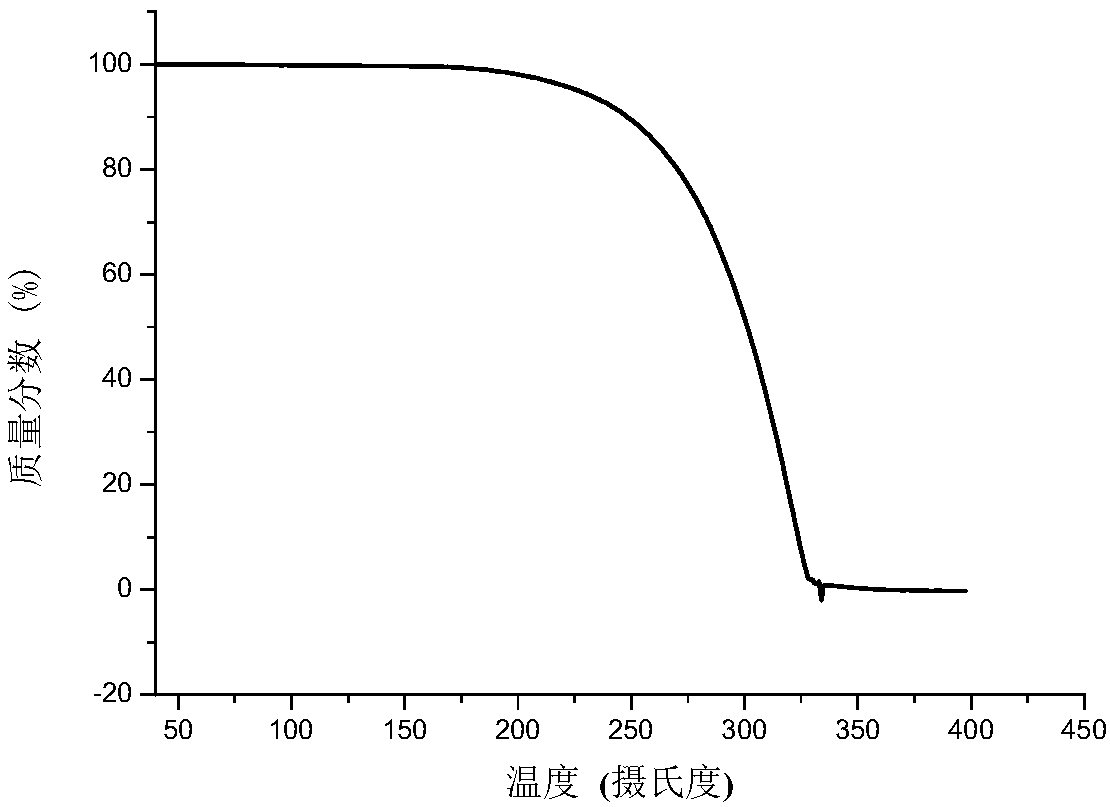
[0058] For the prepared calcidiol and vitamin D 3 The eutectic is characterized by X-ray powder diffraction (XRPD), X-ray single crystal diffraction (S-CXRD), thermogravimetric analysis (TG), differential scanning calorimetry (DSC) and infrared (IR) spectroscopy .
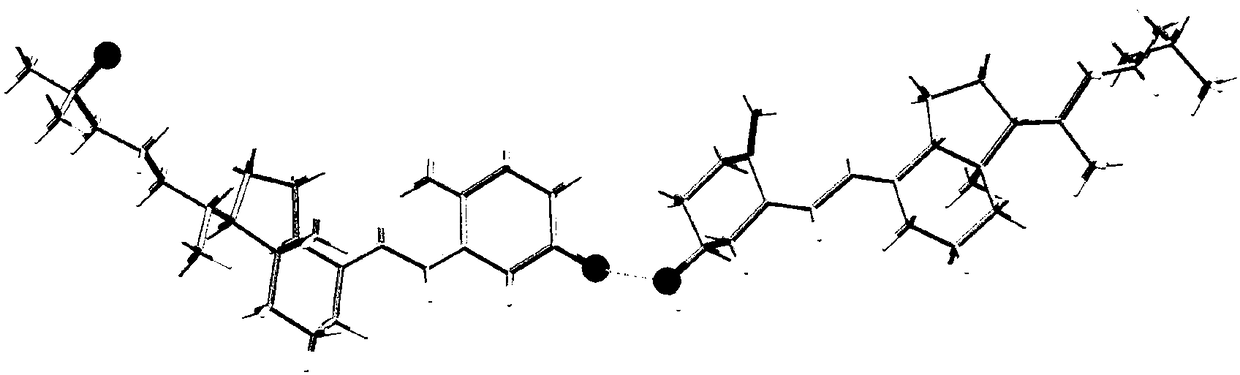
[0059] The X-ray powder diffraction pattern analysis results are attached figure 1 , See attached for X-ray single crystal diffraction analysis results figure 2 , See the results of thermogravimetric analy...
Embodiment 2
[0061] At room temperature, weigh calcidiol (30.48g) and vitamin D 3 (28.85g) powder, completely dissolved in 700mL acetonitrile solution to form calcidiol and vitamin D 3 The unsaturated solution. Cool this unsaturated solution from room temperature to -20 degrees Celsius. After standing for 24 hours, there will be calcifediol and vitamin D. 3 The eutectic powder precipitated. Centrifuge and filter the suspension to obtain calcidiol and vitamin D 3 The eutectic (47.46g).
Embodiment 3
[0063] At room temperature, weigh calcidiol (16.26g) and vitamin D 3 (15.38g) powder, completely dissolved in 300mL acetonitrile solution to form calcidiol and vitamin D 3 The unsaturated solution. Place this unsaturated solution at room temperature and continuously volatilize and concentrate to precipitate crystals, thereby obtaining calcidiol and vitamin D 3 The eutectic. Centrifugal filtration to obtain calcidiol and vitamin D 3 The eutectic (24.63g).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com