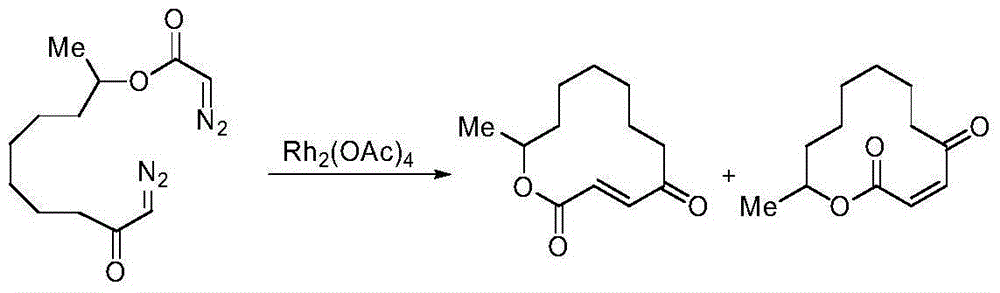
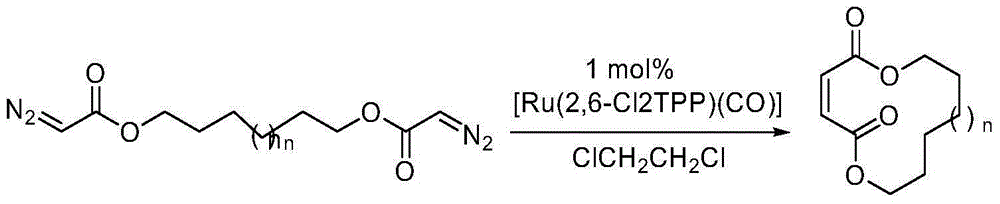
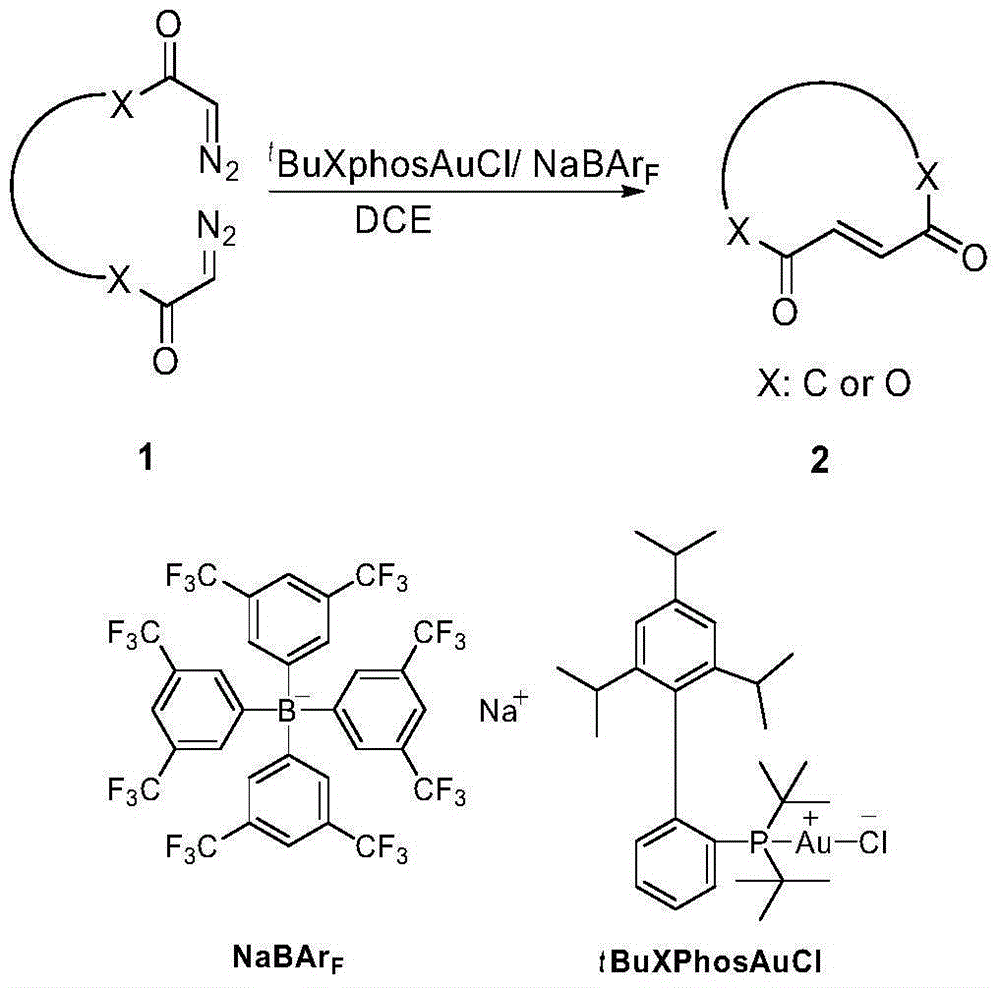
Method for selectively generating trans-macrocycloalkene by gold-catalyzed intramolecular diazo coupling
A selective, macrocyclic alkene technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problem of lack of selectivity of double bond cis-trans isomerism, and achieve high stereoselectivity and operation. Simple, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Synthesis of 2a: add to a dry reaction vial under argon atmosphere t BuXphosAuCl (35mg), NaBAr F (44mg), 1a (772mg), 1,2-dichloroethane (20mL), the reaction was heated up to 80 degrees Celsius and stirred for 12 hours; the reaction solution was cooled, the solvent was evaporated under reduced pressure, and column chromatography gave compound 2a (462mg, Yield:70%) is a white solid, mp:83-85°C; 1 HNMR (400MHz, CDCl 3 )δ6.99(s,2H),6.93-6.75(m,4H),3.96(t,J=5.5Hz,4H),2.67-2.64(t,J=12.0Hz,4H),1.88-1.70(m ,8H),1.51(m,4H). 13 CNMR (100MHz, CDCl 3 )δ202.21, 148.54, 136.57, 120.66, 112.36, 67.94, 41.48, 29.57, 26.33, 26.13.
Embodiment 2
[0025]
[0026] Synthesis of 2b: add to dry reaction vial under argon atmosphere t BuXphosAuCl (35mg), NaBAr F (44mg), 1b (500mg), 1,2-dichloroethane (20mL), the reaction was heated up to 80 degrees Celsius and stirred for 12 hours; the reaction solution was cooled, the solvent was evaporated under reduced pressure, and column chromatography gave compound 2b (252mg, Yield: 65%); 1 HNMR (400MHz, CDCl 3 )δ7.03(s,2H),2.71-2.59(m,4H),1.89-1.71(m,4H),1.46(m,4H),1.28(m,4H). 13 CNMR (100MHz, CDCl 3 )δ203.18, 137.22, 39.12, 24.75, 24.51, 24.18.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com