Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
425 results about "Scandium oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
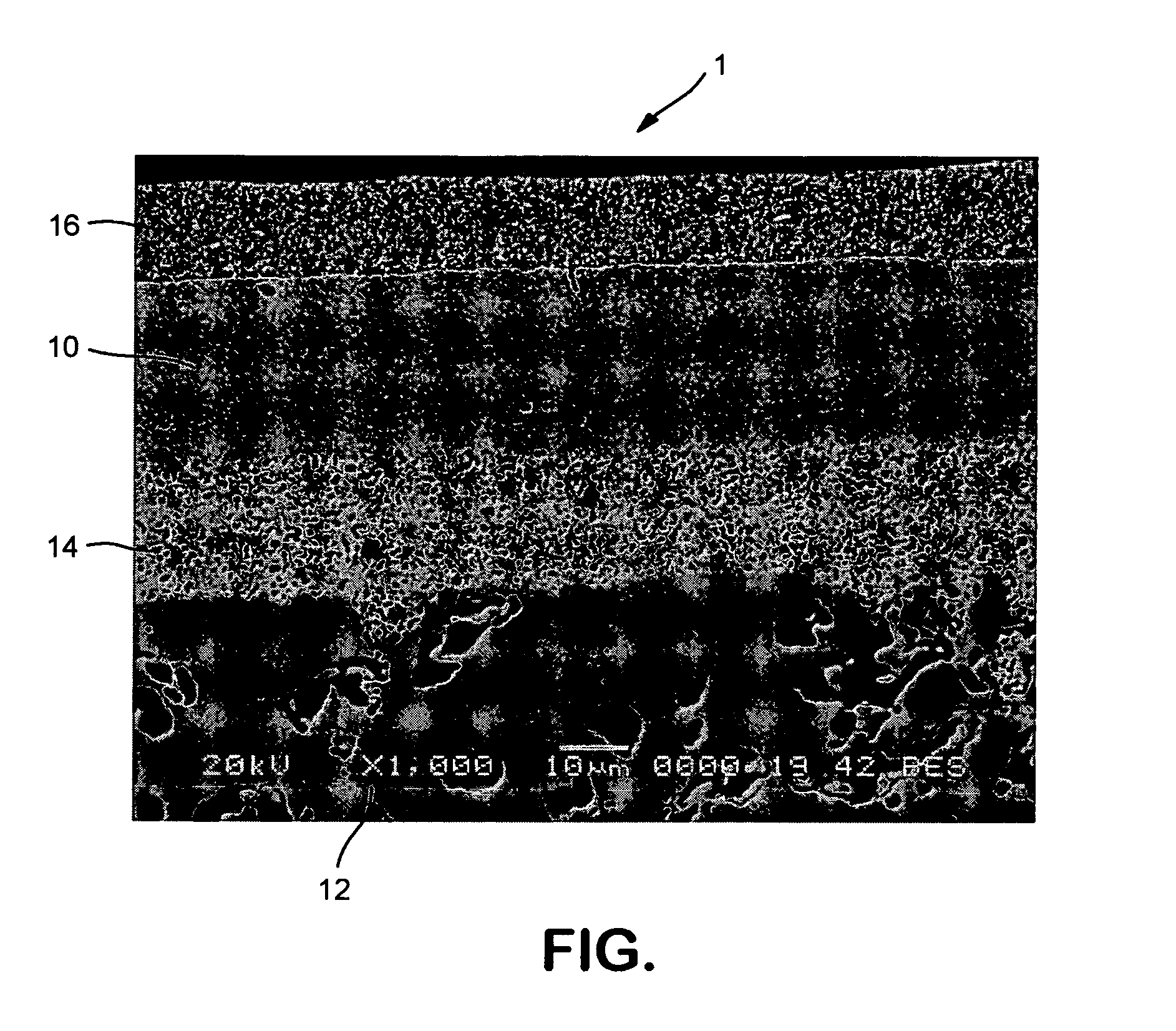
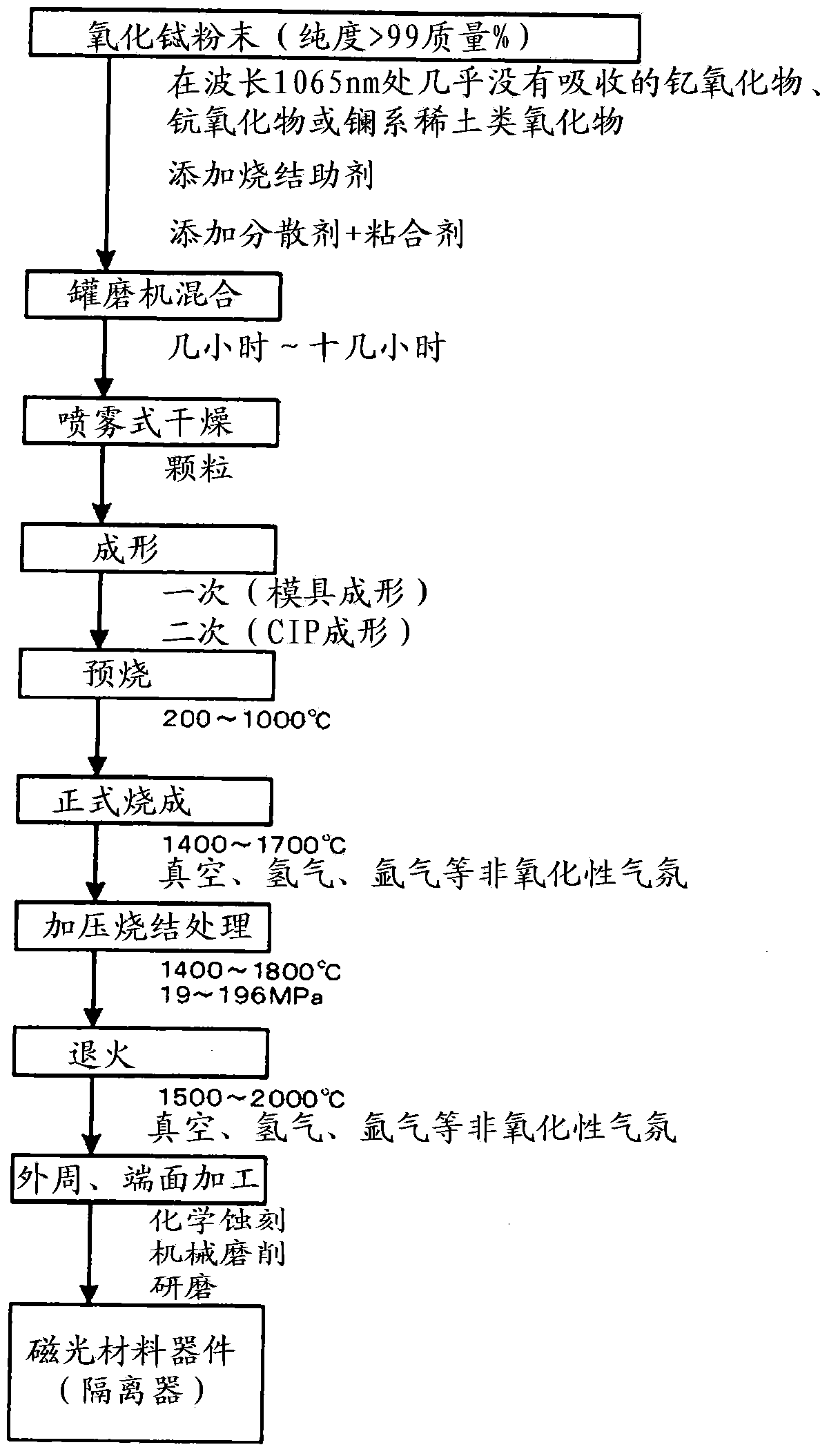
Scandium(III) oxide, Sc₂O₃, or scandia, is a high melting rare earth oxide. It is used in the preparation of other scandium compounds as well as in high-temperature systems (for its resistance to heat and thermal shock), electronic ceramics, and glass composition (as a helper material).
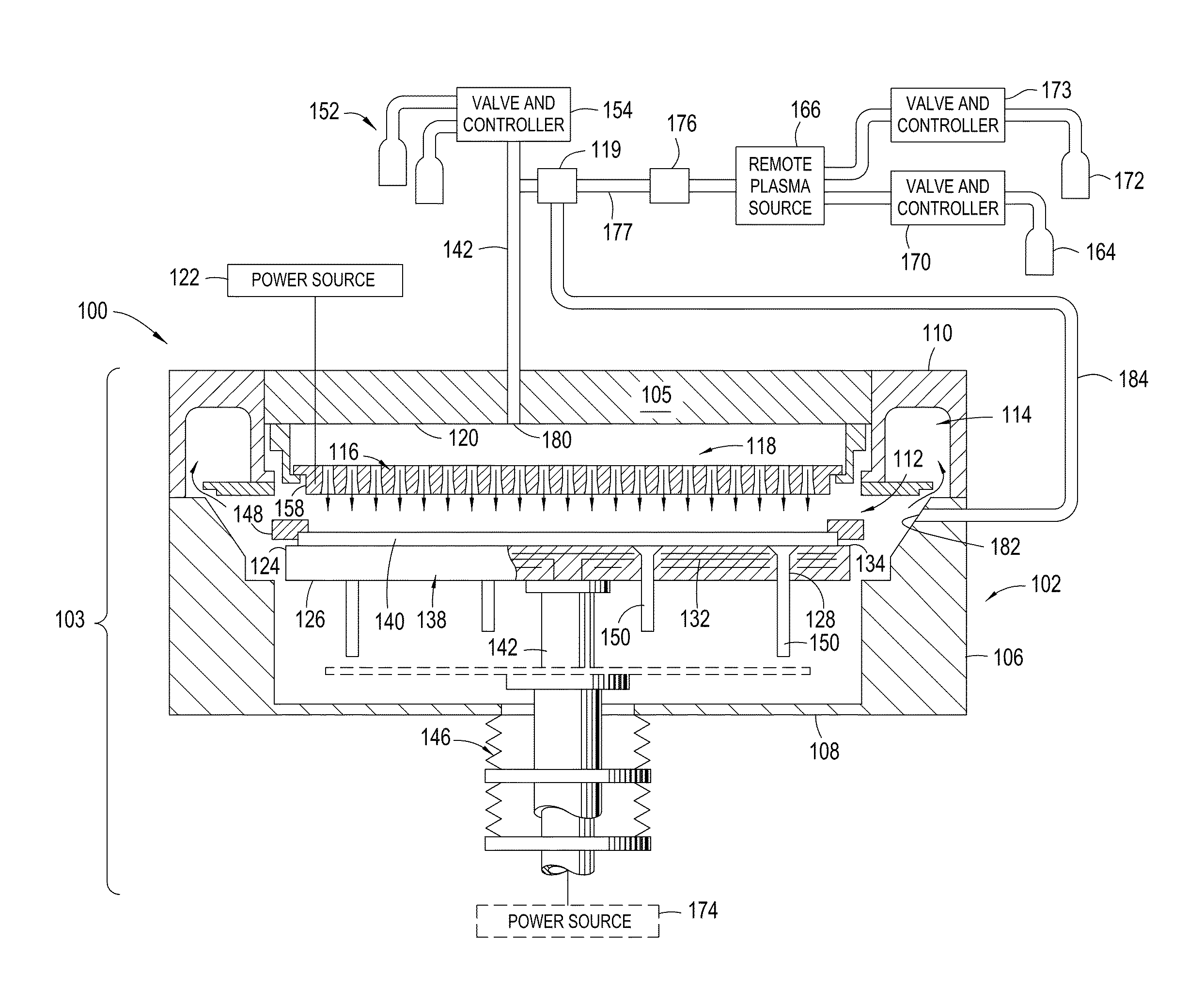
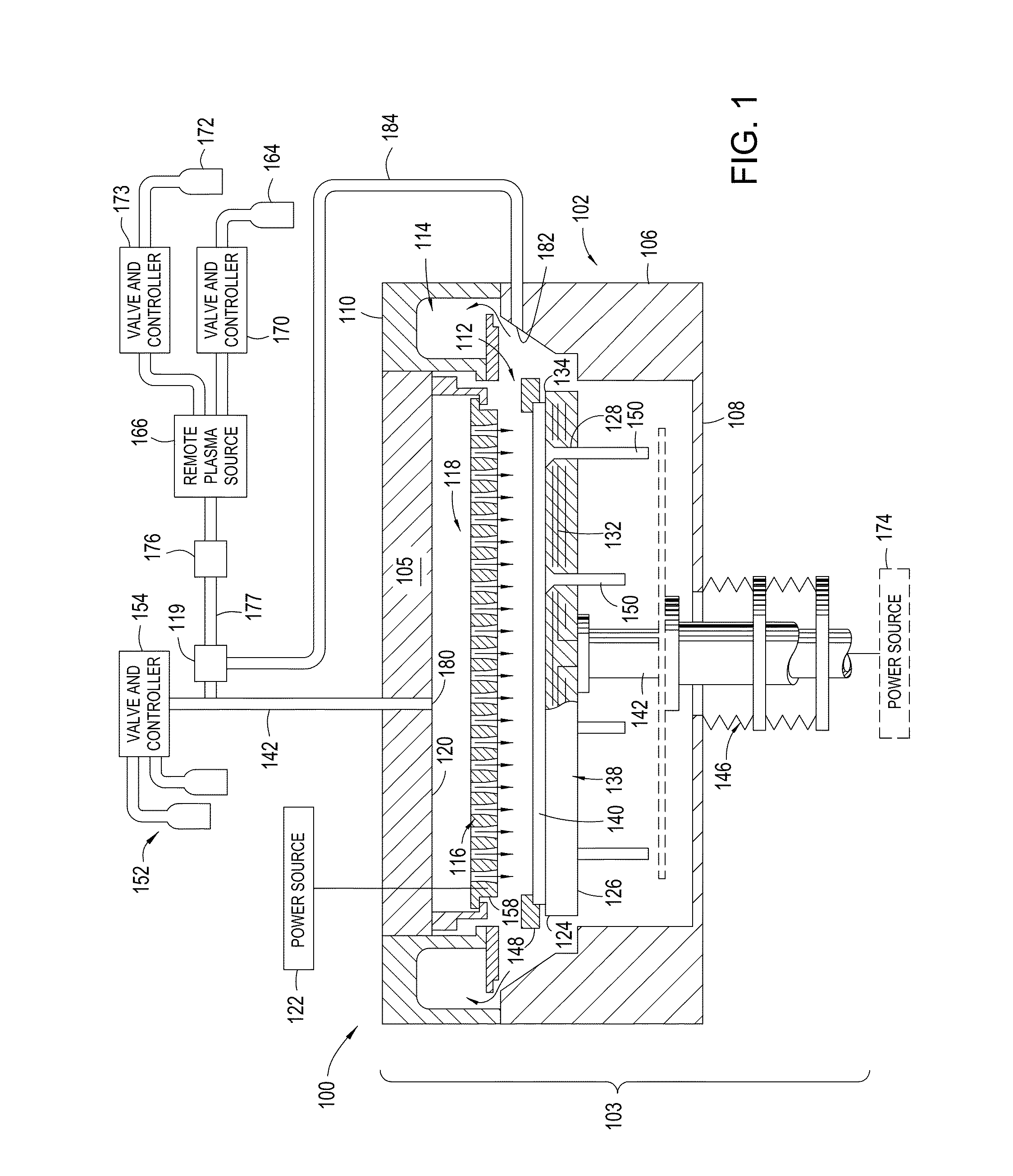
Yttria-based material coated chemical vapor deposition chamber heater
ActiveUS20140263272A1Improve wear resistanceImprove flexural strengthLiquid surface applicatorsMolten spray coatingYTTERBIUM OXIDEFlexural strength
Embodiments of the present invention generally relate to heated substrate supports having a protective coating thereon. The protective coating is formed from yttrium oxide at a molar concentration ranging from about 50 mole percent to about 75 mole percent; zirconium oxide at a molar concentration ranging from about 10 mole percent to about 30 mole percent; and at least one other component, selected from the group consisting of aluminum oxide, hafnium oxide, scandium oxide, neodymium oxide, niobium oxide, samarium oxide, ytterbium oxide, erbium oxide, cerium oxide, and combinations thereof, at a molar concentration ranging from about 10 mole percent to about 30 mole percent. The alloying of yttrium oxide with a compatible oxide improves wear resistance, flexural strength, and fracture toughness of the protective coating, relative to pure yttrium oxide.
Owner:APPLIED MATERIALS INC
Method and apparatus which reduce the erosion rate of surfaces exposed to halogen-containing plasmas
ActiveUS20080264565A1Improve plasma resistanceImprove corrosion resistanceElectric discharge tubesSemiconductor/solid-state device manufacturingYTTERBIUM OXIDEErosion rate
A ceramic article which is resistant to erosion by halogen-containing plasmas used in semiconductor processing. The ceramic article includes ceramic which is multi-phased, typically including two phase to three phases. The ceramic is formed from yttrium oxide at a molar concentration ranging from about 50 mole % to about 75 mole %; zirconium oxide at a molar concentration ranging from about 10 mole % to about 30 mole %; and at least one other component, selected from the group consisting of aluminum oxide, hafnium oxide, scandium oxide, neodymium oxide, niobium oxide, samarium oxide, ytterbium oxide, erbium oxide, cerium oxide, and combinations thereof, at a molar concentration ranging from about 10 mole % to about 30 mole %.
Owner:APPLIED MATERIALS INC
Method and apparatus which reduce the erosion rate of surfaces exposed to halogen-containing plasmas
ActiveUS7696117B2Reduce erosion rateImprove mechanical propertiesElectric discharge tubesSemiconductor/solid-state device manufacturingErosion rateYttrium
A ceramic article which is resistant to erosion by halogen-containing plasmas used in semiconductor processing. The ceramic article includes ceramic which is multi-phased, typically including two phase to three phases. The ceramic is formed from yttrium oxide at a molar concentration ranging from about 50 mole % to about 75 mole %; zirconium oxide at a molar concentration ranging from about 10 mole % to about 30 mole %; and at least one other component, selected from the group consisting of aluminum oxide, hafnium oxide, scandium oxide, neodymium oxide, niobium oxide, samarium oxide, ytterbium oxide, erbium oxide, cerium oxide, and combinations thereof, at a molar concentration ranging from about 10 mole % to about 30 mole %.
Owner:APPLIED MATERIALS INC
LED Apparatus
InactiveUS20120087108A1Reduce feature differencesImprove efficiencySolid-state devicesIlluminated signsLutetiumEngineering
An LED apparatus is disclosed. The LED apparatus includes a substrate, a cup structure, and a dividing structure. The dividing structure divides a containing space formed by the cup structure into a first region and a second region. A first blue-light chip and a first package colloidal are disposed in the first region and a second blue-light chip and a second package colloidal are disposed in the second region. A green-light phosphor is mixed in the second package colloidal to completely convert a monochromatic emission spectrum of a second blue-light band of the second blue-light chip into a monochromatic emission spectrum of a green-light band. The green-light phosphor is selected from one of silicate, oxynitride, lutetium aluminum oxide, and calcium scandium oxide.
Owner:AU OPTRONICS CORP
Method for extracting metal scandium and titanium from red mud
ActiveCN101182601AReduce processing costsEfficient use ofProcess efficiency improvementRed mudHydrolysate
The invention relates to a method for extracting metal scandium and titanium from red mud; the method is used for extracting scandium and titanium from the red mud; the method takes the red mud which is produced through the sinter process of the high-grade ore two portion of clinker in alumina plant as the raw material; the hydrochloric acid with a concentration of 2 percent to 6 percent is used for leaching the calcium and sodium from the red mud to increase the content of the scandium and titanium in the red mud; and then the hydrochloric acid with a concentration of 16 percent to 22 percent is used for a second acid dipping of the red mud and the product of scandium oxide is obtained after the leaching liquid passes the processes of extraction, reverse extraction and baking; the hydrochloric acid with a concentration of 80 percent to 90 percent is used for a third acid dipping of the red mud after the second one and the leaching liquid is added with iron powder for reduction and then the temperature is reduced to crystallize and separate out iron in the pattern of FeSO<4>.7H<2>O; the acid liquid which contains titanium after the iron is removed is added with alkali and heated for hydrolysis; the product of titanium oxide is obtained after the hydrolysate is baked. The method is simple and easy to be carried out and most metal elements in the red mud can be recovered and the cost for processing the red mud is low, all of which are beneficial to the comprehensive utilization of the red mud.
Owner:GUIZHOU BRANCH CHINA ALUMINUM IND
Ceramic bonding composition, method of making, and article of manufacture incorporating the same
A ceramic bonding composition comprises a first oxide and at least a second oxide having a formula of Me2O3; wherein the first oxide is selected from the group consisting of aluminum oxide, scandium oxide, and combinations thereof; Me is selected from the group consisting of yttrium, lanthanum, cerium, praseodymium, neodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, and combinations thereof. The ceramic bonding composition can further comprise silica. An article of manufacture comprising at least two members attached together with the ceramic bonding composition.
Owner:GENERAL ELECTRIC CO
Ceramic-ceramic nanocomposite electrolyte
ActiveUS20050214616A1Improve conductivityImprove ionic conductivityMaterial nanotechnologyFinal product manufactureDopantComposite electrolyte
A ceramic-ceramic nanocomposite electrolyte having enhanced conductivity is provided. The nancomposite electrolyte is formed from chemically stabilized zirconia such as yttria stabilized zirconia or scandia stabilized zirconia and a heterogeneous ceramic dopant material such as Al2O3, TiO2, MgO, BN, or Si3N4 The nanocomposite electrolyte is formed by doping the chemically stabilized zirconia with the ceramic dopant material and pressing and sintering the composite. The resulting electrolyte has a bulk conductivity of from about 0.10 to about 0.50 S / cm at about 600° C. to about 900° C. and may be incorporated into a solid oxide fuel cell.
Owner:UNIV OF DAYTON
Method of producing aluminium scandium alloy by electrolysis
The invention relates to the method for producing the alloy of aluminium and scandium by using oxides of aluminium and scandium as the raw materials. Fused salt electrolysis process is adopted to electrolyze and separate out aluminium and scandium so as to form the alloy. The weight ratio of the electrolyte of fused cryolite is as following: alumina 1%-10%, scandium oxide 0.1%-10%, cryolite as the rest and the unavoidable impurities. The ratio between sodium fluoride and aluminium fluoride is 2-3. The relevant parameters are as following: temperature of electrolysis 900-990 deg.C, operating voltage of the electrobath 3.0V-6.5V and the electrode distance 2.0cm-7.0 cm. The invention possesses the features of no need of high pure metal scandium, shortened technological flow and high metal yield so as to reduce the cost of the alloy greatly.
Owner:GUIZHOU BRANCH CHINA ALUMINUM IND
Optical glass
InactiveCN1450010AHigh coefficient of thermal expansionPrecise without migrationOptical elementsLithium oxideSilicic acid
The present invention relates to an optical glass using silicic acid group as main body, said optic glass has high expansion coefficient, high Young modulus, excellent weatherability and high transmissivity in the infrared wave zone. Said glass composition contains (mole%) 36-66% of silicon dioxide, 0-12% of aluminium oxide, 0-6% of boric oxide, 0-10% of magnesium oxide, 0-16% of calcium oxide, 0-16% of strontium oxide, 0-16% of baria, 0-8% of zinc oxide, 0-32% of lithium oxide, 0-25% of sodium oxide, 0-25% of potassium oxide, 0-20% of cesium oxide, 0-6% of phosphorus pentoxide, 0-8% of scandium oxide and others.
Owner:PICVUE OPTOELECTRONICS INT
Composite oxygen ion transport membrane
ActiveUS7556676B2Closely matchedLow linear expansionSemi-permeable membranesMembranesCeriumOxygen ions
A composite oxygen ion transport membrane having a dense layer, a porous support layer, an optional intermediate porous layer located between the porous support layer and the dense layer and an optional surface exchange layer, overlying the dense layer. The dense layer has electronic and ionic phases. The ionic phase is composed of scandia doped, yttrium or cerium stabilized zirconia. The electronic phase is composed of a metallic oxide containing lanthanum, strontium, chromium, manganese and vanadium and optionally cerium. The porous support layer is composed of zirconia partially stabilized with yttrium, scandium, aluminum or cerium or mixtures thereof. The intermediate porous layer, if used, contains the same ionic and electronic phases as the dense layer. The surface exchange layer is formed of an electronic phase of a metallic oxide of lanthanum and strontium that also contains either manganese or iron and an ionic phase of scandia doped zirconia stabilized with yttrium or cerium.
Owner:PRAXAIR TECH INC
LED apparatus and field sequential display
InactiveCN102427075AImprove efficiencyImprove mass productionSolid-state devicesNon-linear opticsLutetiumDisplay device
An LED apparatus and a field sequential display employing same are disclosed. The LED apparatus includes a substrate, a cup structure, and a dividing structure. The dividing structure divides a containing space formed by the cup structure into a first region and a second region. A first blue-light chip and a first package colloidal are disposed in the first region and a second blue-light chip anda second package colloidal are disposed in the second region. A green-light phosphor is mixed in the second package colloidal to completely convert a monochromatic emission spectrum of a second blue-light band of the second blue-light chip into a monochromatic emission spectrum of a green-light band. The green-light phosphor is selected from one of silicate, oxynitride, lutetium aluminum oxide, and calcium scandium oxide. Characteristic difference among chips of different colors of an LED apparatus can be minimized, thereby improving the entire efficiency.
Owner:AU OPTRONICS CORP
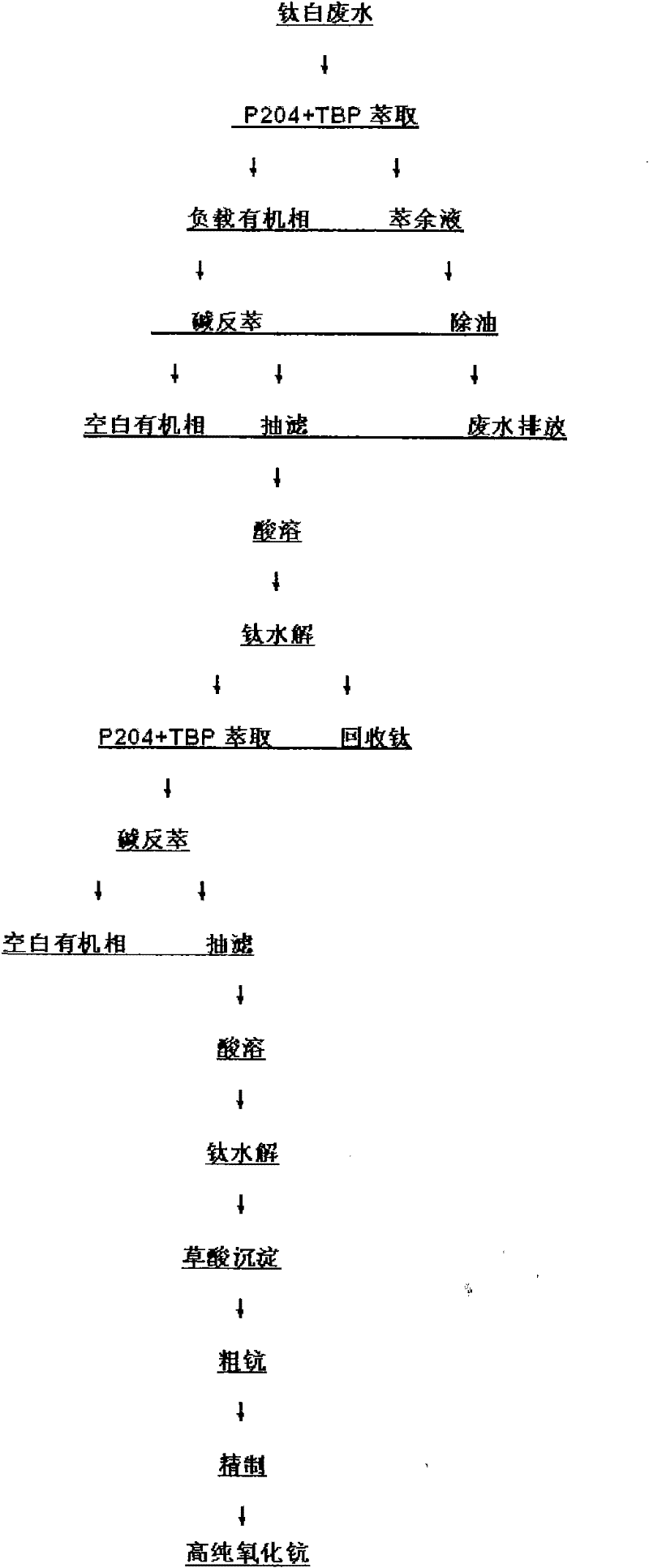
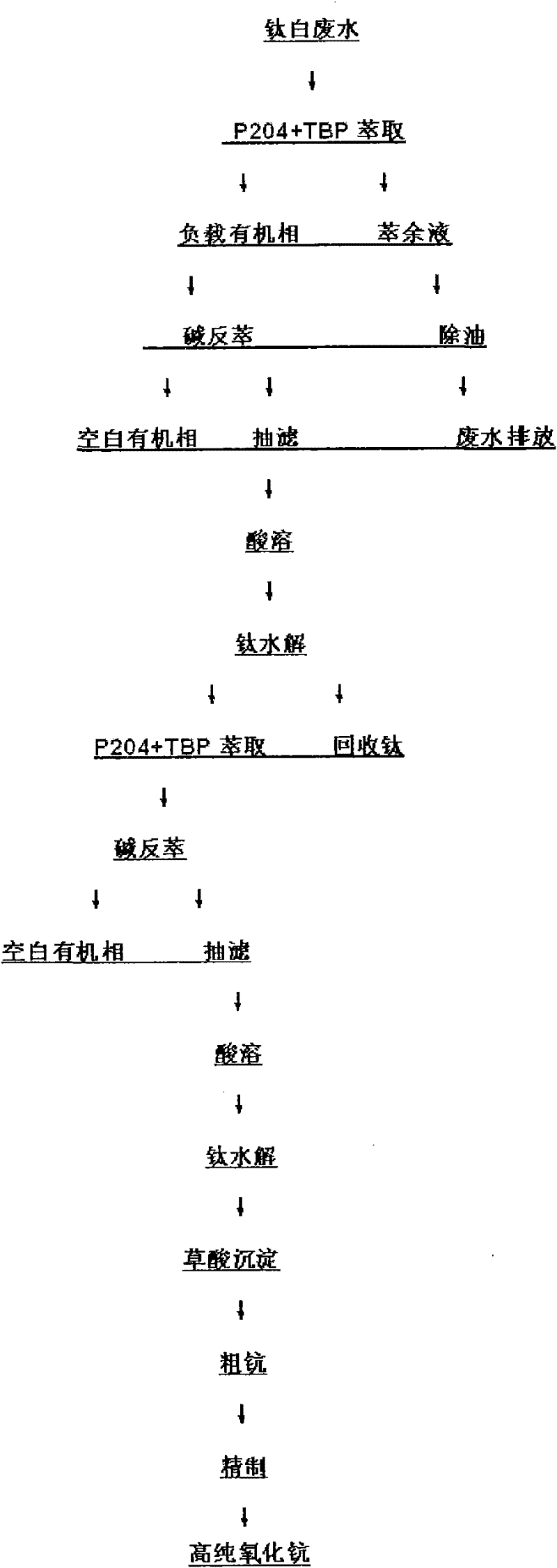
Method for recovering scandium and titanium in titanium white wastewater
ActiveCN102127641AOrganic phase content is smallReduce pollutionProcess efficiency improvementKeroseneEmission standard
The invention discloses a method for recovering scandium and titanium from wastewater in the production of titanium white powder. The method comprises the following steps of: carrying out single stage extraction in titanium white wastewater with P2O4, TBP and kerosene, wherein a phase ratio O / A is 1:50 to 1:10, and balance time is 10-20 min; then, stripping with alkali, filtering and dissolving a stripped substance with acid; heating a solution in a reaction kettle, adjusting a pH value, hydrolyzing titanium, filtering and recovering titanium, repeating the above process to process supernatant fluid, secondarily extracting and dissolving a stripped substance alkali cake with acid; precipitating scandium with oxalic acid and burning to obtain scandium oxide with the purity of 95 percent; and further refining to obtain high-pure scandium oxide. In the invention, titanium is recovered under the condition of ensuring scandium recovery without adopting a titanium washing process of sulfuric acid and hydrogen peroxide, and a hydrolysis mode is adopted to recover titanium, and the recovery cost of scandium is decreased. In addition, a degreasing device and oil-absorbing felt are adopted in waste liquid generated after extraction. The invention has the advantages of environmental protection and simple process, the wastewater contains the organic phase of less than 50 mg / l and reaches an emission standard.
Owner:河南荣佳钪钒科技有限公司
Method for extracting scandium oxide from titanium slag chloride waste
InactiveCN102796876ATo achieve the purpose of preliminary enrichment of scandiumImprove product qualityProcess efficiency improvementSodium hydroxideMaterials science
The invention discloses a method for extracting scandium oxide from titanium slag chloride waste, belonging to the field of comprehensive utilization of vanadium titano-magnetite resources. The method comprises the following steps: a, adding water to titanium slag chloride waste, and leaching; b, adding a reducer to the supernate obtained in the step a, then adding humic acid, regulating the pH value, and filtering to obtain precipitate; c, adding a hydrochloric acid solution to perform acidolysis reaction, and then filtering to obtain a coarse scandium solution; d, respectively performing extraction, washing and back extraction on the coarse scandium solution by taking (2-ethylhexyl)-2-ethylhexyl phosphonate as an extractant, the hydrochloric acid solution as a washing agent and a sodium hydroxide solution as a back extractant, thus obtaining a pure scandium solution; and e, adding oxalic acid to perform scandium precipitation, and calcining to obtain scandium oxide, wherein the grade of the obtained scandium oxide is about 95%. The method disclosed by the invention is high in product quality and simple in process, reasonably utilizes scandium resources in the titanium slag chloride waste, and has favorable economic benefits and social benefits.
Owner:PANZHIHUA UNIV
Desulfurization and novel compositions for same
InactiveUS6914033B2Minimize consumptionMinimizes of saturationOther chemical processesCatalyst activation/preparationSulfurScandium oxide
A composition comprising a promoter and a metal oxide selected from the group consisting of a cerium oxide, a scandium oxide, a lanthanum oxide, and combinations of any two or more thereof, wherein at least a portion of the promoter is present as a reduced valence promoter and methods of preparing such composition are disclosed. The thus-obtained composition is employed in a desulfurization zone to remove sulfur from a hydrocarbon stream.
Owner:CHINA PETROCHEMICAL CORP
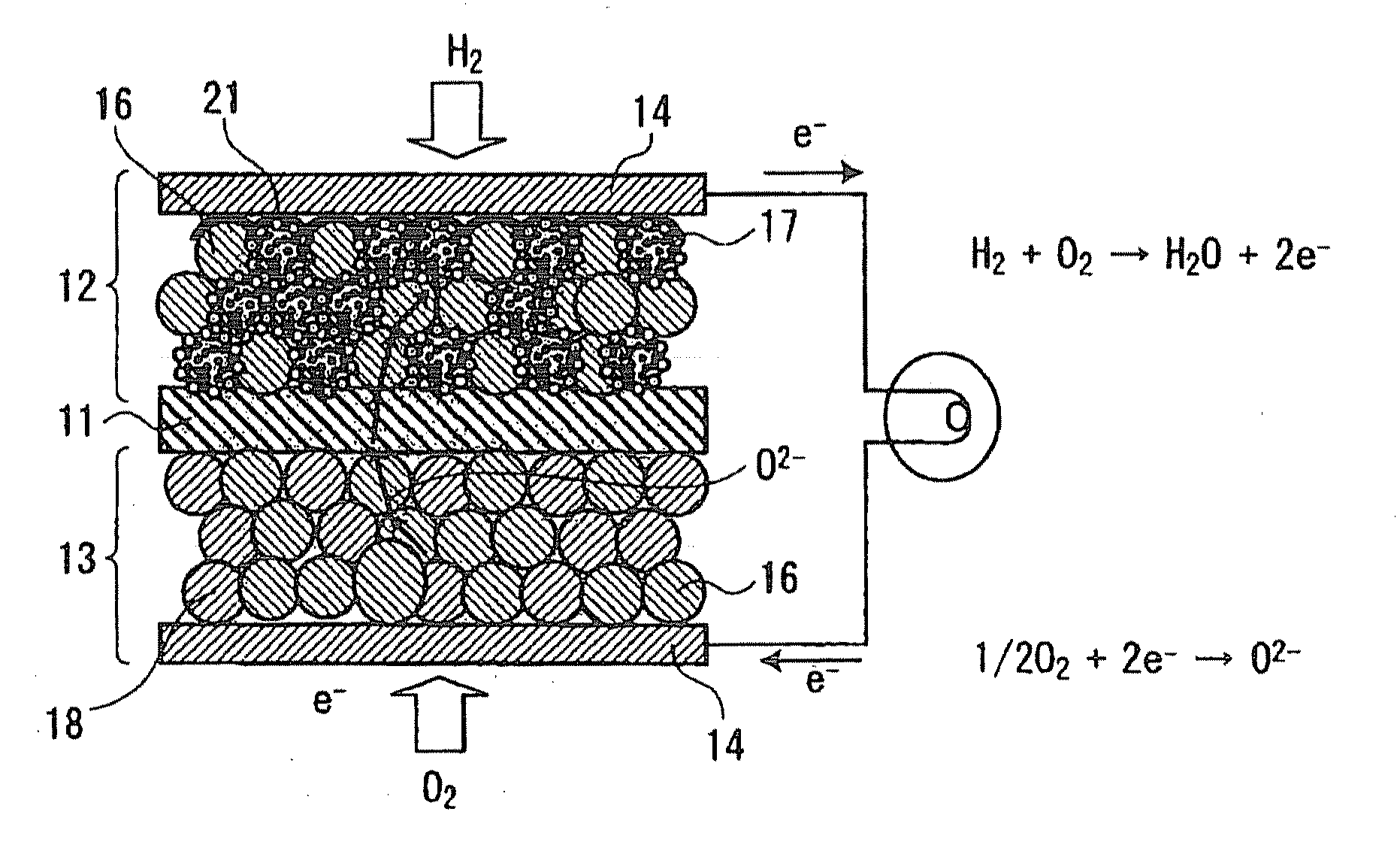
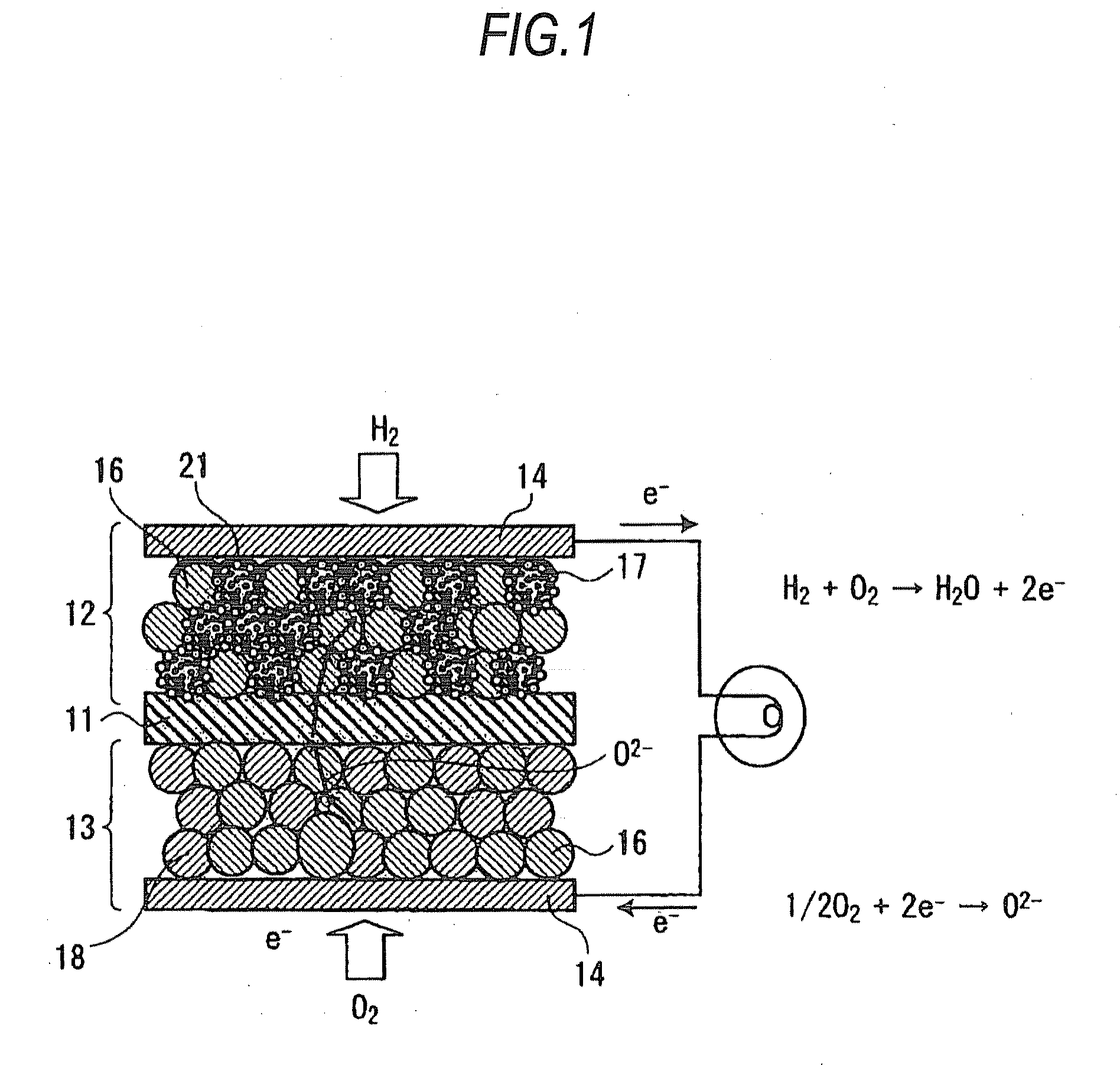
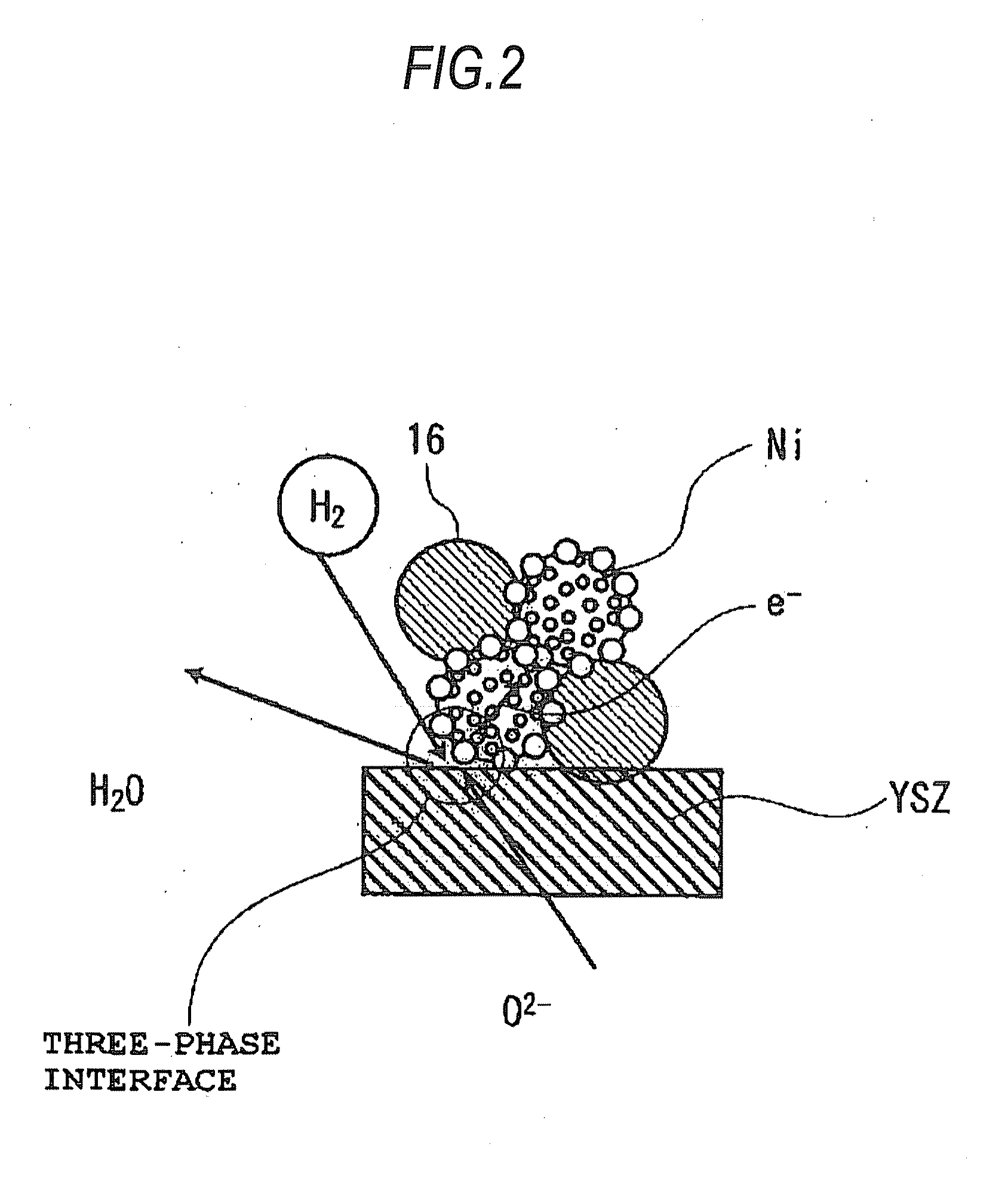
Fuel electrodes for solid oxide electrochemical cell, processes for producing the same, and solid oxide electrochemical cells
InactiveUS20090068533A1High catalytic activityImprove output performanceFuel cells groupingFinal product manufactureYTTERBIUM OXIDEYttrium
A fuel electrode for a solid oxide electrochemical cell includes: an electrode layer including a mixed phase constituted of zirconia stabilized with yttrium oxide, ytterbium oxide, or scandium oxide and of an oxide selected from the group including an aluminum-based oxide and a magnesium-based composite oxide, said oxide having, supported on a surface part thereof, particles of at least one member selected from nickel, cobalt, and nickel-cobalt alloys; a meshy wiring formed on a surface layer part of the electrode layer and made of a material having higher electronic conductivity than the electrode layer; and a current collector which overlies the electrode layer and is in contact with at least the wiring.
Owner:KK TOSHIBA
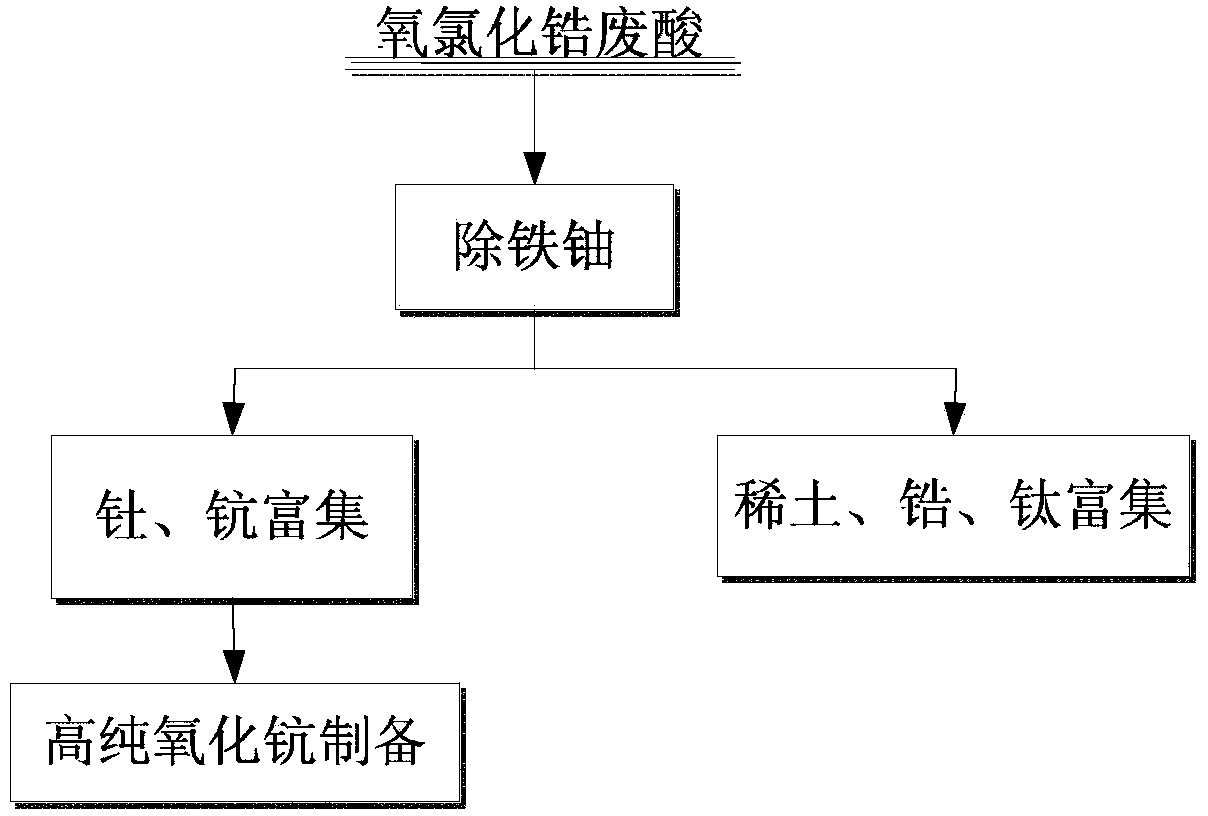
Method for comprehensively recovering multiple elements from zirconium oxychloride liquid waste
The invention provides a method for comprehensively recovering multiple elements from zirconium oxychloride liquid waste. The method comprises the following steps: (1) removing iron and uranium on zirconium oxychloride; (2) gathering scandium and thorium; (3) preparing a high-purity scandium oxide; and (4) gathering rare earth, zirconium and titanium. According to the method provided by the invention, through reasonable configuration of the recovering order of various elements from the zirconium oxychloride liquid waste, scandium enrichment capable of improving the purity by a subsequent purification method can be obtained just by virtue of a cheap extracting agent; and the adverse effects on purity improvement of the subsequently obtained scandium caused by irremovable zirconium and titanium, and radioactive elements thorium and uranium are avoided.
Owner:HUNAN RARE EARTH METAL MATERIAL RES INST
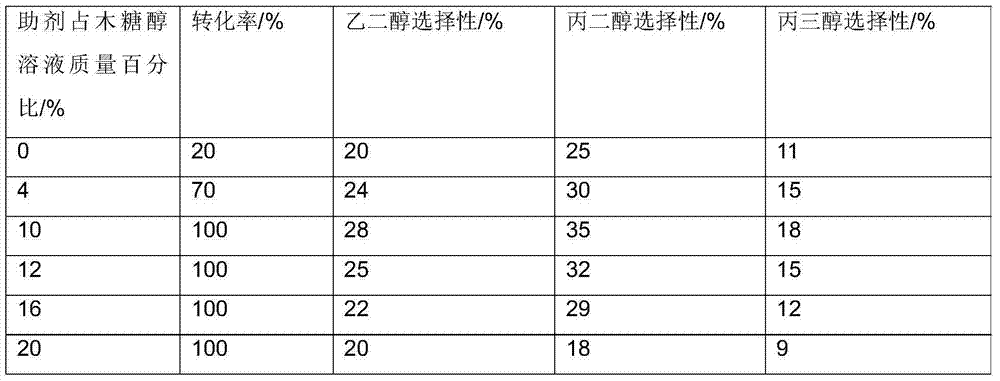
Method for preparation of low carbon alcohol by hydrogenolysis of sugar and sugar alcohol
InactiveCN104710277AEasy to achieve separationOrganic compound preparationPreparation by OH group eliminationGadolinium oxideGlycerol
The invention relates to a method for preparation of low carbon alcohol by hydrogenolysis of sugar and sugar alcohol. The method adopts sugar and sugar alcohol as the raw materials, takes one or more than two of iron, cobalt, nickel, copper, zinc, tin, platinum, ruthenium, palladium, iridium and other transition metals as the catalyst active component, and employs a rare earth oxide like promethium oxide, gadolinium oxide, terbium oxide, holmium oxide, erbium oxide, thulium oxide, cerium oxide, lanthanum oxide, praseodymium oxide, neodymium oxide, scandium oxide, yttrium oxide, dysprosium oxide, europium oxide, samarium oxide, ytterbium oxide, lutecium oxide and the like as the assistant. Under a temperature of 150-320DEG C and an H2 pressure of 1-20MPa, catalytic hydrocracking is carried out in a water solution to obtain ethylene glycol, propylene glycol, glycerol and other low carbon alcohols. The method provided by the invention has the advantages that: the rare earth oxide is added as the assistant to realize hydrocracking of sugar and sugar alcohol, rare earth oxides are insoluble in water and easy to separate, and at the end of reaction, the reaction system has no need for additional acid to neutralize.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for extracting scandium from modified red mud by using composite extractant
InactiveCN102061392ALess acid consumptionEasy extractionProcess efficiency improvementPregnant leach solutionRed mud
The invention relates to a method for extracting scandium from modified red mud by using a composite extractant, belonging to the technical field of extraction and preparation methods of rare-earth metal scandium. The method for extracting the scandium from the modified red mud ensures good extracting effect, high recovery rate and product purity and is suitable for industrialized production. The method comprises the following steps of: a first step: preparing the modified red mud; a second step: leaching the modified red mud by using hydrochloric acids so as to obtain an acid leaching solution; a third step: preparing the composite extractant; a fourth step: mixing the acid leaching solution obtained from the second step with the composite extractant for extraction and separation; a fifth step: washing extracted upper organic phases by using the hydrochloric acids, then washing by using water, and then carrying out back extraction by using a NaOH solution to obtain an Sc(OH)3 solution; a sixth step: precipitating the Sc(OH)3 solution by using oxalic acids, and filtering, and drying; and a seventh step: roasting a solid obtained from the sixth step at high temperature to obtain white scandium oxide powder. The invention can be widely used for the technical field of extraction and preparation of the rare-earth metal scandium.
Owner:TAIYUAN UNIV OF TECH +1
Preparation method of aluminum-scandium master alloy
The invention relates to a preparation method of aluminum-scandium master alloy, which belongs to the technical field of high-performance aluminium alloy raw material preparation. The preparation method is characterized in that: the method adopts the following raw materials: Sc2O3, pure aluminium and a molten salt system, and comprises the following steps: mixing pure aluminium, Sc2O3 and the molten salt system uniformly once and adding the materials in a crucible; putting the crucible in a resistance furnace for heating and smelting, raising the temperature to 950+ / -20 DEG C, and keeping the temperature for 90 to 120 minutes; and casting and sampling after heat preservation. The molten salt system comprises NF4HF, NaF, KCl, NaCl, nNaF AlF3 (sodium cryolite) and nKF AlF3 (kalium cryolite) in a mixing ratio of 10:8:5.4:5.4:3:3; and the molten salt system, pure aluminium and Sc2O3 are added in the crucible in a mass ratio of 30+ / -1.5:100:4+ / -0.1. Compared with the prior art, the method has the advantages that: the prepared aluminum-scandium master alloy has smaller grain size, even distribution of Al3Sc, no obvious agglomeration and small size. When used in subsequent preparation of Sc-containing alloy, the master alloy has excellent refining effects.
Owner:TSINGHUA UNIV
Treatment method of fused salt chlorination slag
The invention provides a treatment method of fused salt chlorination slag. The method comprises the following steps: leaching fused salt chlorination slag by water or a dilute acid solution; then, extracting and back-extracting the leaching liquid by using a blank organic phase I as an extractant and an alkaline solution I as a back-extractant so as to obtain a back-extract I; after adjusting the acidity of the back-extract I by acid, extracting and back-extracting the back-extract I by using a blank organic phase II as the extractant and an alkaline solution II as the back-extractant so as to obtain a residual extraction solution II and a back-extract II; adding a scandium precipitator to the residual extraction solution II and filtering and calcining to obtain scandium oxide; and mixing the back-extract II and a thorium and uranium precipitator, and filtering to obtain a thorium and uranium enriched substance. Compared with existing treatment of fused salt chlorination slag, the method provided by the invention not only extracts scandium with high additional value, but also recovers thorium and uranium in waste residue, so that radioactive contamination is eliminated. Meanwhile, chemical materials used in the treatment method provided by the invention are simple and easy to obtain, and the method is lower in cost and simple in process.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
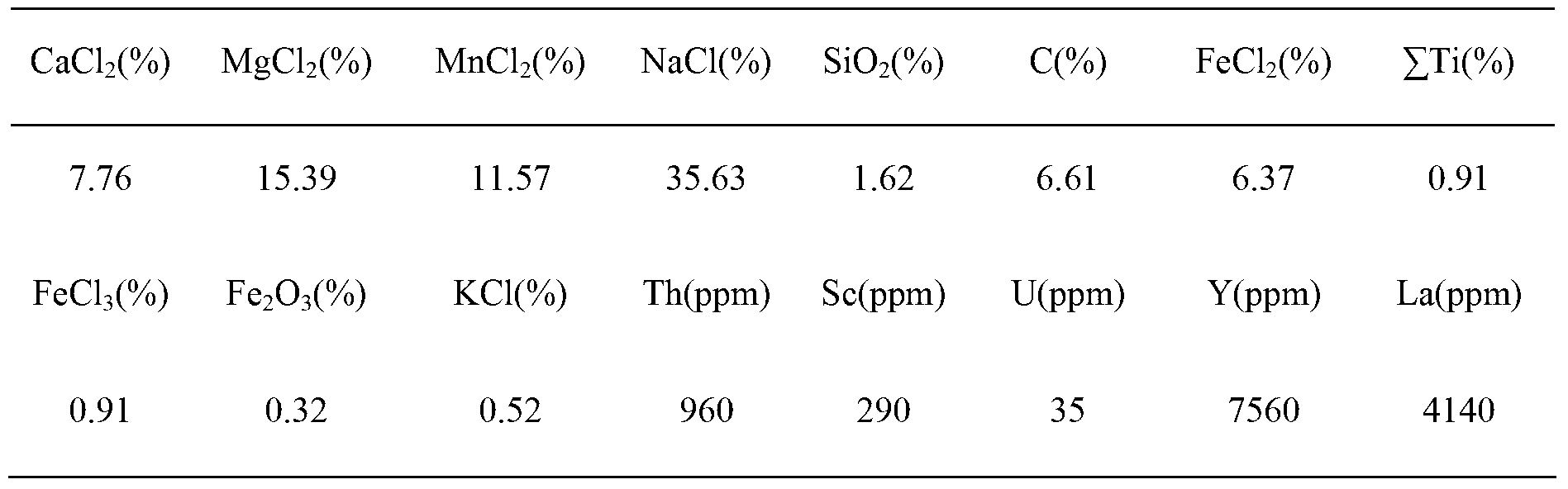
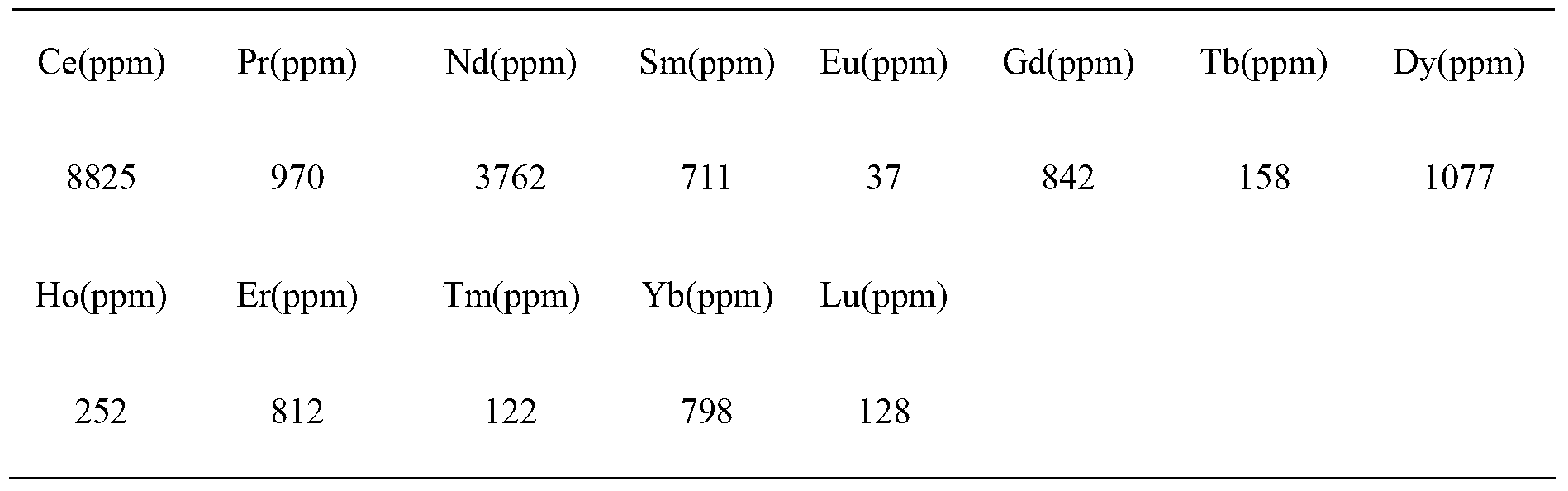
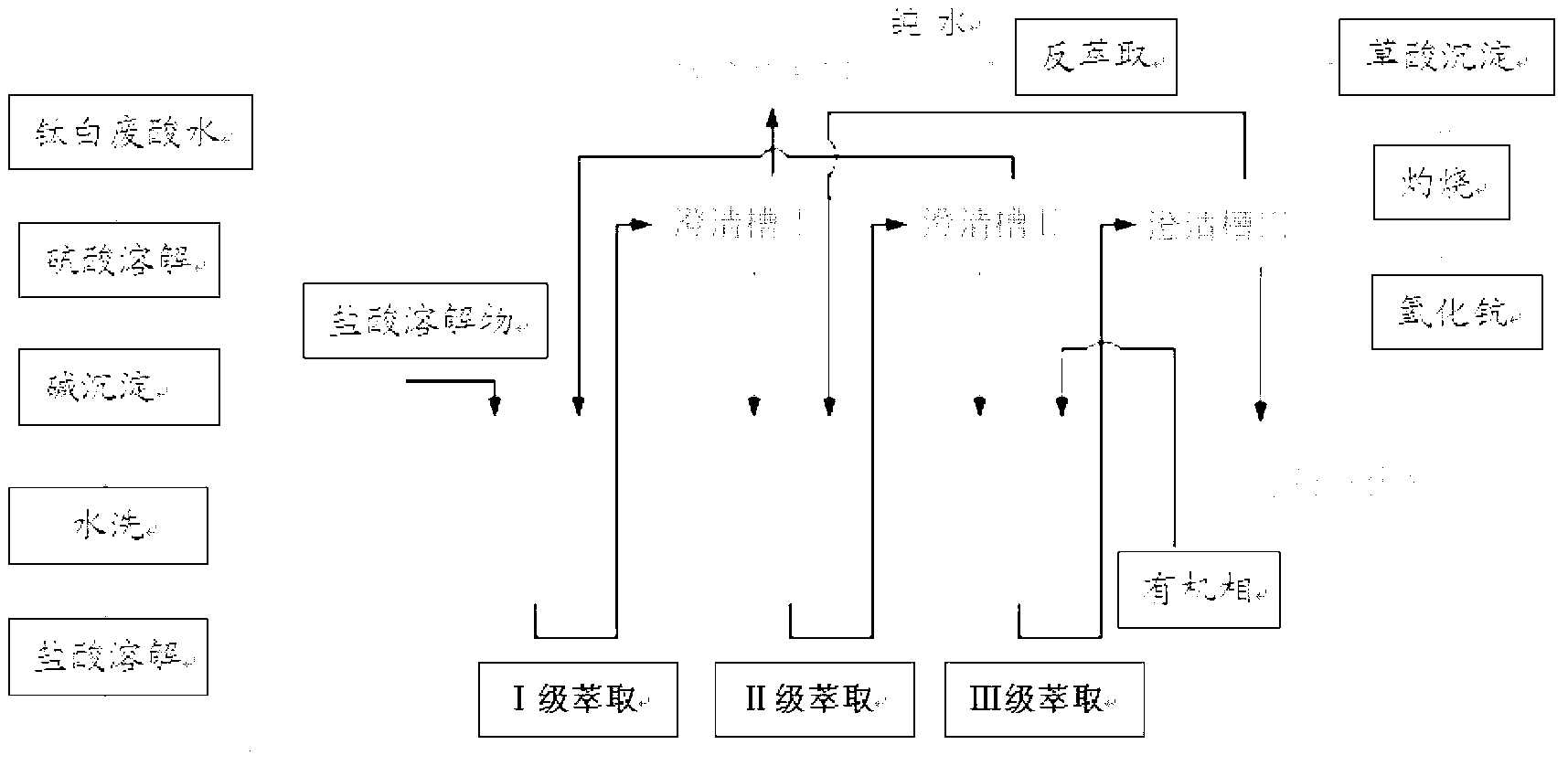
Method for preparing scandium oxide by using rough scandium extracted from waste acid water of titanium dioxide as raw material
The invention relates to a method for preparing scandium oxide by using rough scandium extracted from the waste acid water of titanium dioxide as a raw material. The method comprises the following steps of: sulfuric acid dissolution, alkali deposition, hydrochloric acid dissolution, extraction, back extraction, oxalic acid deposition and firing. Scandium is recovered from a great deal of waste acid water generated in production of the titanium dioxide by using the method provided by the invention, so that not only is the problem of environment pollution solved, but also the waste materials are changed into things of value, and the expensive and rare scandium element is recovered; the purity of the scandium oxide prepared by the invention can be up to as high as over 99.9%, and the recovery rate of the scandium oxide can be up to as high as 80-90%, so that the loss of scandium is greatly reduced; and in addition, the method provided by the invention is simple in preparation process and lower in cost.
Owner:桃江瑞龙金属新材料有限责任公司
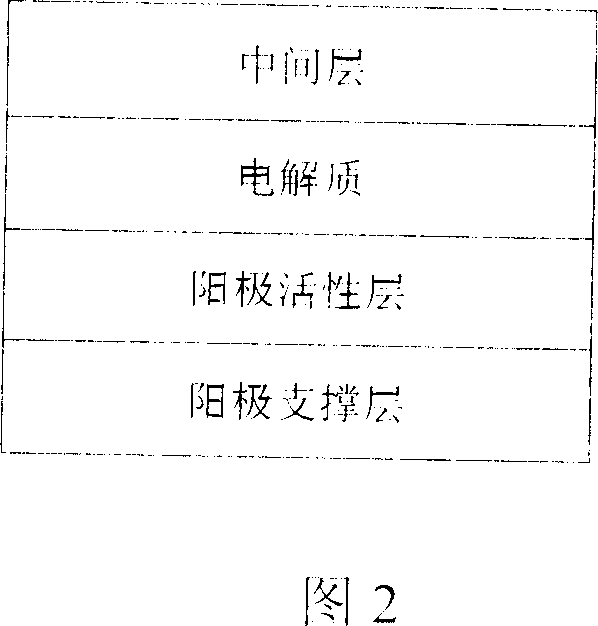
Anode supporting solid electrolyte compound film for solid oxide fuel battery and its preparing method
InactiveCN1925200AEasy to adjustControl thicknessCell electrodesSolid electrolyte fuel cellsFuel cellsGadolinium
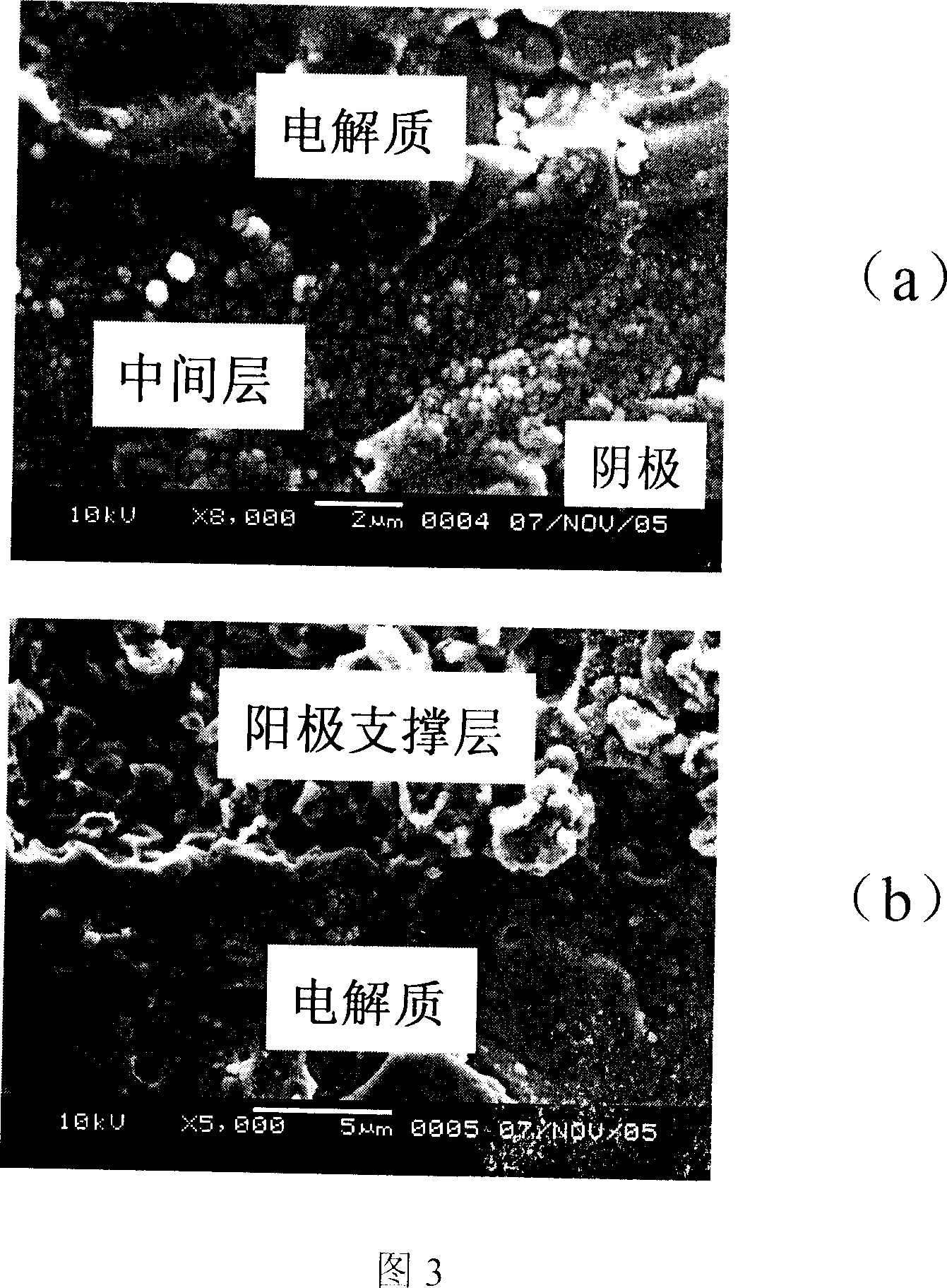
This invention relates to solid oxidation fuel battery anode supportive electrolyte compound film, which comprises the following layers: anode supportive layer by NiO-YSZ of 3mol%Y<2>O<3; anode active layer by NiO-SSZ; solid electrolyte layer of SSZ 9mol% scandium oxide with total stable zircite and middle layer of CGO mixed with 20mol% cerium oxide.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
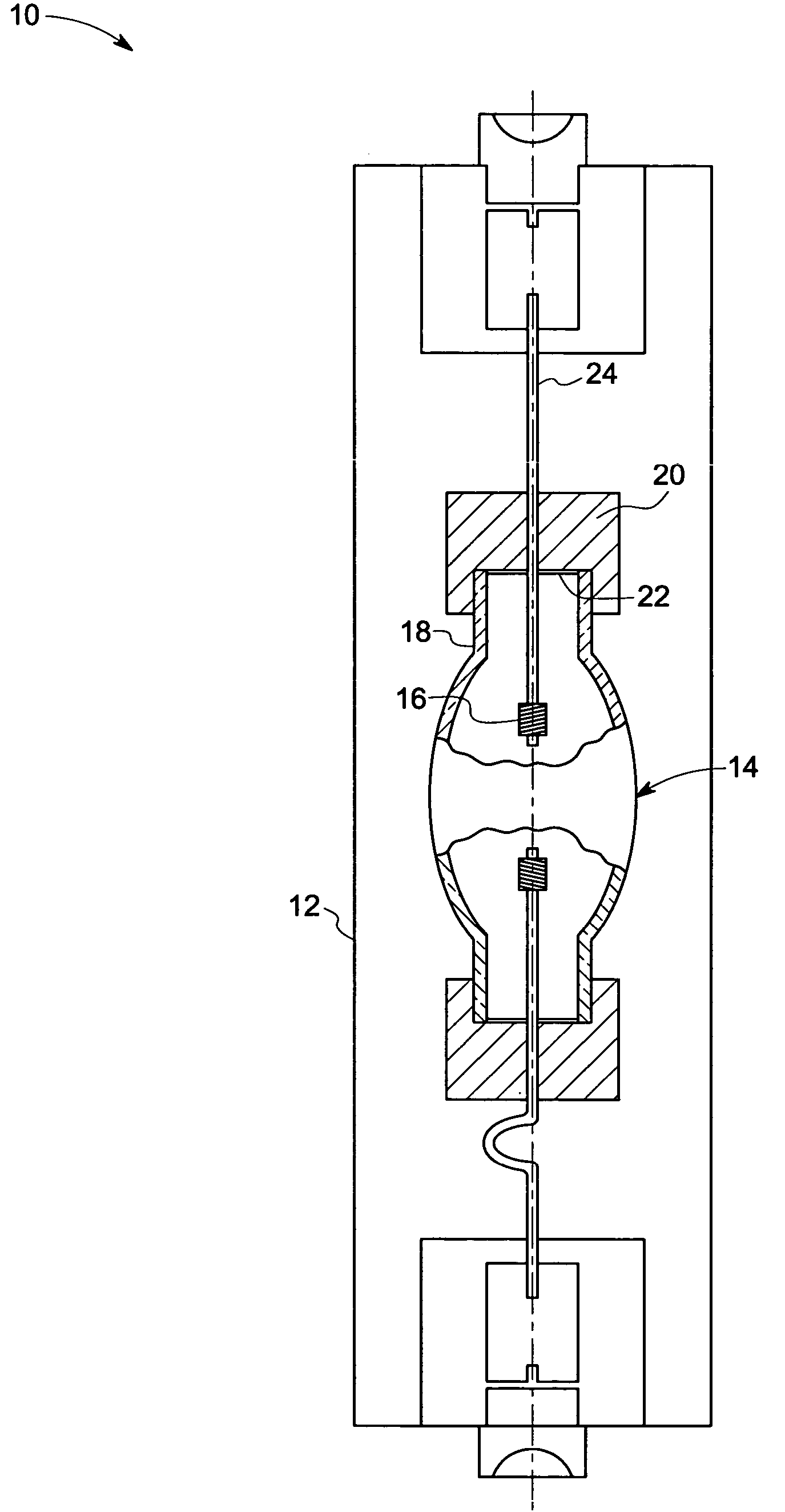
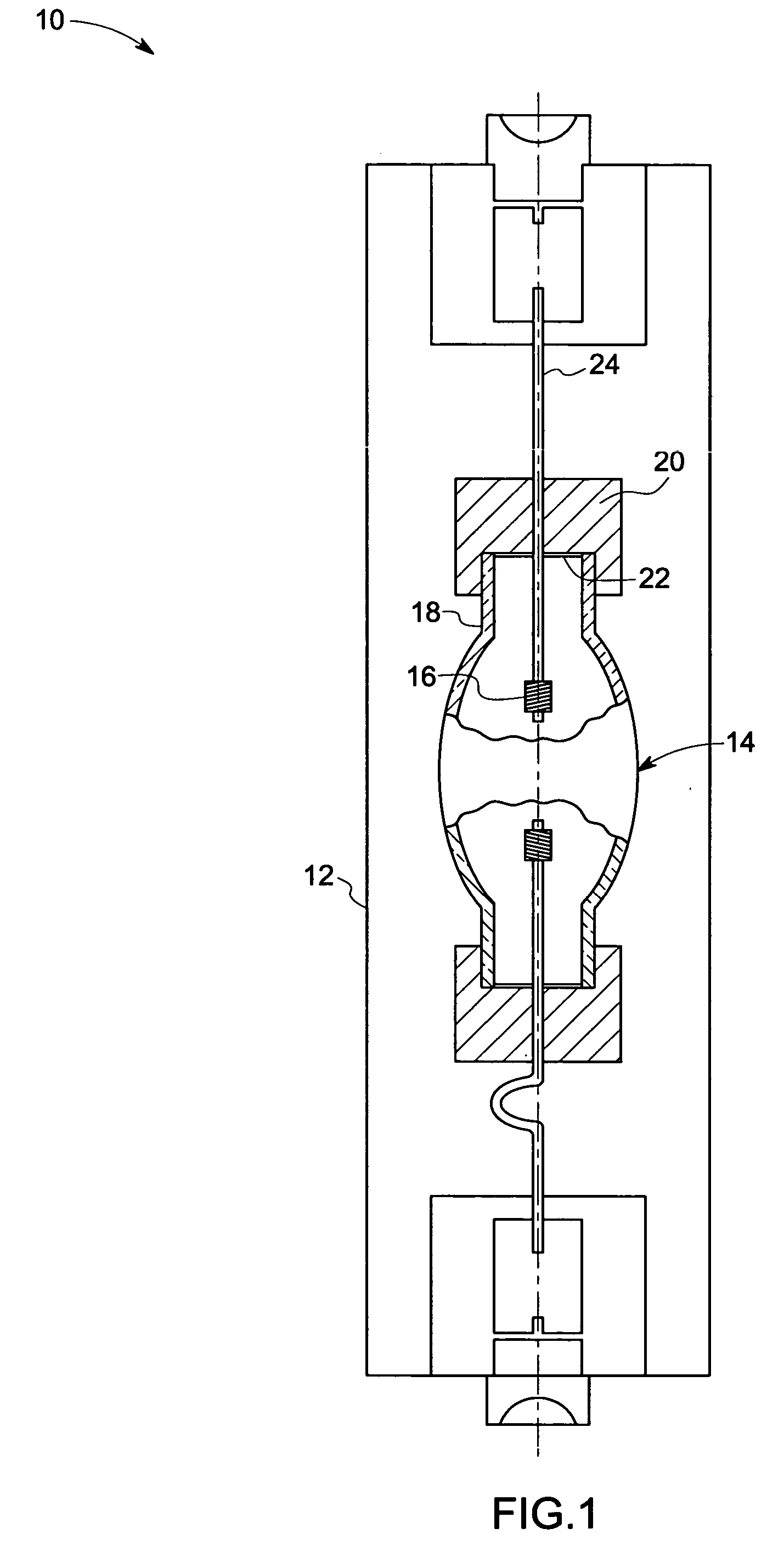
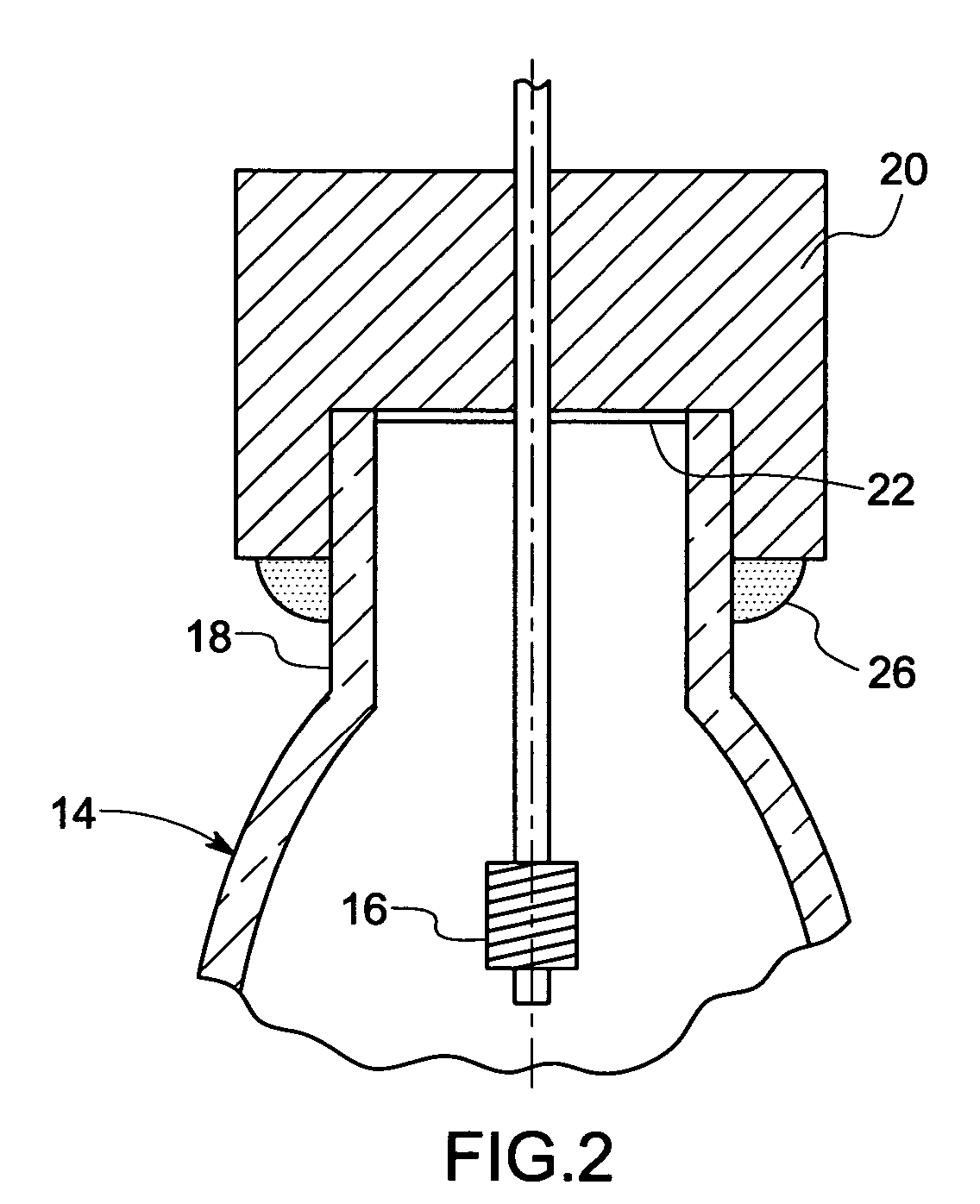
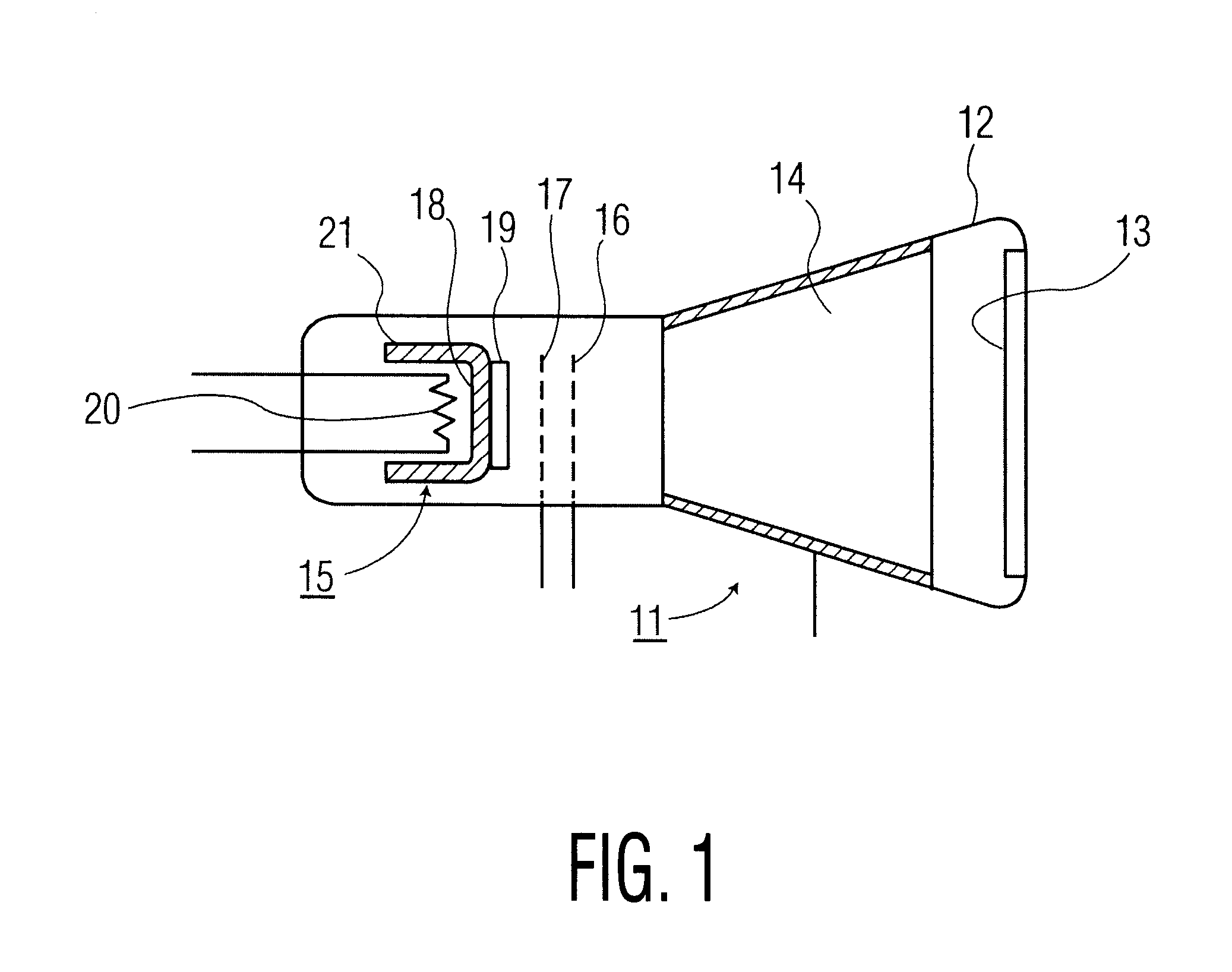
Metal halide lamp and automotive headlamp apparatus
InactiveUS20030222584A1Point-like light sourceDischarge tube main electrodesStable stateMetal-halide lamp
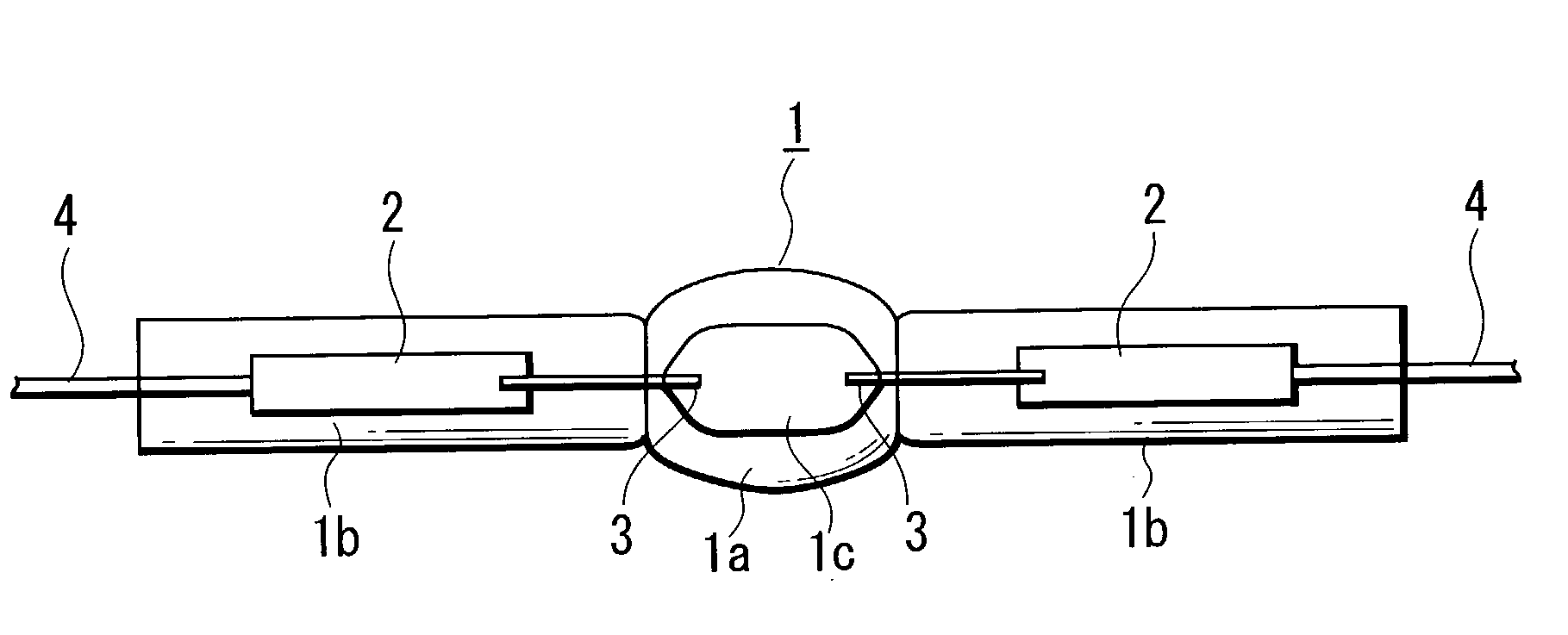
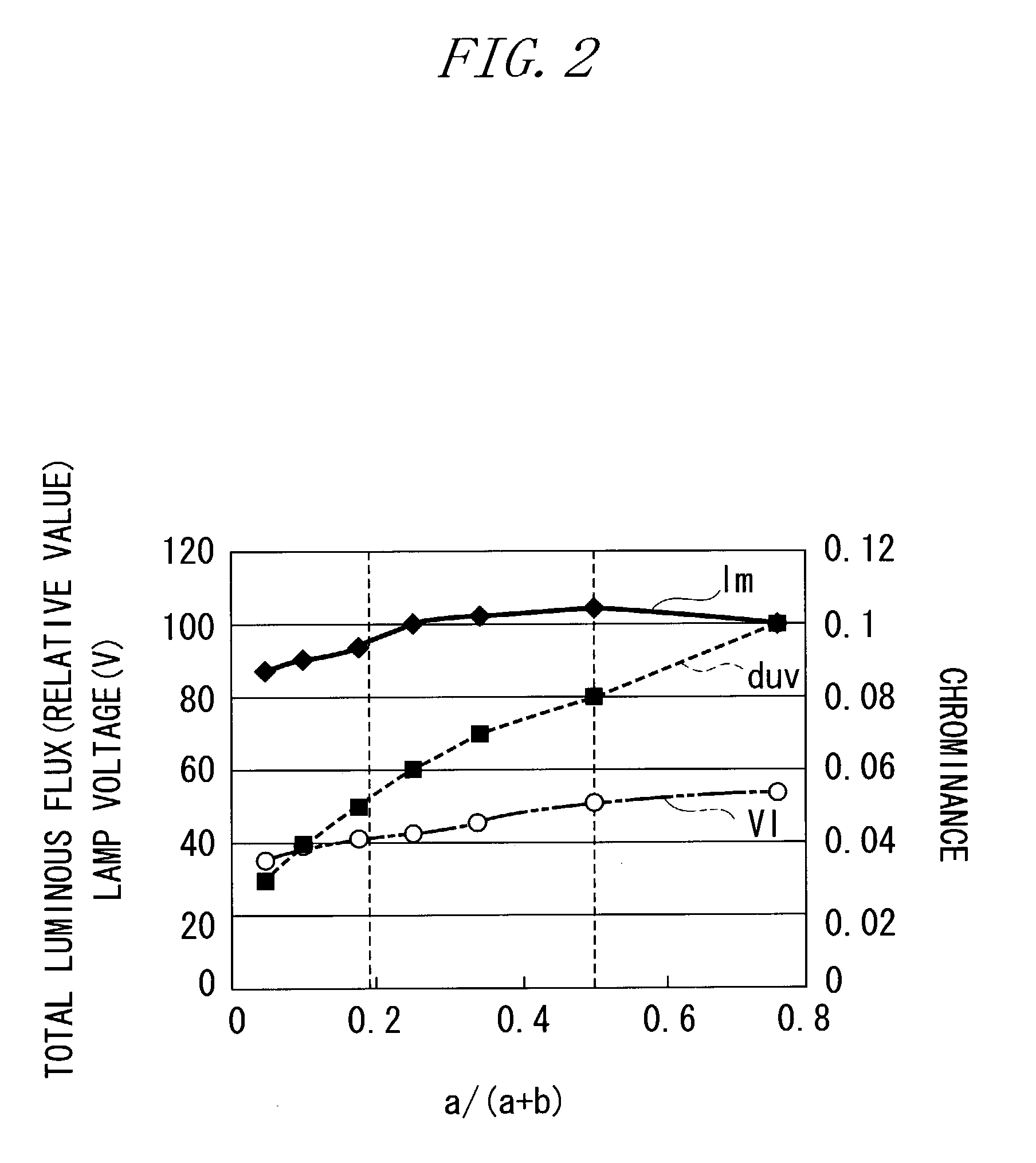
To improve the ratio between first and second halides sealed in a mercury-free lamp, thereby providing a metal halide lamp which has a high luminous efficiency and a low lamp voltage reduction and emits light of an appropriate color and an automotive headlamp apparatus incorporating the same. A metal halide lamp includes a hermetic vessel 1 which is fire resistant and translucent; a pair of electrodes 3, 3 sealed in the hermetic vessel with facing each other at a distant of 5 mm or less; and a discharge medium substantially containing no mercury, sealed in the hermetic vessel 1, and containing first halides mainly including scandium halide and sodium halide, a second halide for mainly providing a lamp voltage and a xenon gas at 5 atmospheres or higher at a temperature of 25° C., the amounts of scandium halide and sodium halide sealed in the hermetic vessel satisfying the formula of 0.25<a / (a+b)<0.5, where reference character a denotes the mass of scandium halide and reference character b denotes the mass of sodium halide, in which, in a stable state, the metal halide lamp is turned on with a lamp power of 60 W or lower.
Owner:HARISON TOSHIBA LIGHTING CORP
Transparent ceramic, method for manufacturing same, and magneto-optical device
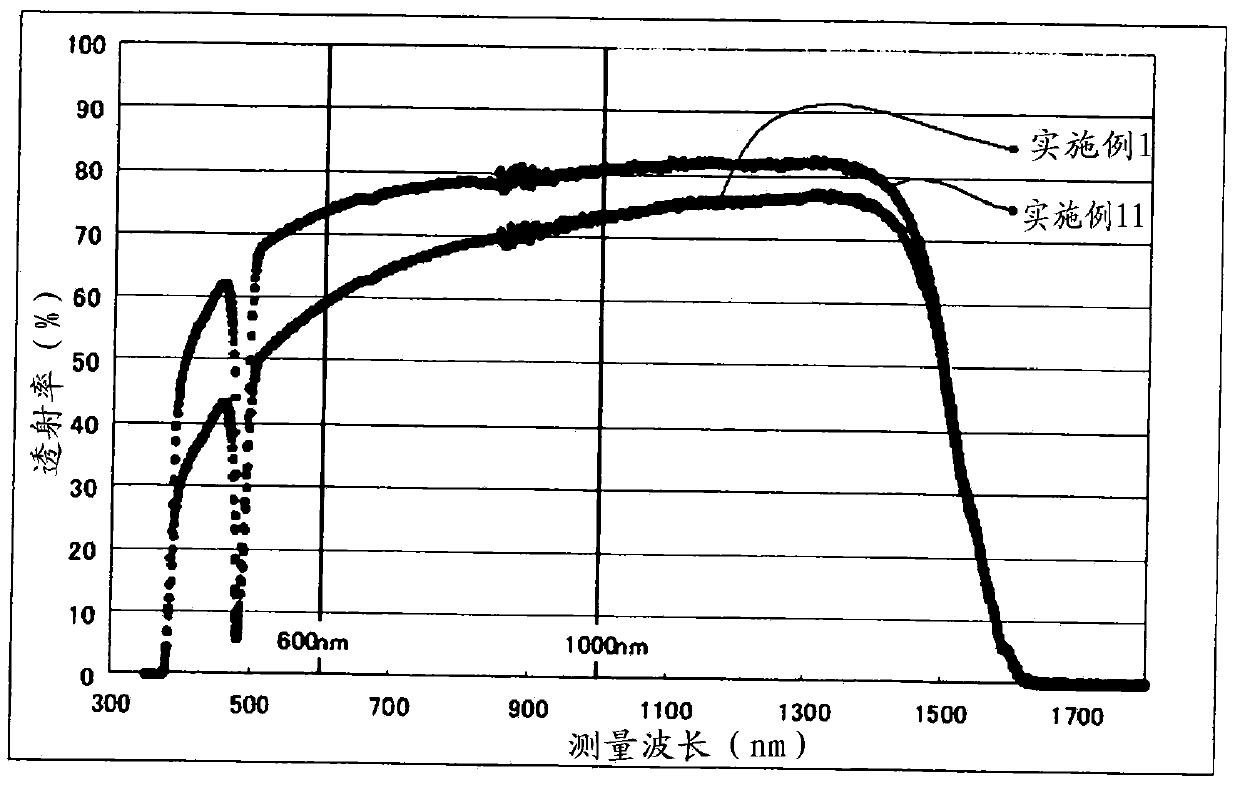
InactiveCN103502180AImprove performanceGood optical performanceRare earth metal compoundsNon-linear opticsInfraredLanthanide
A transparent ceramic having terbium oxide (Tb2O3) in a molar ratio of at least 40%; and at least one oxide selected among an yttrium oxide, a scandium oxide, and a lanthanide rare earth oxide, wherein (1) the crystal structure of the terbium-oxide-based ceramic does not contain a non-cubic-crystal phase, (2) the mean crystal particle diameter is in a range of 0.5 to 100 mum, and (3) the ceramic comprises a sintering auxiliary having no incidence of deposition of a non-cubic-crystal phase in the crystal structure of the terbium-oxide-based ceramic. This transparent ceramic makes a magneto-optical element that performs at least as well as terbium gallium garnet or other existing monocrystal materials. It also makes a functional element for an optical isolator in the infrared region between 500 nm and 1.5 mum having very little scattering and very few birefringence components.
Owner:SHIN ETSU CHEM CO LTD
A kind of preparation method of porous cathode substrate
The invention provides a method for preparing a porous cathode substrate, which belongs to the field of powder metallurgy. The raw material powders used include various powders such as tungsten, iridium, rhenium, and scandium oxide. The various powders are mixed evenly according to a certain weight percentage, and the tungsten content is 40~100%. Mix and disperse the raw material powder particles and the binder evenly to make a granular feed, and the volume fraction of the powder in the feed is 40-75%. The feed material is formed into a green body of a certain shape on an injection molding machine, and the binder in the shaped green body is removed to obtain a porous cathode substrate. The invention has the advantages that the porous cathode substrate with special shape and size requirements can be directly prepared without mechanical processing, the material utilization rate is high, and the cost is low. It can effectively overcome the influence of harmful impurities brought by the traditional process, and improve the electron emission performance, service life and working reliability of the cathode. It can effectively control the pore size, pore distribution, porosity and pore connectivity of the material.
Owner:UNIV OF SCI & TECH BEIJING
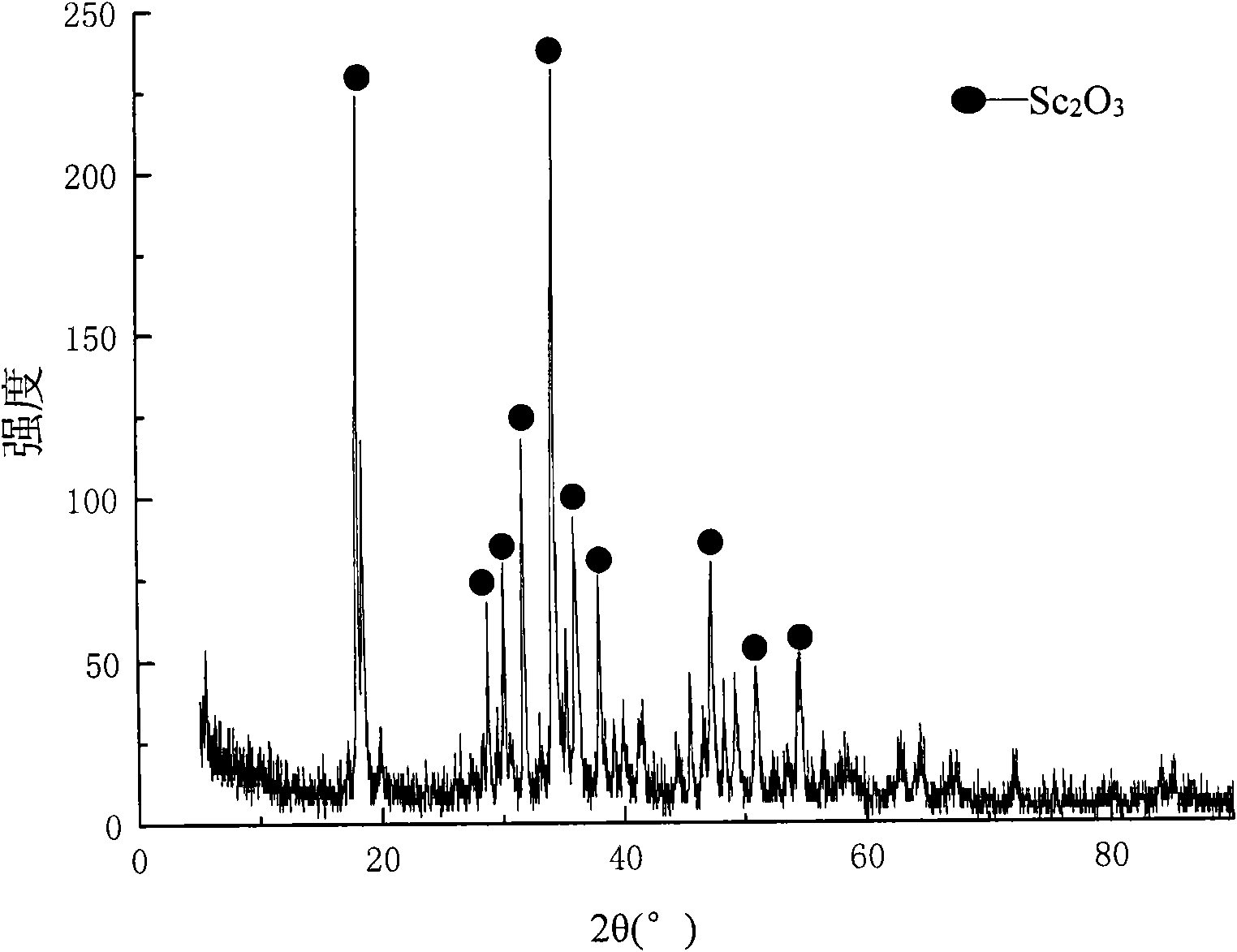
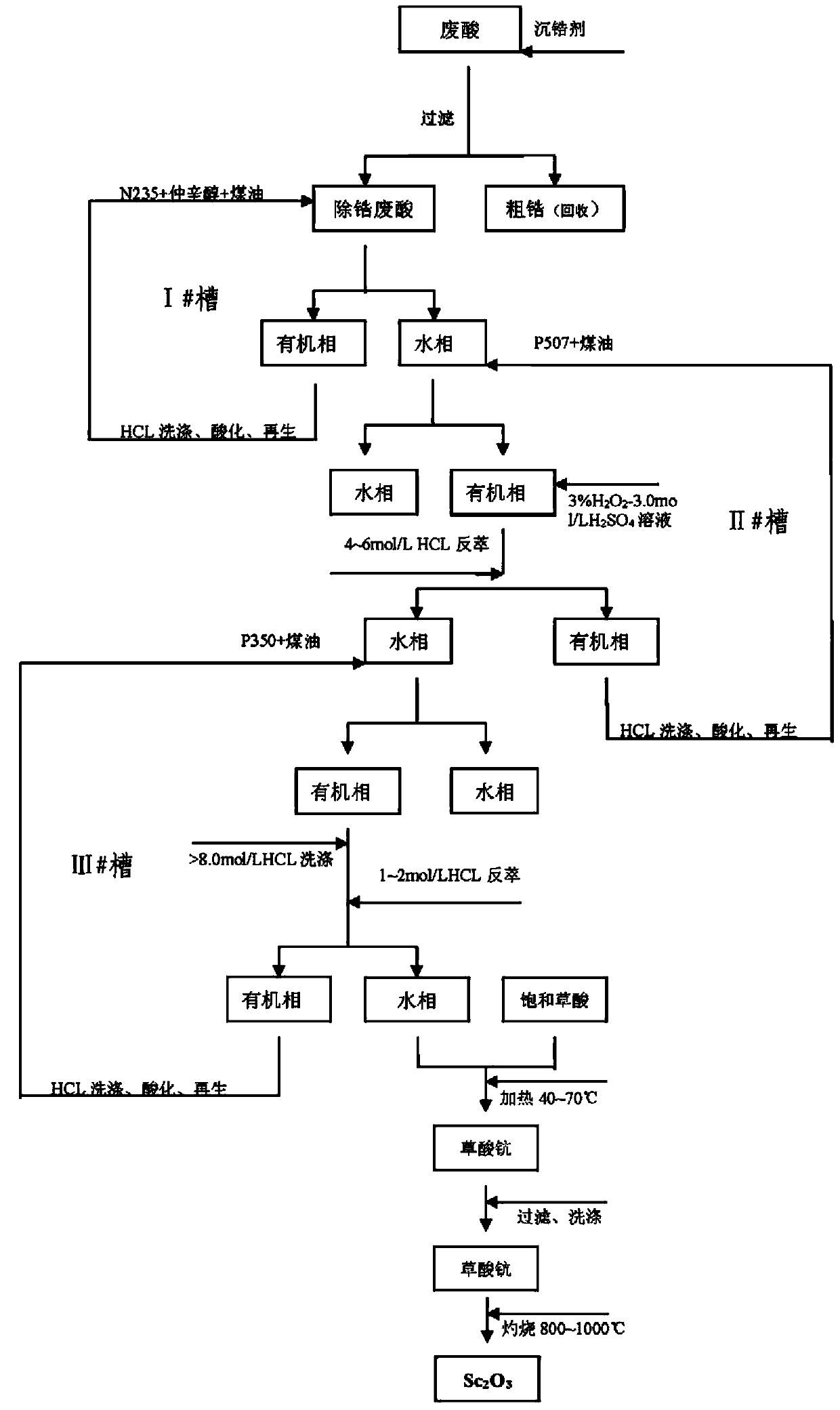
Method for recycling and purifying scandium oxide powder from waste acid in zirconium salt production
ActiveCN103695671AHigh purityPromote environmental protectionProcess efficiency improvementScandium oxideOxalic acid
The invention discloses a method for recycling and purifying scandium oxide powder from waste acid in zirconium salt production. The method comprises the following steps: (S1) performing precipitation separation to remove zirconium from the waste acid liquid in zirconium salt production by use of a zirconium precipitation agent; (S2) performing extraction separation to remove iron from the waste acid after zirconium removal by use of organic-phase trioctyl tertiary amine N235; (S3) extracting and enriching scandium from the waste acid liquid treated by the step (S2) by use of organic-phase mono(2-ethylhexyl) 2-ethylhexyl phosphonate P507; (S4) extracting and purifying scandium from the back extraction liquid of the step (S3) by use of organic-phase di(methylheptyl) methyl phosphonate P350; (S5) precipitating and refining scandium oxide of the back extraction liquid in the step (S4) directly by use of oxalic acid. According to the method disclosed by the invention, scandium is recycled from waste acid and is enriched and further purified and refined to obtain high-purity scandium oxide powder; the method is beneficial to the environment-friendly discharge control and comprehensive utilization of waste acid, and reduces the environmental protection cost of waste acid treatment and up-to-standard discharge of an enterprise; more importantly, the method is used for recycling and preparing expensive high-purity scandium oxide powder.
Owner:江西赛瓷材料有限公司
Electric discharge tube or discharge lamp and scandate dispenser cathode
InactiveUS6348756B1High emission-current densityLow tungsten lossLamp incadescent bodiesGas discharge lampsRheniumChemical reaction
An electric discharge tube or discharge lamp comprising a scandate dispenser cathode which is composed of a cathode body and a coating having an emissive surface, said cathode body including a matrix of at least one refractory metal and / or a refractory alloy and a barium compound which is in contact with the matrix material to supply barium to the emissive surface by means of a chemical reaction with said matrix material, and the coating containing one or more multilayers which may include a bottom layer of tungsten and / or a tungsten alloy, an intermediate layer of rhenium and / or a rhenium alloy and a top layer of scandium oxide, a mixture of scandium oxide and rare-earth metal oxides, a scandate and / or a scandium alloy, is characterized by a long service life and a high emission-current density.
Owner:US PHILIPS CORP
Non-noble metal based electro-catalyst compositions for proton exchange membrane based water electrolysis and methods of making
The invention provides electro-catalyst compositions for an anode electrode of a proton exchange membrane-based water electrolysis system. The compositions include a noble metal component selected from the group consisting of iridium oxide, ruthenium oxide, rhenium oxide and mixtures thereof, and a non-noble metal component selected from the group consisting of tantalum oxide, tin oxide, niobium oxide, titanium oxide, tungsten oxide, molybdenum oxide, yttrium oxide, scandium oxide, cooper oxide, zirconium oxide, nickel oxide and mixtures thereof. Further, the non-noble metal component can include a dopant. The dopant can be at least one element selected from Groups III, V, VI and VII of the Periodic Table. The compositions can be prepared using a surfactant approach or a sol gel approach. Further, the compositions are prepared using noble metal and non-noble metal precursors. Furthermore, a thin film containing the compositions can be deposited onto a substrate to form the anode electrode.
Owner:UNIVERSITY OF PITTSBURGH
Hot pressed sintering high-purity zirconia composite ceramic and preparation method thereof
The invention provides a zirconia composite tough material with adjustable toughness and low price. The tough ceramic is prepared by complex powder of high-purity zirconia doped with at least one of hafnium oxide, yttrium oxide, cerium oxide, calcium oxide, magnesium oxide, aluminum oxide, titanium oxide, silicon oxide, cobalt oxide, iron oxide, scandium oxide, vanadium oxide, manganese oxide, nickel oxide, copper oxide, zinc oxide, niobium oxide, molybdenum oxide, indium oxide, stannic oxide, barium oxide, tantalum oxide, tungsten oxide, lanthanum oxide, praseodymium oxide, neodymium oxide, tellurium oxide, terbium oxide, europium oxide and erbium oxide. The preparation method comprises the following steps: dispersing the zirconia composite powder by using a high energy ball milling method, and adding the dispersed powder into a graphite mold for performing hot pressed sintering treatment, thereby obtaining the high-purity zirconia composite ceramic.
Owner:南京金鲤新材料有限公司
Composite anode of magnesium-modified and nickel-based solid-oxide fuel cell and preparation and application thereof
InactiveCN101771149AThe electrode structure is evenly distributedLower polarization resistanceCell electrodesFuel cell detailsFuel cellsHigh activity
The invention relates to a solid-oxide fuel cell, in particular to a composite anode of a solid-oxide fuel cell and a preparation method thereof. The composite anode comprises the following components by weight percent: 30-69.9 percent of nickel by nickel oxide, 0.01-30 percent of magnesium by magnesium oxide, doped zirconium oxide (zirconium oxide is doped with the yttrium oxide (YSZ)), the scandium oxide doped with the zirconium oxide (ScSZ) and / or the zirconium oxide (CeScSz), wherein the molar percent of the cerium oxide, the zirconium oxide and / or scandium oxide is 0.1-20 percent which accounts for 30-69.9 percent. The composite anode of the solid-oxide fuel cell has low polar polarization resistance, high activity and the like, and improves the output performance of the cell. The novel composite anode can be applied in solid-oxide fuel cells with flat plate type, pipe type and flat pipe type and other various structural modes.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com