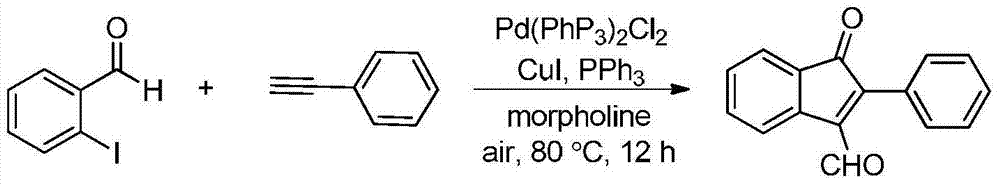
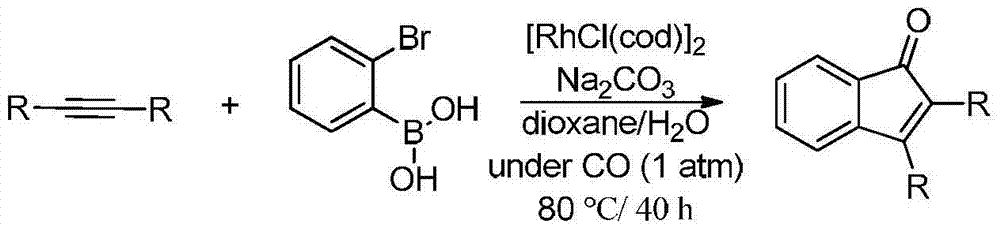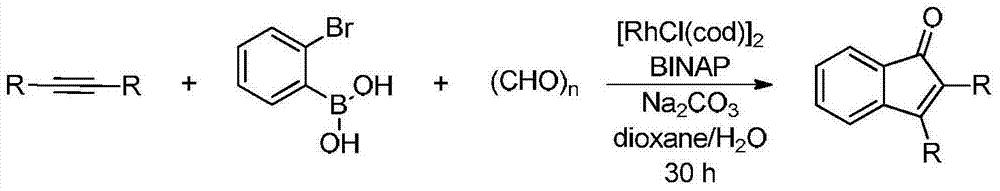Synthetic method of indanone by gold-catalysis
A synthesis method and a gold catalyst technology, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of low yield, few reports on indanone synthesis, long reaction time, etc., and achieve high Reaction yield, wide range of reaction applications, simple and convenient reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Substrate 1 (1mmol) and Ph 3 wxya 2(0.05mmol, 36.9mg) in dry toluene (2mL) was placed in a round-bottomed flask with a rubber stopper, oxygen was continuously fed in and slowly discharged out of the bottle, and reacted at 80°C for 9h. After the reaction is finished, use petroleum ether / ethyl acetate (v / v=100 / 1) as the eluent system, and use 200-300 mesh silica gel as the stationary phase to perform column chromatography separation to finally obtain the target compound I. The reaction yield 75%. Its reaction equation is:
[0034]
[0035] The spectral data of product I is: HRMS (ESI): m / z: [M+H] + 269.0388; 1 H NMR (600MHz, CDCl 3 )δ=10.30(s,1H),7.98(d,J=7.4Hz,1H),7.63(d,J=7.2Hz,1H),7.52–7.45(m,5H),7.34(t,J=7.4 Hz, 1H).
Embodiment 2
[0037] Change the reaction substrate 1 to the reaction substrate 2, change the elution system to petroleum ether / ethyl acetate (v / v=50 / 1), and the others are the same as in Example 1 to obtain the target compound II with a reaction yield of 82%. . Its reaction equation is:
[0038]
[0039] The spectral data of product I is: HRMS (ESI): m / z: [M+H] + 279.1016; 1 H NMR (600MHz, CDCl 3 )δ=10.27(s,1H),7.82(d,J=7.5Hz,1H),7.52(d,J=8.6Hz,2H),7.42(s,1H),7.25(d,J=7.5Hz, 1H), 7.02(d, J=8.6Hz, 2H), 3.88(s, 3H), 2.37(s, 3H).
Embodiment 3
[0041] Change the reaction substrate 1 to the reaction substrate 3, change the elution system to petroleum ether / ethyl acetate (v / v=5 / 1), and the others are the same as in Example 1 to obtain the target compound III with a reaction yield of 82%. . Its reaction equation is:
[0042]
[0043] The spectral data of product I is: HRMS (ESI): m / z: [M+H] + 317.0777; 1 H NMR (600MHz, CDCl 3 )δ=10.25(s,1H),7.57(m,2H),7.55(d,J=8.7Hz,2H),7.03(d,J=8.7Hz,2H),6.70(dd,J=8.2Hz, 2.2Hz, 1H), 3.91(s, 3H), 3.89(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com