Method for synthesizing tetrahydroquinoline containing three continuous chiral centers through asymmetric transfer hydrogenation
A tetrahydroquinoline, asymmetric technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., to achieve the effect of easy-to-obtain raw materials, mild reaction conditions, and convenient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
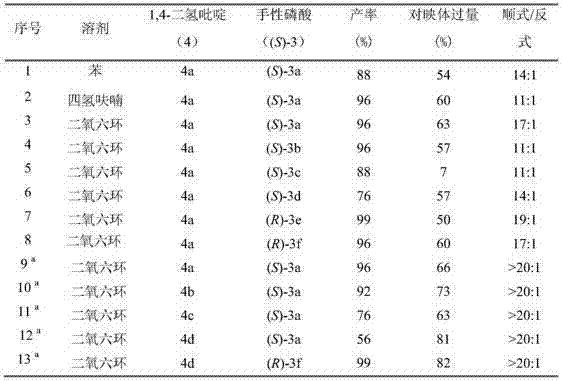
[0038] Embodiment 1: optimization of conditions
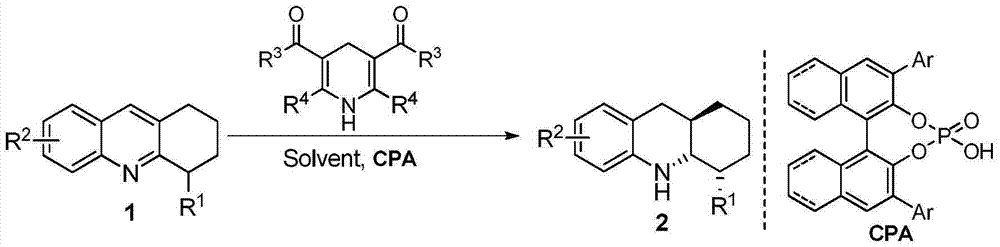
[0039] Under nitrogen protection, add 4-methyl-1,2,3,4-tetrahydroacridine (1a), 1,4-dihydropyridine (4,2.4eq.) and chiral phosphoric acid (CPA-3 , 5mol%), add 3-5 ml of 1,4-dioxane into the Schlenk tube. After stirring and reacting at 25°C for 15-24 hours, direct column chromatography (volume ratio of eluent petroleum ether and ethyl acetate is 30:1) to obtain pure product, the reaction formula is as follows:
[0040]
[0041] The yield is the separation yield. The enantiomeric excess of the product is determined by chiral liquid chromatography, and the diastereomeric excess is determined by crude nuclear magnetic spectrum. See Table 1.
[0042] Table 1. Optimization of asymmetric transfer hydrogenation conditions
[0043]
[0044] a 25°C
Embodiment 2
[0045] Example 2: Synthesis of tetrahydroquinoline compound 2 with three consecutive chiral centers by asymmetric transfer hydrogenation
[0046] Under nitrogen protection, 4-substituted-1,2,3,4-tetrahydroacridine (1), 1,4-dihydropyridine (4d, 2.4eq.) and chiral phosphoric acid ((S)- 3f, 5mol%) into a Schlenk tube with 3 ml of 1,4-dioxane. After stirring and reacting at 25°C for 15-24 hours, direct column chromatography (volume ratio of eluent petroleum ether and ethyl acetate is 30:1) to obtain pure product, the reaction formula is as follows:
[0047]
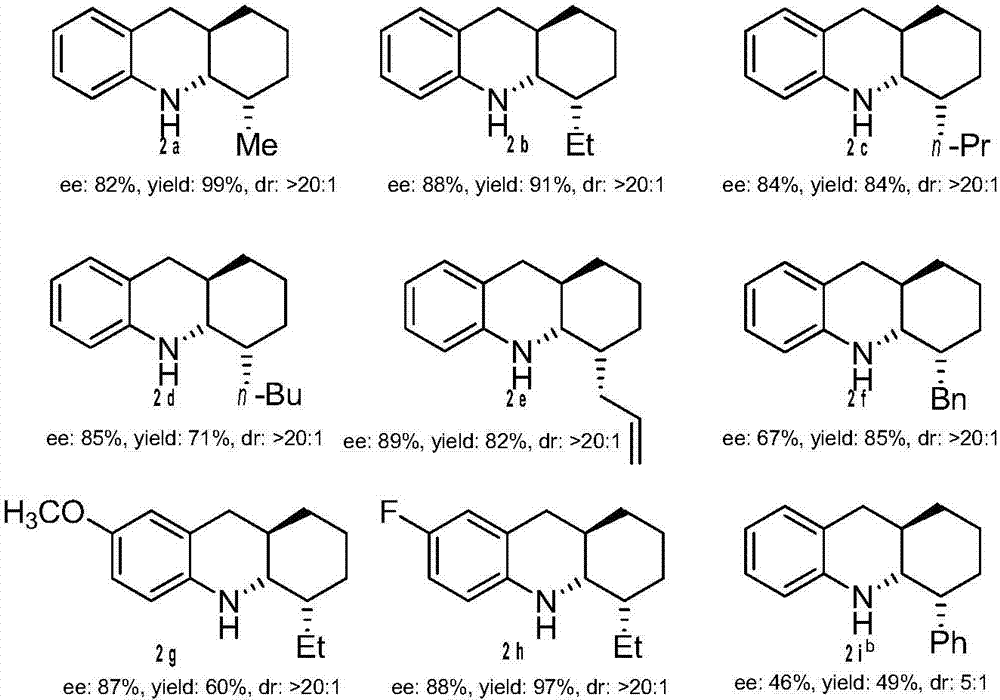
[0048] The productive rate is the separation yield, and the enantiomeric excess of the product is determined by chiral liquid chromatography, and the diastereomeric excess is determined by rough NMR spectrum, see figure 1 .
[0049] (4S,4aS,9aR)-4-Methyl-1,2,3,4,4a,9,9a,10-octahydroacridine(2a):Pale solid,mp=51-53℃,99%yield,R f =0.82(petroleum ether / EtOAc=30:1),83%ee,[α] 21 D =-45.8(c0.90, CHCl 3 ); 1 H NMR (400...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com