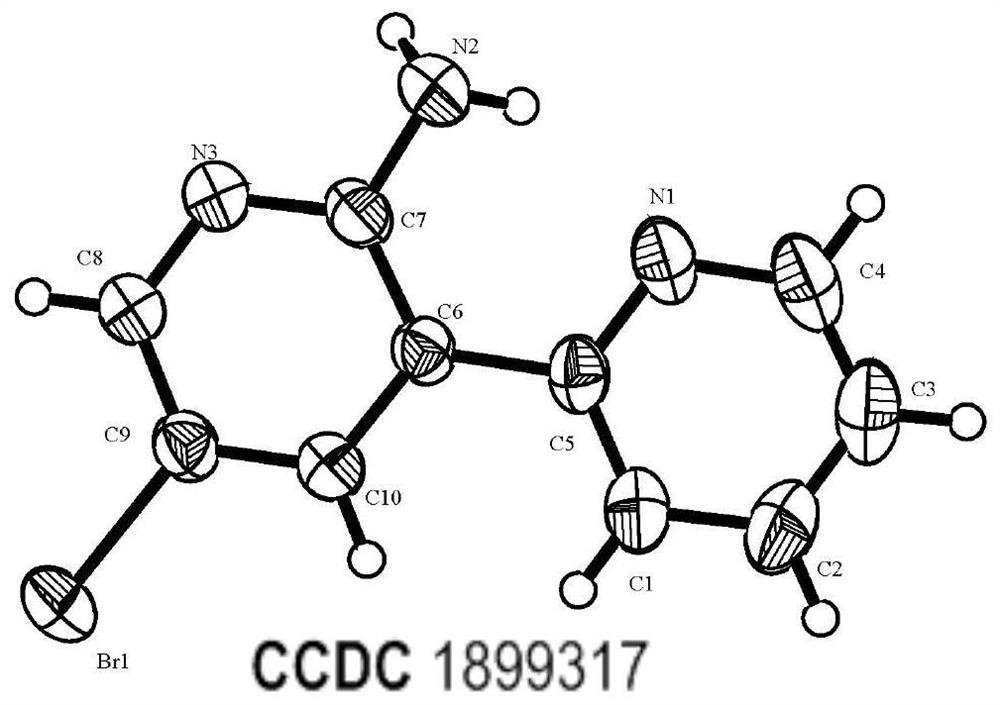
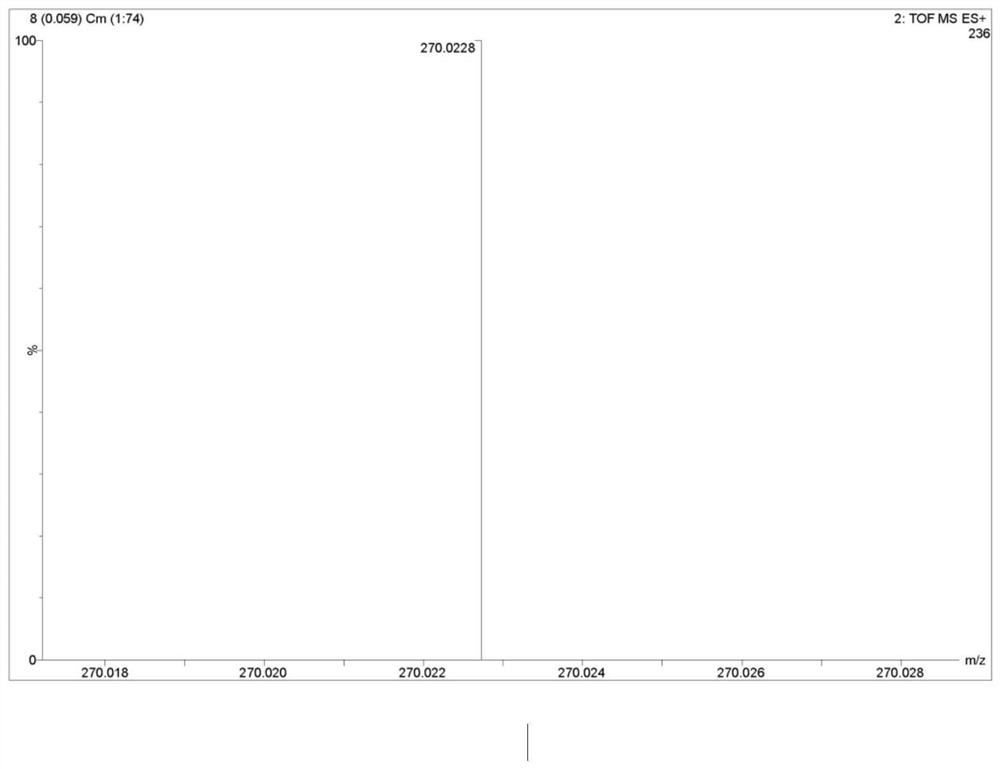
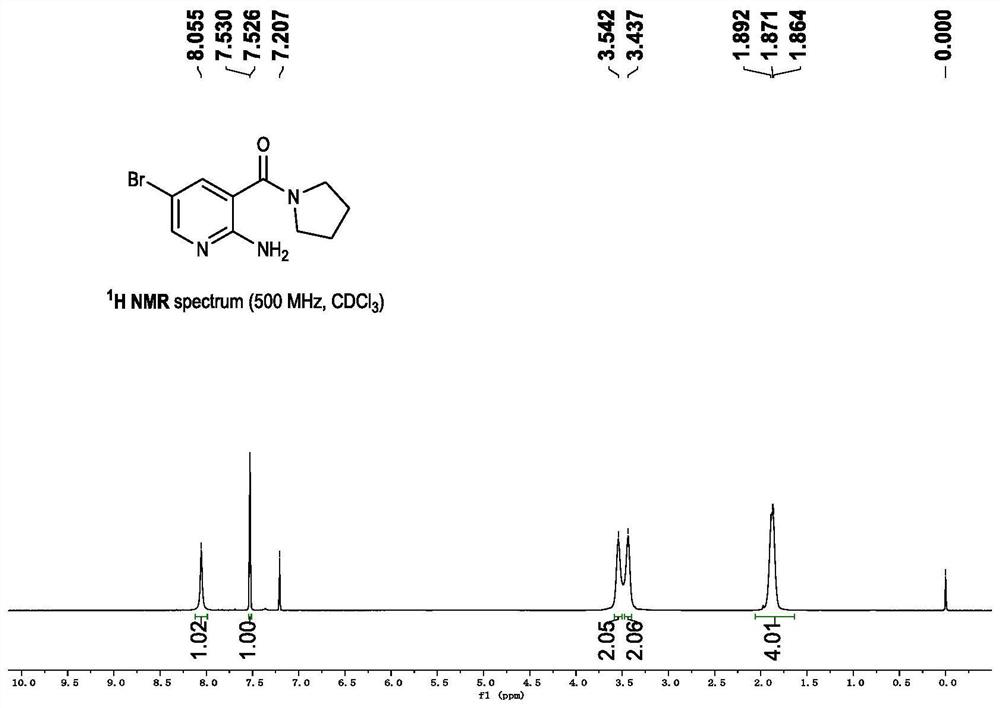
A synthetic method and use of more than 2-amino pyridine derivatives
A technology of aminopyridine and synthesis method, which is applied in the field of synthesis of multi-substituted 2-aminopyridine derivatives, can solve the problems of cumbersome synthesis process, high storage and transportation requirements, and achieve environmental friendliness, high yield, and easy control of reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035]
[0036]
[0037] In the above reaction, the amount of the substrate 1,2,3-triazine compound a, the cyanomethyl compound b and the amount of the base reagent is in a molar ratio: under a nitrogen environment, 1a:1b:base=1.0:1.0:1.5, and the bottom The concentration of compound 1,2,3-triazine a was 1M.
[0038] The operation steps of the reaction are as follows: in a dry sealed reaction tube, 5-bromo-1,2,3-triazine 1a, alkali and cyanomethyl diethyl phosphate 1b are sequentially added. After nitrogen replacement for three times, solvent was added, and the sealed reaction tube was placed under constant temperature for reaction. The reaction was monitored by TLC. After the reaction was completed, the mixture was extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate, concentrated in vacuo, and the target product 1c was obtained after column chromatography.
[0039]For label 16, other conditions remain unchanged, and the...
Embodiment 2-3
[0047] Embodiment 2-3 is the synthesis of C3 substituted 2-aminopyridine
Embodiment 2
[0048] Example 2: Synthesis of 2-aminonicotinic acid ethyl ester (compound 2c)
[0049]
[0050] In a 10 mL dry sealed reaction tube, 1,2,3-triazine 2a (8.1 mg, 0.10 mmol), ethyl cyanoacetate 2b (24.9 mg, 0.22 mmol) and triethylenediamine (22.4 mg, 0.22 mmol) were added sequentially 0.20 mmol). After three times of nitrogen replacement, ethyl acetate (1.0 ml) was added as a solvent, and the sealed reaction tube was placed at room temperature for 3 h. The reaction was monitored by TLC. After the reaction was complete, it was extracted with dichloromethane (3*10 mL). The organic phase was dried over anhydrous sodium sulfate and concentrated in vacuo. After column chromatography, the target product 2c (11.1 mg, 67%) was obtained. NMR is characterized as: 1 H NMR (500MHz, CDCl 3 )δ8.13(dd,J=5.0,2.0Hz,1H),8.06(dd,J=8.0,2.5Hz,1H),6.53(dd,J=7.5,5.0Hz,1H),4.26(q,J =7.0Hz,2H),1.30(t,J=7.0Hz,3H). 13 C NMR (125MHz, CDCl3) δ166.9, 159.4, 153.3, 140.0, 112.5, 106.4, 60.8, 14.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com