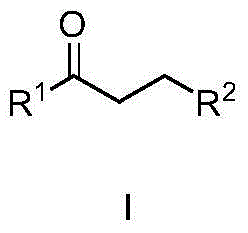
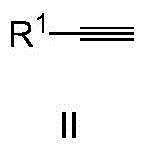Method for synthesizing alpha-alkyl ketone
A technology of alkyl ketone and alkyl, which is applied in the field of synthesis of α-alkyl ketones, can solve the problems of waste salt and achieve broad development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: 1,3-diphenyl-1-propanone
[0027] 1,3-diphenylpropan-1-one
[0028]
[0029] [(IPr)AuCl] (6mg, 0.01mmol, 1mol%), AgOTf (2.6mg, 0.01mmol, 1mol%), phenylacetylene (102mg, 1mmol), 1,4-dioxane (1ml) and water (36ul, 2equiv.) were added to 5ml microwave tubes in turn. The reaction mixture was reacted in a microwave reactor at 120° C. for 1 hour, and then cooled to room temperature. Then add [Cp*IrCl 2 ] 2 (8mg, 0.01mmol, 1mol%), potassium tert-butoxide (34mg, 0.3mmol, 0.3equiv.), benzyl alcohol (130mg, 1.2mmol). The reaction mixture was reacted in a microwave oven at 130° C. for another 2 hours, and then cooled to room temperature. Filtrate, remove the solvent by rotary evaporation, and then obtain the pure target compound by column chromatography (developing solvent: petroleum ether / ethyl acetate), yield: 87%
[0030] 1 H NMR (500MHz, CDCl 3 )δ7.96(d,J=7.6Hz,2H,ArH),7.56(t,J=7.3Hz,1H,ArH),7.46(t,J=7.6Hz,2H,ArH),7.34-7.24(m ,4H,ArH),7.21(t,J=7.2Hz,1H,A...
Embodiment 2
[0031] Example 2: 3-(4-methylphenyl)-1-phenyl-1-propanone
[0032] 1-phenyl-3-p-tolylpropan-1-one
[0033]
[0034] [(IPr)AuCl] (6mg, 0.01mmol, 1mol%), AgOTf (2.6mg, 0.01mmol, 1mol%), phenylacetylene (102mg, 1mmol), 1,4-dioxane (1ml) and water (36ul, 2equiv.) were added to 5ml microwave tubes in turn. The reaction mixture was reacted in a microwave reactor at 120° C. for 1 hour, and then cooled to room temperature. Then add [Cp*IrCl 2 ] 2 (8mg, 0.01mmol, 1mol%), potassium tert-butoxide (34mg, 0.3mmol, 0.3equiv.), 4-methylbenzyl alcohol (146mg, 1.2mmol). The reaction mixture was reacted in a microwave reactor at 130° C. for another 2 hours, and then cooled to room temperature. Filtrate, remove the solvent by rotary evaporation, and then obtain the pure target compound by column chromatography (developing solvent: petroleum ether / ethyl acetate), yield: 86%
[0035] 1 H NMR (500MHz, CDCl 3 )δ7.96(d,J=6.8Hz,2H,ArH),7.55(t,J=7.4Hz,1H,ArH),7.45(t,J=7.4Hz,2H,ArH),7.20-7.06...
Embodiment 3
[0036] Example 3: 3-(3,4-dimethylphenyl)-1-phenyl-1-propanone
[0037] 3-(3,4-dimethylphenyl)-1-phenylpropan-1-one
[0038]
[0039][(IPr)AuCl] (6mg, 0.01mmol, 1mol%), AgOTf (2.6mg, 0.01mmol, 1mol%), phenylacetylene (102mg, 1mmol), 1,4-dioxane (1ml) and water (36ul, 2equiv.) were added to 5ml microwave tubes in turn. The reaction mixture was reacted in a microwave reactor at 120° C. for 1 hour, and then cooled to room temperature. Then add [Cp*IrCl 2 ] 2 (8mg, 0.01mmol, 1mol%), potassium tert-butoxide (34mg, 0.3mmol, 0.3equiv.), 3,4-dimethylbenzyl alcohol (163mg, 1.2mmol). The reaction mixture was microwaved at 130°C for 2 hours and then cooled to room temperature. Filtration, rotary evaporation to remove the solvent, and then column chromatography (developing solvent: petroleum ether / ethyl acetate) to obtain the pure target compound, yield: 91%
[0040] 1 H NMR (500MHz, CDCl 3 )δ7.96(dd,J=8.4Hz and1.3Hz,2H,ArH),7.55(t,J=7.4Hz,1H,ArH),7.45(t,J=7.7Hz,2H,ArH),7.09- 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com