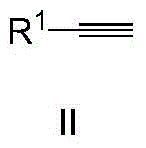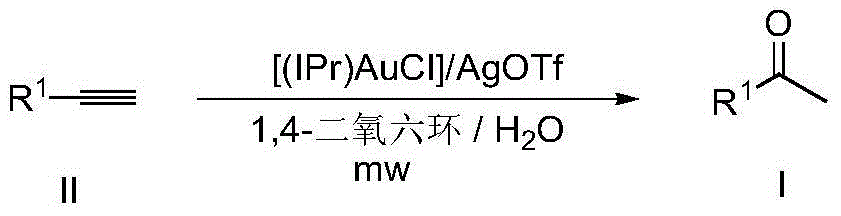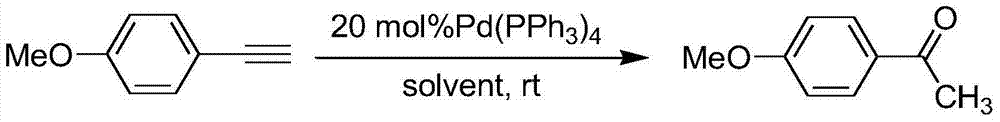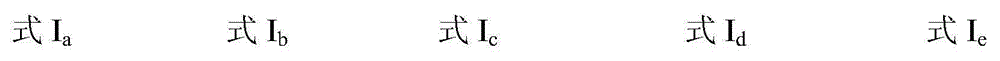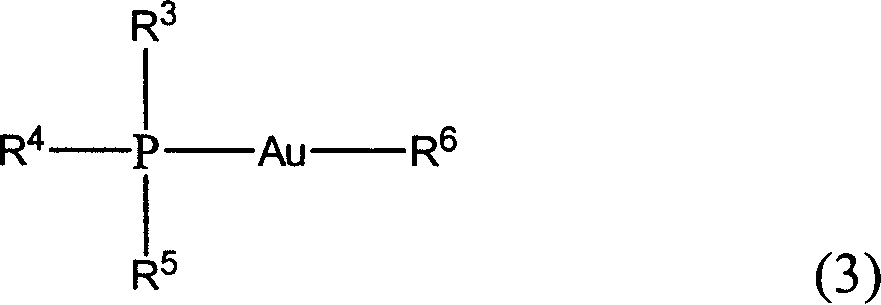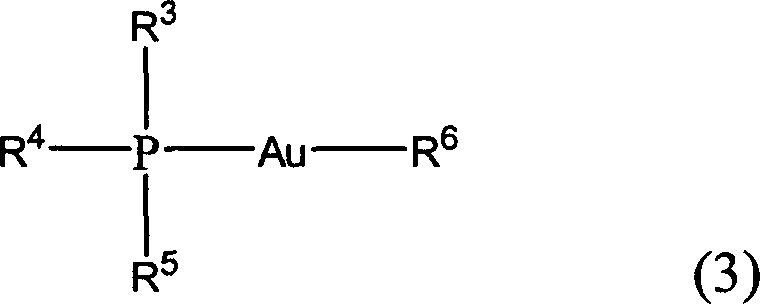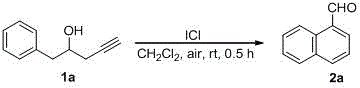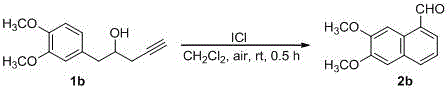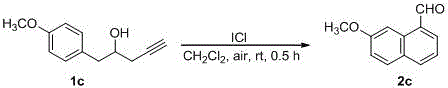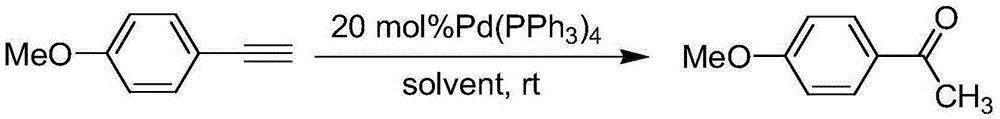Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
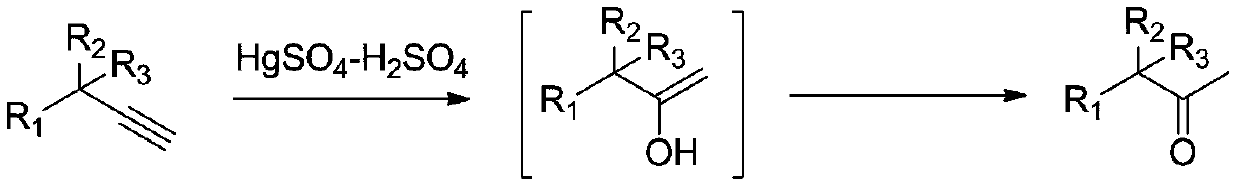
57results about "Preparation by C-C triple bond hydration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydrothermal hydrocatalytic treatment of biomass
A method of hydrothermal hydrocatalytic treating biomass is provided. Lignocellulosic biomass is treated with a digestive solvent to form a pretreated biomass containing soluble carbohydrates. The pretreated biomass is contacted, with hydrogen at a temperature in the range of 150° C. to less than 300° C. in the presence of a pH buffering agent and a supported hydrogenolysis catalyst containing (a) sulfur, (b) Mo or W, and (c) Co, Ni or mixture thereof, incorporated into a suitable support, to form a plurality of oxygenated hydrocarbons.
Owner:SHELL OIL CO
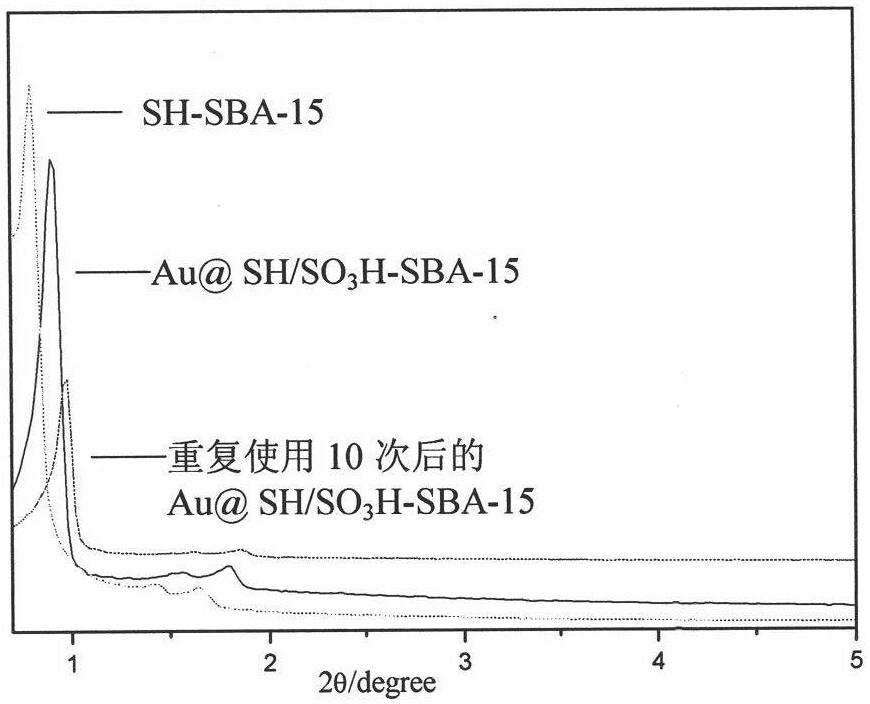
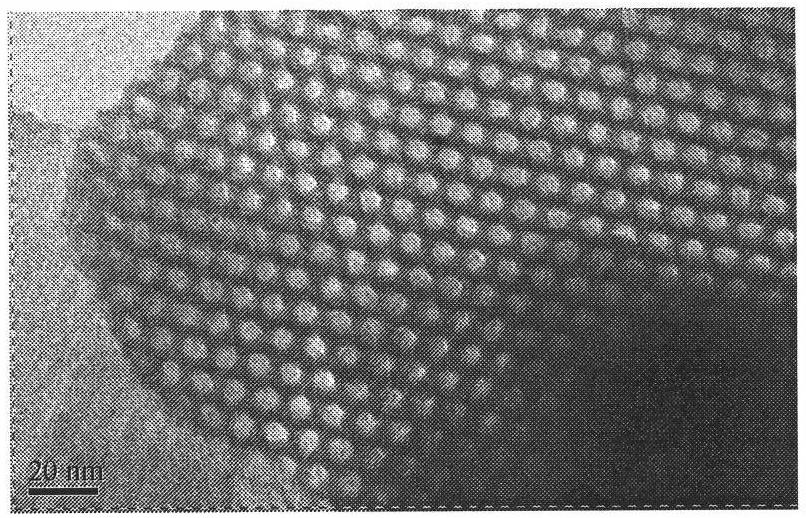
Sulfydryl functionalized ordered mesoporous silicon-immobilized Au heterogeneous catalyst and preparation method and application thereof
InactiveCN101785998AHigh catalytic activityRegular and ordered two-dimensional hexagonal mesoporous structurePreparation by C-C triple bond hydrationMetal/metal-oxides/metal-hydroxide catalystsSilanesStrong acids
The invention relates to sulfydryl functionalized ordered mesoporous silicon-immobilize Au heterogeneous catalyst and a preparation method and application thereof. The preparation method is as follows: mixing surfactant, strong acid, water, ethyl orthosilicate and sulfhydryl silane under heating condition, continuously stirring the reactant mixture, filtering the reactant mixture to get white solid, and sequentially washing and drying the white solid to get a sulfydryl functionalized ordered mesoporous silicon carrier; mixing the carrier with ethanol and HAuCl4 solution, stirring for 24h under room temperature, pump filtering and washing the mixture, and vacuum drying overnight. In this way, the ordered mesoporous sulfydryl functionalized SBA-15-immobilize Au heterogeneous catalyst can be formed. The preparation process is simple and the heterogeneous catalyst prepared through the method has efficient and long-lasting catalytic activity, can improve the reaction speed, reduce the production cost and improve the quality of the product, and can be recycled for use, thereby reducing environment pollution.
Owner:SHANGHAI NORMAL UNIVERSITY
Process for producing carbonyl compound
InactiveUS20050143597A1Carboxylic acid nitrile preparationOrganic compound preparationHydration reactionOrganic solvent
The present invention relates to a process which comprises efficiently proceeding a hydration reaction of an alkyne in aspects of turnover numbers of a catalyst, yield and speed to thereby industrially and advantageously produce the corresponding carbonyl compound. The present invention provides a process for producing a carbonyl compound, which comprises reacting an alkyne compound with water in the presence of a gold catalyst which is an organogold complex compound and acid in an organic solvent.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Method for synthesizing methyl ketone
InactiveCN104557499AShort reaction timeMeet the requirements of green chemistryPreparation by C-C triple bond hydrationCarbonyl compound preparation by hydrolysisAmyl methyl ketoneEvaporation
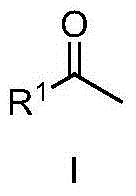
The invention discloses a method for synthesizing methyl ketone. The method comprises the following steps: adding alkyne, [(IPr)AuCl], AgOTf, a solvent, namely 1,4-dioxane and water in a reaction container, performing microwave reaction on a reaction mixture for 1h at 120 DEG C and cooling to room temperature; filtering, performing rotary evaporation to remove the solvent, and then separating by a column to obtain a target compound. The method disclosed by the invention adopts a microwave technology, and the reaction time is shortened; furthermore, the reaction only generates a byproduct, namely water, so that the reaction is in line with the requirements of green chemistry and has broad development prospects.
Owner:NANJING UNIV OF SCI & TECH
Zinc catalyst for catalyzing acetylene hydration reaction and preparation method of zinc catalyst
PendingCN108993576AEvenly distributedSmall particlesMolecular sieve catalystsPreparation by C-C triple bond hydrationMolecular sieveHydration reaction
The invention relates to a zinc catalyst for catalyzing an acetylene hydration reaction and a preparation method of the zinc catalyst. The method for preparing the zinc catalyst for catalyzing an acetylene hydration reaction comprises the following steps of (1) preparing a modified carrier: adding a modifying agent to a molecular sieve precursor liquid, performing uniform mixing, performing filtering, performing drying, performing calcining and performing roasting to obtain the modified carrier; (2) preparing the catalyst precursor salt liquid: enabling zinc salt to completely dissolve in water to obtain the catalyst precursor salt liquid; and (3) preparing the catalyst: dropwise adding the catalyst precursor salt liquid to the modified carrier, performing immersing for 12h, and performingdrying to obtain the zinc catalyst. According to the zinc catalyst for catalyzing an acetylene hydration reaction and the preparation method of the zinc catalyst, the preparation cycle is short, andthe operation is simple; and the prepared zinc catalyst is a non-mercury catalyst for the acetylene hydration reaction, so that mercury pollution to environment can be avoided. The prepared zinc catalyst is high in activity and high in selectivity in the acetylene hydration reaction.
Owner:SHIHEZI UNIVERSITY
Catalyzing system for preparing acetaldehyde by acetylene liquid-phase hydratation and application thereof
InactiveCN108311174AAvoid dependenceReduce harmful effectsOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by C-C triple bond hydrationSolventCuprous chloride
The invention provides a catalyzing system for preparing acetaldehyde by acetylene liquid-phase hydratation and application thereof, and belongs to the technical field of chemistry. The catalyzing system comprises cuprous chloride, inorganic acid, sulfo organic acid, a solvent, an inorganic chloride or organic nitrogen containing hydrochloride, wherein the ratio of cuprous chloride to sulfo organic acid to inorganic acid to solvent is (2-10mol): (0.1-1mol): (0-0.6mol): 1L; the ratio of the inorganic chloride or organic nitrogen containing hydrochloride to solvent is (2-10mol): 1L. According tothe catalyzing system and the application thereof, the catalyzing system is capable of effectively catalyzing hydratation; and moreover, a mercury catalyst in existing liquid-phase acetylene hydratation can be completely replaced by a catalyzing system utilizing cuprous chloride as an active component, so that the independence of acetylene liquid-phase hydratation on the mercury catalyst is solved; and the harmful influence of the mercury catalyst on environment and human body can be reduced.
Owner:SHIHEZI UNIVERSITY
Preparation method of p-methoxyacetophenone
InactiveCN103772177ALow priceReduce generationPreparation by C-C triple bond hydrationSide productSolvent
The invention provides a preparation method of p-methoxyacetophenone. The preparation method comprises the steps of sequentially adding p-methoxyphenyl acetylene, tetrakis (triphenyl phosphine)palladium, hydrochloric acid and a solvent into a reactor, wherein the mole rate of methoxyphenyl acetylene to hydrochloric acid is 1:1.2; reacting at room temperature for 48h; evaporating the solvent; purifying through filtration column chromatography to obtain a light yellow solid product, namely pure p-methoxyacetophenone, wherein ethyl acetate is used as an eluting agent for column chromatography purification, and the volume ratio of ethyl acetate to petroleum ether is 1:5. Methoxyphenyl acetylene is used as a raw material, so that the raw material is low in price; an experiment can be carried out at room temperature, the operation process is simple, and few side products are generated in the experiment and are easy to separate; the preparation method is novel in design, is a good design scheme and has a certain market promotion prospect.
Owner:ZHENGZHOU UNIV
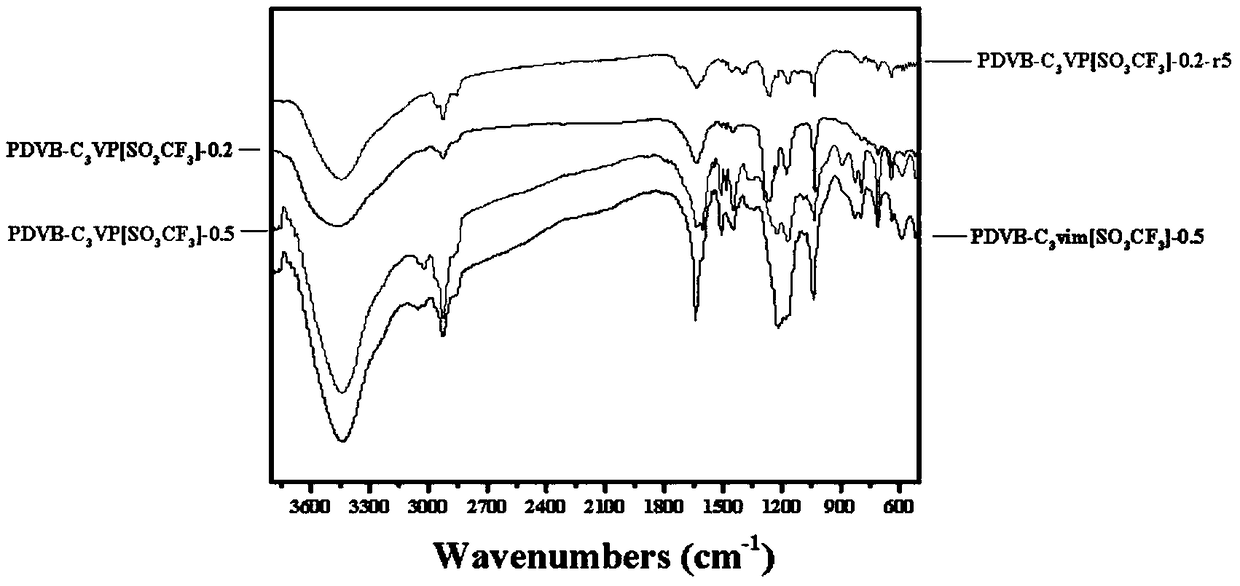
Porous polymer solid acid catalyst for alkyne hydration reaction
InactiveCN108620124AIncreased BET surface areaHigh acid strengthOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by C-C triple bond hydrationHydration reactionSolid acid
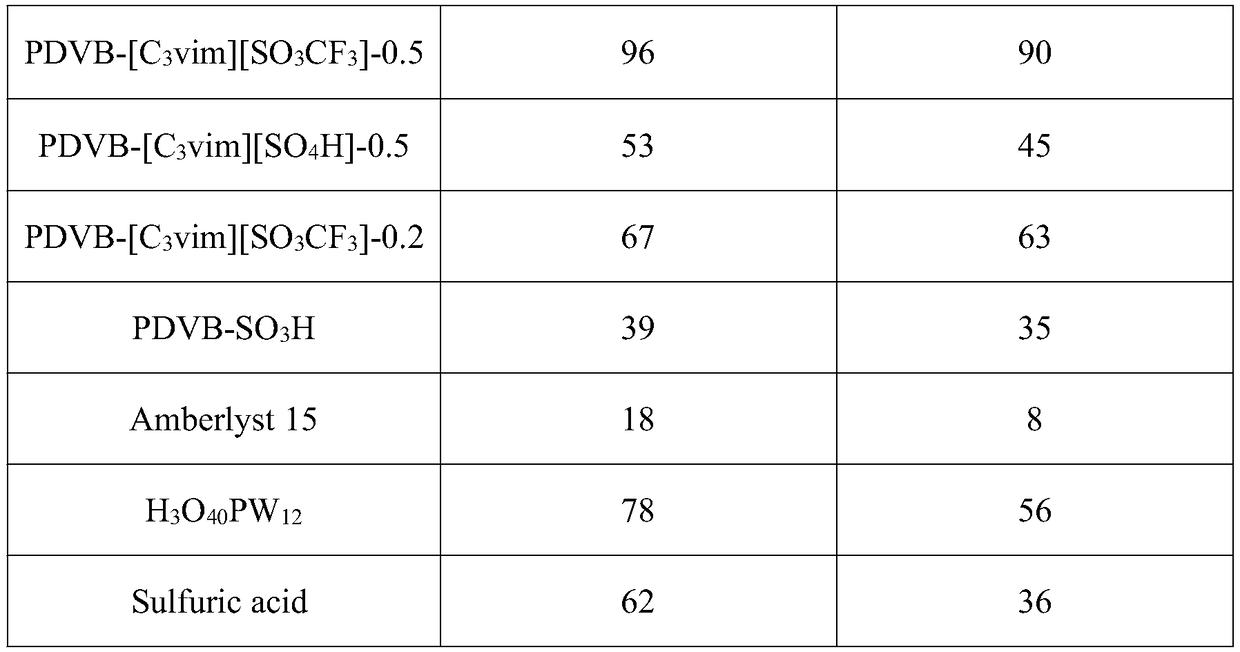
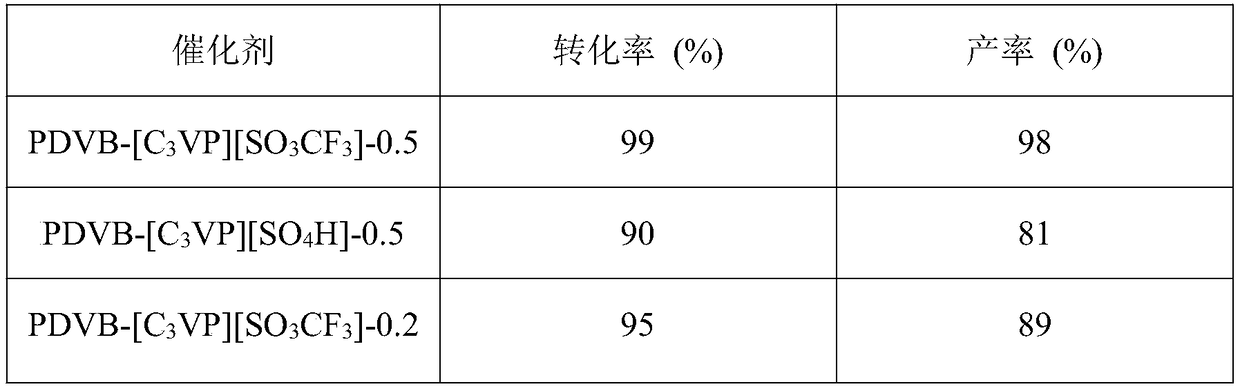
The invention belongs to the technical field of alkyne hydration catalysts and particularly relates to a porous polymer solid acid catalyst for alkyne hydration reaction. Solid acid takes lipid as a solvent, thermal copolymerization of the solvent is performed on sodium p-styrenesulfonate through divinyl benzene (DVB) and 1-vinyl imidazole (vim) or 4-vinylpyridine (VP), the solid acid is reacted with 1,3-propane sultone to form quaternary ammonium salt, and the porous polymer solid acid catalyst is finally formed through ion exchange with acid. The porous polymer solid acid has a large BET surface area, layered nanopores and enhanced acid strength, and controllable structure, easiness in operation, large-scale production and wide application prospect are achieved.
Owner:SHAOXING UNIVERSITY
Method for producing acetophenone compound employing arylethynylene hydration reaction
InactiveCN104496770AHigh yieldMild reaction conditionsPreparation by C-C triple bond hydrationSolventChemistry
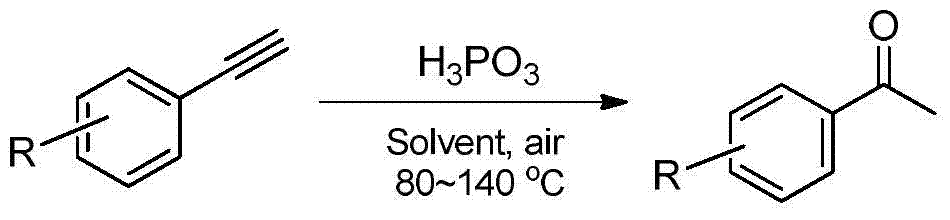
The invention provides a synthesis method of an acetophenone compound. The method comprises the following steps: by adopting mild, efficient, cheap and available phosphorous acid as a catalyst, and adopting water or organic solvent / water as a solvent by a reaction system, producing the acetophenone compound by taking phenylacetylene and derivatives thereof as reaction substances. The method has the advantages that the catalyst is cheap and available, and can be repeatedly utilized; the reaction system is an oil / water two-phase system; the obtained target product is easy to separate, relatively high in selectivity and productive rate, and mild in reaction condition; and the problems that a lewis acid catalyst is sensitive to water, and the product is not easy to separate and the like in the method for producing the acetophenone compound employing the phenylacetylene and the derivatives thereof are solved.
Owner:HUNAN UNIV
Method for preparing 9-fluorenone with fluorene
InactiveCN105801389ANo changeEasy to separateOrganic compound preparationPreparation by C-C triple bond hydrationChemical synthesisFluorenone
The invention discloses a method for preparing 9-fluorenone with fluorine.Fluorine serves as the raw material, alkali serves as a catalyst, an organic solvent containing aromatic nucleus and water serve as solvents, crown ether serves as a phase transfer agent, and 9-fluorenone is prepared.The preparation method includes the step that industrial fluorine and high-purity fluorine are used for preparing industrial 9-fluorenone and high-purity 9-fluorenone respectively.Under the conditions, the fluorine conversion rate can reach 100%, and selectivity of fluorenone is 100%.After a reaction solution is cooled and crystal fluorenone is separated out, filter liquid containing the solvents, alkali, crown ether, fluorine and fluorenone is directly recycled, the solvents in reaction tail gas are absorbed with high-boiling-point solvents, and the chemical synthesis technology saves energy and is environmentally friendly.
Owner:BAOSHUN TECH CO LTD +1
Zinc-based catalyst used for catalyzing acetylene hydration reaction and preparation method thereof
PendingCN111185222ASimple processImprove efficiencyMolecular sieve catalystsCatalyst activation/preparationHydration reactionPtru catalyst
The invention relates to a zinc-based catalyst used for catalyzing an acetylene hydration reaction and a preparation method thereof. The preparation method for the zinc-based catalyst used for catalyzing the acetylene hydration reaction comprises the following steps: (1) completely dissolving zinc salt into deionized water so as to obtain a precursor solution; (2) soaking a carrier into the precursor solution, carrying out stirring for 10-14 hours, then carrying out standing for 10-14 hours, and carrying out drying so as to obtain a catalyst; and (3) performing plasma treatment, i.e., carryingout plasma treatment on the catalyst in different plasma atmospheres so as to obtain the zinc-based catalyst used for catalyzing the acetylene hydration reaction. The zinc-based catalyst used for catalyzing the acetylene hydration reaction and the preparation method thereof provided by the invention have the following advantages: the preparation process is simple and short in time; the preparation process is clean and free of pollution; the obtained catalyst is high in efficiency, good in activity in the acetylene hydration reaction and high in selectivity; and the prepared catalyst is a non-mercury catalyst, is used for the acetylene hydration reaction, and can solve the problems of environmental pollution and harm to the human health due to a volatile mercury catalyst.
Owner:SHIHEZI UNIVERSITY
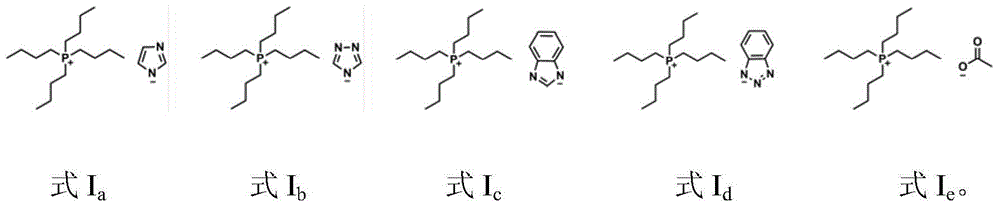
Catalytic system for synthesizing alpha-hydroxy ketone by alkynol hydration reaction
ActiveCN104437621AHigh catalytic activityWide applicabilityOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by C-C triple bond hydrationHydration reactionAcetylenic alcohol
The invention discloses a catalytic system for synthesizing alpha-hydroxy ketone by alkynol hydration reaction. The catalytic system consists of an ionic liquid and a CO2 gas, wherein the ionic liquid is selected from at least one of ionic liquids as shown in formulas Ia-Ie described in the specification. In the catalytic system, the amount of substances of the ionic liquid is 0.01-0.5mol, and the pressure of the gas is 0.1-8MPa. The invention further provides a method of preparing alpha-hydroxy ketone compounds by utilizing the catalytic system. The catalytic system is suitable for a reaction system for catalyzing alkynol hydration reaction to synthesize alpha-hydroxy ketone compounds. The catalytic system is relatively high in catalytic activity, mild in reaction condition, and free of metals. The main catalyst ionic liquid is easy to synthesize and recycle, and has a relatively strong application value.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Method for synthesizing alpha-alkyl ketone
InactiveCN104557500AMeet the requirements of green chemistryPreparation by C-C triple bond hydrationIridiumEvaporation
The invention discloses a method for synthesizing alpha-alkyl ketone. The method comprises the following steps: adding alkyne, [(IPr)AuCl], AgOTf, 1,4-dioxane and water in a reaction container, performing microwave reaction on a reaction mixture for 1h at 120 DEG C and cooling to room temperature; further adding [Cp*IrCl2]2, alkali and alcohol into the reaction mixture, performing microwave reaction on the reaction mixture for 2h at 130 DEG C and cooling to room temperature; filtering, performing rotary evaporation to remove a solvent, and then separating by a column to obtain a target compound. The method disclosed by the invention is started from chemical raw materials which are easy to obtain, namely alkyne, water and alcohol, alpha-alkyl ketone is obtained under the participation of gold and iridium catalysts, and the reaction only generates water as a byproduct. Therefore, the reaction is in line with the requirements of green chemistry and has broad development prospects.
Owner:NANJING UNIV OF SCI & TECH
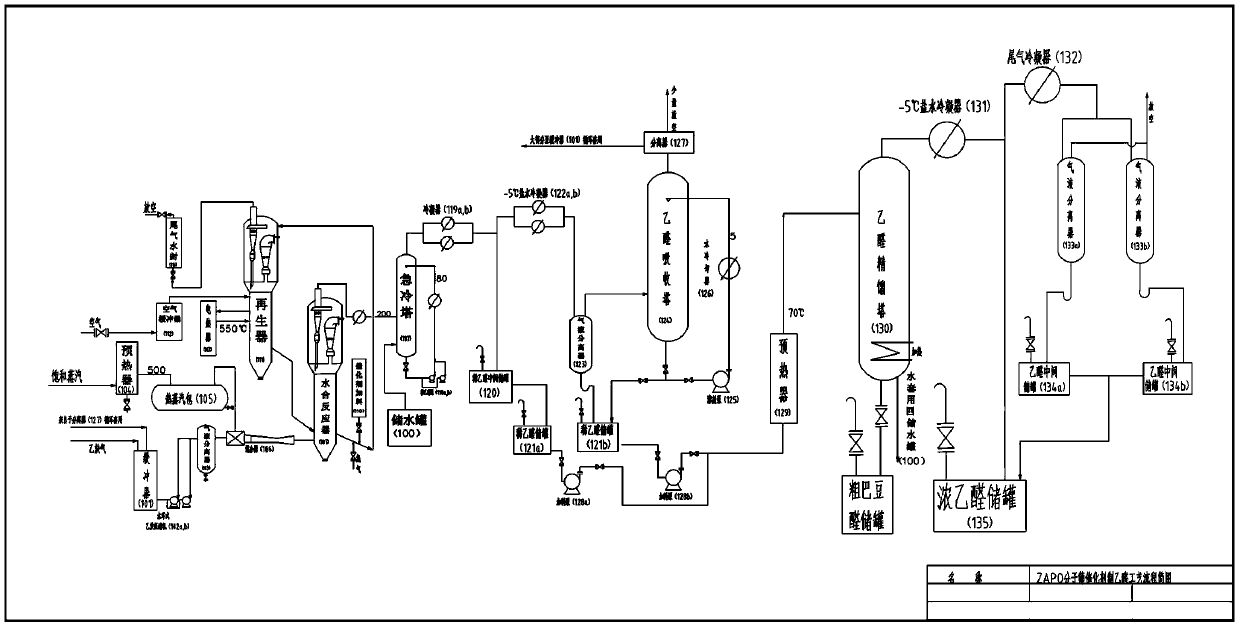
Preparation method for producing acetaldehyde by catalyzing acetylene with ZAPO molecular sieve
PendingCN111574344AAvoid dependenceAvoid harmOrganic compound preparationPreparation by C-C triple bond hydrationMolecular sieveHydration reaction
The invention discloses a preparation method for producing acetaldehyde by catalyzing acetylene with a ZAPO molecular sieve. The method comprises the following steps: preheating feed gas acetylene andwater, carrying out ZAPO molecular sieve catalysis and continuous hydration in a fluidized bed to obtain acetaldehyde, separating, absorbing and rectifying to obtain an acetaldehyde product, and continuously regenerating the catalyst for use. The method is simple, stable and efficient in process, solves the problem of dependence of acetaldehyde production through acetylene hydration on mercury catalysts, avoids harm of mercury to human bodies and the environment, and has high production and use value.
Owner:BOZUN INVESTMENT GRP LTD
Ketone synthesis method through alkyne hydrolysis
InactiveCN106032348AHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationOrganic synthesisRoom temperature
The invention discloses a ketone synthesis method through alkyne hydrolysis. The method comprises the following steps: adding alkyne, a catalyst [(IPr)AuCl], a solvent methanol, and water into a reactor, carrying out reactions for several hours at a temperature of 110 to 120 DEG C, cooling to the room temperature, carrying out rotary evaporation to remove the solvent, and performing column separation to obtain target compounds. Compared with conventional ionic gold catalyst, the provided method directly uses gold chloride [(IPr)AuCl] as the catalyst, alkyne is hydrolyzed into ketone, the yield is high, the selectivity is complete, and thus the method has an important meaning for organic synthesis and environment protection.
Owner:NANJING UNIV OF SCI & TECH
Process for producing carbonyl compound
InactiveCN1639099AOrganic-compounds/hydrides/coordination-complexes catalystsOrganic chemistry methodsHydration reactionOrganic solvent
The present invention relates to a method for hydrating alkynes, which enables the hydration of alkynes to proceed efficiently in terms of catalyst turnover, productivity and speed, and thereby industrially advantageously produces the corresponding carbonyl compounds. The present invention provides a method for producing a carbonyl compound comprising reacting an alkyne compound with water in an organic solvent in the presence of a gold catalyst, the gold catalyst being an organogold complex, and an acid.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Synthesizing method of fused-ring aryl substituted formaldehyde compound
ActiveCN105601480AAtom economy is highMild conditionsOrganic compound preparationPreparation by C-C triple bond hydrationAir atmosphereAryl
The invention discloses a synthesizing method of a fused-ring aryl substituted formaldehyde compound, and belongs to the technical field of synthesis technologies of aldehyde compounds. According to the technical scheme, the synthesizing method of the fused-ring aryl substituted formaldehyde compound particularly comprises the steps that a 1-phenyl-4-pentyne-2-alcohol compound or a 1-(naphthalene-1-base)-4-pentyne-2-alcohol compound is dissolved in solvent, accelerant is added, a reaction is conducted in an air atmosphere at 0-40 DEG C, and the fused-ring aryl substituted formaldehyde compound is obtained, wherein the solvent adopts tetrahydrofuran or acetonitrile or dichloromethane, and the accelerant adopts iodine monochloride. The synthesizing method starts from the raw materials which are simple and easy to prepared, and by means of a one-pot cascade reaction, a 1-naphthaldehyde compound or a 1-phenanthrenecarboxaldehyde compound is obtained directly, that is to say, fused-ring aryl and aldehyde groups are built simultaneously in the one-pot cascade reaction; operation is convenient, the condition is mild, atoms are high in economic efficiency, substrates are wide in application scope, and the synthesizing method of the fused-ring aryl substituted formaldehyde compound is suitable for industrial production.
Owner:HENAN NORMAL UNIV
Preparation method of copper-based catalyst and application of copper-based catalyst in acetylene hydration reaction
ActiveCN112871188AGood dispersionEffective anchoringMolecular sieve catalystsCatalyst activation/preparationHydration reactionPtru catalyst
The invention discloses a preparation method of a copper-based catalyst and application of the copper-based catalyst in acetylene hydration reaction. The preparation method of the copper-based catalyst comprises the following steps: dissolving a copper-containing precursor and a base metal additive in a solvent, stirring to uniformly mix, dropwise adding the mixed solution onto a porous solid carrier at 25-35 DEG C, carrying out equivalent-volume impregnation under the action of an electrostatic field for 3-5 hours, and then drying at 35-120 DEG C for 6-10 hours to obtain the copper-based catalyst, and the base metal auxiliary agent is a salt of one or more metals selected from Bi, Ba, Fe, Mn, Zn, K, Ca and Ni. The invention provides the application of the prepared copper-based catalyst in acetylene hydration reaction to generate acetaldehyde, and the copper-based catalyst has the advantages of high conversion rate and good stability.
Owner:ZHEJIANG UNIV OF TECH
1-(8-arylnaphthyl)phosphine ligand and preparation method thereof as well as phosphine-gold compound and application
InactiveCN108383873ASimple methodOvercome the defect of narrow substrate scopeOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsArylOrganic synthesis
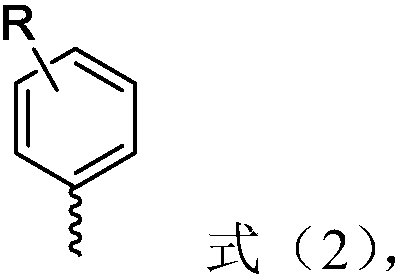
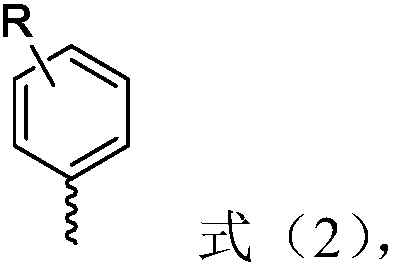
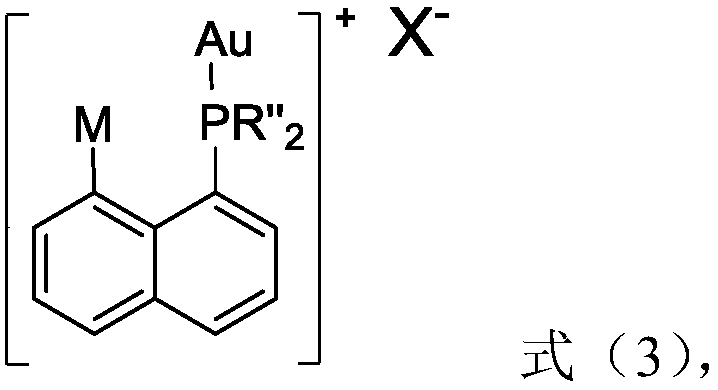
The invention relates to the field of organic synthesis and discloses a 1-(8-arylnaphthyl)phosphine ligand and a preparation method thereof as well as a phosphine-gold compound and application. The ligand is shown as a formula (1), wherein each of two R'' is independently selected from phenyl and cyclohexyl and M is selected from substituted or unsubstituted heteroaryl and substituted or unsubstituted aryl; a heteroatom of the heteroaryl is a nitrogen atom; a substituent group of the heteroaryl is selected from at least one of methyl, methoxyl, trifluoromethyl, -Cl and -Br; the substituted aryl is shown as a formula (2). The 1-(8-arylnaphthyl)phosphine ligand can be directly prepared and the method is simple; the defect of a preparation method of the 1-(8-arylnaphthyl)phosphine ligand in the prior art that an applicable range of a substrate is narrow is overcome and the new 1-(8-arylnaphthyl)phosphine ligand is prepared; the yield of the method provided by the invention is high. The formula (1) and the formula (2) are shown in the description.
Owner:HUAZHONG NORMAL UNIV
Preparation method of isotope 13C-marked aldehyde
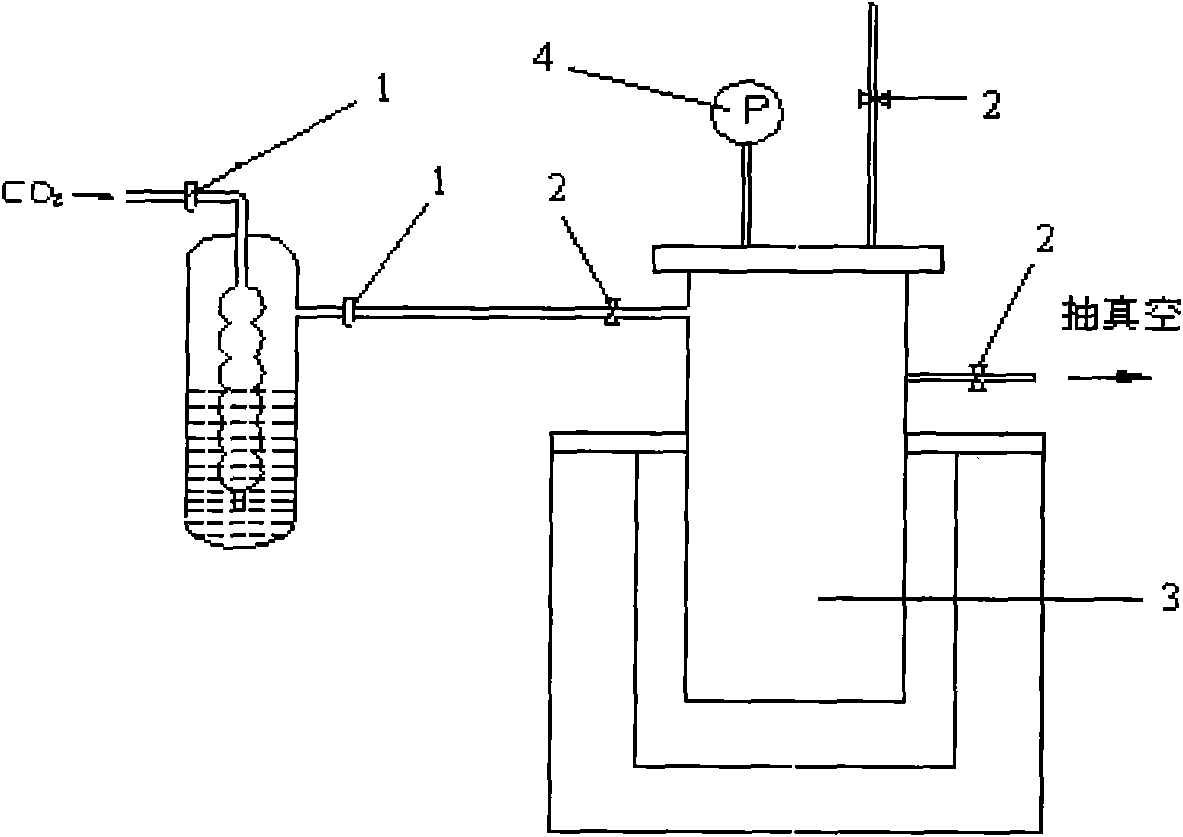
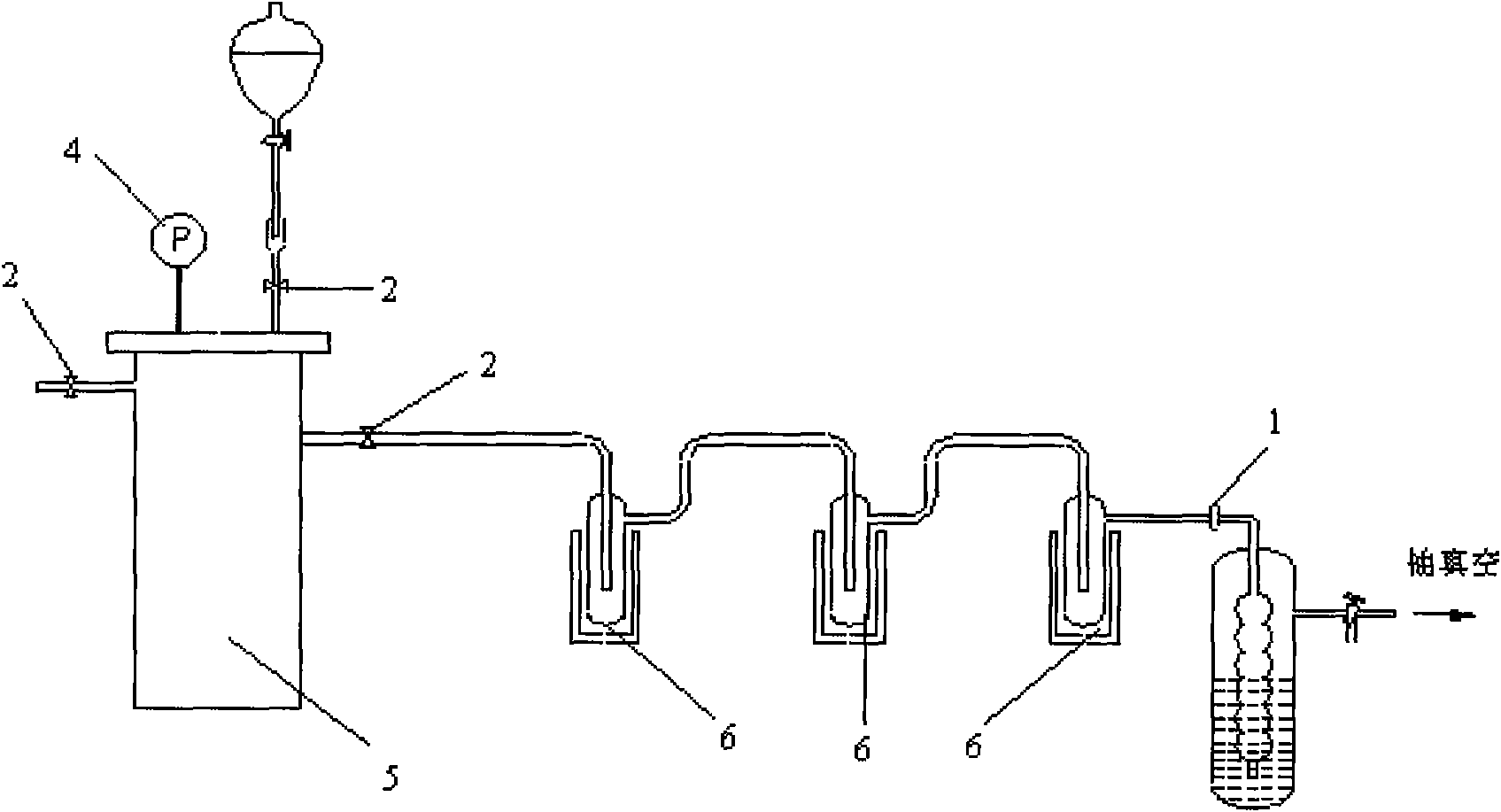
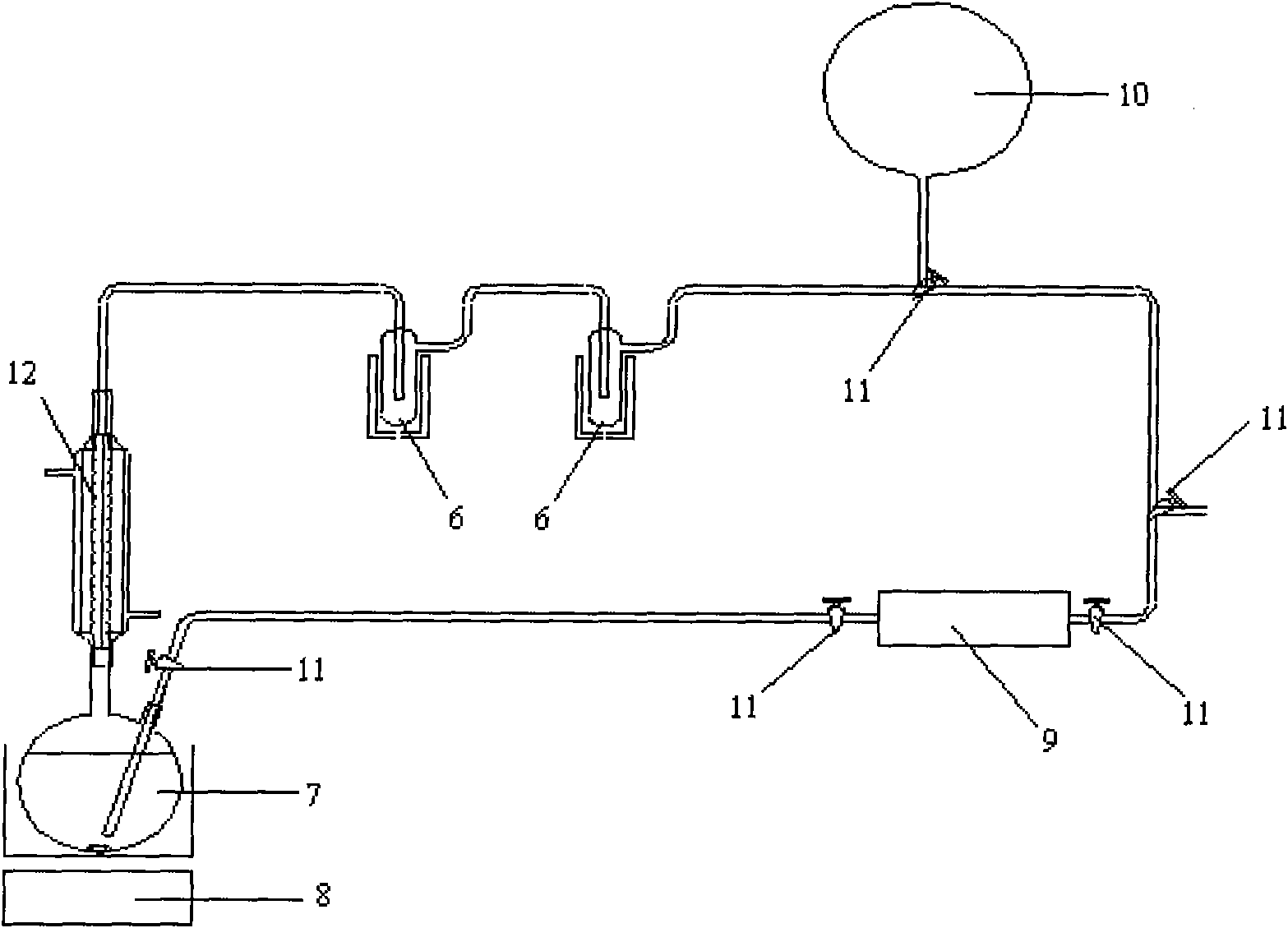
InactiveCN101768063AOptimize the synthetic routeSimple processPreparation by C-C triple bond hydrationHydration reactionVacuum pump
The invention relates to a preparation method of isotope 13C-marked aldehyde. The method includes the following steps that firstly, metallic carbide is generated by active metal and carbon dioxide, namely, the active metal is put in a reactor, the vacuum degree and the temperature of the reactor are controlled, 13 carbon dioxide and the active metal are put in the reactor to generate the metallic carbide; secondly, the metallic carbide is hydrolyzed to generate acetylene, and the by-product hydrogen is discharged by a vacuum pump; the acetylene is catalyzed and hydrated to generate aldehyde, namely, a catalyst system is added to the reactor provided with a cold trap and a constant flow pump and then hydrated to generate the 13C-marked aldehyde which is liquefied in the cold trap and collected. Compared with the prior art, the invention selects proper synthetic precursors, self-designs the reactor and optimizes synthetic path and technique. The method has a simple process, the preparation is easy, the purity of the product aldehyde is more than 99 percent, and the abundance of the isotope 13C is equal to or more than 98 atom percent.
Owner:SHANGHAI RES INST OF CHEM IND
Composite AuAgPd catalyst for hydrolysis and oxidation of alkynol and its preparation method
ActiveCN107670663AOrganic compound preparationCatalyst activation/preparationNano catalystHigh activity
The invention discloses a novel supported multi-metal nano-catalyst AuAgPd@HT and application of the same to a synthesis process of bisphenol F and hydrolysis of alkynol. According to the invention, Au nanoparticles, Au@HT, AuAg@HT and AuAgPd@HT are separately prepared in steps. The novel supported multi-metal nano-catalyst provided by the invention has the advantages of high activity, recyclability and long service life. Therefore, the design and synthesis of the supported multi-metal nano-catalyst have good industrial application prospects.
Owner:JIANGNAN UNIV
Method for preparing cinnamyl aldehyde compounds
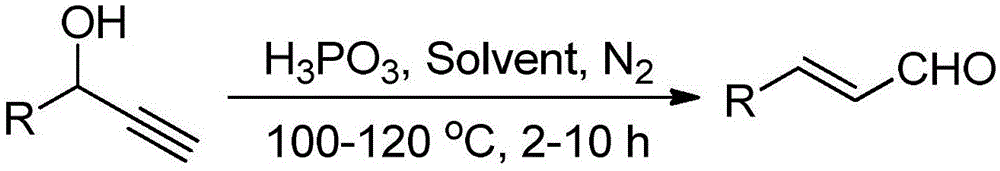
InactiveCN105130726ASimple response systemRaw materials are cheap and easy to getAmino preparation from aminesOrganic compound preparationPhosphorous acidNitrogen gas
The invention provides a method for preparing cinnamyl aldehyde compounds. According to the method, under the nitrogen condition, propargyl alcohol is adopted as a raw material, a phosphorous acid aqueous solution is adopted as a medium, and the cinnamyl aldehyde compounds are synthesized in organic solvents. According to the method, the easily-obtained raw material and the cheap phosphorous acid aqueous solution are adopted, a reaction is conducted under the nitrogen condition, no ligand or peroxide or valuable catalyst or microwave radiation or other special reaction conditions are adopted, the reaction condition is simple, the product is high in selectivity and productivity, and good industrial application prospects are achieved.
Owner:HUNAN UNIV
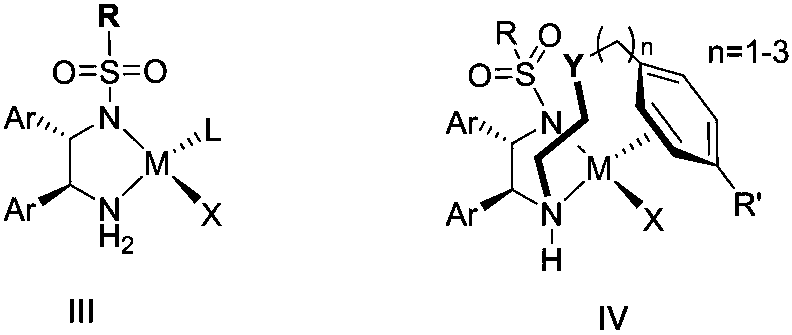
Polysubstituted chiral (1-ethoxy)benzene and asymmetric synthesis method thereof
ActiveCN108046995ARaw materials are easy to getSimple and fast operationOrganic compound preparationOrganic chemistry methodsHydration reactionKetone
The invention relates to polysubstituted chiral (1-ethoxy)benzene. The specific structure of the polysubstituted chiral (1-ethoxy)benzene is as shown in a formula II. The invention also discloses a 'two-step one-pot' synthetic method of the polysubstituted chiral (1-ethoxy)benzene. The 'two-step one-pot' synthetic method is characterized by taking polyacetylenyl substituted benzene (I) as a raw material, and comprises the following steps: step (1) taking fluorine-containing alcohol and water as solvents, carrying out hydration reaction under the catalysis of trifluoromethanesulfonic acid, thusgenerating an intermediate-ketone; step (2) directly adding a complex of mono-sulfonyl chiral diamine and metal ruthenium, rhodium or iridium as a catalyst in a reaction system, adding alkali, supplementing hydrogen, and carrying out asymmetric hydrogenation reaction, thus obtaining a product II; or directly adding the complex of the mono-sulfonyl chiral diamine and the metal ruthenium, rhodium or iridium as the catalyst in the reaction system, using a mixture of sodium formate or formic acid and triethylamine as a hydrogen source, and carrying out the asymmetric transfer hydrogenation reaction, thus obtaining the product II. According to the 'two-step one-pot' synthetic method disclosed by the invention, the operation is simple, the raw material is easy to obtain, and enantioselectivityand diastereoselectivity are very high. (The formula II is shown in the description).
Owner:CHINA THREE GORGES UNIV
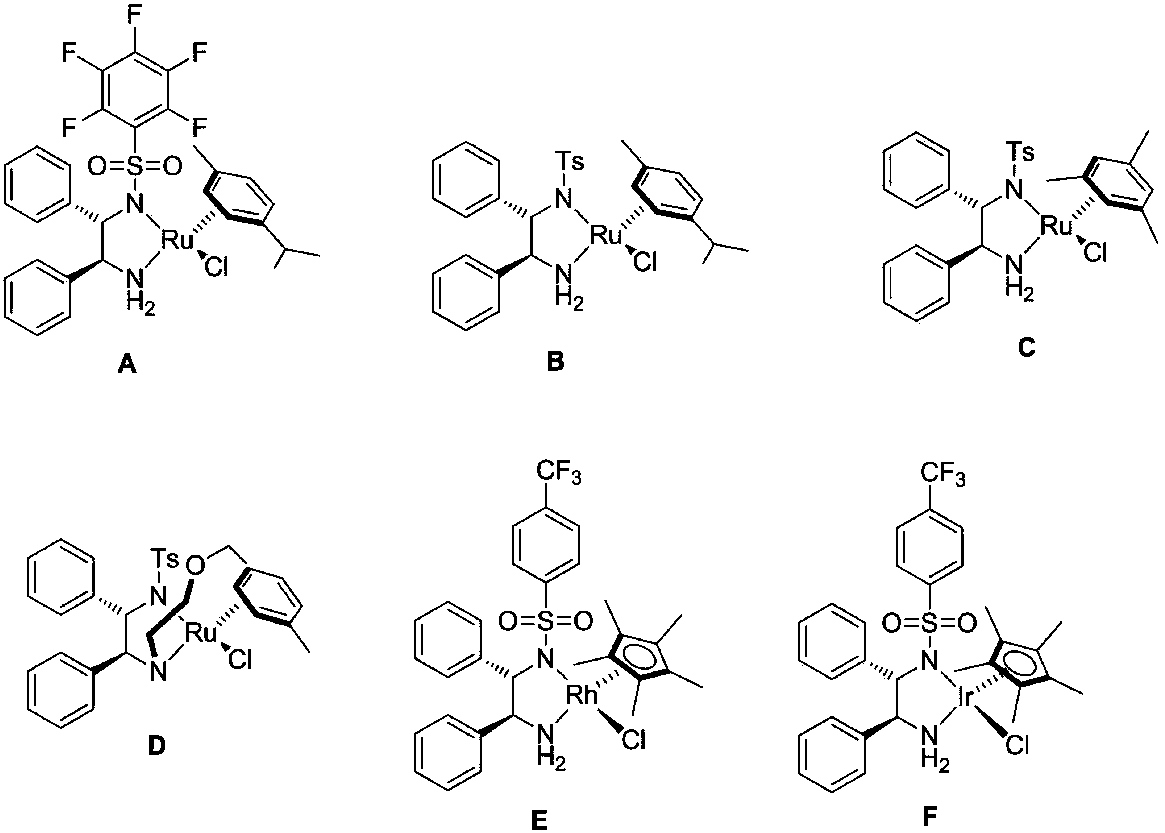
Method for direct conversion of aromatic alkyne into chiral alcohol through one-pot process
ActiveCN108101740ASimple and fast operationMild reaction conditionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydration reactionIridium
The invention relates to a method for direct conversion of aromatic alkyne into chiral alcohol through a one-pot process. The method uses cheap and easily-available alkyne I as a raw material, adoptsa two-step one-pot strategy for direct synthesis of chiral alcohol II, and comprises the following concrete steps: step 1) with fluorine-containing alcohol and water as solvents, allowing the alkyne Ito generate a hydration reaction under the catalysis of trifluoromethanesulfonic acid so as to generate an intermediate namely ketone; and step 2) directly adding a complex of monossulfonyl chiral diamine and metal ruthenium or rhodium or iridium as a catalyst into a reaction system, and with a mixture of a sodium formate aqueous solution or formic acid-triethylamine as a hydrogen source, carrying out an asymmetric transfer hydrogenation reaction so as to obtain a product II. The method provided by the invention has the following advantages: operation is simple and convenient; reaction conditions are mild; and a substrate has wide application range and high enantioselectivity. Concretely, the method provided by the invention has a general reaction formula which is described in the specification.
Owner:CHINA THREE GORGES UNIV
Production of oxygenates from a methane conversion process
Methods and systems are provided for converting methane in a feed stream to acetylene. The method includes processing acetylene as an intermediate stream to form a stream having oxygenates. The hydrocarbon stream is introduced into a supersonic reactor and pyrolyzed to convert at least a portion of the methane to acetylene. The reactor effluent stream may be treated to convert acetylene to oxygenates through subsequent reactors.
Owner:UOP LLC
Method of preparing methyl ketone through cobalt catalysis
ActiveCN106986754ALow costReduce pollutionGroup 4/14 element organic compoundsOrganic compound preparationReaction temperatureAlkyne
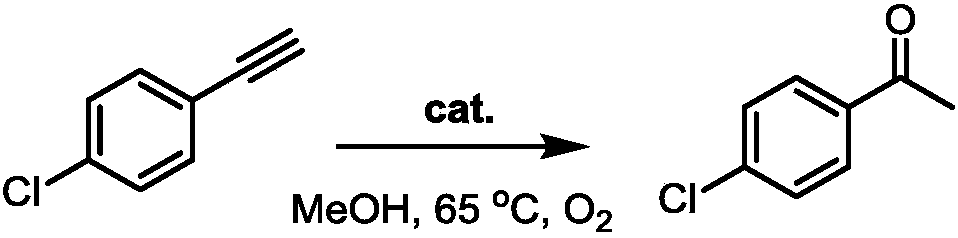
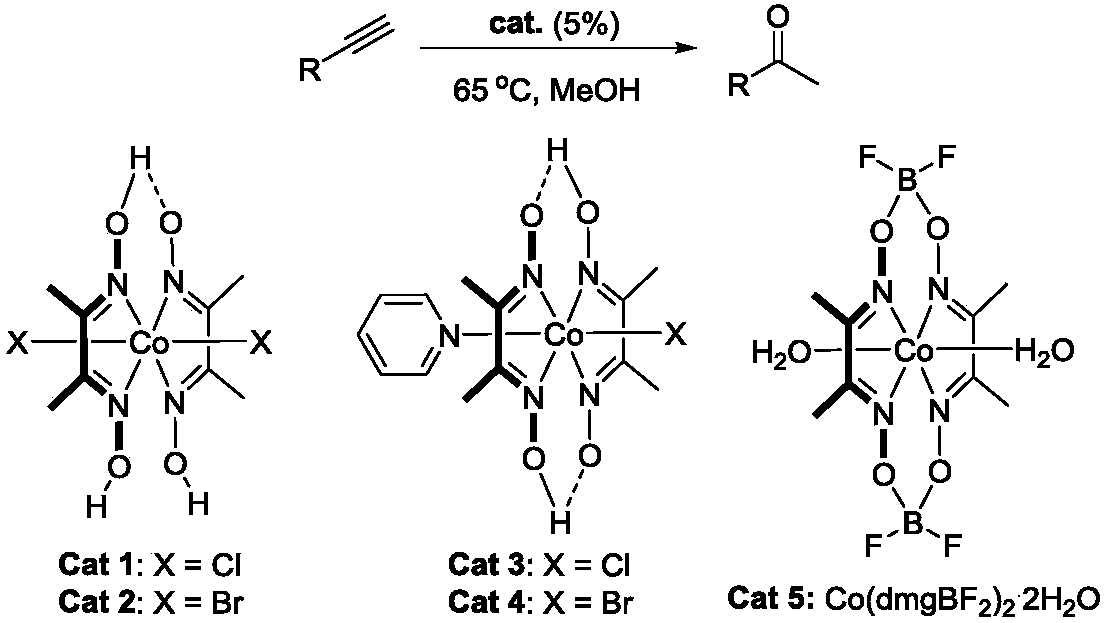
The invention particularly discloses a method of preparing methyl ketone through cobalt catalysis and aims at the defect that the prior art for preparing methyl ketone uses expensive catalysts, is harsh in reaction conditions, causes environmental pollution and cannot realize large-scale production. Triple bonds in alkyne are activated through lewis acid of metal cobalt coordinated with oxime, so that alkyne can be efficiently hydrolyzed into methyl ketone. Methanol is used as a solvent, concentration of reaction substance is 0.25mol / L, a catalyst is added into a reaction system according to 5% of molar weight of raw materials, reaction temperature is 65 DEG C, and alkyne can be efficiently hydrolyzed into methyl ketone. Catalysis performance can be on a par with other catalysts. Reaction temperature is comparatively mild, reaction speed is high, and no acidic substance needs to be added in the process of reaction. Environmental pollution is lowered to minimum extent, the operation process of reaction is simplified to maximum extent, and safety of an operator and feasibility of reaction are guaranteed to maximum extent. Therefore, the method has more obvious industrialization trend.
Owner:LANZHOU UNIVERSITY
Synthetic method of methyl ketone compound
PendingCN111170837AImprove conversion rateHigh yieldPreparation by C-C triple bond hydrationHydration reactionPtru catalyst
The invention relates to the technical field of organic synthesis, and provides a synthesis method for a methyl ketone compound. The synthesis method comprises the following steps: mixing terminal alkyne, an organic solvent, an acid and water, and carrying out a hydration reaction to obtain a methyl ketone compound. According to the synthetic method provided by the invention, the use a catalyst containing metal ions and an oxidizing agent can be avoided, the raw materials are directly subjected to the hydration reaction in the presence of acid and water, and the complicated operation of removing metal ions is avoided in the post-treatment process of the produced methyl ketone compound; the method provided by the invention is high in raw material conversion rate and relatively high in product yield and product purity; the synthesis reaction process is simple and convenient to operate, green and environment-friendly, and suitable for large-scale industrial production; the synthetic method provided by the invention is mild in reaction conditions and easy to control. Results of an embodiment of the invention show that when the method is used for preparing the methyl ketone compound, yield can reach 96.4%, and product purity reaches 99.2%.
Owner:DALIAN CHEMPHY CHEM
Method for Producing Asymmetric Conjugated Diyne Compound and Method for Producing Z,Z-Conjugated Diene Compound Using the Same
ActiveUS20160229829A1High yieldLow costOrganic compound preparationOrganic chemistry methodsOrganic solventHydroxylamine Hydrochloride
Provided are a method for efficiently producing an asymmetric conjugated diyne from an inexpensive and safe alternative compound to hydroxylamine hydrochloride and a method for producing a Z,Z-conjugated diene compound from the asymmetric conjugated diyne compound thus obtained. More specifically, provided is a method for producing an asymmetric conjugated diyne compound comprising a step of subjecting a terminal alkyne compound (1): HC≡C—Z1—Y1 to a coupling reaction with an alkynyl halide (2) Y2—Z2—C≡C—X by using sodium borohydride in water and an organic solvent in the presence of a copper catalyst and a base to obtain the asymmetric conjugated diyne compound (3): Y2—Z2—C≡C—C≡C—Z1—Y1. In addition, provided is a method for producing a Z,Z-conjugated diene compound by reducing the resulting asymmetric conjugated diyne compound, or the like.
Owner:SHIN ETSU CHEM IND CO LTD
A kind of preparation method of p-methoxyacetophenone
InactiveCN103772177BLow priceReduce generationPreparation by C-C triple bond hydrationFiltrationSolvent
The invention provides a preparation method of p-methoxyacetophenone. The preparation method comprises the steps of sequentially adding p-methoxyphenyl acetylene, tetrakis (triphenyl phosphine)palladium, hydrochloric acid and a solvent into a reactor, wherein the mole rate of methoxyphenyl acetylene to hydrochloric acid is 1:1.2; reacting at room temperature for 48h; evaporating the solvent; purifying through filtration column chromatography to obtain a light yellow solid product, namely pure p-methoxyacetophenone, wherein ethyl acetate is used as an eluting agent for column chromatography purification, and the volume ratio of ethyl acetate to petroleum ether is 1:5. Methoxyphenyl acetylene is used as a raw material, so that the raw material is low in price; an experiment can be carried out at room temperature, the operation process is simple, and few side products are generated in the experiment and are easy to separate; the preparation method is novel in design, is a good design scheme and has a certain market promotion prospect.
Owner:ZHENGZHOU UNIV
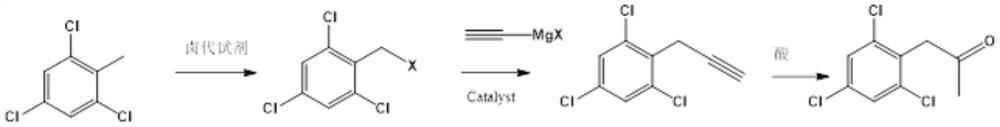
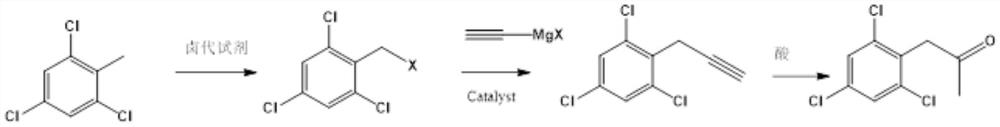
Preparation method of 2, 4, 6-trichlorophenyl substituted acetone
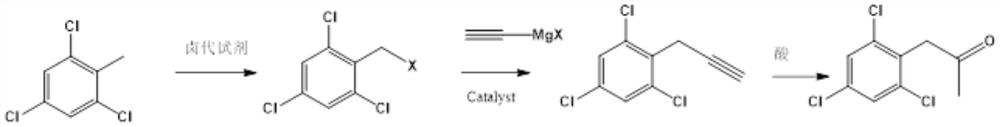
ActiveCN113004131ARich sourcesObvious cost advantagePreparation by C-C triple bond hydrationHalogenated hydrocarbon preparationPtru catalystWater chlorination
The invention relates to a preparation method of 2, 4, 6-trichlorophenyl substituted acetone, which comprises the following steps: adding 2, 4, 6-trichlorotoluene and a chlorination reagent into an organic solvent at a certain temperature to prepare 2, 4, 6-trichlorophenyl benzyl chloride, then coupling with ethynyl magnesium halide under the action of a metal catalyst, and then carrying out acid catalysis and water addition to finally obtain the product 2, 4, 6-trichlorophenyl substituted acetone. The method provided by the invention has the advantages of many raw material sources, obvious cost advantage, safer and more convenient preparation method, high total yield and less three wastes, and is beneficial to industrialization.
Owner:江苏先导药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com