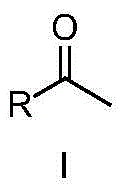
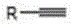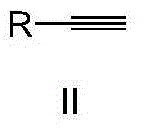Ketone synthesis method through alkyne hydrolysis
A hydrolysis reaction and alkyne technology, applied in the field of ketone synthesis, can solve the problems of affecting the reaction selectivity, sensitivity to light, etc., and achieve the effect of complete selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: acetophenone
[0019] Acetophenone
[0020]
[0021] Catalyst [(IPr)AuCl] (3.1 mg, 0.5 mol%), phenylacetylene (1 mmol), methanol (1 ml) and water (0.5 ml) were sequentially added to a 25 ml reactor. After the reaction mixture was reacted at 110°C for 6 hours, it was cooled to room temperature. Rotary evaporation removes solvent, then obtains pure target compound by column chromatography (developing solvent: sherwood oil / ethyl acetate), productive rate: 99%
[0022] 1HNMR (500MHz, CDCl3) δ7.99-7.94(m, 2H, ArH), 7.59-7.54(m, 1H, ArH), 7.46(t, J=7.65Hz, 2H, ArH), 2.61(s, 3H, CH3); 13C NMR (125MHz, CDCl3) δ198.03, 136.94, 132.95, 128.40, 128.13, 26.40
Embodiment 2
[0023] Embodiment 2:4-methylacetophenone
[0024] 1-p-tolylethanone
[0025]
[0026] Catalyst [(IPr)AuCl] (3.1 mg, 0.5 mol%), 4-methylphenylacetylene (1 mmol), methanol (1 ml) and water (0.5 ml) were sequentially added to a 25 ml reactor. After the reaction mixture was reacted at 110°C for 6 hours, it was cooled to room temperature. Rotary evaporation removes solvent, then obtains pure target compound by column chromatography (developing solvent: sherwood oil / ethyl acetate), productive rate: 95%
[0027] 1 HNMR (500MHz, CDCl 3 )δ7.85(d, J=8.05Hz, 2H, ArH), 7.25(d, J=8.35Hz, 2H, ArH), 2.57(s, 3H, CH 3 ),2.40(s,3H,CH 3 ); 13 C NMR (125MHz, CDCl 3 )δ197.60, 143.65, 134.49, 129.01, 128.20, 26.23, 21.37.
Embodiment 3
[0028] Embodiment 3: 3-methylacetophenone
[0029] 1-m-tolylethanone
[0030]
[0031] Catalyst [(IPr)AuCl] (3.1 mg, 0.5 mol%), 3-methylphenylacetylene (1 mmol), methanol (1 ml) and water (0.5 ml) were sequentially added to a 25 ml reactor. After the reaction mixture was reacted at 110°C for 6 hours, it was cooled to room temperature. Rotary evaporation removes solvent, then obtains pure target compound by column chromatography (developing solvent: sherwood oil / ethyl acetate), productive rate: 96%
[0032] 1H NMR (500MHz, CDCl3) δ7.78-7.73 (m, 2H, ArH), 7.40-7.33 (m, 2H, ArH), 2.59 (s, 3H, CH3), 2.41 (s, 3H, CH3); 13C NMR (125MHz, CDCl3) δ198.29, 138.22, 137.06, 133.74, 128.67, 128.32, 125.47, 26.52, 21.20.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com