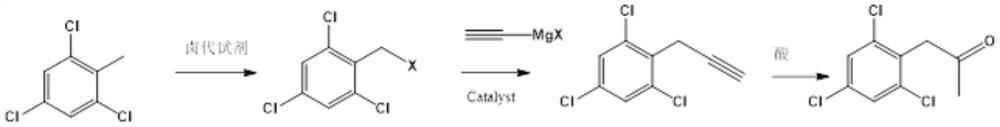
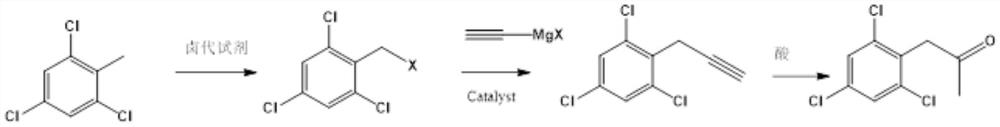
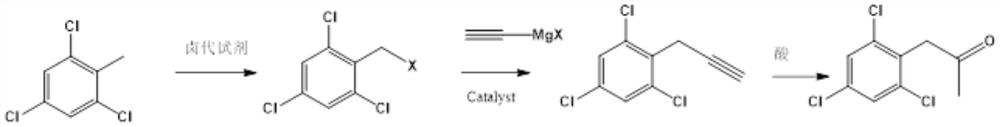
Preparation method of 2, 4, 6-trichlorophenyl substituted acetone
A technology of trichlorophenyl propyne and trichlorophenyl, which is applied in the field of preparation of 2,4,6-trichlorophenyl substituted acetone, can solve the problems of low reaction yield, environmental pollution, high price, etc. Achieve the effects of safe preparation method, obvious cost advantage, and favorable industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (Example 1) 2,4,6-trichlorophenyl substituted acetone
[0029]
[0030] Preparation of the target product in a small test:
[0031] In a 500ml four-neck flask equipped with a condenser, add 200mL dichloroethane, 0.2mol2,3,6-trichlorotoluene, 0.22molNBS in sequence under nitrogen protection, stir well and then slowly raise the temperature to 80°C, and then keep warm in the material The normalized content of 2,3,6-trichlorotoluene in liquid chromatography is 98% The target object 2,3,6-trichlorobenzyl bromide, the yield is 92%.
[0032] In a 500ml four-necked flask equipped with a condenser, add 200mLTHF, 0.2mol2,3,6-trichlorobenzyl bromide, 0.005mol anhydrous nickel chloride in sequence under nitrogen protection, stir well and cool down to 0°C, then dropwise add 0.21mol ethynylmagnesium bromide THF solution, after the dropwise addition, keep warm until the normalized content of 2,3,6-trichlorobenzyl bromide in the material is 95%, with a yield of 98%.
Embodiment 2
[0033] (Example 2) 2,4,6-trichlorophenyl substituted acetone
[0034]
[0035] Try to prepare the target product:
[0036] In a 3000L enamel kettle equipped with a graphite condenser, 1500L of dichloroethane, 390kg of 2,3,6-trichlorotoluene, and 390kg of NBS were sequentially pumped under nitrogen protection, stirred evenly, and slowly raised to 80°C, and then kept warm for 2 , 3,6-trichlorotoluene liquid chromatography normalized content98% Target 2,3,6-trichlorobenzyl bromide 490kg, yield 90%.
[0037] In a 5000l enamel kettle equipped with a graphite condenser, 1000LTHF, 540kg2,3,6-trichlorobenzyl bromide, 650g anhydrous nickel chloride were sequentially pumped under nitrogen protection, stirred evenly and cooled to 0°C, and then added dropwise 268kg of ethynylmagnesium bromide THF solution, after the dropwise addition, keep warm until the normalized content of 2,3,6-trichlorobenzyl bromide in the material is 96% of 2,4,6-trichlorophenylpropyne, with a yield of 90%.
...
Embodiment 3
[0039] (Example 3) 2,4,6-trichlorophenyl substituted acetone
[0040]
[0041] Preparation of the target product in a small test:
[0042] Partial results of the optimization of the first-step halogenation parallel reaction for halogenation reagents are as follows:
[0043] serial number Halogenating reagent solvent yield 1 Thionyl chloride chlorobenzene 54% 2 NCS Dichloroethane 83% 3 Chlorine Chloroform 88% 4 Bromine benzene 90%
[0044] Partial results of parallel optimization of the second-step coupling reaction for metal catalysts are as follows:
[0045] serial number catalyst yield 1 Ferrous chloride 92% 2 Cuprous bromide 53% 3 Triphenylphosphine nickel chloride 95% 4 nickel sulfate 88%
[0046] The results of the parallel optimization of the third step water addition reaction on the acid catalyst are as follows:
[0047] serial number acid solvent yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com