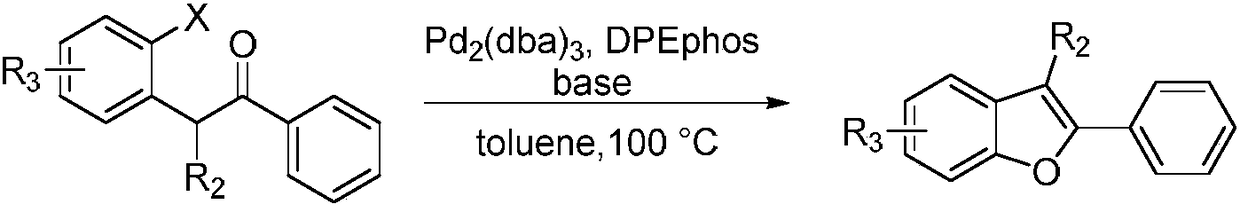
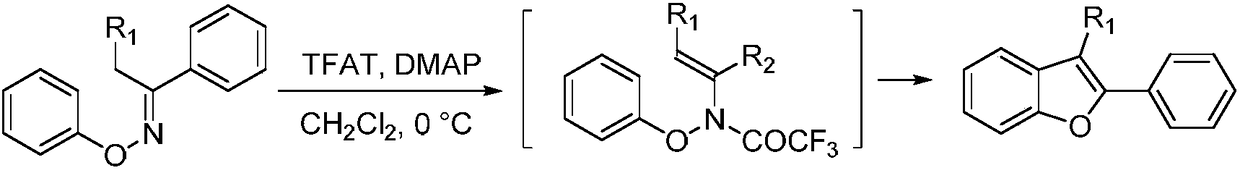
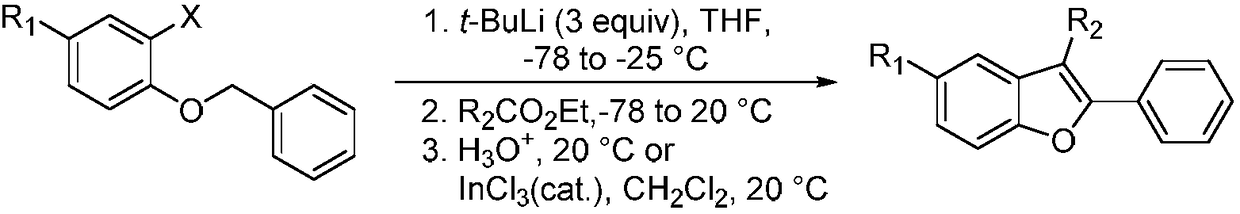Method for synthesizing 2-phenyl-3-methylbenzofuran compounds
A synthesis method and technology of benzofuran methyl phenyl ether are applied in the field of synthesis of 2-phenyl-3-methyl benzofuran compounds, can solve the problems of serious environmental pollution, expensive catalyst and the like, and achieve high reaction Yield, wide range of reaction applications, simple and convenient reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Substrate 1 (1 mmol) was dissolved in mesitylene (2 mL), the reaction mixture was heated to 180 ° C, and stirred for 4 hours. After the reaction was completed, the target product a was separated by flash column chromatography with a yield of 35 %. Its reaction equation is:
[0031]
[0032] The spectral data of product a is: 1 H NMR (600MHz, CDCl 3 )δ7.46(d, J=7.4Hz, 2H), 7.42(d, J=7.1Hz, 2H), 7.39(t, J=8.4Hz, 5H), 7.33(m, 2H), 7.25(m, 5H), 7.08(d, J=2.2Hz, 1H), 6.97(dd, J=8.5, 2.2Hz, 1H), 6.32(d, J=2.2Hz, 1H), 6.29(d, J=2.2Hz, 1H), 5.93(s,1H), 5.12(s,2H), 5.04(s,2H), 5.02(s,2H), 2.09(s,3H); 13 C NMR (150MHz, CDCl 3 )δ161.7,158.6,157.3,156.7,155.6,143.9,137.1,136.8,136.7,128.8,128.7,128.6,128.3,128.1,127.8,127.7,127.6,127.1,124.1,119.7,115.8,112.2,100.4,97.3,94.7 ,94.2,70.8,70.6,70.30,9.26; HRMS(ESI):m / z:Calcd.for C 36 h 30 o 5 [M+H] + 543.2166, Found 543.2173.
Embodiment 2
[0034] Substrate 1 (1 mmol) was dissolved in toluene (2 mL), ZnCl was added 2 (0.2mmol, 27mg), the reaction mixture was heated to 140°C and stirred for 4 hours. After the reaction was completed, the target product a was separated by flash column chromatography with a yield of 40%. Its reaction equation is:
[0035]
[0036] The spectral data of product a is: 1 H NMR (600MHz, CDCl3 )δ7.46(d, J=7.4Hz, 2H), 7.42(d, J=7.1Hz, 2H), 7.39(t, J=8.4Hz, 5H), 7.33(m, 2H), 7.25(m, 5H), 7.08(d, J=2.2Hz, 1H), 6.97(dd, J=8.5, 2.2Hz, 1H), 6.32(d, J=2.2Hz, 1H), 6.29(d, J=2.2Hz, 1H), 5.93(s,1H), 5.12(s,2H), 5.04(s,2H), 5.02(s,2H), 2.09(s,3H); 13 C NMR (150MHz, CDCl 3 )δ161.7,158.6,157.3,156.7,155.6,143.9,137.1,136.8,136.7,128.8,128.7,128.6,128.3,128.1,127.8,127.7,127.6,127.1,124.1,119.7,115.8,112.2,100.4,97.3,94.7 ,94.2,70.8,70.6,70.30,9.26; HRMS(ESI):m / z:Calcd.for C 36 h 30 o 5 [M+H] + 543.2166, Found 543.2173.
Embodiment 3
[0038] Substrate 1 (1 mmol) was dissolved in dichloromethane (2 mL), silica gel (1 g) was added, dichloromethane was evaporated to dryness by distillation under reduced pressure, the reaction mixture was heated to 140° C., and the reaction was stirred for 4 hours. After the reaction was completed, The target product a was separated by flash column chromatography with a yield of 75%. Its reaction equation is:
[0039]
[0040] The spectral data of product a is: 1 H NMR (600MHz, CDCl 3 )δ7.46(d, J=7.4Hz, 2H), 7.42(d, J=7.1Hz, 2H), 7.39(t, J=8.4Hz, 5H), 7.33(m, 2H), 7.25(m, 5H), 7.08(d, J=2.2Hz, 1H), 6.97(dd, J=8.5, 2.2Hz, 1H), 6.32(d, J=2.2Hz, 1H), 6.29(d, J=2.2Hz, 1H), 5.93(s,1H), 5.12(s,2H), 5.04(s,2H), 5.02(s,2H), 2.09(s,3H); 13 C NMR (150MHz, CDCl 3 )δ161.7,158.6,157.3,156.7,155.6,143.9,137.1,136.8,136.7,128.8,128.7,128.6,128.3,128.1,127.8,127.7,127.6,127.1,124.1,119.7,115.8,112.2,100.4,97.3,94.7 ,94.2,70.8,70.6,70.30,9.26; HRMS(ESI):m / z:Calcd.for C 36 h 30 o 5 [...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com