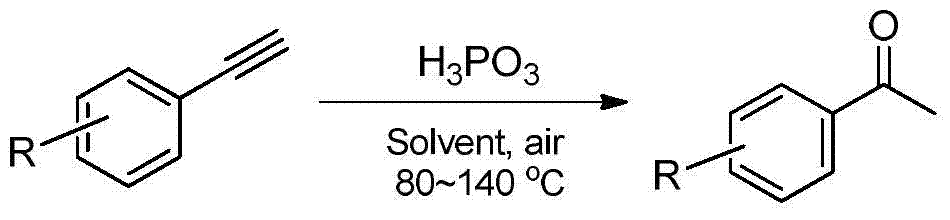
Method for producing acetophenone compound employing arylethynylene hydration reaction
A synthesis method and compound technology, applied in the preparation of carbon-carbon triple bond hydration, organic chemistry, etc., can solve problems such as environmental pollution, pollution, and intractability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0024] Formation of acetophenone
[0025] Add 1 mmol of phosphorous acid into the reactor, then add 82 mg of water to prepare a 50% phosphorous acid aqueous solution, and then add 1 mmol of phenylacetylene. Heat to 80°C, keep stirring for 8h, stop the reaction, cool to room temperature, extract with ethyl acetate, dry, and distill off the solvent under reduced pressure. The crude product is separated by column chromatography to obtain the target product with a yield of 64%. 1 H NMR (400MHz, CDCl 3 , TMS): δ7.80 (d, J=7.60Hz, 2H), 7.40 (t, J=7.20Hz, 1H), 7.29 (t, J=7.60Hz, 2H), 2.43 (s, 3H).
[0026] Add the aqueous phase saved during the previous extraction step into the reactor, and then add 1 mL of an organic solvent (eg, n-hexane), and 1 mmol of phenylacetylene. Heat to 80°C, keep stirring for 15h, stop the reaction, cool to room temperature, extract with ethyl acetate, dry, and distill off the solvent under reduced pressure. The crude product is separated by column chrom...
Synthetic example 2
[0028] Formation of m-methylacetophenone
[0029] Add 1.2 mmol of phosphorous acid into the reactor, and then add 98.4 mg of water to prepare a 50% phosphorous acid aqueous solution, and then add 1 mmol of m-methylphenylacetylene. Heat to 100°C, keep stirring for 16h, stop the reaction, cool to room temperature, extract with ethyl acetate, dry, and distill off the solvent under reduced pressure. The crude product is separated by column chromatography to obtain the target product with a yield of 75%. 1 H NMR (400MHz, CDCl 3 , TMS): δ7.69 (s, 2H), 7.33–7.23 (m, 2H), 2.51 (s, 3H), 2.33 (s, 3H).
Synthetic example 3
[0031] Formation of p-methylacetophenone
[0032] Add 1.6 mmol of phosphorous acid into the reactor, and then add 131.2 mg of water to prepare a 131.2% phosphorous acid aqueous solution, and then add 1 mmol of p-methylphenylacetylene. Heat to 110°C, keep stirring for 12 hours, stop the reaction, cool to room temperature, extract with ethyl acetate, dry, and distill off the solvent under reduced pressure. The crude product is separated by column chromatography to obtain the target product with a yield of 69%. 1 H NMR (400MHz, CDCl 3 , TMS): δ7.75 (d, J=8.1Hz, 2H), 7.14 (d, J=8.0Hz, 2H), 2.46 (s, 3H), 2.30 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com