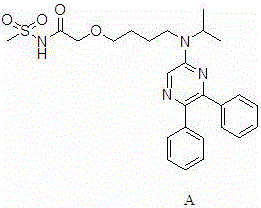
Preparation method of selexipag
A compound and reaction technology are applied in the field of preparation of medicines for treating pulmonary arterial hypertension, can solve the problems of high boiling point of solvent N-methylpyrrolidone, unsuitable for large-scale industrial production, central nervous system dysfunction and the like, and achieve reactivity and selectivity. High, no toxic side effects, quick response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method for selexipag, comprising the steps of: sequentially adding 1,4-dioxane (700 mL), a compound represented by formula I (60 g, 0.166 mol), a compound represented by formula II (85.44 g, 0.498mol) and potassium tert-butoxide (111g, 0.996mol), stirred at 30-35°C for 3h, TLC detected that the reaction was complete, and the reaction was stopped. Suction filtration, the filtrate was evaporated under reduced pressure to remove the solvent, the residual liquid was diluted with water (300mL), the pH was adjusted to 6-7 with 1N hydrochloric acid aqueous solution, extracted with dichloromethane (3×50mL), and the organic layer was separated with water (300mL ), dried over anhydrous magnesium sulfate, decolorized with activated carbon (15g), removed the solvent by rotary evaporation under reduced pressure, recrystallized the residual solution with a mixed solution (THF:n-hexane volume ratio = 1:1.1) (700mL), and dried under reduced pressure to obtain 68.4g light ...
Embodiment 2
[0034] A method for preparing selexipag, comprising the steps of: sequentially adding 1,4-dioxane (700 mL), a compound represented by formula I (60 g, 0.166 mol), a compound represented by formula II (107.57 g, 0.498mol) and potassium tert-butoxide (111g, 0.996mol), stirred at 25-30°C for 3h under the protection of nitrogen, the reaction was complete by TLC detection, and the reaction was stopped. Filtrate with suction, remove the solvent by rotary evaporation of the filtrate under reduced pressure, dilute the residual liquid with water (300 mL), adjust the pH to 6-7 with 1N hydrochloric acid, extract with dichloromethane (3×50 mL), separate the organic layer with water ( 300mL), washed with anhydrous magnesium sulfate, decolorized with activated carbon (15g), removed the solvent by rotary evaporation under reduced pressure, recrystallized the residual solution with a mixed solution (THF:n-hexane volume ratio = 1:1.5) (700mL), dried under reduced pressure, Obtained 69.07g ligh...
Embodiment 3
[0038]A preparation method for selexipag, comprising the steps of: sequentially adding 1,4-dioxane (300 mL), a compound represented by formula I (20 g, 0.055 mol), a compound represented by formula II (47.52 g, 0.22mol) and potassium tert-butoxide (49.36g, 0.44mol), stirred at 23-28°C for 3h, TLC detected that the reaction was complete, and stopped the reaction. Filtrate with suction, remove the solvent by rotary evaporation of the filtrate under reduced pressure, dilute the residual liquid with water (100 mL), adjust the pH to 6-7 with 1N hydrochloric acid aqueous solution, extract with dichloromethane (3×20 mL), separate the organic layer with water ( 100mL), washed with anhydrous magnesium sulfate, decolorized with activated carbon (5g), removed the solvent by rotary evaporation under reduced pressure, recrystallized the residual solution with a mixed solution (THF:n-hexane volume ratio = 1:1.3) (200mL), dried under reduced pressure, 22.86 g of light yellow powdery solid wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com