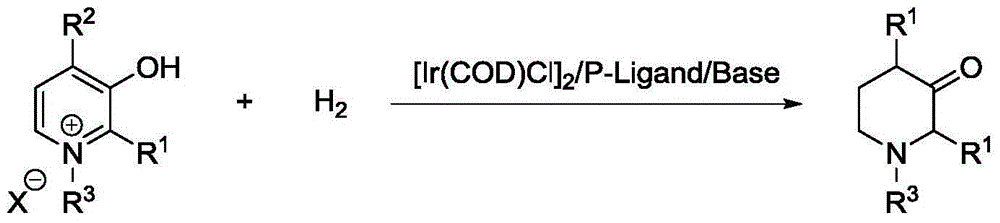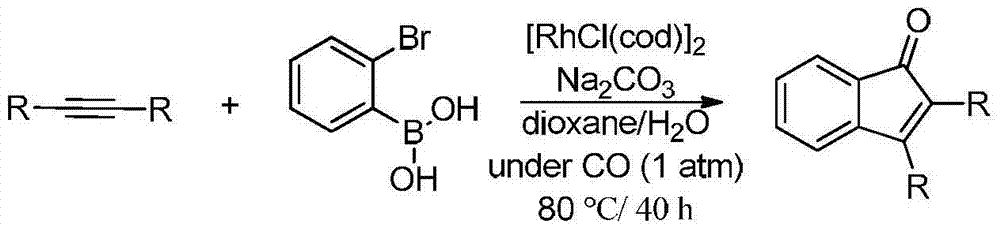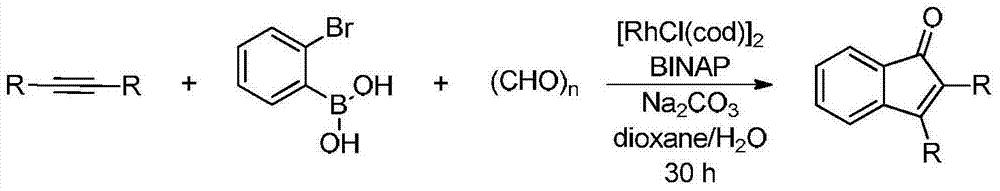Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
121 results about "1,2-Dichloroethane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water (at a boiling point of 70.5 °C or 158.9 °F or 343.6 K) and other chlorocarbons.
Method and device for producing 1,2-dichlorethane by means of direct chlorination
ActiveUS7579509B2Excellent part-load performanceReduce stressFlow mixersMixing methodsLiquid stateStatic mixer
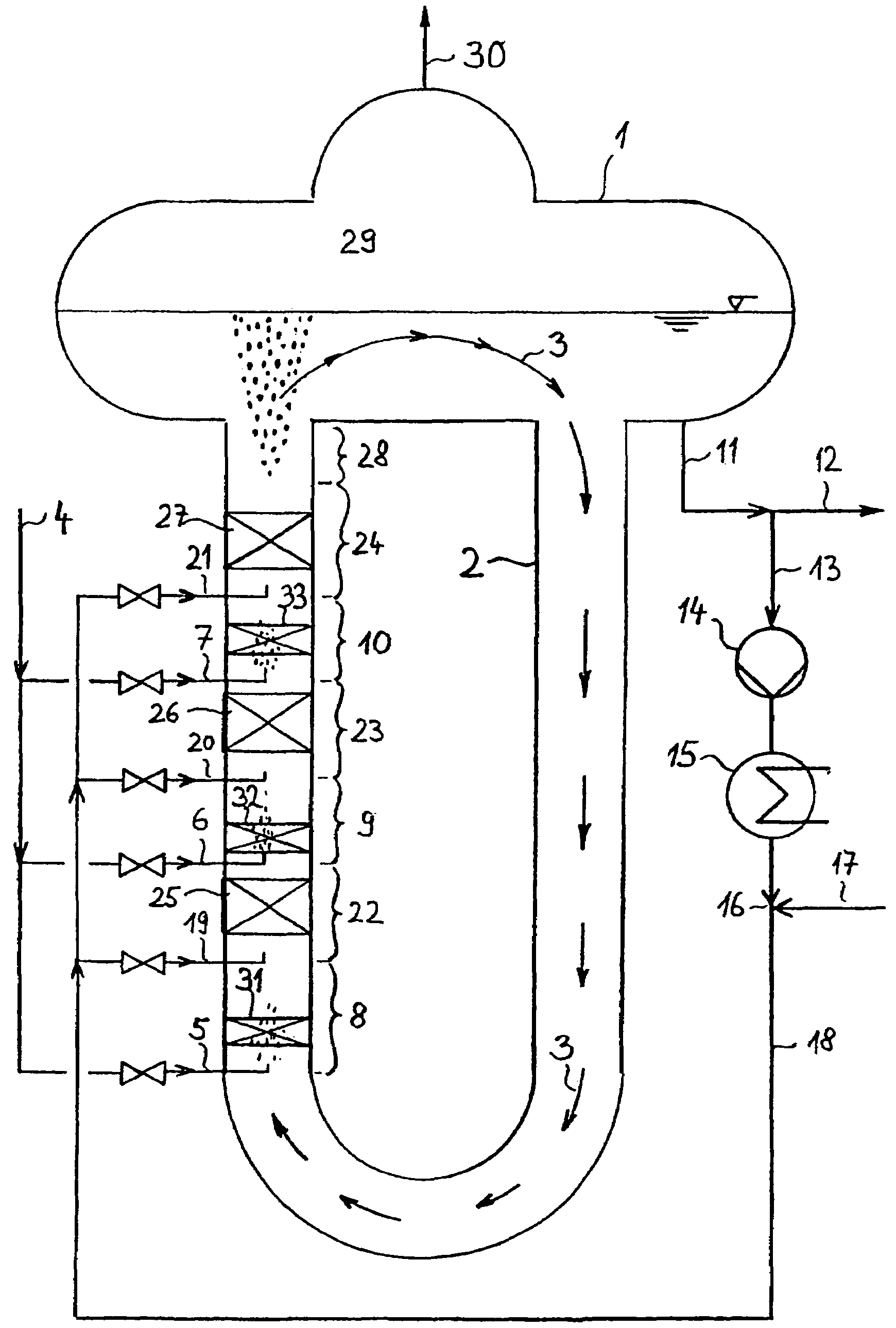
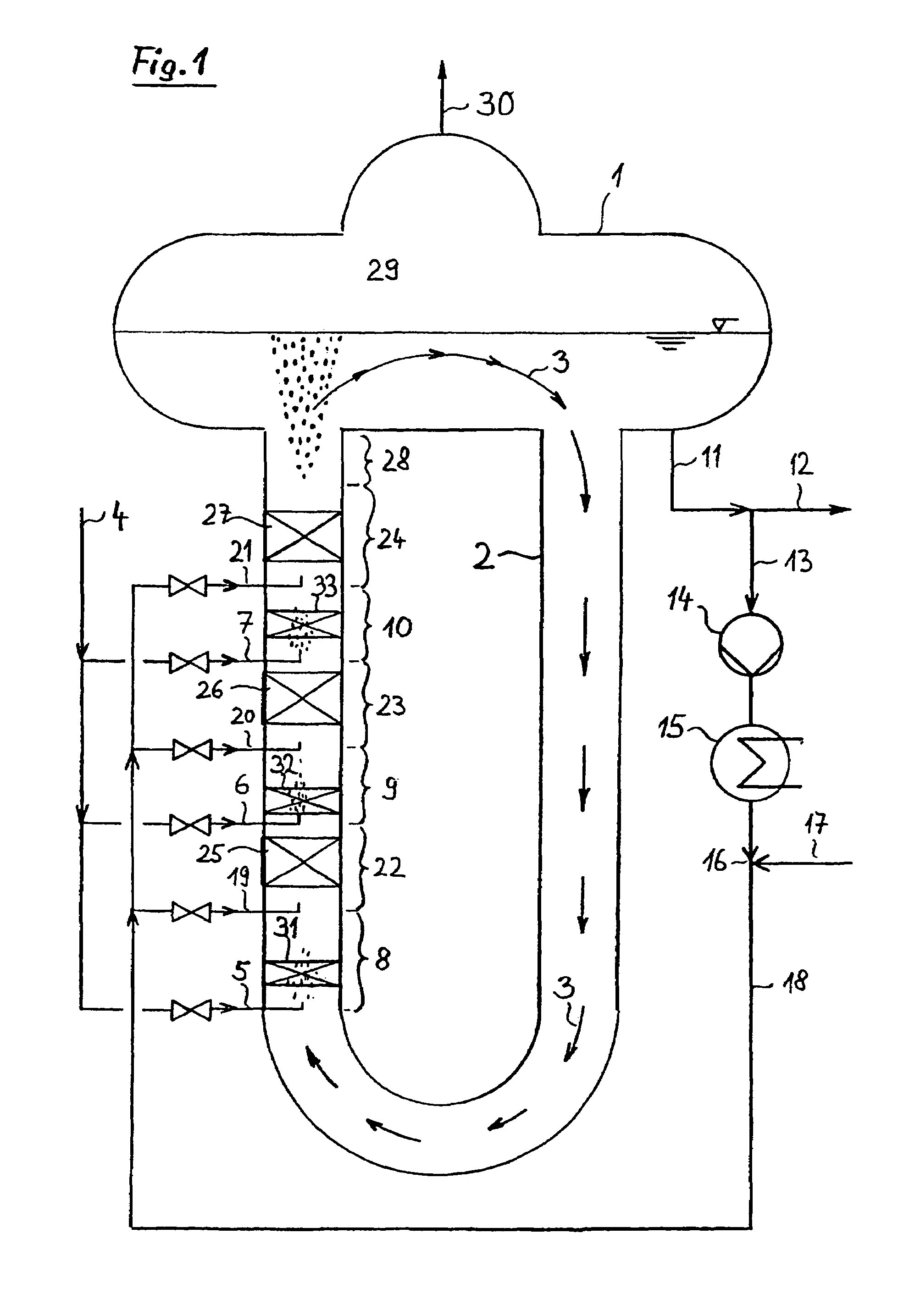
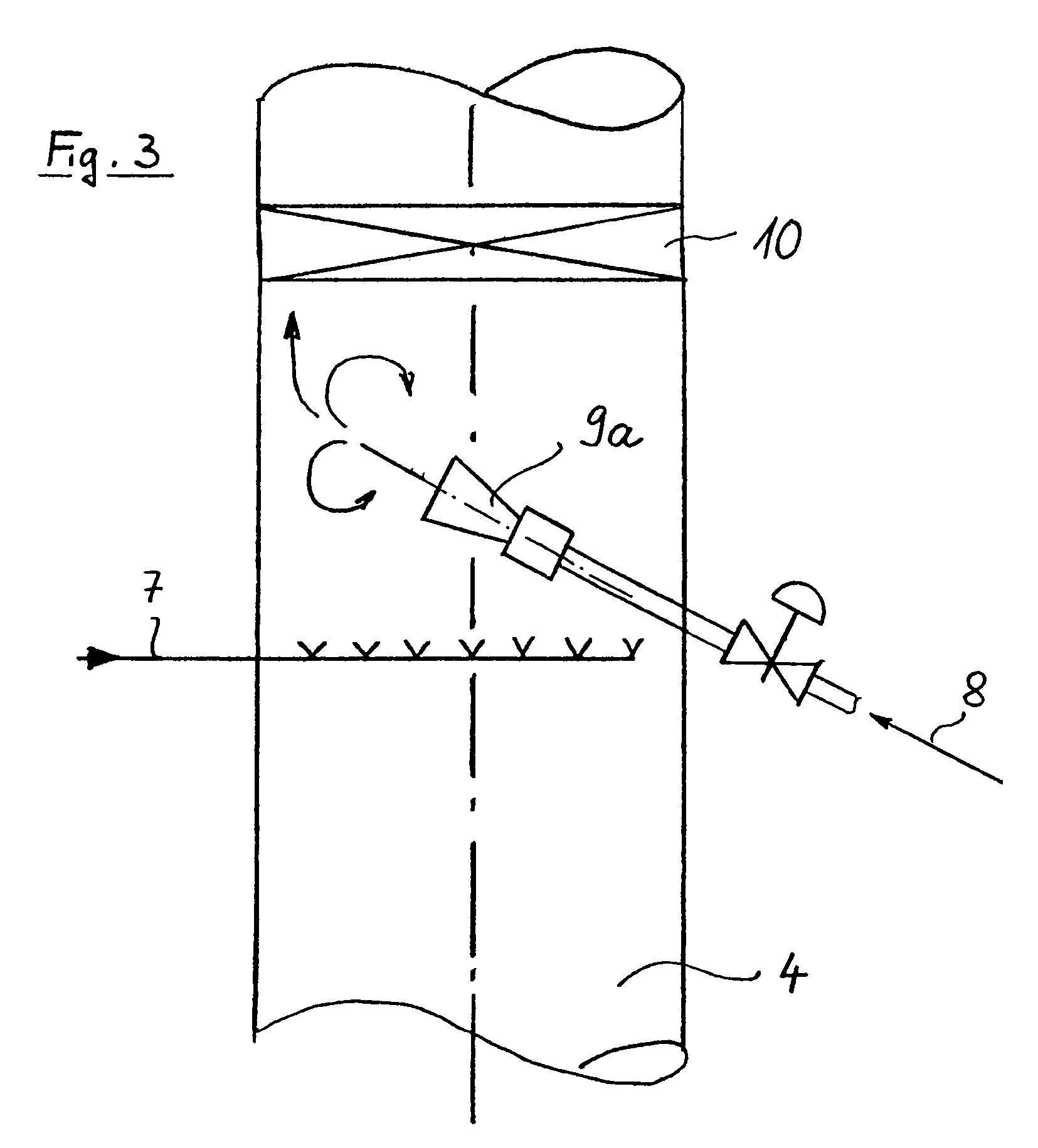
The invention refers to a process for the production of high-purity 1,2-dichloroethane from dissolved chlorine and dissolved ethylene, which are brought into contact with each other in a circulating liquid reaction fluid, which mainly consists of 1,2-dichlorethane and a catalyst and flows through at least one vertically arranged loop-type reaction section, both legs of the loop being connected to an overhead degassing vessel from where the reaction product is withdrawn either in gaseous or in liquid state or in both gaseous and liquid state, and numerous admixing sections being arranged in the leg of the loop in which the liquid rises, and each of these admixing sections having one upstream feed point for dissolved or gaseous ethylene and one downstream feed point for dissolved chlorine and, if required, the admixing sections featuring static mixers.
Owner:UHDE GMBH +1
Method for producing 1,2-dichloroethane by means of direct chlorination
ActiveUS7671244B2Increase capacityDouble the conversion rate according to reactionGaseous chemical processesPreparation by hydrogen halide split-offGas phaseEthane Dichloride
The invention relates to a method for producing high-purity 1,2-dichloroethane from dissolved chlorine and dissolved ethylene which are brought into contact with each other using a circulating liquid reaction medium which essentially consists of 1,2-dichloroethane and a catalyst and passes through at least one reaction loop. The two limbs of the loop are connected to a gas-phase stripping container which is arranged at the top and from which the reaction product is outwardly transferred either in a gaseous or liquid form or both in a gaseous form and in a liquid form. The addition points for the addition of chlorine and dissolved ethylene are arranged in the limb of the loop in which the liquid rises. The addition point for dissolved chlorine is always arranged downstream of the ethylene addition point. At least one addition point for liquid 1,2-dichloroethane follows each chlorine addition point, and the addition of the liquid 1,2-dichloroethane is carried out under kinetic energy which is high enough to enable a vigorous mixture of 1,2-dichloroethane, dissolved chlorine and ethylene to be carried out. Preferably, the liquid 1,2-dichloroethane is added by means of at least one jet mixer.
Owner:UHDE GMBH
Method for the detection of a cannabinoid, detection kit, and developing solvent
The invention relates to a method and detection kit for the detection of a cannabinoid in a cannabis sample. The method is based on TLC and the method and kit enables everyone to avoid laborious, time consuming and expensive analytical research and determine the cannabinoid content. Good results are obtained with a developing solvent with at least 25 vol. % chloroform and at least 25 vol. % 1,2-dichloro ethane.
Owner:GLAS RONALD JOHANNES
Ionic liquid functionalized ultra-crosslinking polymer as well preparation method and application thereof
The invention relates to a novel functionalized organic porous polymer and particularly relates to an ionic liquid functionalized ultra-crosslinking polymer and a preparation method thereof. The ionic liquid functionalized ultra-crosslinking polymer is prepared by reacting the following components in a molar ratio: 0.5-10% of a crosslinking agent, 10-70% of an imidazole ionic liquid and 10-80% of a monomer, wherein the used solvent is 1,2-dichloroethane; the monomer used in the ionic liquid functionalized ultra-crosslinking polymer is one or a combination of two or more of benzene, biphenyl, pyrrole, thiophene, furan, 1,3,5-triphenyl benzene or p-chloromethyl styrene, preferably, p-chloromethyl styrene. The porous polymer can be applicable to gas adsorption, especially capture and separation of CO2 gas.
Owner:HANGZHOU REWARD TECHNOLOGY CO LTD
Process for the manufacture of 1,2-dichloroethane
InactiveUS8058490B2Low costHigh purityPreparation by hydrogen halide split-offChemical recyclingCatalytic oxidationEthane Dichloride
Process for the manufacture of 1,2-dichloroethane (DCE) starting from a stream of ethane which is subjected to a catalytic oxydehydrogenation (ODH) thus producing a gas mixture containing ethylene which is then dried and conveyed to a chlorination reactor supplied with a flow of chlorine so that at least 10% of the ethylene is converted to DCE. The stream of products derived from the chlorination reactor is then conveyed to an oxychlorination reactor in which the majority of the balance of ethylene is converted to DCE.
Owner:SOLVAY SA
Catalyst applied to preparation of vinyl chloride by catalytic cracking of 1,2-dichloroethane as well as preparation method and application of catalyst
InactiveCN104289247AGood dispersionLow cracking temperaturePreparation by hydrogen halide split-offPhysical/chemical process catalystsActivated carbonEthane Dichloride
The invention relates to a nitrogen-doped active carbon catalyst applied to preparation of vinyl chloride by catalytic cracking of 1,2-dichloroethane and in particular relates to active carbon, wherein the inner surface and the outer surface of the active carbon are doped with nitrogen; the doping content of nitrogen is 0.1-10wt%; furthermore, meal is loaded on the nitrogen-doped active carbon catalyst and exists in the form of a metal compound; the loading content of the metal compound is 0.1-10wt%. According to the catalyst, the preparation method is simple, the process is short, materials are easily available, and the cost is relatively low; the nitrogen doped in the active carbon has a small possibility of losing and inactivating; the nitrogen is doped in the nitrogen-doped active carbon catalyst loaded with the metal compound, so that the dispersion of the metal component on the active carbon can be effectively improved; the catalytic activity is improved; the cracking temperature of dichloroethane can be greatly reduced under the catalytic action of the catalyst prepared by the method.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
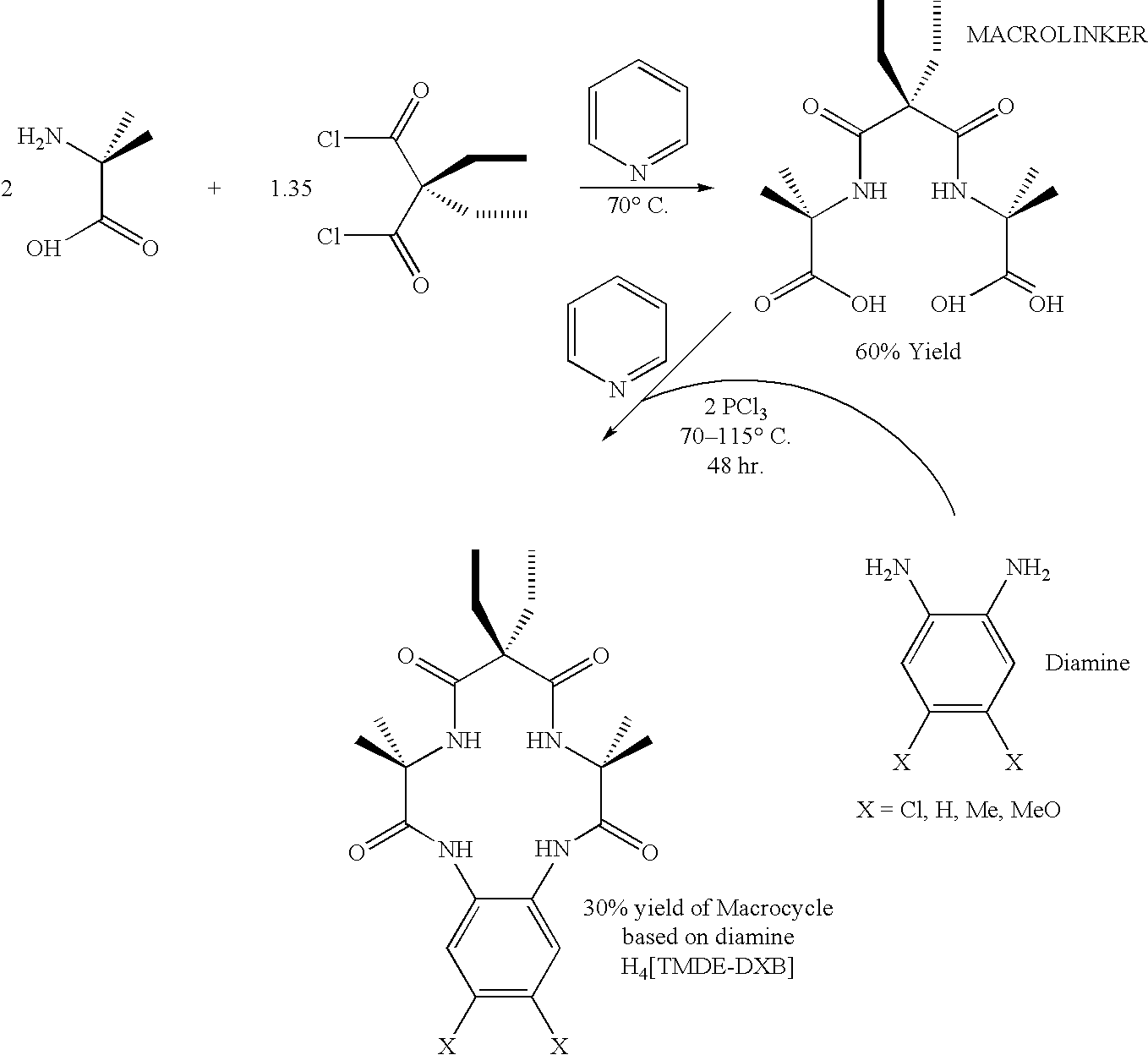
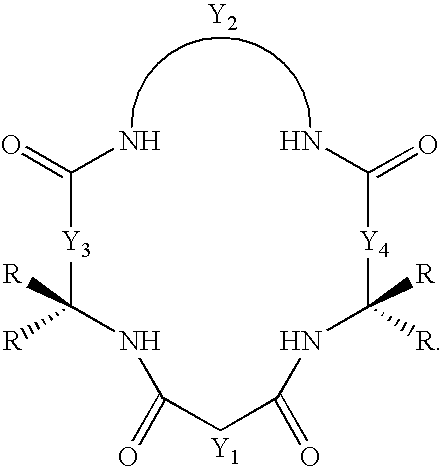
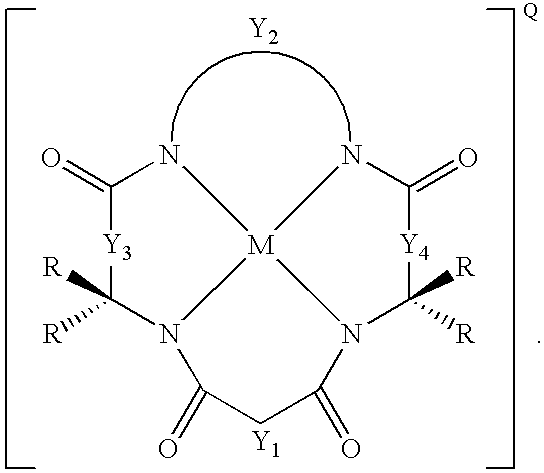
Synthesis of macrocyclic tetraamido compounds and new metal insertion process
An improved method of synthesizing a macrocyclic tetraamido compound includes protecting the amino portion of an amino carboxylic acid to form a protected amino carboxylic acid; exposing the protected amino carboxylic acid to a first solvent, preferably a hydrocarbon solvent, such as toluene or 1,2-dichloroethane, dichloromethane, dibromomethane and 1,2-dibromoethane. The carboxylic acid portion of the protected amino carboxylic acid is then converted to an activated carboxylic acid by one of esterification or acid halide formation, to form a protected amino activated carboxylic acid derivative. The protected amino activated carboxylic acid derivative is reacted with a diamine in the presence of a second solvent, such as THF or ,2-dichloroethane, dichloromethane, dibromomethane and 1,2-dibromoethane, to form a protected diamide diamine intermediate. Following deprotection, the diamide diamine intermediate is reacted with an activated diacid, such as an activated malonate, oxalate or succinate derivative to form the macrocyclic tetraamido compound. The macrocyclic tetraamido compound may further be complexed with a transition metal.
Owner:CARNEGIE MELLON UNIV
Catalysts for oxychlorination of ethylene to 1,2-dichlorethane
InactiveUS20060129008A1Satisfactory performanceSolution to short lifeHalogenated hydrocarbon preparationMetal/metal-oxides/metal-hydroxide catalystsCopper atomEthane Dichloride
Catalysts for oxychlorination of ethylene to 1,2-dichlorethane, comprising compounds of copper and magnesium supported on gamma alumina, wherein the copper, expressed as metal, is present in a quantity from 7 to 12% by weight and the Mg / Cu ratio is from 0.05 to 1, wherein the distribution of copper in the catalyst particle is such that the ratio X / Y between the concentration of the copper atoms on the surface given by the Al / Cu ratio (X) on the surface (20-30 nm layer) and the concentration given by the Al / Cu ratio (Y) referred to the entire particle is greater than 1.3 and can reach 3.
Owner:SUD CHEM CATALYSTS ITAL SRL
Method for preparing amide compound
InactiveCN102746077AMild reaction conditionsMeet the requirementsOrganic compound preparationSulfonic acid esters preparationSodium iodidePotassium iodine
The invention discloses a method for preparing an amide compound, which comprises the steps of configuration of reaction system. Methyl ketone, amine, catalyst, an oxidizing agent and solvent are use, wherein the general formula of the methyl ketone is RCOCH3, and R is selected from one kind of C6-C14 aryl group, C2-C8 alkenyl group, and five-or six-membered heterocyclic group; the amine is primary amine; the catalyst is selected from one kind of potassium iodide, iodine, tetramethyl ammonium iodide, tetrabutyl ammonium iodide, tetrahexyl ammonium iodide, bismuth iodide, lithium iodide, phenyl trimethyl ammonium iodide, benzyl trimethyl ammonium iodide or sodium iodide; the oxidizing agent is tert-butyl hydroperoxide; the solvent is selected from one kind of water, dichloromethane, ethyl acetate, toluene, 1,2-dichloroethane, 1,1,1-trichloroethane, acetonitrile, and isopropanol. The methyl ketone, the catalyst and the amine are added into the reaction system under the temperature of 0 DEG C and stirred for 5 minutes, and then the oxidizing agent is added to react for 2-48 hours to obtain the amide compound. The method provided by the invention is green, moderate, high in selectivity and wide in application range.
Owner:云梦星联物流有限公司
Asymmetric hydrogenation synthetic method of chiral aromatic amine compound with high steric hindrance
InactiveCN103570484AThe hydrogenation reaction conditions are mildSmooth responseOrganic compound preparationCarboxylic acid amides preparationIridiumPotassium iodine
The invention relates to an asymmetric hydrogenation synthetic method of a chiral aromatic amine compound of iridium-catalyzed imine with high steric hindrance. The catalyst adopted is a catalyst generated by in-situ reaction of a 1, 5-cyclooctadiene iridium chloride dimer and a chiral phosphine-phosphoramidite ligand. The reaction can be carried out under the following condition: the additives comprise iodine, potassium iodide and tetrabutyl ammonium iodide; the temperature is 0-200 DEG C; the solvent is dichloromethane, 1, 2-dichloroethane to the like; the pressure is 10-100 barometric pressures; the time is 12-48 hours; and the proportion of a primer and the catalyst can reach 100000 / 1. The method provided by the invention has the characteristics of mild reaction condition, high activity, high stereoselectivity, wide application range of the primer and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
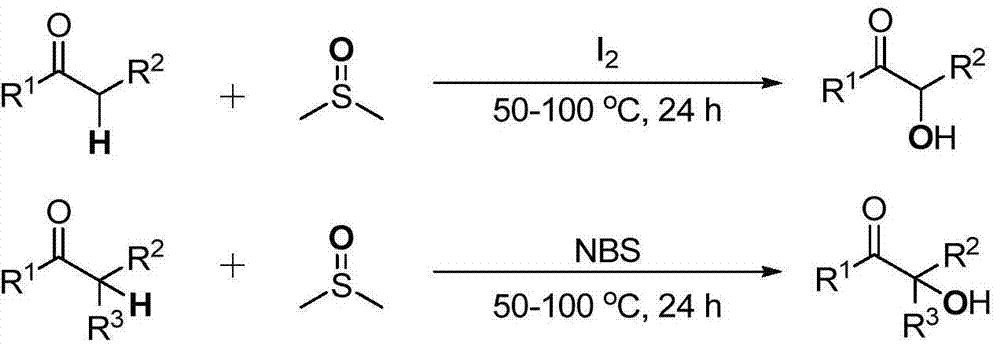
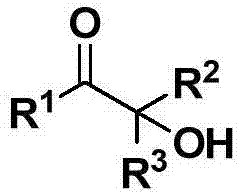
Cheap and efficient synthesis method of alpha-hydroxyketone compound
ActiveCN104710256AEmission reductionHigh yieldOrganic compound preparationHydroxy group formation/introductionSynthesis methodsEthyl acetate
The invention discloses a cheap and efficient synthesis method of an alpha-hydroxyketone compound. The synthesis method is characterized in that a carbonyl compound undergoes an oxidation hydroxylation reaction at 10-120DEG C under normal pressure with iodine simple substance, N-bromosuccimide, copper bromide, bromine simple substance, hydrogen bromide, N-iodosuccimide or hydrogen iodide as a catalyst, sulfoxide as an oxidant, water or sulfoxide as a hydroxy source and sulfoxide, ethyl acetate, N,N-dimethyl formamide, acetonitrile, toluene, 1,4-dioxane, 1,2-dichloroethane, tetrahydrofuran or H2O as a solvent, and converts into the alpha-hydroxyketone compound in a high selectivity manner. Compared with traditional synthesis methods, the method disclosed in the invention has the advantages of simple operation, high yield, simple conditions, easy purification, small waste discharge amount, simple reaction apparatus, and easy industrial production. The method has wide applicability and can be used for synthesizing various alpha-hydroxyketone compounds.
Owner:QINGDAO RUIJI MEDICAL TECH CO LTD
1, 2-dichloroethane selective hydrodechlorination reaction catalyst and preparation method and application thereof
InactiveCN105148907AGood dispersionImprove utilization efficiencyHydrocarbon from halogen organic compoundsMetal/metal-oxides/metal-hydroxide catalystsFiltrationEthane Dichloride
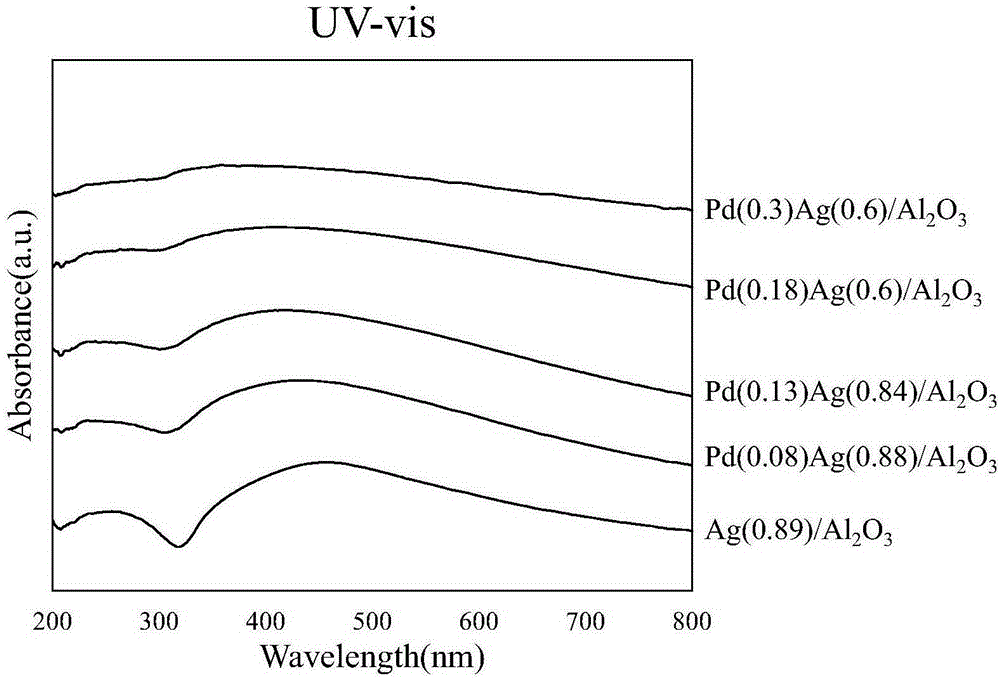
The invention relates to a 1, 2-dichloroethane selective hydrodechlorination reaction catalyst and a preparation method and application thereof. The catalyst uses oxide C as a carrier, metals A and B are loaded respectively through an impregnation method and a replacement method and an A-B / C catalyst is obtained, wherein the metal A is Ag or Cu, the metal B is Pd or Pt, and the oxide C is Al2O3, SiO2, TiO2 or ZrO2. The preparation method of the catalyst comprises the steps of adding oxide C into metal A salt solution for impregnation and evaporative drying, and then performing drying and roasting to obtain A / C; feeding H2 into A / C for reduction, adding deoxidized metal B salt solution into inert gas for replacement reaction, agitating for 0.5 to 3h, performing filtration to obtain solids and drying the solids to obtain an A-B / C catalyst. The A-B / C catalyst is used for 1, 2-dichloroethane selective hydrodechlorination reaction to obtain hydrocarbons dominated by ethylene, and the selectivity of ethylene is greater than 80 percent.
Owner:NANJING UNIV
1, 2-dichloroethane cracking catalyst, as well as preparation method and application thereof
InactiveCN102773086ASimple preparation processMaterials are readily availablePreparation by hydrogen halide split-offMetal/metal-oxides/metal-hydroxide catalystsActivated carbonReaction temperature
The invention relates a preparation method and an application of a 1, 2-dichloroethane cracking catalyst, and the catalyst can be used in the process of producing chloroethylene by cracking 1, 2-dichloroethane for optimizing and improving the process. Specifically, activated carbon is taken as a carrier, the main body catalyst is attached on the carrier through an immersion method, and the obtained catalyst is used in the process of producing the chloroethylene by cracking the 1, 2-dichloroethane on a fixed bed reactor. The catalyst can show the advantages of low reaction temperature, low power consumption, high conversion rate of the dichloroethane, high selectivity and yield of the chloroethylene, long service life of the catalyst, smooth operation and the like when the catalyst is used in the process of producing the chloroethylene by cracking the 1, 2-dichloroethane via long-term continuous operation of the fixed bed reactor.
Owner:新疆化工设计研究院有限责任公司
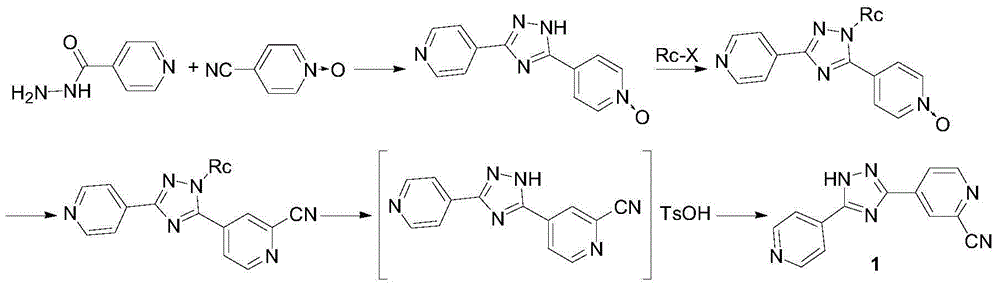
Preparation method for topiroxostat
The invention belongs to the field of medicine and chemical engineering, and particularly relates to a preparation method for topiroxostat. 2-chloro-4-[(5-pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]-pyridine is subjected to a cyanation reaction under the action of a cyanation reagent in the presence of a catalyst, base and a ligand to obtain topiroxostat. The preparation method comprises the following steps: 4-cyanopyridine-N-oxide is taken as a starting material, 1,2-dichloroethane is taken as a solvent, triethylamine is taken as base, phosphorus oxychloride is used as a chlorinated reagent, and chlorination is conducted to obtain 2-chloro-4-cyanopyridine; 2-chloro-4-cyanopyridine and isoniazide are in a methanol solvent, sodium methoxide is taken as a catalyst, and close-loop condensation is performed to obtain 2-chloro-4-[(5-pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]-pyridine. The preparation method has the advantages that a safe and cheap cyanogroup source is selected, a hypertoxic cyanation reagent is avoided, the environmental harm is reduced, the product yield is high, the purity is high, and the suitability for industrial mass production is high.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
Selective hydrogenation dechlorination catalyst for 1,2-dichloroethane, as well as preparation method and application thereof
InactiveCN102658127AImprove stabilityGood choiceCatalyst activation/preparationHydrocarbon from halogen organic compoundsContinuous lightEthane Dichloride
The invention discloses a bimetallic catalyst prepared by a continuous light deposition method, and a method for degrading 1,2-dichloroethane by selective hydrogenation dechlorination. The bimetallic catalyst is A-B / TiO2 type catalyst obtained by sequentially depositing binary metal on a titanium dioxide carrier by light deposition, wherein A is Pd, Pt, Au or Rh, and B is Ag, Cu or Ni. The catalyst is prepared by the steps of: adding TiO2, A salt solution and methyl alcohol into a photoreactor, filling N2 into the photoreactor under the dark condition, and carrying out reaction for 3-6h under the ultraviolet illumination; filtering, washing and then drying; and loading the metal B in the same way to prepare the catalyst. 1,2-dichloroethane is degraded by hydrogenation through selective catalysis of the A-B / TiO2 type catalyst, thus being converted into hydrocarbon taking ethylene as main product. The catalyst has technical feasibility, is free from secondary pollution, is used for degrading 1,2-dichloroethane with remarkable effect, and has good economic benefit and environmental benefit.
Owner:NANJING UNIV
Catalyst used in process of preparing vinyl chloride from 1,2-ethylene dichloride and preparation method of catalyst
ActiveCN102247884APreparation by hydrogen halide split-offMolecular sieve catalystsRare-earth elementFluidized bed
The invention provides a catalyst used in the process of preparing vinyl chloride from 1,2-ethylene dichloride and a preparation method of the catalyst. The catalyst consists of 10.0 to 80.0 weight percent of zeolite and 20.0 to 90.0 weight percent of inorganic oxide substrate, wherein the zeolite is a zeolite socony mobil-5 (ZSM-5) molecular sieve which comprises a rare earth element and has a melt flow index (MFI) structure; the rare earth element is 1 to 15 weight percent based on RE2O3; RE represents the rare earth element; and the inorganic oxide substrate can be a pure oxide or a complex of a plurality of oxides. In the preparation method, the catalyst is prepared by a spray drying forming method. Compared with the conventional thermal cracking technology, the method has the advantages that: energy consumption can be greatly reduced, production cost is reduced, and the catalyst is suitable for a fluidized bed reaction device.
Owner:天津渤化化工发展有限公司
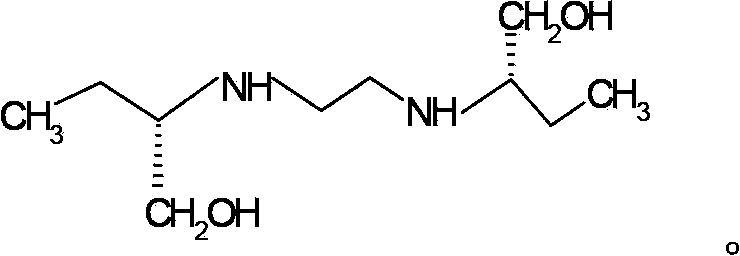
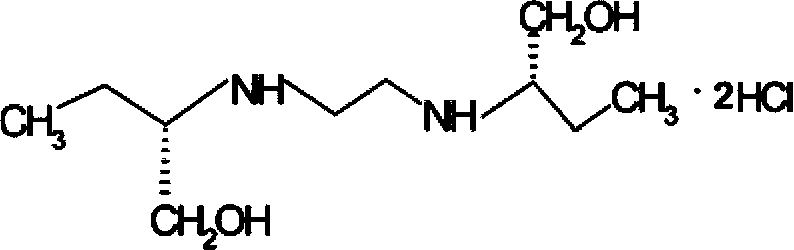
Methods for preparing ethambutol and ethambutol hydrochloride
ActiveCN103772214AEasy to recycleHigh recovery rateOrganic compound preparationAmino-hyroxy compound preparationOrganic solventBoiling point
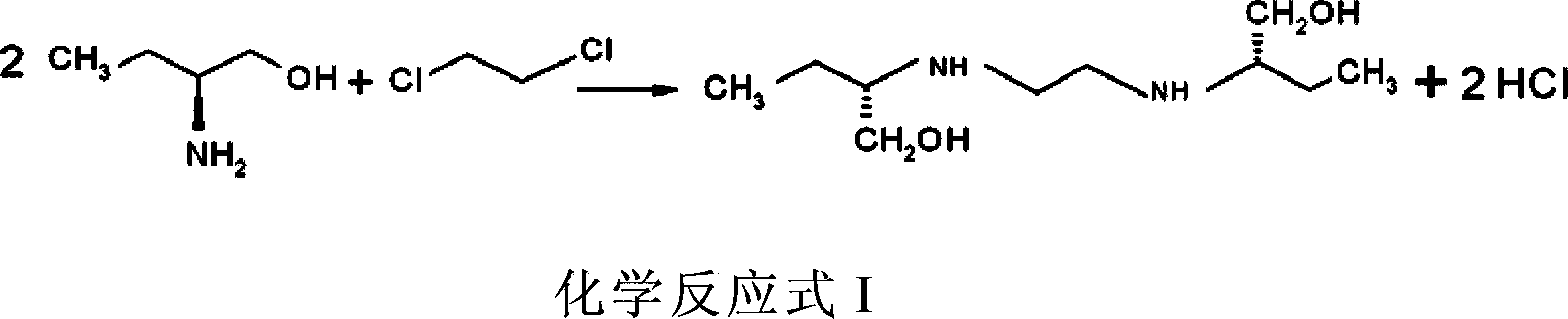
The invention provides methods for preparing ethambutol and ethambutol hydrochloride. The method for preparing the ethambutol comprises the step of utilizing (S)-2-aminobutanol and 1,2-dichloroethane to perform condensation reaction to prepare the ethambutol, wherein the condensation reaction is carried out in a low-boiling organic solvent, and HCl produced in the ammonia gas neutralization reaction process is utilized. Through the utilization of the method, the ethambutol of which the yield coefficient is improved can be obtained, so that the ethambutol hydrochloride of which the yield coefficient is improved can be obtained. Besides, the methods are simple in technology, safe, stable, low in cost and super-high in practical value in the industry.
Owner:NEW FOUNDER HLDG DEV LLC +2
Method for recovering ferrocene derivatives and ammonium perchlorate from composite solid propellant
The invention provides a method for recovering ferrocene derivatives and ammonium perchlorate from a composite solid propellant, which belongs to the field of fabrication of composite solid propellants. The method comprises an immersion step of immersing the composite solid propellant in dichloromethane, 1,2-dichloroethanes or N,N-dimethylacetamide, a distillation step of distilling an organic solvent so as to obtain a crude product of ferrocene derivatives, a purification step of purifying the crude product of ferrocene derivatives through recrystallization or sublimation, an extraction step of using a saturated solution of ammonium perchlorate as an extractant and a recrystallization step for obtaining ammonium perchlorate. According to the invention, the method of organic solvents is utilized in the invention for separation and recovery the active components of ammonium perchlorate and ferrocene derivatives, and a high recovery rate is achieved; implementation process of the invention is simple, and the method is easy to form a flow and has a good industrial prospect.
Owner:BEIJING UNIV OF CHEM TECH +1
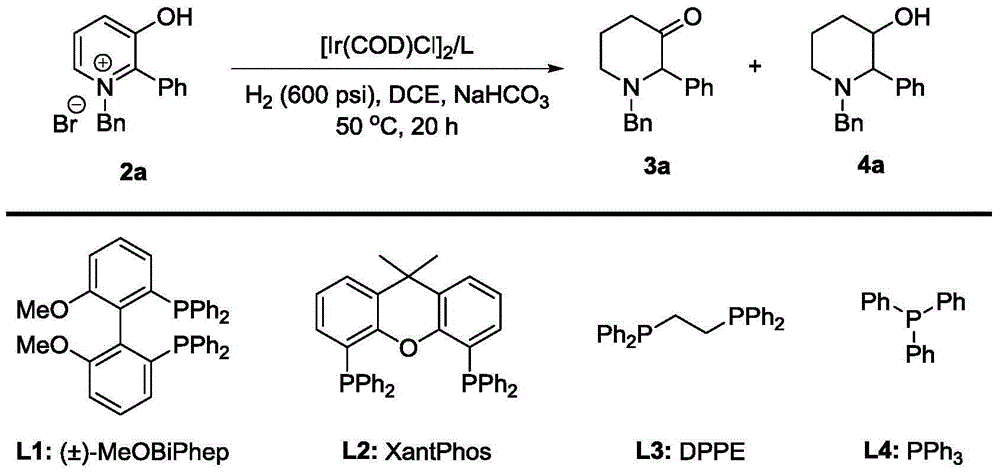
Method for synthesis of 3-piperidone derivatives through iridium catalytic hydrogenation
A method for synthesis of 3-piperidone derivatives through iridium catalytic hydrogenation is provided, wherein a used catalyst is a triphenylphosphine complex of iridium. A reaction can be carried out under the following conditions: the temperature is 40-60 DEG C; the solvent is 1,2-dichloroethane; the pressure is 20-50 atmospheric pressures; the ratio of a substrate to a catalyst is 100 to 1; and the catalyst is a complex of chloro(1,5-cyclooctadiene)iridium(I) dimer and triphenylphosphine. Hydrogenation of 3-hydroxypyridine benzyl bromide salt can obtain the 3-piperidone derivatives with excellent chemoselectivity, the highest yield can reach 97%, and the chemical selectivity of ketones and alcohols is greater than 20:1. The method has the advantages of simple and convenient operation, easily obtained raw materials, high chemoselectivity, and good yield, and provides an atom economic and environment-friendly route for synthesis of a series of 3-piperidone derivatives.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
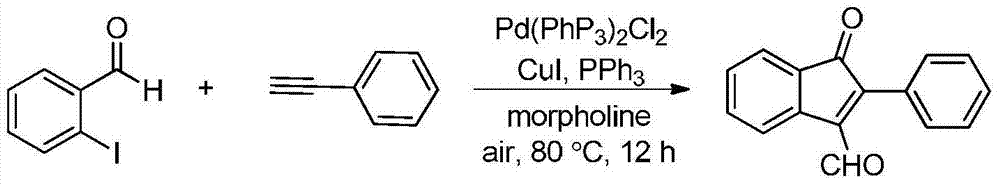
Synthetic method of indanone by gold-catalysis
InactiveCN104326892AWide range of reaction applicationsEasy access to substratesOrganic compound preparationCarbonyl compound preparationGeneral equationChloroform
The invention provides a synthetic method of indanone. The general equation of reaction is defined in the specification. Reaction substrates of the method are different substituted 1,5-eneyne. A catalyst is Ph3PAuCl, Ph3PAuNTf2, HAuCl4, NaAuCl4, Ph3PAuOTf, Ph3PAuSbF6 or IPrAuCl. A medium required by the reaction is methanol, toluene, dichloromethane, 1,2-dichloroethane, chloroform or tetrahydrofuran. The reaction is implemented by microwave heating which promotes the reaction, and also can be implemented by heating and stirring. According to the reaction, cyclization and oxidation can be cascaded in the presence of a gold catalyst to generate a series of indanone-containing compounds. The method provided by the invention has characteristics of simple operation, wide range of application, few by-products, high yield and green reaction.
Owner:SHENYANG PHARMA UNIVERSITY
Method for synthesizing p-fluorobenzoyl chloride
InactiveCN104592013AMeet purity requirementsRaw materials are cheap and easy to getOrganic compound preparationCarboxylic compound preparationDistillationChloride
The invention discloses a method for synthesizing p-fluorobenzoyl chloride. The method is characterized by comprising the following steps: adding bis(trichlormethyl) carbonate into a reaction kettle filled with parafluorobenzoic acid and 1,2-dichloroethane, reacting in the presence of a catalyst, filtering, and performing reduced pressure distillation, thereby obtaining p-fluorobenzoyl chloride. The selected acylating chlorination reagents are green and environment-friendly, the raw materials are low in price and readily available, the synthetic process is mild in conditions, the purity of the finally obtained product can be 99.9 percent, and the product has wide application prospects and industrial values.
Owner:DONGGUAN DONGYANG SOLAR SCI RES & DEV CO LTD
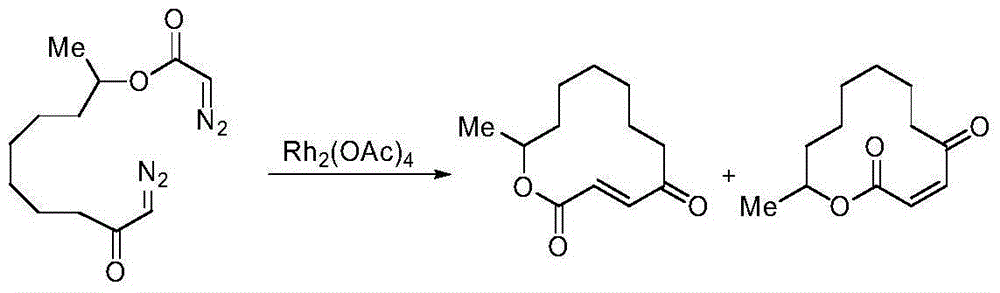
Method for selectively generating trans-macrocycloalkene by gold-catalyzed intramolecular diazo coupling
ActiveCN105037319AEasy to operateHigh yieldOrganic compound preparationCarbonyl compound preparationCouplingArgon atmosphere
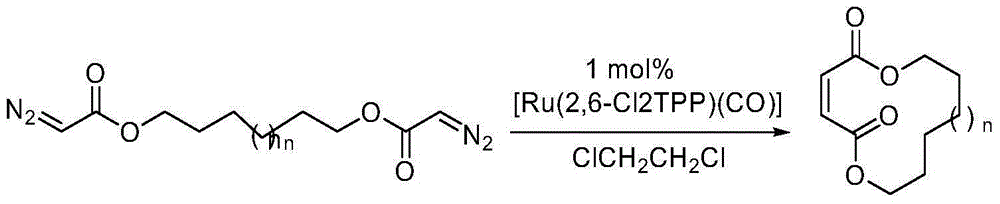
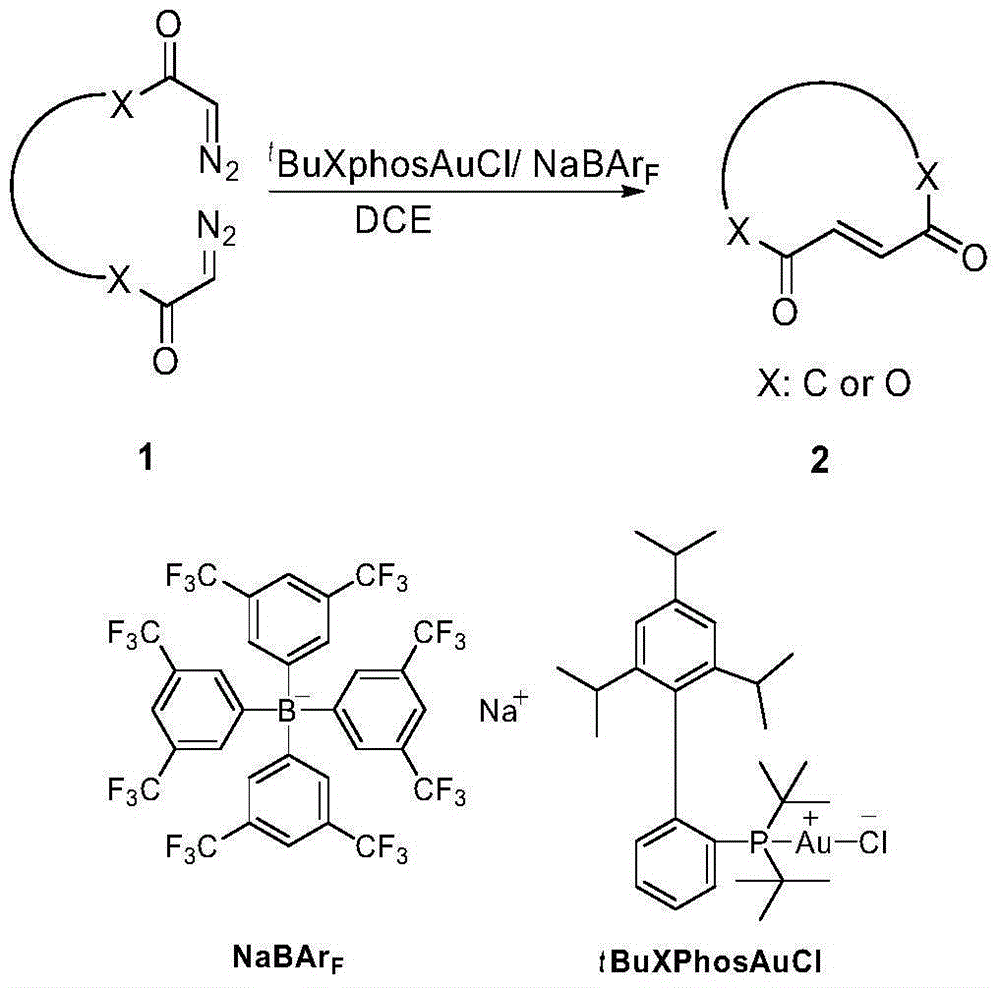
The invention discloses a method for selectively generating trans-macrocycloalkene by gold-catalyzed intramolecular diazo coupling and belongs to the field of organic synthesis. The method comprises the following steps: adding a catalyst, NaBArF, 1,2-dichloroethane and a diazo compound into a dry reaction flask in an argon atmosphere; raising the temperature to 80 DEG C; and carrying out a reaction for 12 hours to obtain the trans-macrocycloalkene with high yield and high selectivity. The method has the advantages of being simple to operate, high in yield and good in stereoselectivity.
Owner:CHANGZHOU UNIV
Method for preparing vinyl chloride from 1,2-dichloroethane
InactiveCN102249844AReduce energy consumptionReduce manufacturing costPreparation by hydrogen halide split-offChemical recyclingCatalytic pyrolysisMolecular sieve
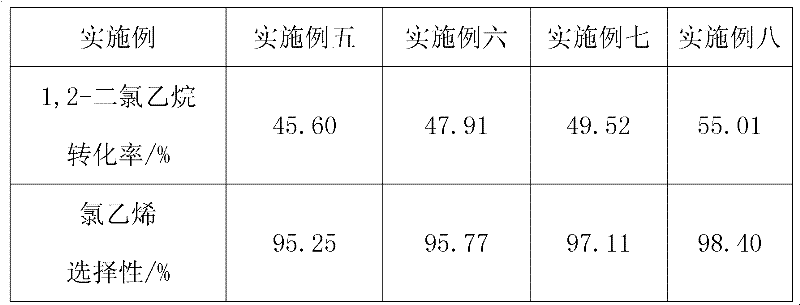
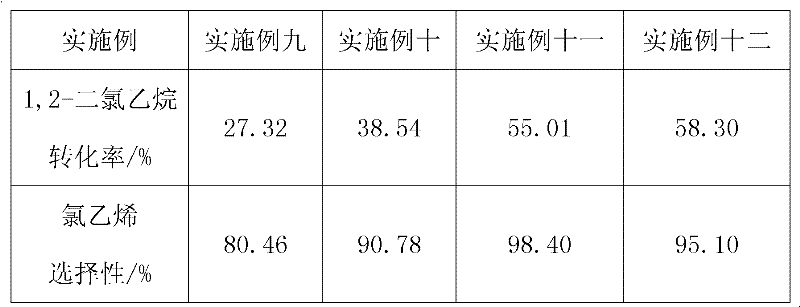
The invention provides a method for preparing vinyl chloride by adopting a SAPO-34 molecular sieve catalyst containing metals for catalytic pyrolysis of 1,2-dichloroethane. The method is characterized in that: the metals comprise Fe, Mg, Zn and Mn; the catalyst reacts at a reaction temperature of 280-400 DEG C under reaction pressure of 0.1-2 MPa, wherein weight space velocity of the 1,2-dichloroethane is 0.2-5h<-1>. According to the present invention, conversion rate of the 1,2-dichloroethane is 55%; selectivity of the vinyl chloride is more than 98%; the catalyst can be recycled repeatedly; due to carrying out reaction at the low temperature, the energy consuming are substantially reduced, the production cost is decreased; the method has a good industrial prospect.
Owner:TIANJIN DAGU CHEM CO LTD
Method for determining (4R, 6R) -6-aminoethyl-2,2-dimethyl-1,3-dioxane-4- tert-butyl acetate content
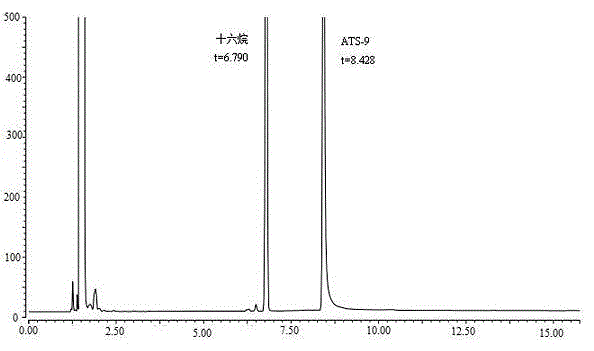
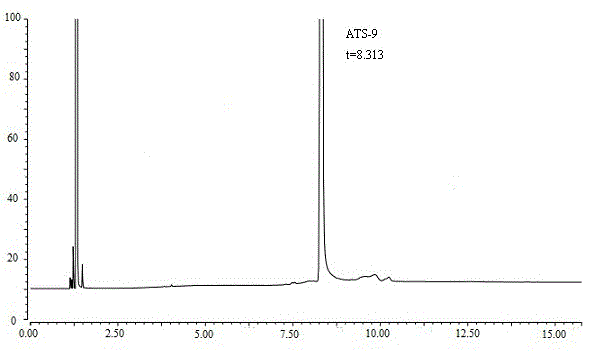
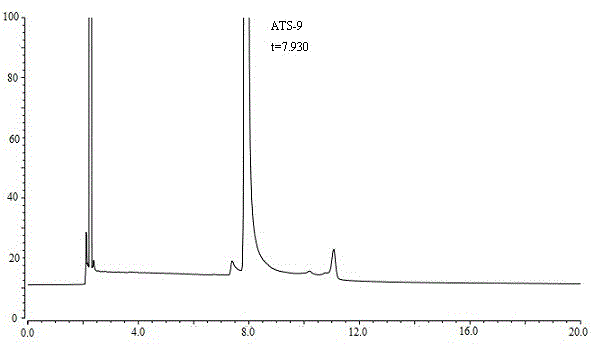
ActiveCN105136921AAccurate contentAccurate detectionComponent separationCapillary gas chromatographyN-hexadecane
The present invention discloses a method for determining (4R, 6R) -6--aminoethyl-2,2-dimethyl-1,3-dioxane-4- tert-butyl acetate content. The method is as follows: ATS-9 is diluted with 1,2-dichloroethane, an Agilent DB-624 capillary gas chromatography column is used, a hydrogen flame ionization detector is used, appropriate temperature programing, vaporization chamber temperature and detector temperature are set, nitrogen is used as a carrier gas, n-hexadecane is used as an internal standard for detection, and ATS-9 content in a to-be-tested solution can be calculated by internal standard method according to the gas chromatogram. The ATS-9 and related substances and the internal standard can be effectively separated, and the method is rapid, accurate, simple and well reproducible, and lays the foundation for the establishment of ATS-9 quality standards.
Owner:HENAN ZHIWEI BIOMEDICINE CO LTD
Process for the preparation of 1,2-dichloroethane free crystals of zonisamide
InactiveUS20040138474A1Improve securityNervous disorderOrganic chemistryAlcoholAzeotropic distillation
A process for the preparation of crystals of zonisamide containing residual 1,2-dichloroethane of not more than 5 ppm, which comprises adding an aqueous C2-4 alcohol to crystals of zonisamide containing residual 1,2-dichloroethane of more than 5 ppm, removing said 1,2-dichloroethane by azeotropic distillation, followed by collecting the precipitated crystals from the residual mixture.
Owner:UENO YOSHIKAZU +1
Bis(pinacolato)diboron production process
ActiveCN102558209AHigh reaction conversion rateImprove securityGroup 3/13 element organic compoundsBoron trichlorideReaction temperature
The invention discloses a bis(pinacolato)diboron production process which is characterized in that boron trichloride is used as a raw material and is aminated with dimethylamine gas in an n-hexane system, the desalinated reaction solution and boron tribromide react at the room temperature, the prepared intermediate and magnesium are coupled in toluene, 1, 2-dichloroethane solution of pinacol is dropped into the desalinated reaction solution, and the target product is prepared via transesterification. The invention has the advantages that: n-hexane is used as a reaction solvent for the first two steps, the conversion rate of reaction is increased, and the ultra low temperature can be avoided; the safety of the process is improved due to use of magnesium; and the yield of the product is increased in transesterification through controlling the reaction temperature.
Owner:HAIMEN RUIYI MEDICAL TECH
Environmentally friendly preparation technology of 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane
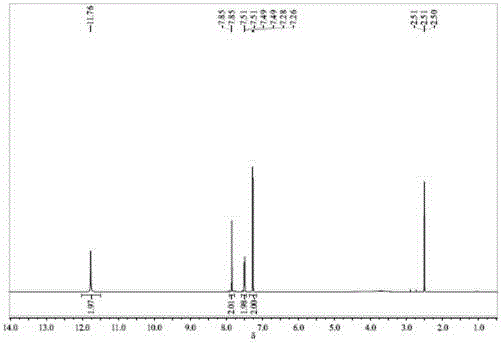
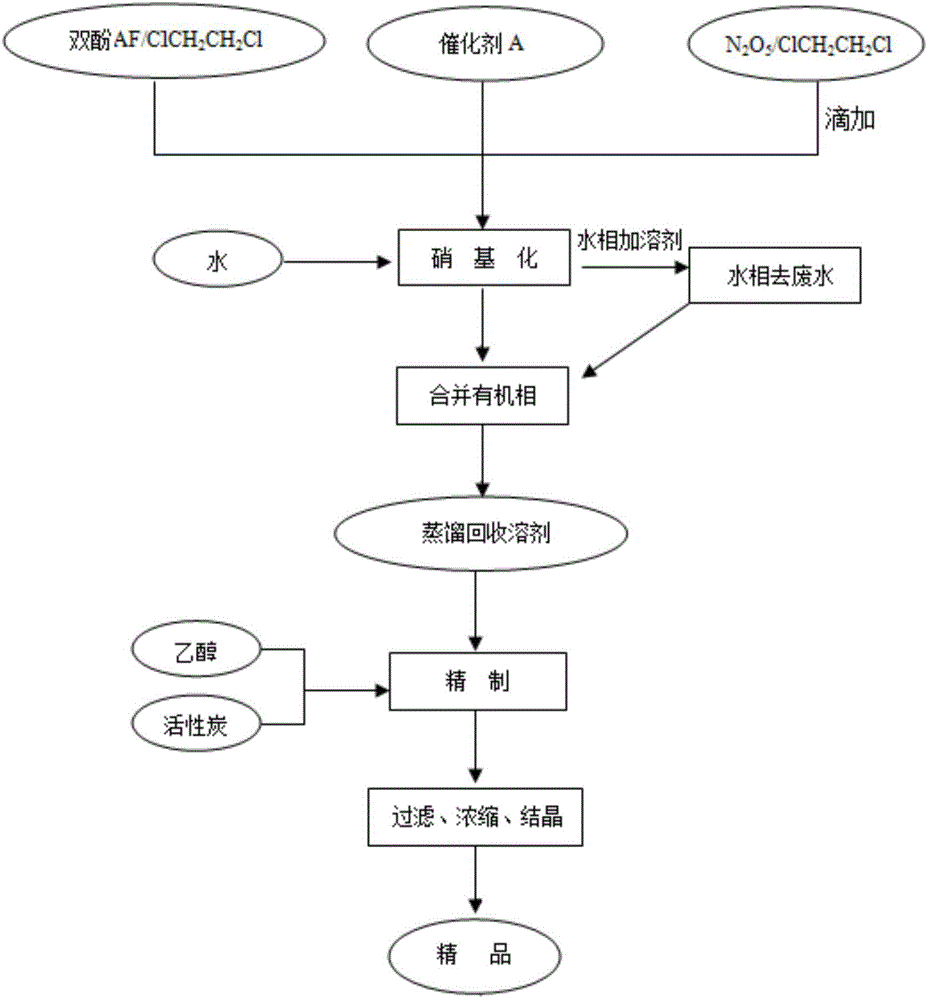
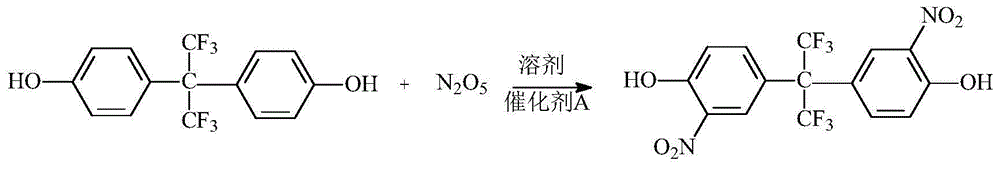
ActiveCN105753710AEliminate pollutionReduce generationNitro compound preparationEthane DichlorideDinitrogen pentoxide
The invention relates to an environmentally friendly preparation technology of 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane. The technology comprises the following steps: preparing a 1,2-dichloroethane solution of bisphenol AF; preparing a 1,2-dichloroethane solution of N2O5; carrying out a nitration reaction to generate 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane; filtering the 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane, washing the 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane, and layering the 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane to obtain a lower layer organic phase; carrying out dehydration drying, filtering the obtained material, and carrying out vacuum distillation to obtain crude 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane; and re-crystallizing the crude 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane, cooling the re-crystallized 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane, filtering the cooled 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane, and carrying out vacuum drying to obtain purified 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane. The preparation technology adopts dinitrogen pentoxide (N2O5) as a nitration agent and allows the reaction to be carried out a low temperature, so pollution of waste acids is thoroughly eliminated, and toxic, harmful and highly risky byproducts are reduced, and the nitration reaction is clean and environmentally friendly.
Owner:LIANYUNGANG TETRAFLUOR NEW MATERIALS
Preparation method of pazufloxacin intermediate
The invention relates to a preparation method of a pazufloxacin intermediate, the preparation method is as folows: in a suitable solvent and at a suitable temperature, under the effects of a phase transfer catalyst and an inorganic alkali, a compound II reacts with 1, 2 -dichloroethane for cyclopropanation, the reaction system is direct heated and refluxed for hydrolysis reaction, a pazufloxacin intermediate compound IV is obtained by postprocessing; the weight ratio of inorganic alkali to compound II is 1.5-2.5:1; the ratio of solvent to compound II is 14-16mL:1g. Compared with the prior art, according to the preparation method, a specified concentration and the specific amount of the alkali is used, a two-step method is performed by a 'one pot' method, the unit operation is simplified; and in the preparation process, the three wastes are less, the environmental protection pressure is low, the yield is high, and the method is suitable for industrialized production.
Owner:ZHEJIANG HAISEN PHARMACY CO LTD
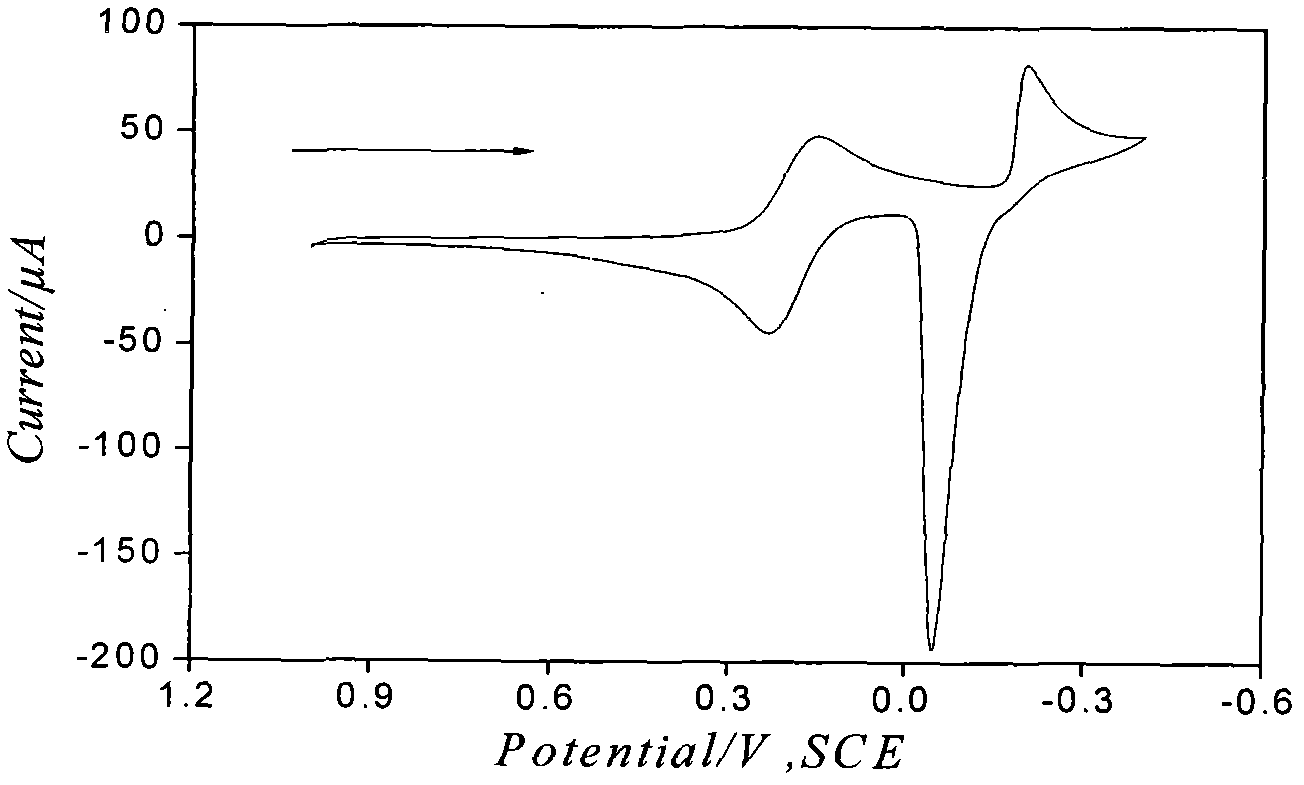
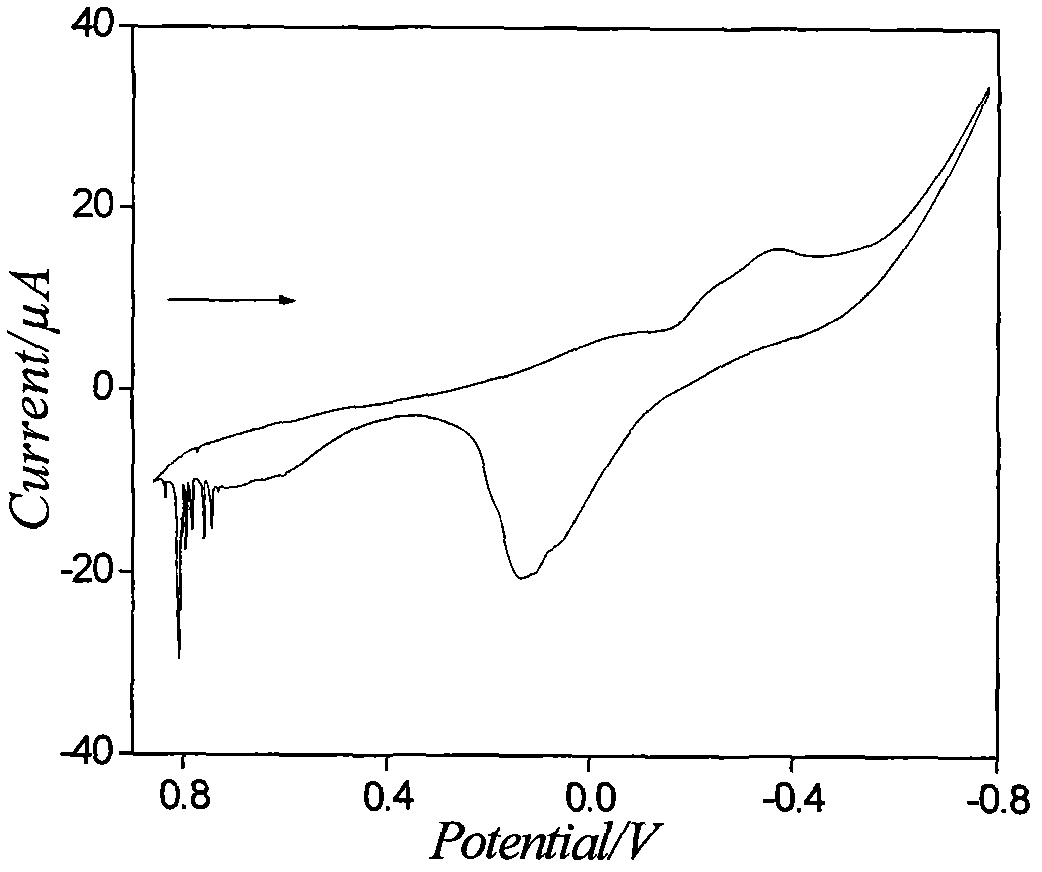
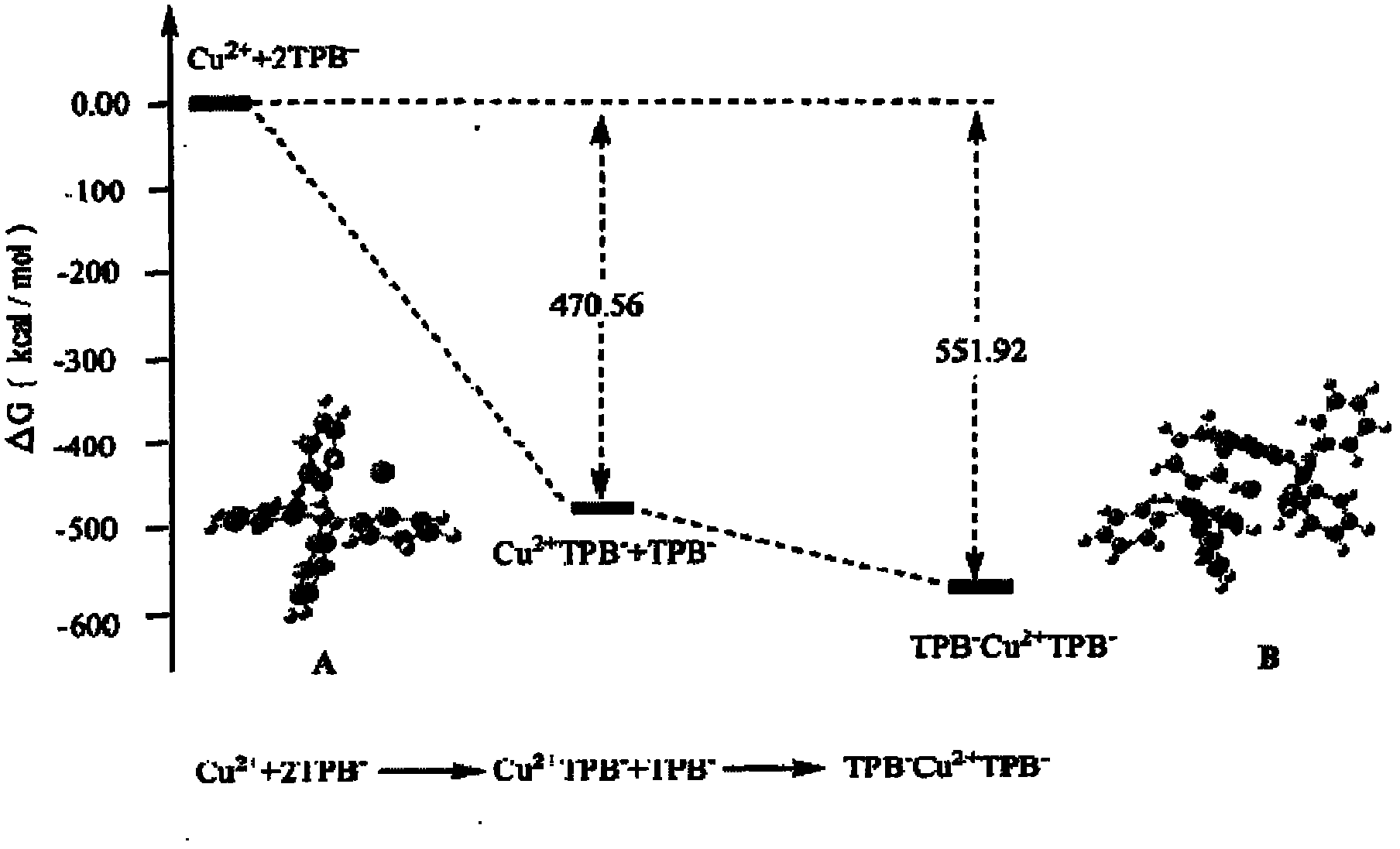
Electrochemical detection method for ion pairs formed by Cu2+ and TPB- in water/1,2-dichloroethane system
InactiveCN102680558ASimple experimental designHigh sensitivityMaterial electrochemical variablesGibbs free energyDensity functional theory
The invention discloses an electrochemical detection method for ion pairs formed by Cu2+ and TPB- in a water / 1,2-dichloroethane system, which comprises the following steps: performing cyclic voltammetry scanning on platinum working electrode, counter electrode and reference electrode on a water / 1,2-dichloroethane interface, thereby obtaining an irregular current cyclic voltammogram, wherein a test proves that the irregular current is caused by the ion pairs formed by Cu2+ and TPB- on a liquid / liquid interface; and meanwhile, utilizing a density functional theory to calculate minimal gibbs free energy of the ion pairs TPB-Cu2+TPB- formed by Cu2+ and TPB-, thereby obtaining the composition of the most stable ion pairs. The electrochemical detection method has the characteristics that the adopted instrument is simple; the consumption of drugs is less; the sensitivity is high; the test operation is easily performed; and the electrochemical detection method has an application prospect in researching the structure of the liquid / liquid interface and biochemistry by detecting the ion pairs formed by Cu2+ and TPB-.
Owner:NORTHWEST NORMAL UNIVERSITY
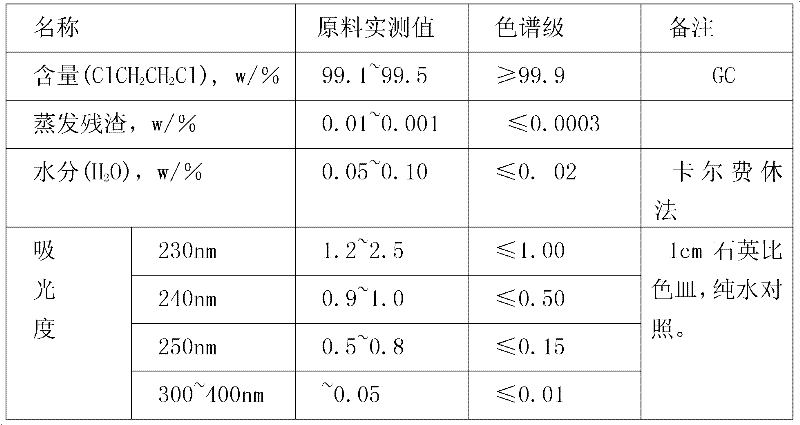
Method for purifying chromatographic grade organic solvent 1,2-dichloroethane
InactiveCN102417435AImprove yieldEasy to separateHalogenated hydrocarbon separation/purificationPurification methodsSorbent
The invention relates to a method for purifying a chromatographic grade organic solvent 1,2-dichloroethane. The method comprises the following steps of: allowing a raw material, namely 1,2-dichloroethane to pass through an absorption column loaded with activated carbon and acidic alumina so as to remove chloride and some acid impurities, dehydrating by using a drying agent, rectifying, and filtering to obtain a high performance liquid chromatography grade 1,2-dichloroethane product. In the method, the 1,2-dichloroethane with the content of about 99.2 percent is taken as the raw material and the activated carbon and the acidic alumina are taken as adsorbents for adsorption separation so as to remove impurities from the raw material, anhydrous calcium chloride is used as the drying agent so as to remove the moisture from the raw material, and a 1,2-dichloroethane product with the purity of 99.9 percent is obtained by the steps of adsorption, drying, rectification and purification; the yield is improved to be more than 93 percent; and various other indexes can all meet chromatographic index requirements.
Owner:天津市康科德科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com