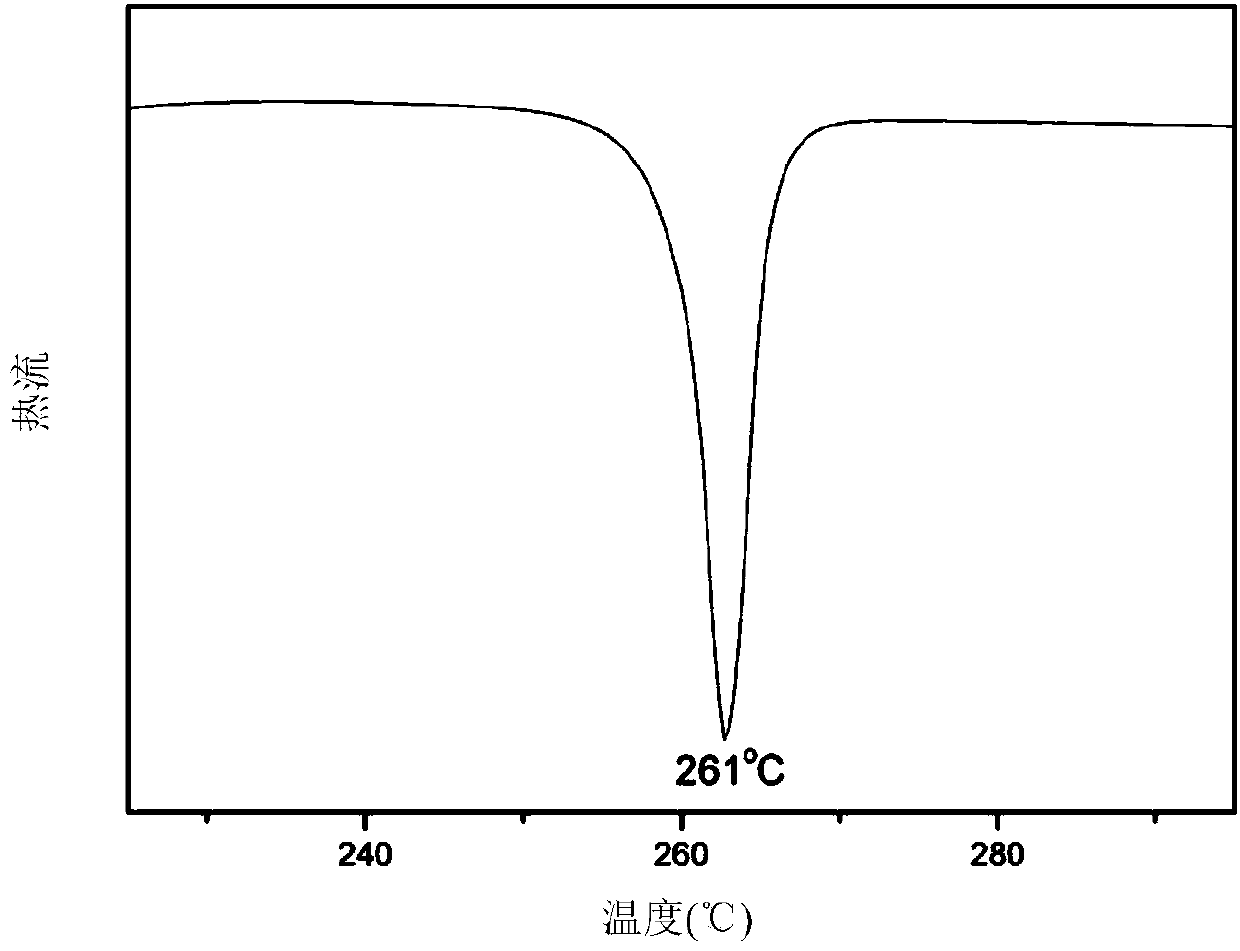
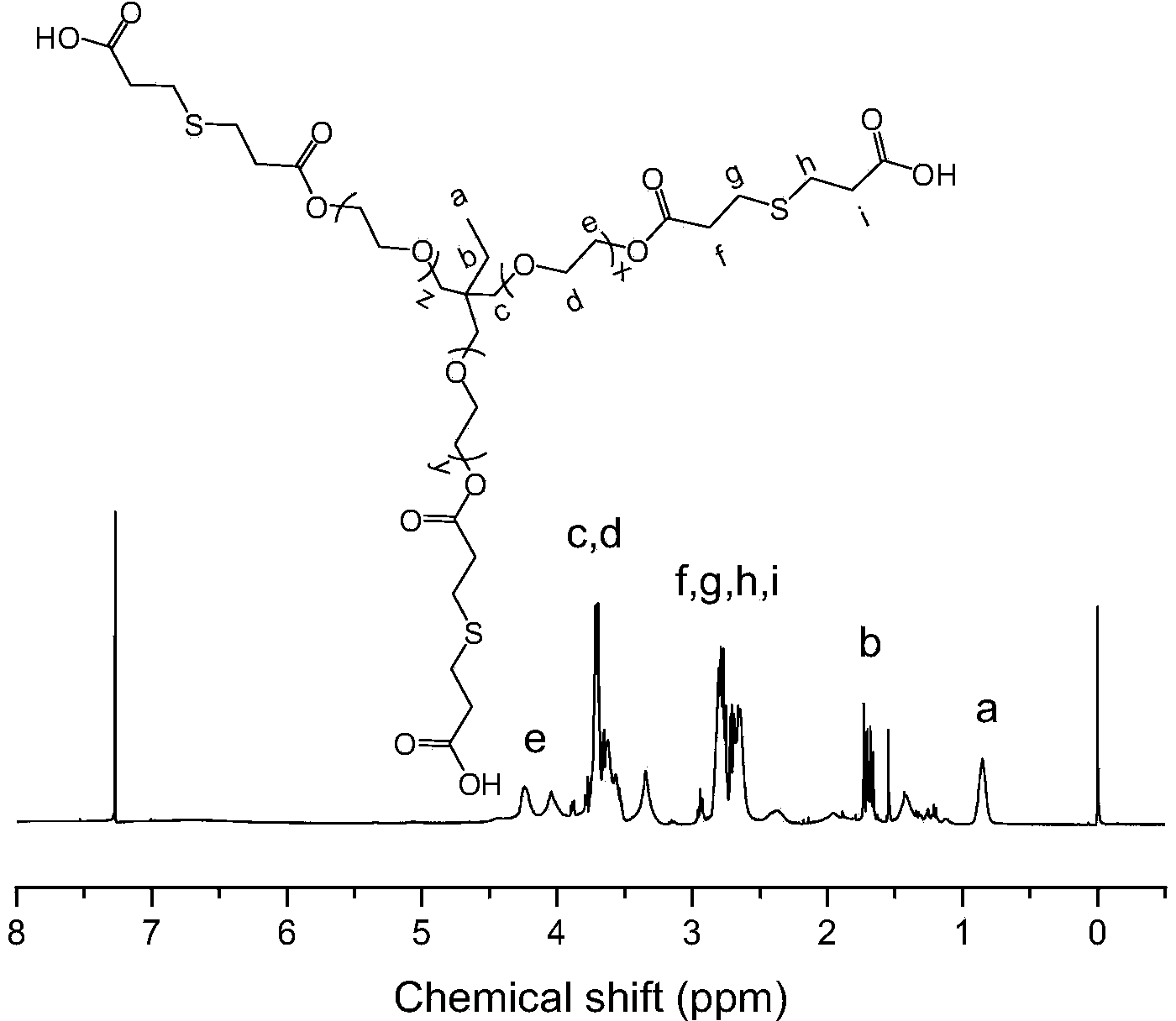
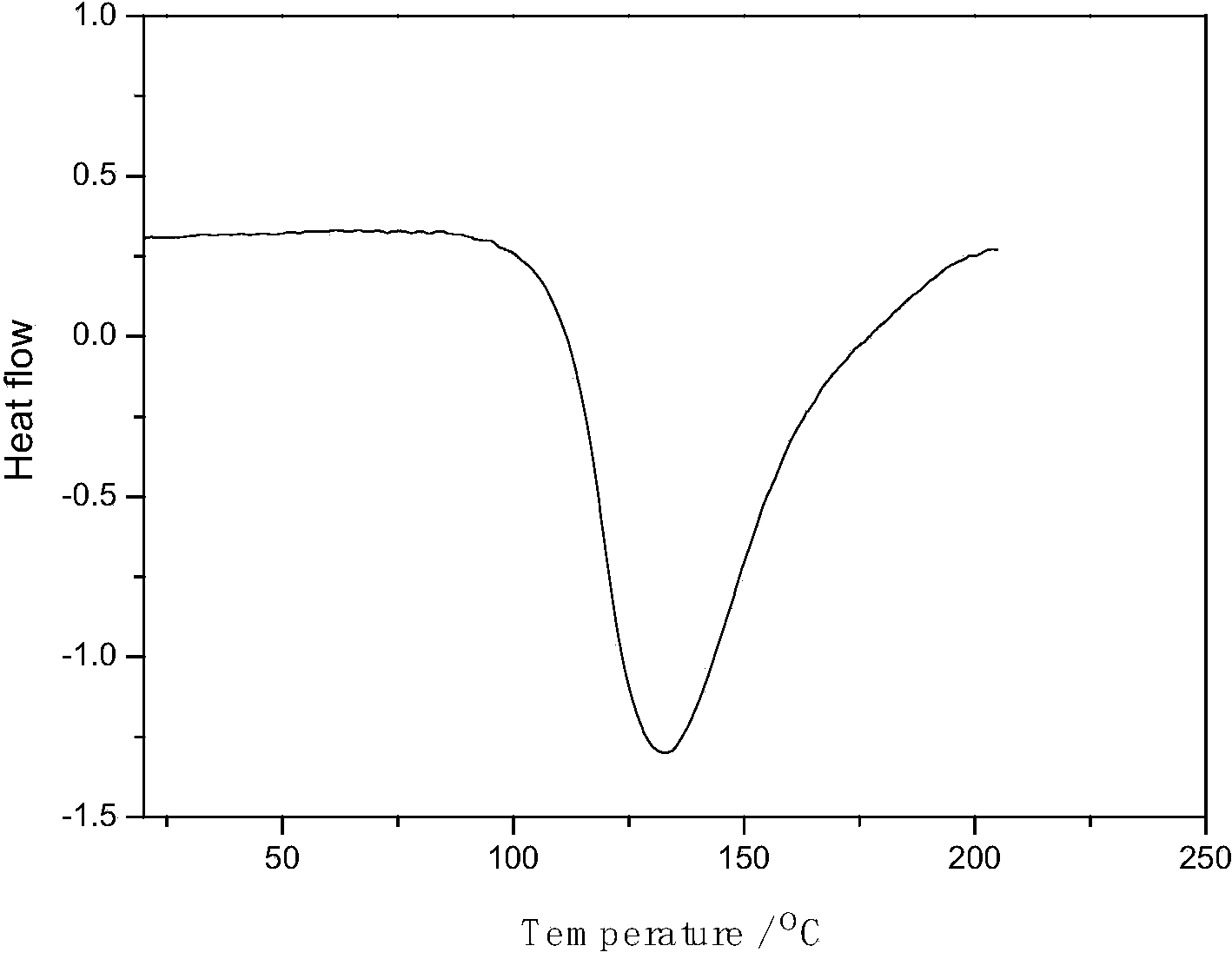
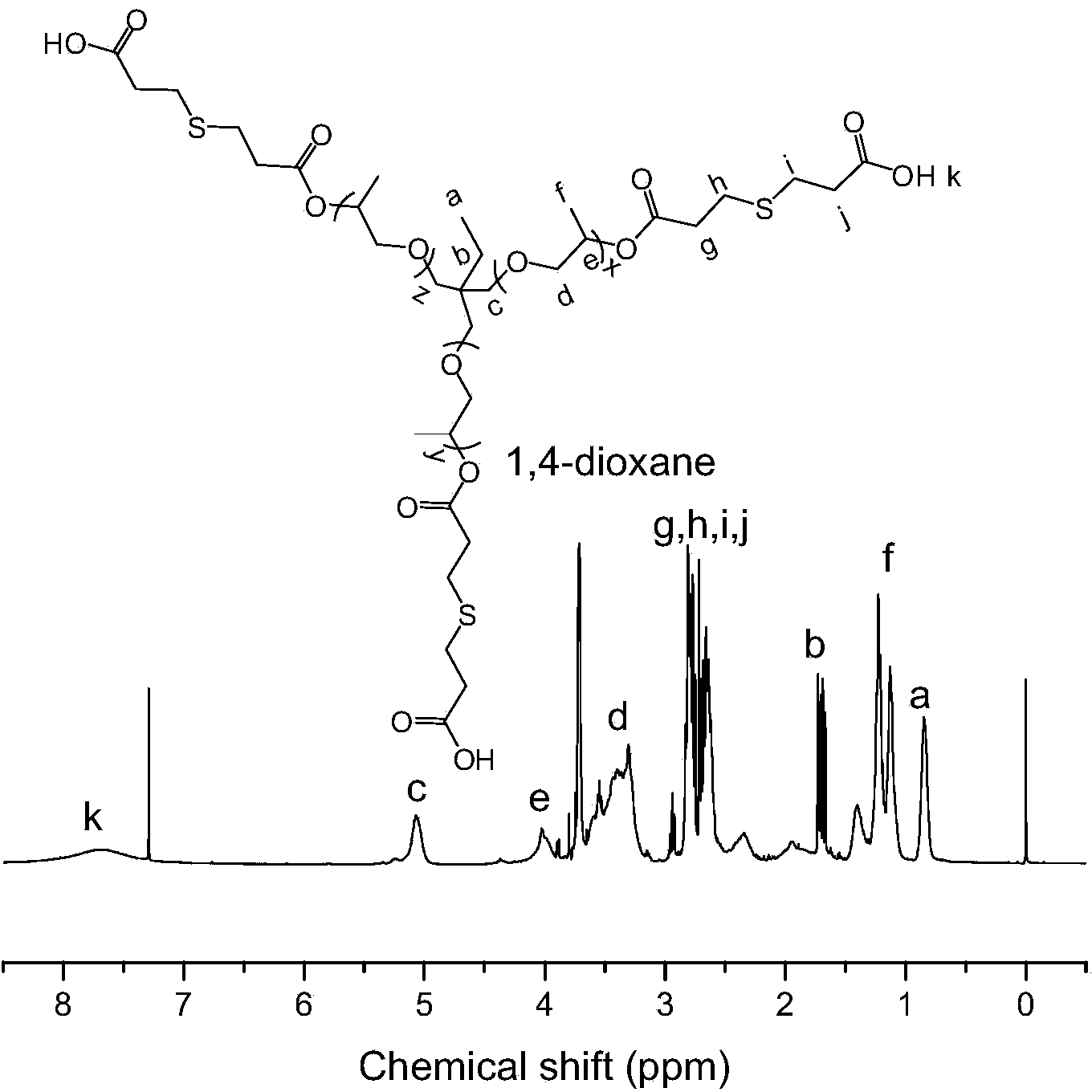
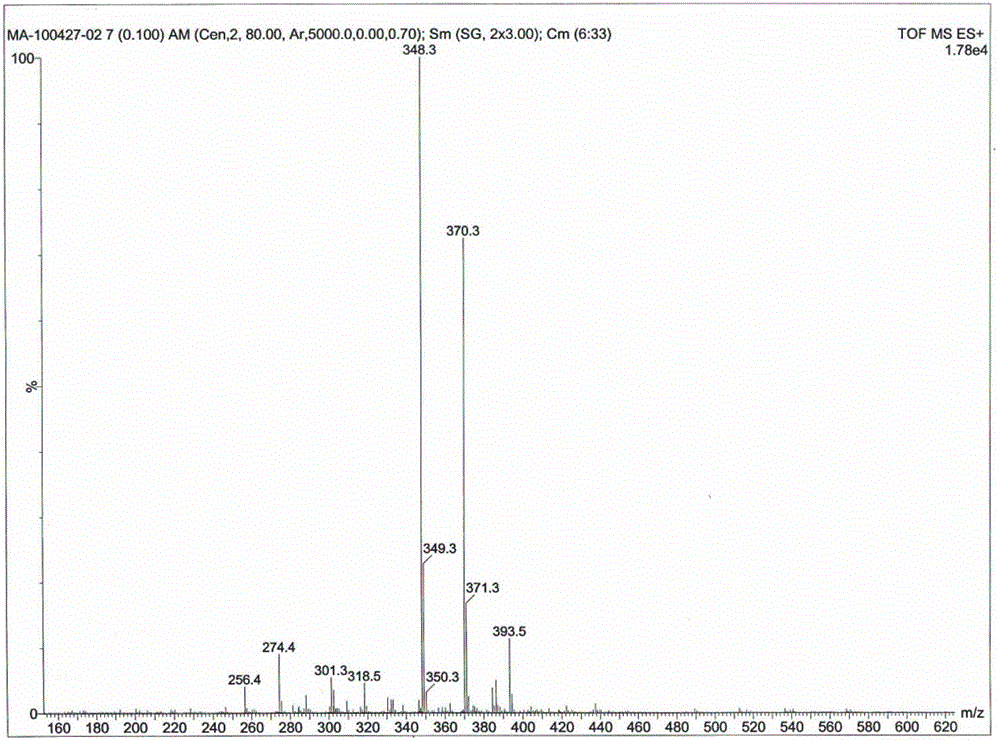
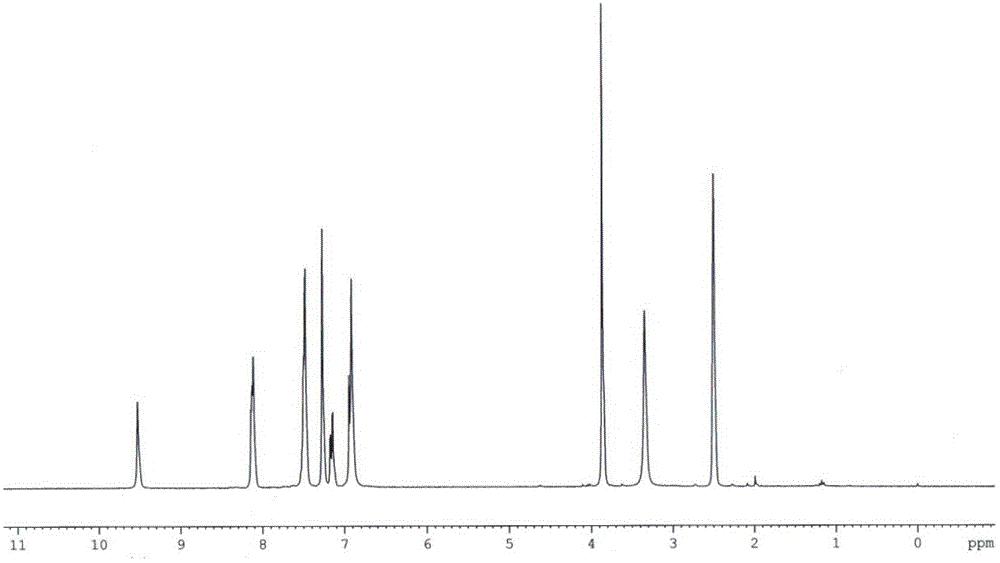
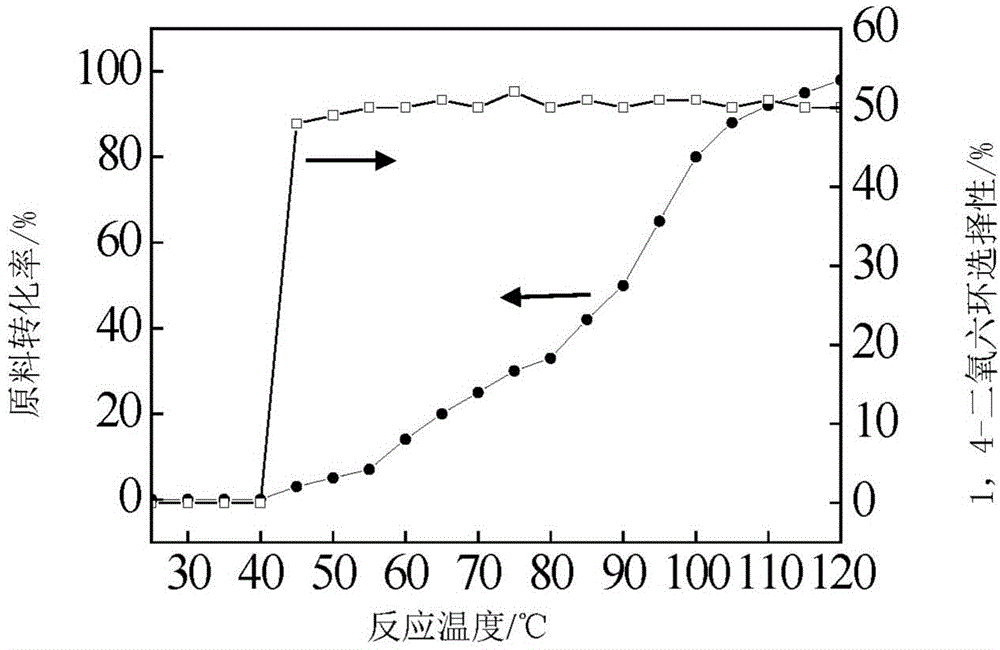
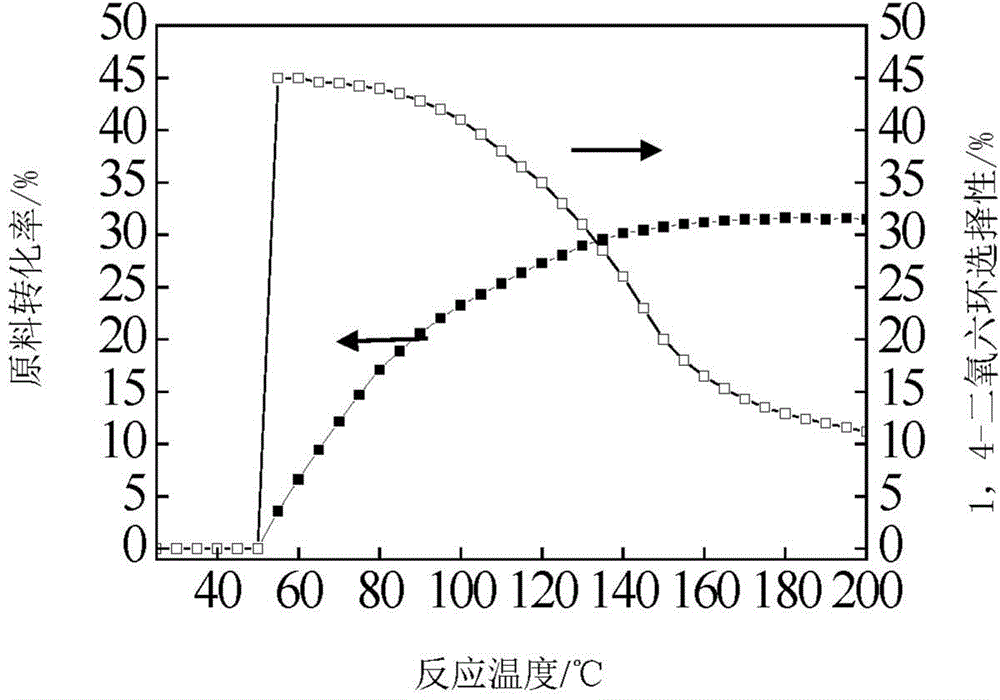
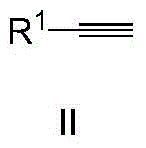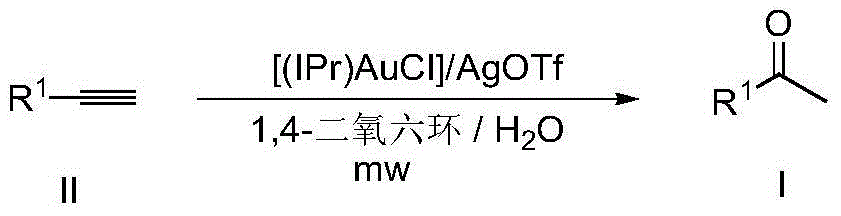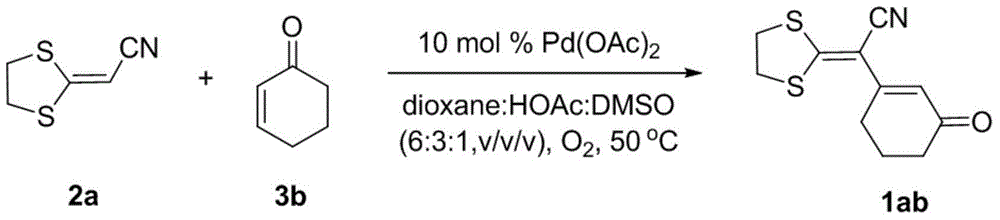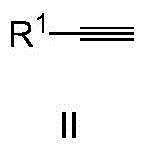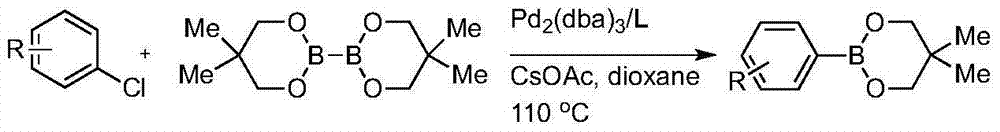Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
94 results about "1,4-Dioxane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
1,4-Dioxane (/daɪˈɒkseɪn/) is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers (1,2- and 1,3-) are rarely encountered.
Sulfide functionalized covalent organic frame material and synthesis method thereof
ActiveCN103694469AHas a mesoporous structureHigh reaction yieldWater/sewage treatment by sorptionSynthesis methodsSolvent
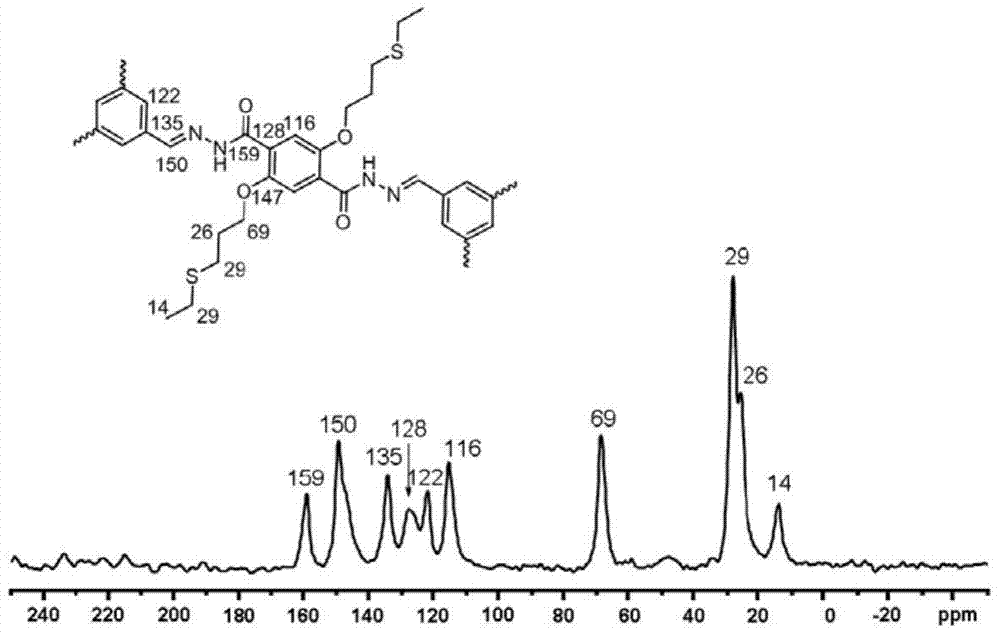
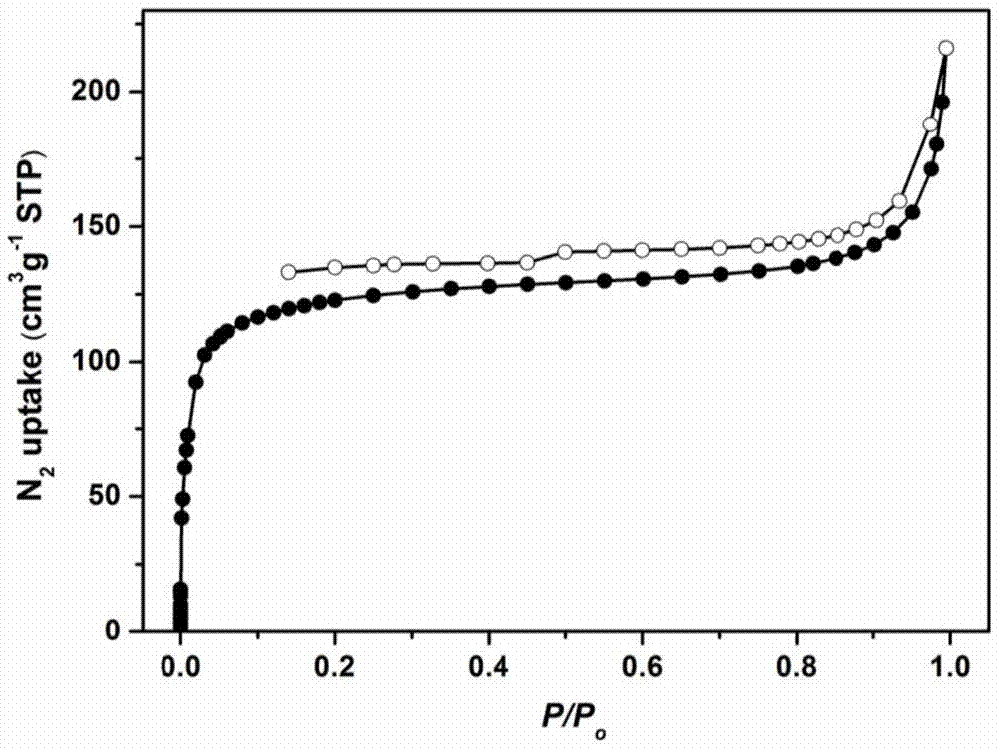
The invention discloses a sulfide functionalized covalent organic frame material, The material has a structural unit shown in the specification, and is obtained by the following steps: adding 2,5-Bis(3-(ethylthio)propoxy)-terephthalohydrazide and benzenetricarboxaldehyde into a mixed solvent of 1,4-dioxane and mesitylene, adding an aqueous acetic acid solution into the above mixture and reacting at 120 DEG C for 1-3 days. The material has a mesoporous structure, has BET specific surface area of 470-480 m<2>?g<-1>, has good selective identification for mercury ions, and can be used for removal of the mercury ions.
Owner:LANZHOU UNIVERSITY
Fluorescent probe reagent for concurrent selection and determination of multiple metal ions, and preparation and appliance
InactiveCN105482812APreparation conditions are easy to controlReduce distractionsOrganic chemistryColor/spectral properties measurementsOrganic synthesisPhotochemistry
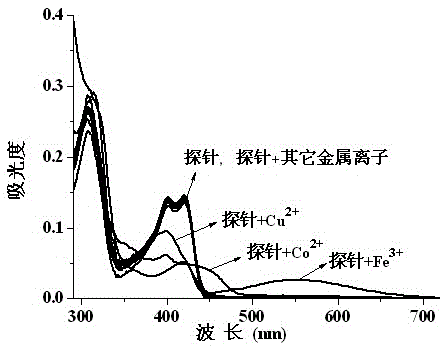
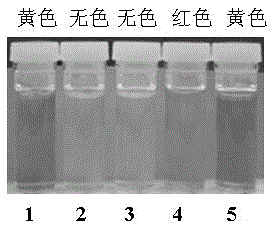
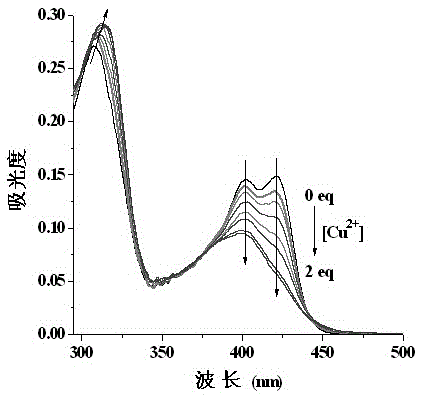
The invention discloses a probe reagent for concurrent selection and determination of multiple metal ions, and preparation and appliance, and belongs to the field of organic synthesis and analytical chemistry. Tri (2-aminoethyl) amine serves as a parent, wherein rhodamine B is connected to an amino chain, 2-hydroxy-1-naphthaldehyde groups are connected to other two amino chains respectively, and thus a tripod structured rhodamine-hydroxyl naphthalene derivative probe is prepared. In 1,4-dioxane / water (19 / 1, v / v, pH=7) solution, the probe respectively detects Cu2+, Co2+ and Fe3+ by utilizing rate absorption of different wavelengths, and the detection does not interfere with each other; in acetonitrile / water (19 / 1, v / v) solution, fluorescence emission of different wavelengths under different pHs is utilized, the probe respectively detects Zn2+, Al3+, Hg2+ and Cu2+, and the detection does not interfere with each other; under an ultraviolet lamp of 365 nm, Zn2+, Al3+ and Hg2+ are detected to show blue, pink and orange red fluorescence respectively, and through -Zn2+ mixture detection by the probe, Cu2+ shows blue fluorescence vanishing. The probe structure is as follows.
Owner:GUIZHOU UNIV
Preparation method of segmented copolymer from vinylidene chloride copolymer and polyethylene glycol
The invention discloses a preparation method of a segmented copolymer from a vinylidene chloride copolymer and polyethylene glycol which comprises the steps of: reacting polyethylene glycol (PEG) and a reversible addition fragmentation transfer radical polymerization (RAFT) chain transfer reagent in dichloromethane under the action of a catalyst 4-dimethylamino pyridine to obtain a PEG macromolecular RAFT reagent; and performing RAFT polymerization on vinylidene chloride or vinylidene chloride and a comonomer in the PEG macromolecular RAFT reagent under the action of azo-bis-iso-butyrynitrile initiator and with 1,4-dioxane as a solvent to obtain a segmented copolymer from a vinylidene chloride copolymer and polyglycol with large molecular weight and narrow molecular weight distribution width. The invention provides a novel method for preparing a vinylidene chloride segmented copolymer, and the segmented copolymer composed of a vinylidene chloride copolymer and polyglycol prepared by the invention has a potential application preparation of hydrophilic modified vinylidene chloride films and vinylidene chloride polymer based mesoporous carbon.
Owner:ZHEJIANG UNIV
Bioactive bone repair material containing williams elder twig as well as preparation method and application thereof
InactiveCN104689373AEnhanced osteogenic propertiesHigh speedAdditive manufacturing apparatusProsthesisLoss rateAdditive ingredient
The invention discloses a bioactive bone repair material containing williams elder twig as well as a preparation method and application thereof, and belongs to the field of bone repair materials. The preparation method of the bioactive bone repair material containing williams elder twig comprises the following steps: adding 4g of PLGA and 2g of TCP into 40ml of 1,4-dioxane, and dissolving and stirring for 24 hours; and adding 2mg to 0.4g of williams elder twig active components into 10ml of 1,4-dioxane, dissolving, then adding 1ml of a williams elder twig dissolved solution into an obtained solution, and then stirring for 2 hours to obtain the bioactive bone repair material containing williams elder twig. The bioactive bone repair material containing williams elder twig, disclosed by the invention, can be subjected to three-dimensional printing to form an artificial bone by virtue of a low-temperature fused deposition production and molding technology; and an obtained artificial bone material can be used for releasing an estrogen-like traditional Chinese medicine namely williams elder twig in an absorption process, and can be used for slowing down the bone loss rate of patients suffering osteoporosis; and meanwhile, the bioactive bone repair material containing williams elder twig has biocompatibility and bone inducing ability.
Owner:STOMATOLOGY AFFILIATED STOMATOLOGY HOSPITAL OF GUANGZHOU MEDICAL UNIV
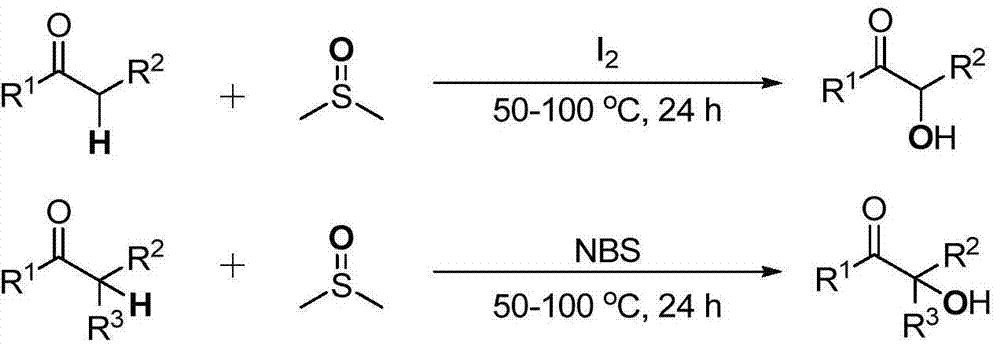
Cheap and efficient synthesis method of alpha-hydroxyketone compound
ActiveCN104710256AEmission reductionHigh yieldOrganic compound preparationHydroxy group formation/introductionSynthesis methodsEthyl acetate
The invention discloses a cheap and efficient synthesis method of an alpha-hydroxyketone compound. The synthesis method is characterized in that a carbonyl compound undergoes an oxidation hydroxylation reaction at 10-120DEG C under normal pressure with iodine simple substance, N-bromosuccimide, copper bromide, bromine simple substance, hydrogen bromide, N-iodosuccimide or hydrogen iodide as a catalyst, sulfoxide as an oxidant, water or sulfoxide as a hydroxy source and sulfoxide, ethyl acetate, N,N-dimethyl formamide, acetonitrile, toluene, 1,4-dioxane, 1,2-dichloroethane, tetrahydrofuran or H2O as a solvent, and converts into the alpha-hydroxyketone compound in a high selectivity manner. Compared with traditional synthesis methods, the method disclosed in the invention has the advantages of simple operation, high yield, simple conditions, easy purification, small waste discharge amount, simple reaction apparatus, and easy industrial production. The method has wide applicability and can be used for synthesizing various alpha-hydroxyketone compounds.
Owner:QINGDAO RUIJI MEDICAL TECH CO LTD
Polybenzimidazole with side groups and ether bonds and preparation method and application thereof
InactiveCN103435804AImprove solubilityGood dimensional stabilityFuel cell detailsSolubilityPolymer science
The invention belongs to the technical field of high polymer material and synthesis of a high polymer material. The preparation method comprises the following steps: carrying out the reaction between bisphenol compound and p-dihalobenzonitrile, re-crystallizing acetone to obtain dinitrile monomer with side groups and ether bonds, adding potassium hydroxide into the dinitrile monomer solution with side groups and ether bonds to carry out hydrolysis, re-crystallizing 1,4-dioxane to obtain diacid monomer with side groups and ether bonds, dissolving the diacid monomer with side groups and ether bonds and 3,3'-diaminobenzidine monomer into phosphorus pentoxide-methane sulfonic acid solution for reaction, and thus obtaining the polybenzimidazole with side groups and ether bonds after treatment. The product can be used for preparing proton conductive membranes of fuel cells. The polybenzimidazole with side groups and ether bonds prepared by the method provided by the invention has good solubility in organic solvent, is convenient to prepare membranes with high performance, and better improved in size and oxidation stability of membrane material and methanol permeability while the proton conductivity of a blend membrane is basically kept.
Owner:JILIN UNIV
Preparation method of fluorine-containing high-molecular surfactant
InactiveCN103483513AHigh molecular weight distributionNarrow molecular weight distributionCritical micelle concentrationEmulsion polymerization
The invention discloses a preparation method of a fluorine-containing high-molecular surfactant, and the preparation method comprises the following steps: enabling an initiator, methacrylic acid-N,N-ethyl dimethylamide, a chain transfer agent and 1,4-dioxane to react at 60-90 DEG C in the presence of the argon to obtain polymethylacrylic acid-N,N-ethyl dimethylamide; adding the initiator, hexafluorobutyl acrylate and 1,4-dioxane, and reacting for 2-8 hours at 65-95 DEG C in the presence of argon; precipitating in n-hexane; drying to obtain the fluorine-containing high-molecular surfactant. By adopting an RAFT polymerization technology, the prepared fluorine-containing high-molecular surfactant contains a fluorine-containing unit similar to the structure of a fluorine-containing monomer, has the characteristics of narrow molecular weight distribution, adjustable structure, low critical micelle concentration, good emulsibility, low foamability and the like, and has broad application prospect in emulsion polymerization.
Owner:广州市科灵精细化工有限公司
Epoxy curing agent synthesized through mercapto-vinyl addition
The invention discloses an epoxy curing agent synthesized through mercapto-vinyl addition. The epoxy curing agent is synthesized through carrying out addition reaction on a C=C double-bond containing acrylate or vinyl ether compound with difunctionality or polyfunctionality and a mercapto compound under the condition of light or heat initiation. A preparation method of the epoxy curing agent comprises the steps of adding a C=C double-bond containing difunctional or polyfunctional compound, the mercapto compound, a proper volume of solvent (such as 1,4-dioxane) and a free radical initiator or photoinitiator into a three-mouthed flask, reacting for certain time under the condition of heating or lighting, and removing the solvent, thereby obtaining the product. The epoxy curing agent overcomes the disadvantages of the existing modified amine and polymercaptan epoxy curing agents that the odor is strong, the toxicity is high, the heat release quantity is large during curing and the like; and the epoxy curing agent has the advantages of light color and luster, high index of refraction, low toxicity and the like and accordingly is particularly suitable for serving as a curing agent of epoxy encapsulation materials of electronic products, such as LED (Light Emitting Diode).
Owner:YANTAI UNIV
Preparation method of nitrogen functionalized microporous carbon nanoparticle
The invention relates to a preparation method of a nitrogen functionalized microporous carbon nanoparticle. The preparation method comprises the following steps: weighing m-phenylenediamine, terephthalaldehyde, 1,4-dioxane and 3mol / L acetum according to the mass ratio of 1: 0.6-2.5: 24-192: 1.7-6.7 and mixing uniformly; putting the mixed solution in a stainless steel reaction kettle and carrying out heat treatment at 120 DEG C for 2-4 days to obtain a nitrogen-containing polymer; and carrying out inert gas shielding in a tube furnace, heating the nitrogen-containing polymer at a heating rate of 0.5-10 DEG C / min to 700-900 DEG C for carbonization and finally naturally cooling to the room temperature to obtain the nitrogen functionalized microporous carbon nanoparticle. The prepared nitrogen functionalized microporous carbon nanoparticle has the advantages of high specific surface area, regular micropores, nitrogen functional group and spherical geometrical morphology; and when used as an electrode of a super capacitor, the nitrogen functionalized microporous carbon nanoparticle is capable of improving the high-current charging / discharging performance of the electrode while effectively keeping the high specific capacitance.
Owner:TONGJI UNIV
Cellulose triacetate aerogel and preparation method
The invention provides a cellulose triacetate aerogel and its preparation method. The cellulose triacetate aerogel is prepared by a method including the following steps: adding cellulose triacetate particles into 1,4-dioxane, heating and stirring to fully dissolve cellulose triacetate so as to form a colorless and transparent solution; adding an anhydrous alcohols solvent into the solution while heating and stirring and fully stirring and dispersing to form a colorless and transparent solution again; injecting the solution into a forming mold, standing, naturally cooling to room temperature to form wet gel and aging for some time; and carrying out supercritical drying by using carbon dioxide to remove the solvent so as to obtain the cellulose triacetate aerogel. Coagulation bath is not required for regeneration by the preparation technology. After formation of gel, supercritical drying can directly be carried out without tedious and time-consuming solvent displacement time. The cellulose triacetate prepared by the above method has advantages of low density, high specific surface area, good toughness and good formability.
Owner:LASER FUSION RES CENT CHINA ACAD OF ENG PHYSICS
Method for synthesizing methyl ketone
InactiveCN104557499AShort reaction timeMeet the requirements of green chemistryPreparation by C-C triple bond hydrationCarbonyl compound preparation by hydrolysisAmyl methyl ketoneEvaporation
The invention discloses a method for synthesizing methyl ketone. The method comprises the following steps: adding alkyne, [(IPr)AuCl], AgOTf, a solvent, namely 1,4-dioxane and water in a reaction container, performing microwave reaction on a reaction mixture for 1h at 120 DEG C and cooling to room temperature; filtering, performing rotary evaporation to remove the solvent, and then separating by a column to obtain a target compound. The method disclosed by the invention adopts a microwave technology, and the reaction time is shortened; furthermore, the reaction only generates a byproduct, namely water, so that the reaction is in line with the requirements of green chemistry and has broad development prospects.
Owner:NANJING UNIV OF SCI & TECH
Synthesis and curing method of carborane benzo oxazine resin
ActiveCN104327105AEasy curingImprove thermal stabilityGroup 3/13 element organic compoundsAdhesivesAir atmosphereStructural formula
The present invention designs a synthesis and curing method of carborane benzo oxazine resin, and a carborane-containing benzo oxazine prepolymer has the structural formula as shown in the specification, wherein Cb is o-carborane, m-carborane or p-carborane; R is alkyl, aryl and alkylaryl. The resin is prepared by using carborane bisphenol, formaldehyde and amine as raw materials and 1, 4-dioxane as a solvent. The prepolymer can be cured by simple heating in air atmosphere. The curing process is very simple and does not require strict pollution control measures. A curing product has good thermal stability and high temperature carbon residue rate, and can be used for the study of high temperature resistant materials.
Owner:BEIJING UNIV OF CHEM TECH
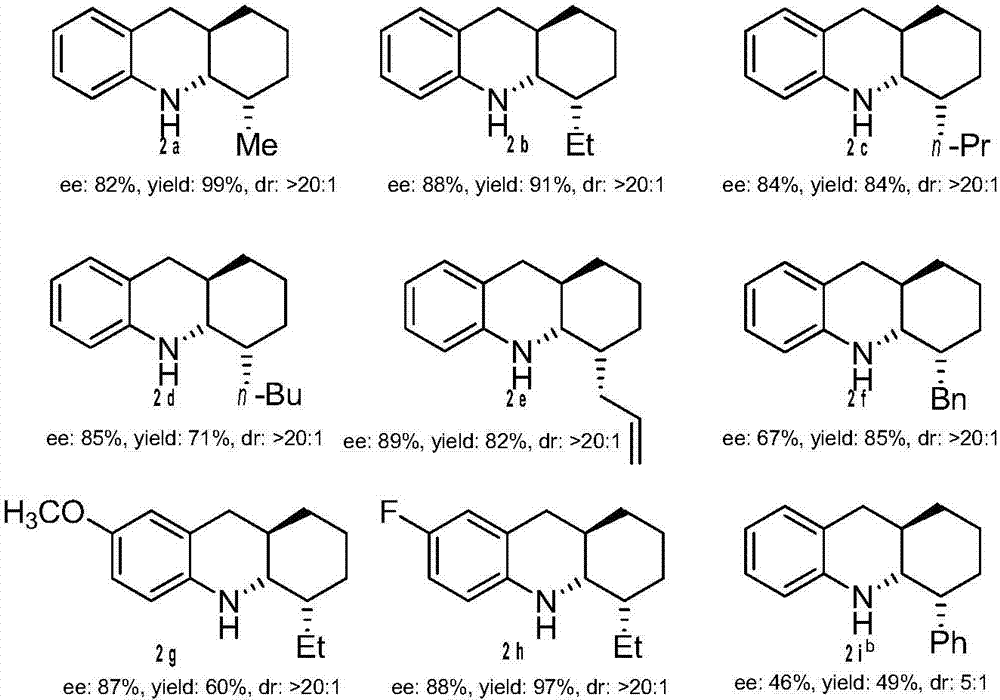
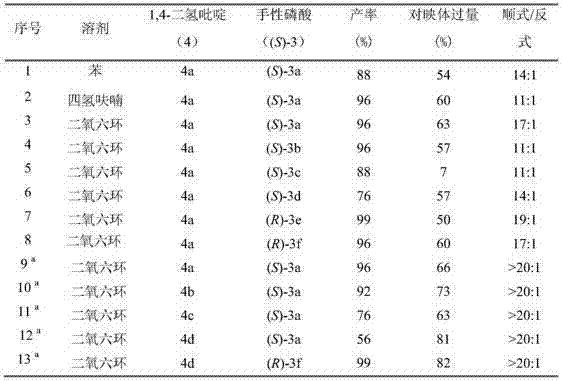
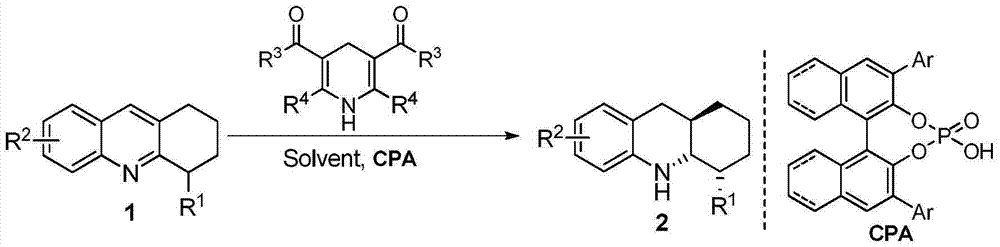
Method for synthesizing tetrahydroquinoline containing three continuous chiral centers through asymmetric transfer hydrogenation
ActiveCN104710359ARaw materials are easy to obtainHigh purityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDihydropyridineHydrogen
The invention relates to a method for synthesizing tetrahydroquinoline containing three continuous chiral centers through asymmetric transfer hydrogenation, wherein chiral phosphoric acid is adopted as a catalyst and substituted 1,4-dihydropyridine is adopted as a hydrogen source in the used catalysis system. The reaction can be performed under the following conditions that: the temperature is 0-70 DEG C, the solvent is 1,4-dioxane, and a ratio of the substrate to the catalyst is 20 / 1. According to the present invention, simple and easily-available 4-substituted-1,2,3,4-tetrahydroacridine is subjected to transfer hydrogenation to obtain the corresponding tetrahydroquinoline compound containing three continuous chiral centers, wherein the diastereoselectivity can be more than 20:1, and the enantiomeric excess can achieve 89%; and the method of the present invention has characteristics of simple and practical operation, high diastereoselectivity / enantioselectivity and high yield, and the reaction has characteristics of green atom economy and environment-protection.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
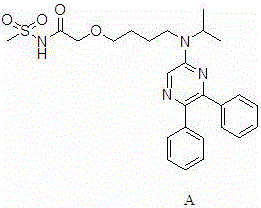
Preparation method of selexipag
ActiveCN106008364AReasonable designHigh reactivityOrganic chemistryPotassium tert-butoxideOrganic solvent
The invention discloses a preparation method of selexipag, and relates to the technical field of preparation of drugs for treating pulmonary hypertension. The preparation method comprises the following steps: adding a compound as shown in the formula I and a compound as shown in the formula II in an organic solvent; adding alkali; stirring at the reaction temperature of 10-40 DEG C and carrying out Williamson reaction; synthesizing a compound as shown in the formula A, namely the selexipag. The organic solvent is 1-4-dioxane; the alkali is sodium tert-butoxide, potassium tert-butoxide or mixed alkalis of sodium tert-butoxide and potassium tert-butoxide. A synthetic route is reasonable in design, reaction conditions are mild, the cost is low, special equipment is not required, a product is high in purity and high in yield, and aftertreatment is simple and convenient, so that the preparation method of the selexipag is suitable for industrial production.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
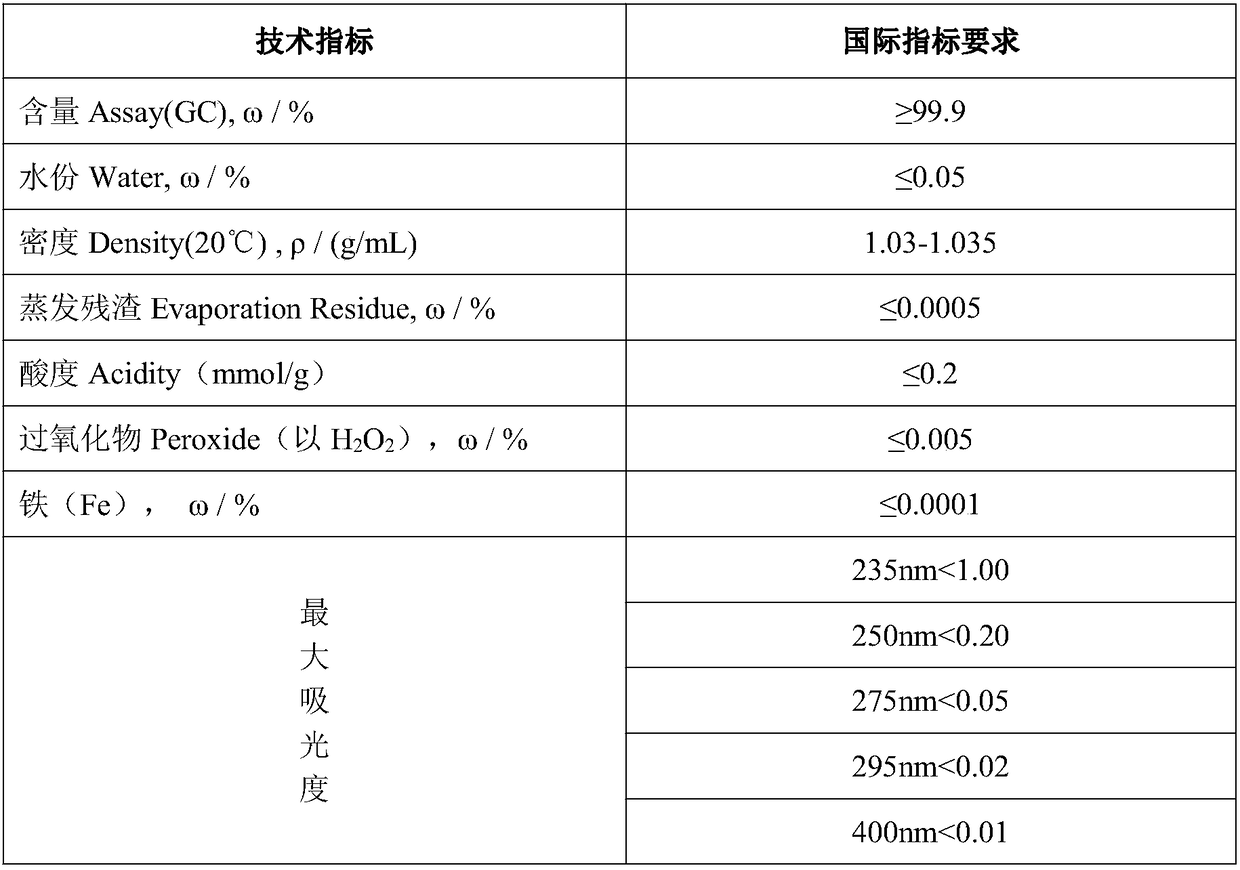
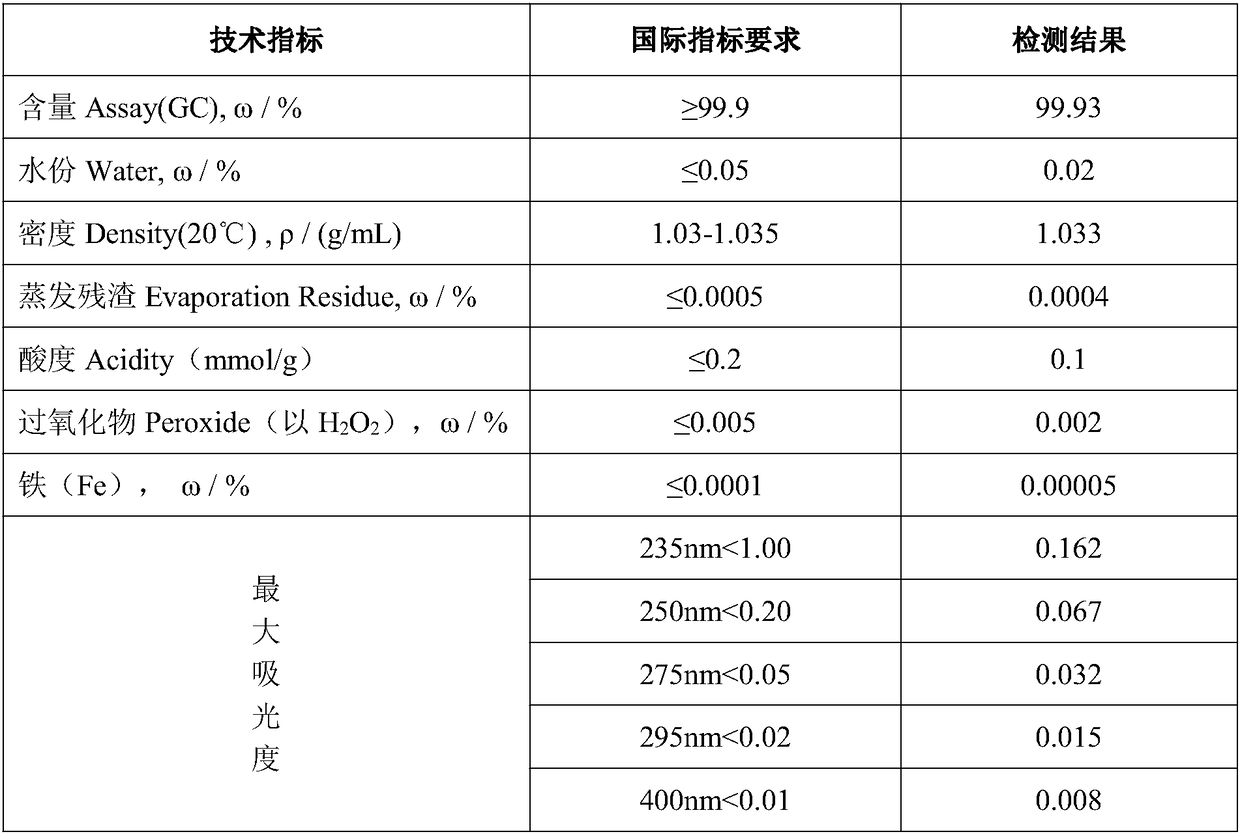
Purification method of chromatographic pure 1,4-dioxane
InactiveCN109438413AReach the index requirement of chromatographically pure 1,4-dioxaneReach the index requirement of pure 1,4-dioxaneOrganic chemistryPurification methodsALUMINUM HYDRIDE
The invention provides a purification method of chromatographic pure 1,4-dioxane. The method comprises the following steps of (1) eliminating 2-methyl-1,3-dioxolame impurities through hydrolysis; (2)performing adsorption: injecting the hydrolyzed 1,4-dioxane raw materials into a serially connected cation exchange resin adsorption column and active carbon adsorption column; adsorbing some aldehydes impurities in the 1,4-dioxane raw materials; (3) removing peroxides through the adsorption by a lithium aluminum hydride adsorption column; (4) performing drying and dewatering by a 4A molecular sieve; (5) performing rectification. According to the method, 1,4-dioxane industrial products with the content being 98.0 percent are used as raw materials; some adsorbable impurities can be eliminated through hydrolysis, the cation exchange resin adsorption column and the active carbon adsorption column; then, through heating treatment, the peroxides in the products are eliminated through the entering into a lithium aluminum hydride filling column. The method has the advantages that the reaction is complete; the safety is high. After the rectification, the final product content reaches 99.9 percent or higher; the moisture is less than or equal to 0.02 percent.
Owner:天津康科德医药化工有限公司
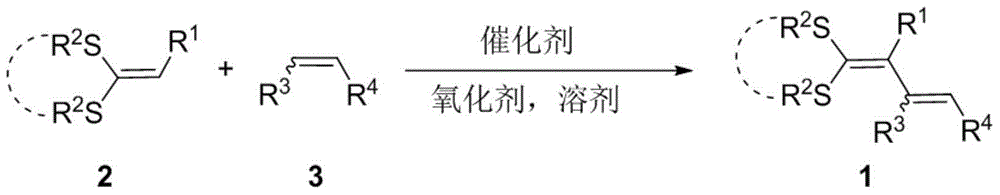
Method for preparing 1,3-diolefins
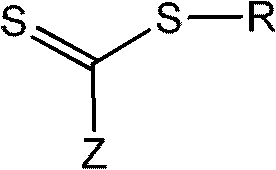
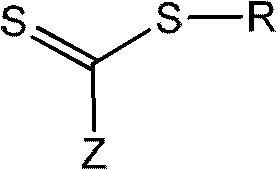
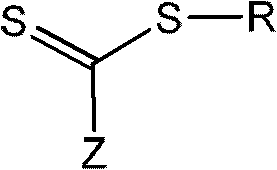
The invention discloses a method for preparing 1,3-diolefins. In a ternary mixed solvent of 1,4-dioxane, acetic acid and dimethyl sulphoxide, an oxidative cross-coupling reaction is carried out for ketene dithioacetals with alpha-cyano or ester and olefin which are catalyzed by divalent palladium salt in a condition with oxygen or air which is used as an oxidizing agent, in order to generate 1,3-diolefins. Compared with the reported synthetic method of 1,3-diolefins, the method has the advantages of easily available raw materials, simple operation, mild synthetic reaction condition, and high atom economy.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
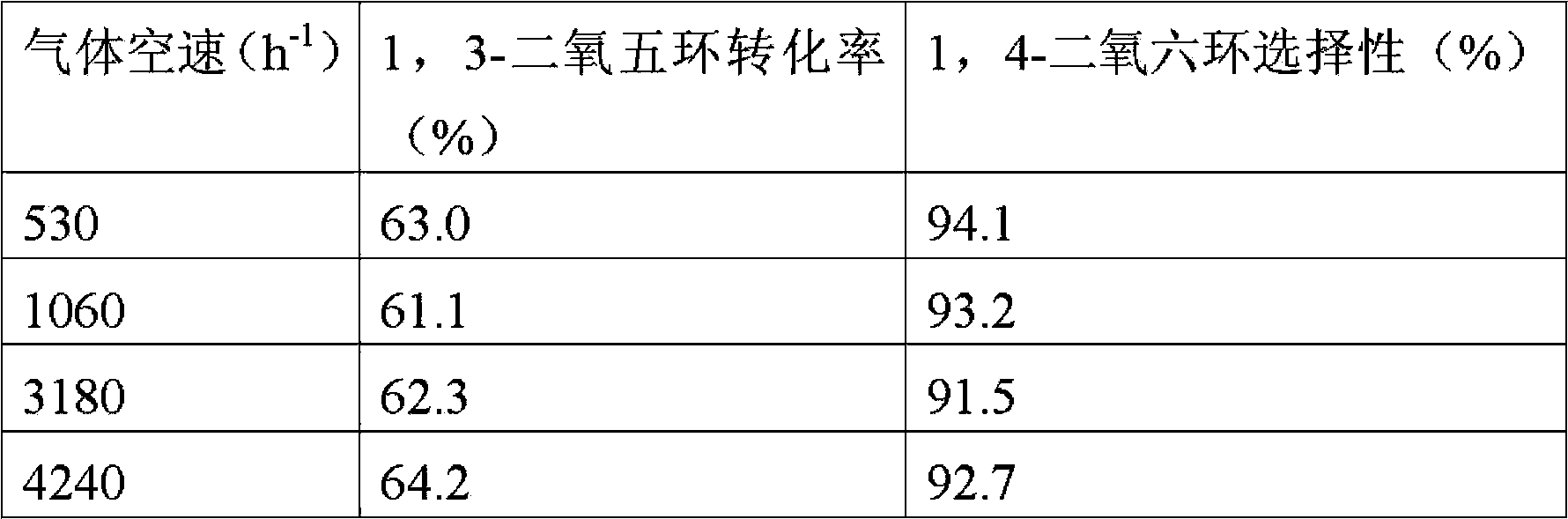
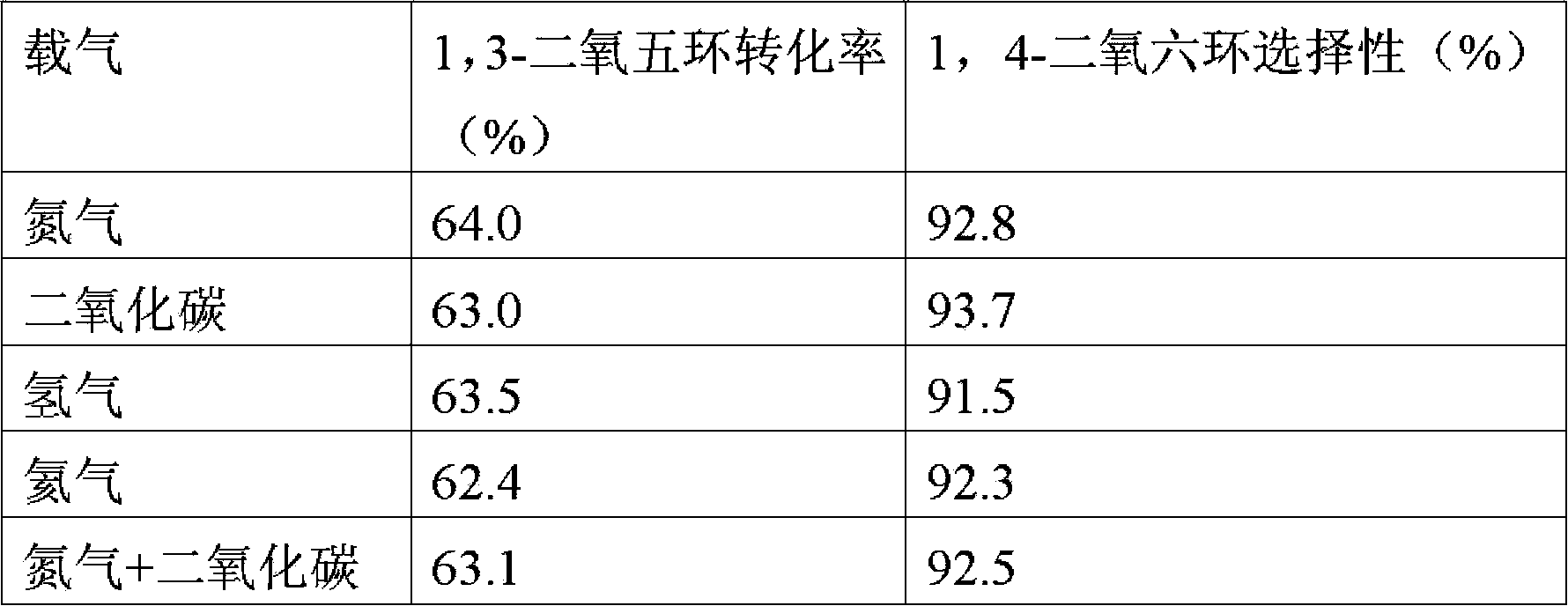
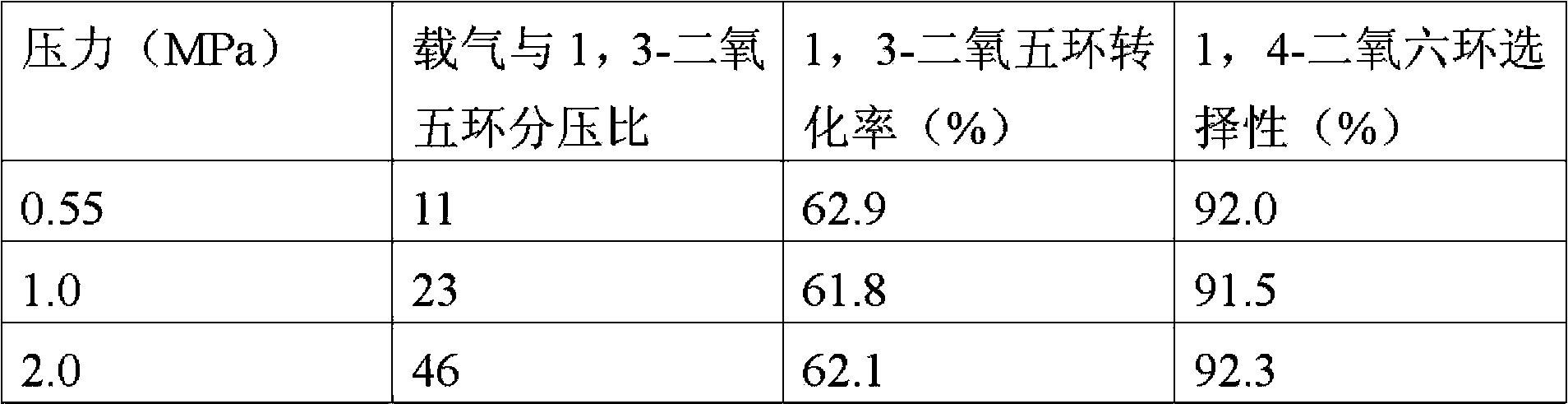
Method used for preparing 1,4-dioxane from 1,3-dioxolane
The invention provides a method used for preparing 1,4-dioxane from 1,3-dioxolane. According to the method, raw material gas containing 1,3-dioxolane and carrier gas is delivered into a reactor filled with a solid acid catalyst, and reaction is carried out at a reaction temperature of 120 to 160 DEG C, under a reaction pressure of 0.1 to 2.0MPa, and with gaseous hourly space velocity of 500 to 4500 / h so as to obtain 1,4-dioxane, wherein partial pressure ratio of the carrier gas to 1,3-dioxolane in the raw material gas ranges from 1.0 to 50. The method is capable of realizing production of 1,4-dioxane under gas-solid reaction conditions; reaction conditions are mild; and separation of products from the catalyst is simple.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Anti-tumor 2-amino nicotinonitrile, application and preparation method thereof
InactiveCN102875462ARapid responseSpeed upOrganic active ingredientsOrganic chemistryBrain cancersAcetophenone
The invention provides a novel anti-tumor drug which is represented by the molecular formula (I) and specifically relates to anti-tumor 2-amino nicotinonitrile. The invention further provides a synthetic method of the anti-tumor drug. The method includes dissolving 2mmol of vanillic aldehyde or 2mmol of syringaldehyde, 2mmol of acetophenone and 2mmol of malononitrile in 1, 4-dioxane, then adding 4mmol of ammonium acetate, heating a mixture at the temperature of 120 DEG C for reaction for 20 minutes by using a microwave with the length of 300W as an assistant, adding ice water to the reaction mixture to separate out a product, and finally subjecting the mixture to recrystallization to obtain the 2-amino nicotinonitrile. The synthetic method of the anti-tumor drug is simple, and the anti-tumor drug can be rapidly and efficiently synthesized by means of a one-pot four-component reaction assisted by the microwave. The anti-tumor drug which is represented by the molecular formula (I) is capable of obviously inhibiting activities of proliferation of liver cancer cells, gastric cancer cells, breast cancer cells, pancreatic cancer cells, brain cancer cells, lung cancer cells and ovarian cancer cells.
Owner:CHINA PHARM UNIV +1
Method for preparing 1, 4-dioxane
InactiveCN105985312AEasy to separatePrevent the occurrenceOrganic chemistryWastewaterDimethoxyethane
The invention provides a method for preparing anhydrous high-purity 1, 4-dioxane from glycol dimethyl ether through one step. Through the method, high-purity 1, 4-dioxane is prepared from the glycol dimethyl ether raw material at a low temperature at a high conversion rate. In the whole reaction process, water does not react and product water is not produced so that 1, 4-dioxane-containing industrial wastewater is prevented thoroughly. The glycol dimethyl ether raw material has a high conversion ratio at a low temperature, catalyst stability is good, product 1, 4-dioxane selectivity is high and the whole process is simple and environmentally friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Acrylamide copolymer oil-dispelling agent with sulfitobetaine structure and synthesis method
InactiveCN104403052APromote hydrationStrong hydration abilityDrilling compositionSynthesis methodsSulfite
The invention discloses an acrylamide copolymer with a sulfitobetaine structure and a synthesis method of a monomer with a sulfitobetaine structure. The acrylamide copolymer is composed of the following monomers in parts by weight: 55 to 75 parts of acrylamide, 24 to 38 parts of acrylic acid, and 1 to 7 parts of 2-(3-methylacrylamidepropyldimethylamino)ethyl sulfurous acid inner salts, and is synthesized in the presence of an initiator namely 0.1 to 0.5 part of NaSO3-(NH4)2S2O8 in a water solution with a pH value of 5 to 9 at a temperature of 30 to 40 DEG C for 6 to 10 hours. The monomer with a sulfitobetaine structure is prepared by carrying out reflux reactions between 1 part of N-(3-dimethylaminopropyl)methacrylamide and 1 to 1.2 parts of ethylene sulfite in 1,4-dioxane for 2 to 5 hours.
Owner:SOUTHWEST PETROLEUM UNIV
Method for synthesizing alpha-alkyl ketone
InactiveCN104557500AMeet the requirements of green chemistryPreparation by C-C triple bond hydrationIridiumEvaporation
The invention discloses a method for synthesizing alpha-alkyl ketone. The method comprises the following steps: adding alkyne, [(IPr)AuCl], AgOTf, 1,4-dioxane and water in a reaction container, performing microwave reaction on a reaction mixture for 1h at 120 DEG C and cooling to room temperature; further adding [Cp*IrCl2]2, alkali and alcohol into the reaction mixture, performing microwave reaction on the reaction mixture for 2h at 130 DEG C and cooling to room temperature; filtering, performing rotary evaporation to remove a solvent, and then separating by a column to obtain a target compound. The method disclosed by the invention is started from chemical raw materials which are easy to obtain, namely alkyne, water and alcohol, alpha-alkyl ketone is obtained under the participation of gold and iridium catalysts, and the reaction only generates water as a byproduct. Therefore, the reaction is in line with the requirements of green chemistry and has broad development prospects.
Owner:NANJING UNIV OF SCI & TECH
Method for synthesizing 1,4-dioxane
ActiveCN101948461ALow price of inorganic alkaliLow costOrganic chemistryAlkaline earth metalReaction temperature
The invention discloses a method for synthesizing 1,4-dioxane by reacting 2-chlorine-ethoxyethanol with a caustic alkali such as the low-concentration solution of the hydroxide of an alkali metal or an alkaline-earth metal. The molecular ratio of the 2-chlorine-ethoxyethanol to the hydroxide of the alkali metal or the alkaline-earth metal is 1.10-1.40:1; and the mass fraction of hydroxide aqueoussolution is 5 to 25 percent. The method comprises the following steps of: adding the 2-chlorine-ethoxyethanol into a stainless steel stirred reactor; controlling the reaction temperature to be between 85 and 190 DEG C and the reaction time to be 1 to 4 hours; rectifying obtained reaction solution; collecting azeotropic distillate of the 1,4-dioxane and water; adding an entrainer; separating water; and obtaining a 1,4-dioxane product of which the purity is over 98 percent. The method solves the problems of storage and transportation difficulties of ethylene oxide, corrosion caused by strong acidic catalyst, more byproducts and the like in a 1,4-dioxane process which takes an ethylene oxide gas as a raw material, so that the production of the 1,4-dioxane gets rid of the dependence on ethylene oxide and the like; and production cost is reduced.
Owner:锦西化工研究院有限公司
Preparation method of zingiber corallinum hance essential oil/hydroxypropyl-beta-cyclodextrin clathrate and preparation method of zingiber corallinum hance essential oil/hydroxypropyl-beta-cyclodextrin clathrate injection
InactiveCN102847102APrevent volatilizationPrevent oxidative deteriorationAntibacterial agentsPharmaceutical delivery mechanismWater bathsRoom temperature
The invention relates to a preparation method of a zingiber corallinum hance essential oil / hydroxypropyl-beta-cyclodextrin clathrate and a preparation method of a zingiber corallinum hance essential oil / hydroxypropyl-beta-cyclodextrin clathrate injection. The preparation method of the zingiber corallinum hance essential oil / hydroxypropyl-beta-cyclodextrin clathrate comprises the following steps of weighing hydroxypropyl-beta-cyclodextrin of which the weight is 4-7 times the weight of zingiber corallinum hance essential oil, dissolving zingiber corallinum hance essential oil into absolute methanol or absolute DMF or absolute 1,4-dioxane to obtain a zingiber corallinum hance essential oil solution, mixing the zingiber corallinum hance essential oil solution and the hydroxypropyl-beta-cyclodextrin solution by stirring, carrying out stirring clathration in a water-bath having a temperature of 40 to 80 DEG C for 2 to 20 hours, cooling to a room temperature, and filtering by a filter membrane to obtain a zingiber corallinum hance essential oil / hydroxypropyl-beta-cyclodextrin clathrate aqueous solution. The preparation method of the zingiber corallinum hance essential oil / hydroxypropyl-beta-cyclodextrin clathrate injection comprises the following step of diluting the zingiber corallinum hance essential oil / hydroxypropyl-beta-cyclodextrin clathrate aqueous solution by injection water into the zingiber corallinum hance essential oil / hydroxypropyl-beta-cyclodextrin clathrate injection having a required concentration. The zingiber corallinum hance essential oil / hydroxypropyl-beta-cyclodextrin clathrate and the zingiber corallinum hance essential oil / hydroxypropyl-beta-cyclodextrin clathrate injection avoid volatilization and oxidation deterioration of zingiber corallinum hance essential oil, and can stabilize zingiber corallinum hance essential oil content.
Owner:重庆灵方生物技术有限公司
Recrystallization method of rabeprazole sodium
ActiveCN105085481AHigh purityLow impurity contentOrganic chemistry methodsCyclohexanoneOrganic solvent
The invention belongs to the field of pharmaceutical chemistry and specifically relates to a recrystallization method of rabeprazole sodium. A mixed solvent of a ketone organic solvent and a cyclic ether organic solvent is used for crystallization, wherein the ketone organic solvent is preferably selected from acetone, methyl ethyl ketone and cyclohexanone; and the cyclic ether organic solvent is preferably selected from tetrahydrofuran and 1,4-dioxane. The rabeprazole sodium prepared according to the method has high purity, contains less impurities and accords with crude drug standards.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
New method of synthesizing 2,4-di-substituted benzothiazole
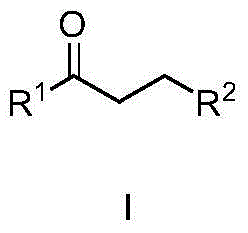
A new method of synthesizing 2,4-di-substituted benzothiazole is disclosed, wherein R and R' both can be aryl groups, alkyl groups and heterocyclic ring groups. The method includes the steps of mixing a substituted thioamide substrate and a fluorine-containing alkali with a reaction solvent 1,4-dioxane, adding a known benzyne precursor slowly into the reaction solution to prepare 2,4-di-substituted benzothiazole, and performing column chromatographic purification to obtain a finish product of which the yield is 75%. The new method is low in raw material cost and the raw material is easy to obtain. The new method is simple in operation, is less in synthetic steps, is high in yield and has important application value in the fields of pharmaceutical chemistry and organic synthesis.
Owner:CHONGQING UNIV
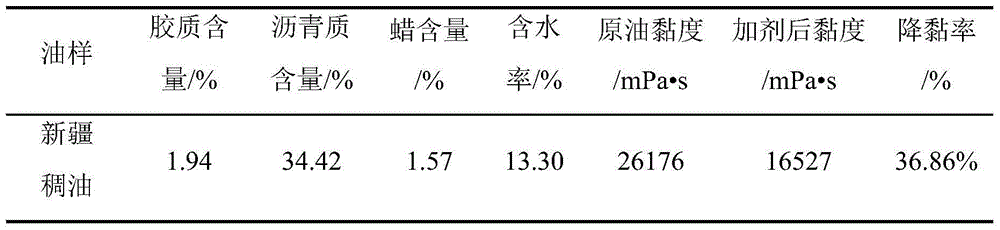
Low-molecular hyperbranched oil-soluble thickened oil viscosity reducer and preparation method thereof
ActiveCN104629704AMild reaction conditionsEasy to controlPipeline systemsDrilling compositionSolubilityReaction temperature
The invention relates to a low-molecular hyperbranched oil-soluble thickened oil viscosity reducer for thickened oil recovery and gathering and transportation and viscosity reduction and a preparation method of the viscosity reducer. The defect that the conventional viscosity reducer is poor in viscosity reduction effect and high in cost is overcome. According to the technical scheme, the viscosity reducer comprises the following raw materials in percentage by mole: 10.0-15.8 percent of ethidene diamine, 42.0-50.0 percent of formaldehyde, 10.0-11.1 percent of acetophenone and 30.0-33.3 percent of butyl acrylate, wherein the amount of a solvent 1,4-dioxane accounts for 25-50 percent of the total mass of the previous four monomers. The preparation method comprises the following steps: adding the ethidene diamine, acetophenone, butyl acrylate and the solvent 1,4-dioxane into a flask; additionally adding the formaldehyde and residual solvent into a constant pressure titration funnel, introducing nitrogen protection into the flask, stirring, reacting at the temperature of 60-80 DEG C, and dripping the formaldehyde solution in the funnel, wherein the reaction time is 6-9 hours; and finally, performing rotary evaporation on the reaction solution, extracting, and drying, thereby obtaining the viscosity reducer. The viscosity reducer is good in viscosity reduction effect and high in oil solubility and can be mixed with a solvent.
Owner:SOUTHWEST PETROLEUM UNIV
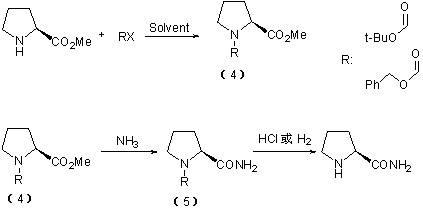
Method for preparing L-prolinamide and intermediate thereof
ActiveCN102432616AShort reaction cycleComplete conversion of the reactionOrganic chemistryCost of treatmentImpurity
The invention relates to a method for preparing chiral drug intermediate L-prolinamide and an intermediate thereof. The method comprises the following steps of: firstly, reacting L-proline as an initial raw material with triphosgene, diphosgene or phosgene in solvents of anhydrous tetrahydrofuran, 1,4-dioxane, dichlormethane, chloroform, acetonitrile and the like to synthesize L-proline carbamyl chloride of an intermediate state; secondly, further condensing in the presence of triethylamine to generate L-proline-N-carboxyl-anhydride; and finally, performing ammonolysis, and thus obtaining the L-prolinamide. By adopting the method, the yield of the L-prolinamide is improved, the generation quantity of impurities is reduced, the whole reaction time is shortened, cost of treatment on discharge of three wastes and sewage is reduced, the production cost of the L-prolinamide is comprehensively reduced, and industrialized production is easier to realize.
Owner:HEBEI BOLUNTE PHARMA
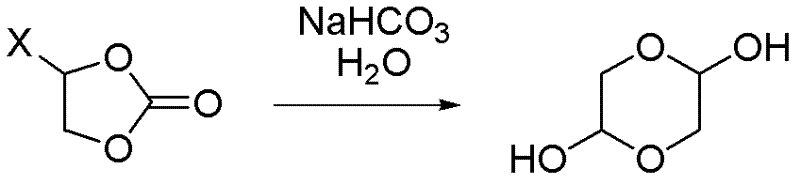
Synthetic method of 1,4-dioxane-2,5-diol
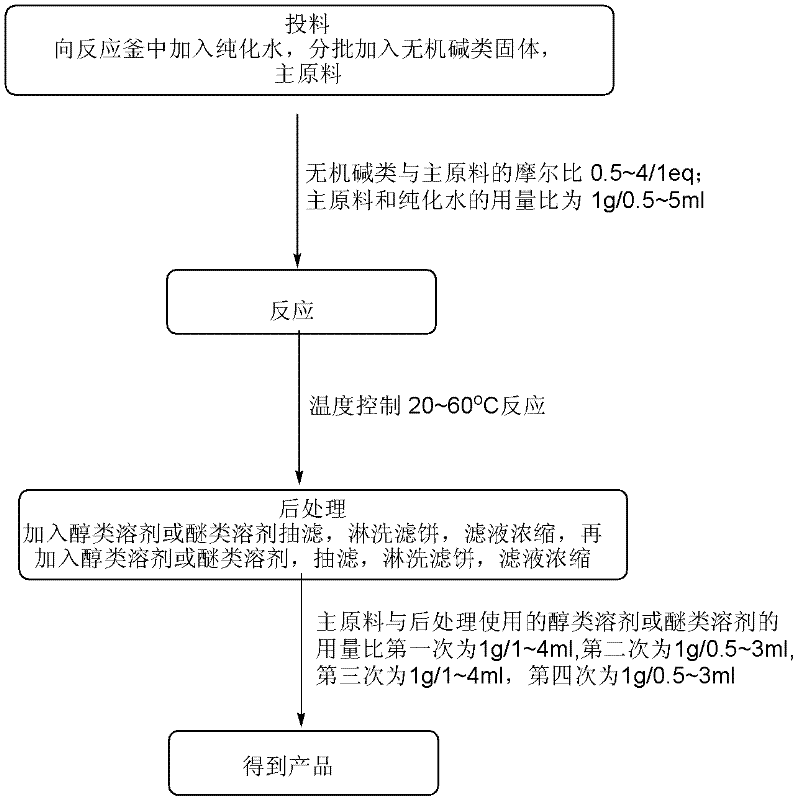
ActiveCN102351838ALow priceMeet the needs of large-scale productionOrganic chemistryChemical reactionCompound (substance)
A synthetic method of 1,4-dioxane-2,5-diol is characterized in that concrete preparation comprises the following steps of: (1) feeding; (2) reacting; (3) post-processing; (4) preparation of the product. The invention has following advantages: 1, raw materials adopted in the invention are easily available and are cheap in price; the raw materials are all commercial raw materials or raw materials which are easy to prepare, and can satisfy the requirement of large scale production; 2, by the adoption of a simple hydrolysis reaction and simultaneously a batch charging method, the solvent amount is less, the energy and post-processing time are saved, the operation is simple, the synthetic method is suitable for large scale industrial production; 3, the condition of chemical reactions adopted in the invention is mild; there is no high temperature or low temperature reaction during the whole reaction process; during the production process, the technology is reasonable, the production is safe and reliable, and the cost is low; there is no environmental pollution; the synthetic method is suitable for large scale industrial production.
Owner:ASYMCHEM LAB TIANJIN +1
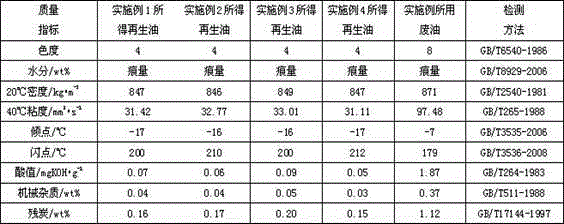
Method for regenerating waste oil by extracting novel combination solvent
InactiveCN104130853ACoordination selectivityCoordinated Extraction CapabilitiesTreatment with plural serial refining stagesLubricant compositionBenzeneOrganic solvent
The invention provides a method for regenerating waste oil by extracting a novel combination solvent, which comprises the following steps: 1)taking waste oil and placing in a container, performing settlement in a thermotank, drying, taking a supernatant oil; 2)adding an organic solvent in the supernatant oil after filtering in the step 1), wherein the organic solvent is composed of a solvent a and a solvent b, a is one, two or three of benzene, toluene and xylene; b is one or two of tetrahydrofuran and 1,4-dioxane; and 3)extracting and refining to obtain an oil-containing mixture, sending to the thermotank for settlement, and taking the supernatant oil for distillation to obtain the reclaimed oil. In the invention, while extracting and refining, the solvent has good extracting effect to the waste oil. The experiment result displays that the regeneration method of the waste oil has the advantages of high yield and good reclaimed oil quality. In addition, the regeneration method comprises the characteristics of simple operation, mild technology condition, cycle use of the solvent, low cost and high economic benefit.
Owner:CHONGQING TECH & BUSINESS UNIV
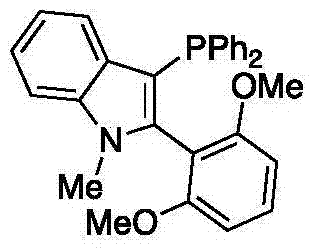
Preparation method of high-sterically-hindered arylborate compound
InactiveCN104327106AAir stabilizationEasy to synthesizeGroup 3/13 element organic compoundsCaesium acetateAldehyde
The invention discloses a preparation method of a high-sterically-hindered arylborate compound. The preparation method includes following steps: in the presence of a catalyst of a catalyst tri(dibenzalacetone)dipalladium with a phosphine ligand (wherein the phosphine ligand is 3-diphenylphosphine-2-(2,6-dimethoxylphenyl)-N-methylindole), adding an aryl chloride, bis(neopentyl glycolato)diboron, and an additive ceseium acetate to a 1,4-dioxane solution; and carrying out a reaction at 100-130 DEG C for 12-48 hours to obtain the arylborate compound. In the invention, the employed substrate is stable, is low in cost and is easy to obtain and the catalyst is unique, is easy to prepare and is suitable for the reaction of the high-sterically-hindered aryl chloride. The system is compatible of existences of functional groups, such as an ester group, an aldehyde group, methoxyl and the like so that range of the substrate is greatly developed. The catalyst system is stable, is high in catalytic activity, is wide in suitable scope, is good in selectivity and is mild in reaction conditions. The high-sterically-hindered arylborate compound can be widely applied in cross coupling reaction catalyzed by transition metal, thereby preparing various compounds, such as biaromatic hydrocarbons. The preparation method has a great application potential in synthesis of natural medicines and drug intermediates.
Owner:THE HONG KONG POLYTECHNIC UNIV SHENZHEN RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com