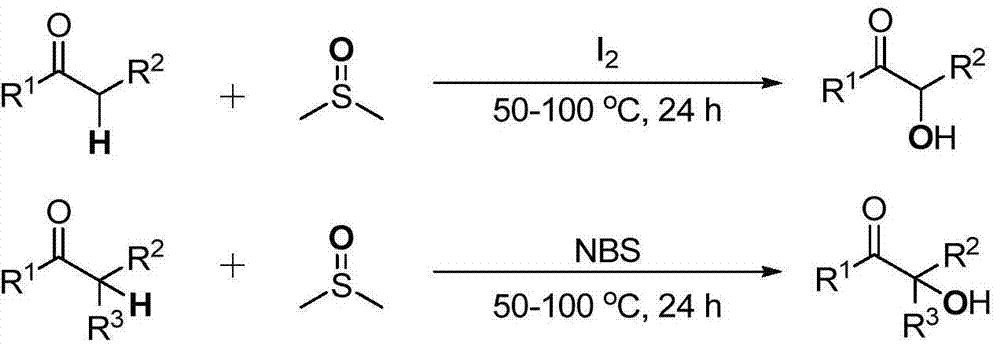
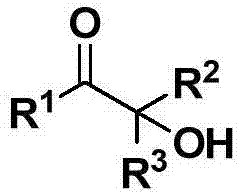
Cheap and efficient synthesis method of alpha-hydroxyketone compound
A carbonyl compound and compound technology, which is applied in the field of synthesis of α-hydroxy ketone compounds, can solve the problems of narrow substrate application range and complicated operation, and achieves the effects of wide applicability, simple reaction equipment and less waste discharge.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0036] The preparation of embodiment 12-hydroxyl-1-phenylacetone
[0037]
[0038] a): get a 25mL Schlenk reaction tube, add iodine simple substance (I 2 ) 26mg (0.1mmol) as catalyst, 1-phenylacetone 68mg (0.5mmol), dimethyl sulfoxide (DMSO) 1mL as oxidant, hydroxyl source and solvent, stirred at 60°C for 24 hours. After the reaction was completed, 15 mL of ethyl acetate and 3 mL of brine were added, extracted three times with ethyl acetate, the organic phases were combined, and separated by column chromatography to obtain 54 mg of pure 2-hydroxy-1-phenylacetone with a yield of 72%. (optimal system)
[0039] b): Take a 25mL Schlenk reaction tube, add N-bromosuccinimide (NBS) 18mg (0.1mmol) as a catalyst, 1-phenylacetone 68mg (0.5mmol), dimethyl sulfoxide (DMSO) 1 mL was used as oxidizing agent, hydroxyl source and solvent, and stirred at 60°C for 24 hours. After the reaction was completed, 15 mL of ethyl acetate and 3 mL of brine were added, extracted three times with et...
Embodiment 22
[0076] The preparation of embodiment 22-hydroxyl-1-p-methyl phenylacetone
[0077]
[0078] Get a 25mL Schlenk reaction tube, add iodine simple substance (I 2 ) 26mg (0.1mmol) as catalyst, p-methyl phenylacetone 75mg (0.5mmol), dimethyl sulfoxide (DMSO) 1mL as oxidant, hydroxyl source and solvent, stirred at 60°C for 24 hours. After the reaction, 15 mL of ethyl acetate and 3 mL of brine were added, extracted three times with ethyl acetate, the organic phases were combined, and separated by column chromatography to obtain 62 mg of pure 2-hydroxy-1-p-methylphenylacetone with a yield of 76%.
[0079] 1 H NMR (400MHz, CDCl 3 ):δ7.83(d,J=8.4Hz,2H),7.29(d,J=8.0Hz,2H),5.17-5.10(m,1H),3.86(d,J=8.0Hz,1H),2.42 (s,3H),1.44(d,J=7.2Hz,3H); 13 C NMR (100MHz, CDCl 3 ):δ201.8,144.9,130.6,129.4,128.6,69.0,22.3,21.6ppm; MS(70ev):m / z(%):91.1(50),119.0(100),164.0(M + ,1).
Embodiment 32
[0080] The preparation of embodiment 32-hydroxyl-1-p-methoxyphenylacetone
[0081]
[0082] Get a 25mL Schlenk reaction tube, add iodine simple substance (I 2 ) 26mg (0.1mmol) as catalyst, p-methoxyphenylacetone 83mg (0.5mmol), dimethyl sulfoxide (DMSO) 1mL as oxidant, hydroxyl source and solvent, stirred at 60°C for 24 hours. After the reaction, 15 mL of ethyl acetate and 3 mL of brine were added, extracted three times with ethyl acetate, the organic phases were combined, and separated by column chromatography to obtain 70 mg of pure 2-hydroxy-1-p-methoxyphenylacetone with a yield of 78%.
[0083] 1 H NMR (400MHz, CDCl 3 ): δ7.92(d, J=8.4Hz, 2H), 6.97(d, J=9.2Hz, 2H), 5.14-5.07(m, 1H), 3.91(s, 1H), 3.88(s, 3H) ,1.44(d,J=6.4Hz,3H); 13 C NMR (100MHz, CDCl 3 ):δ200.5,164.0,130.9,125.9,113.9,68.7,55.4,22.4ppm; MS(70ev):m / z(%):77.0(20),135.0(100),180.0(M + ,3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com