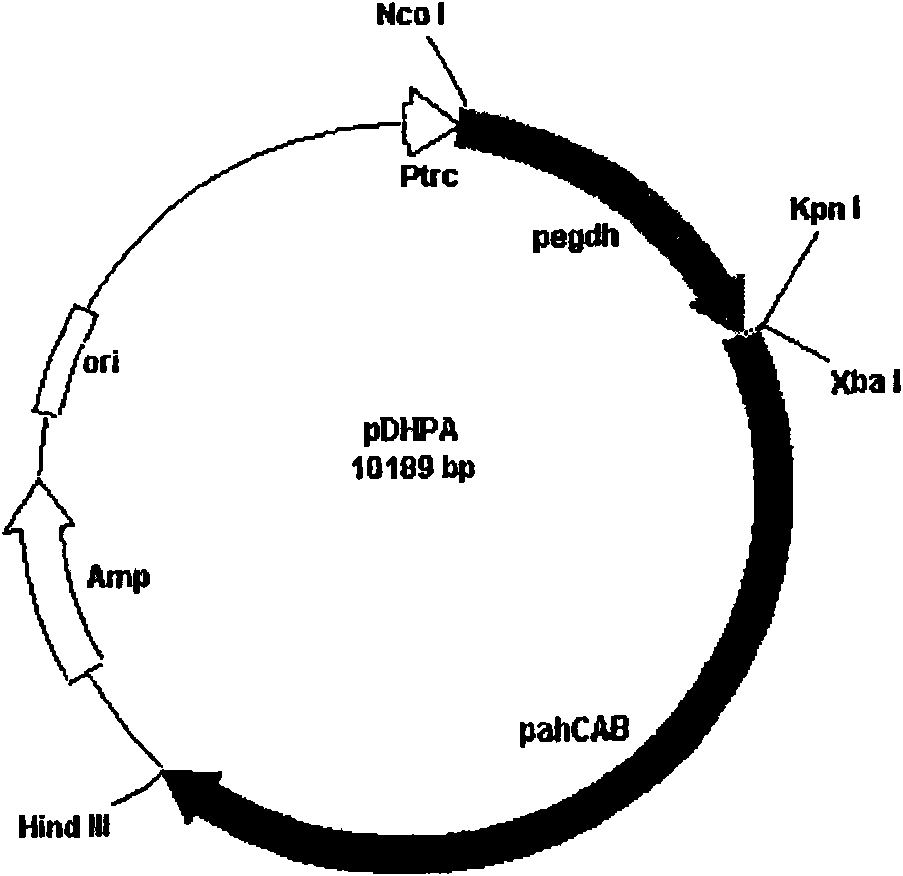
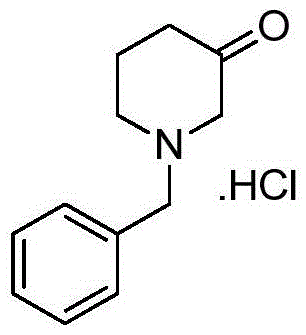
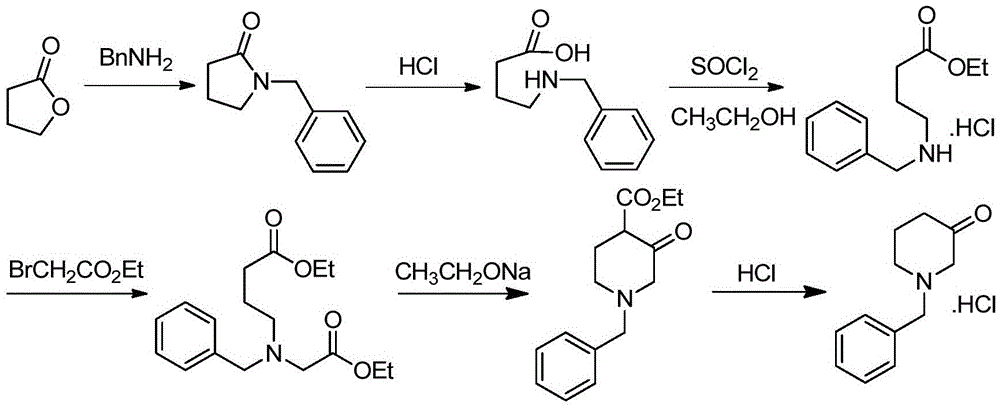
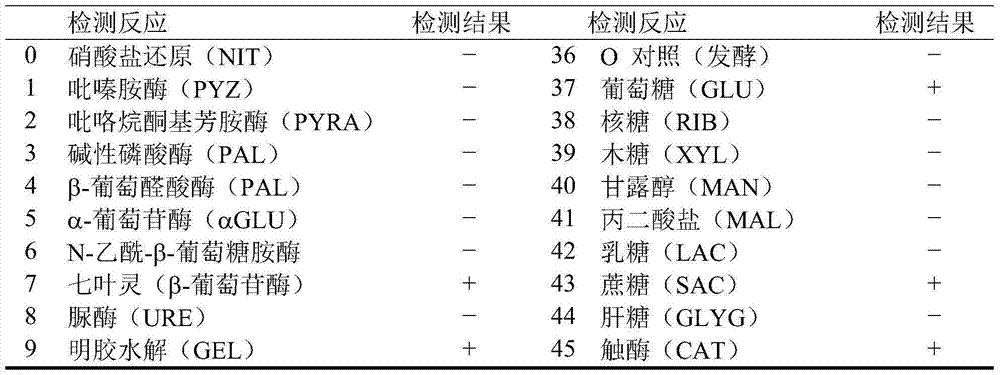
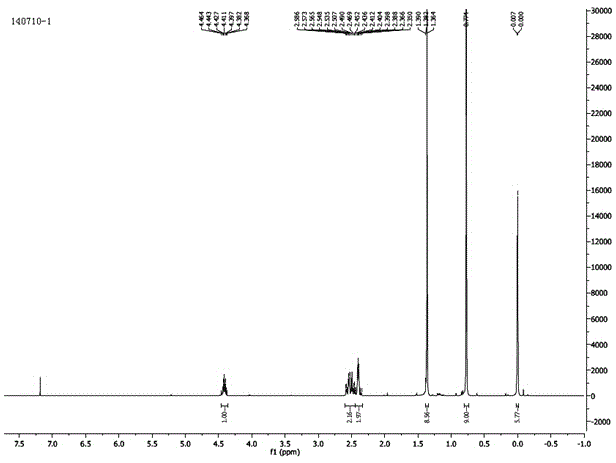
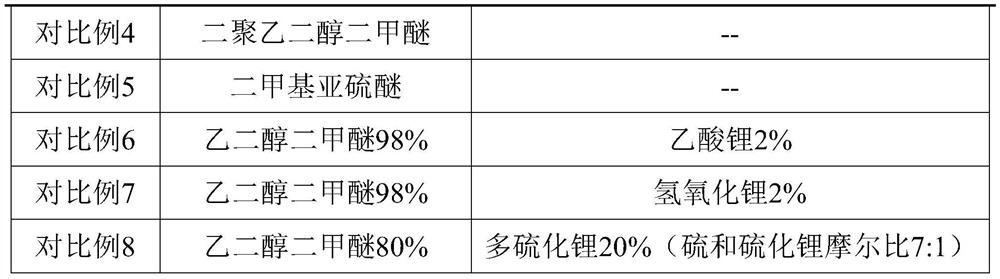
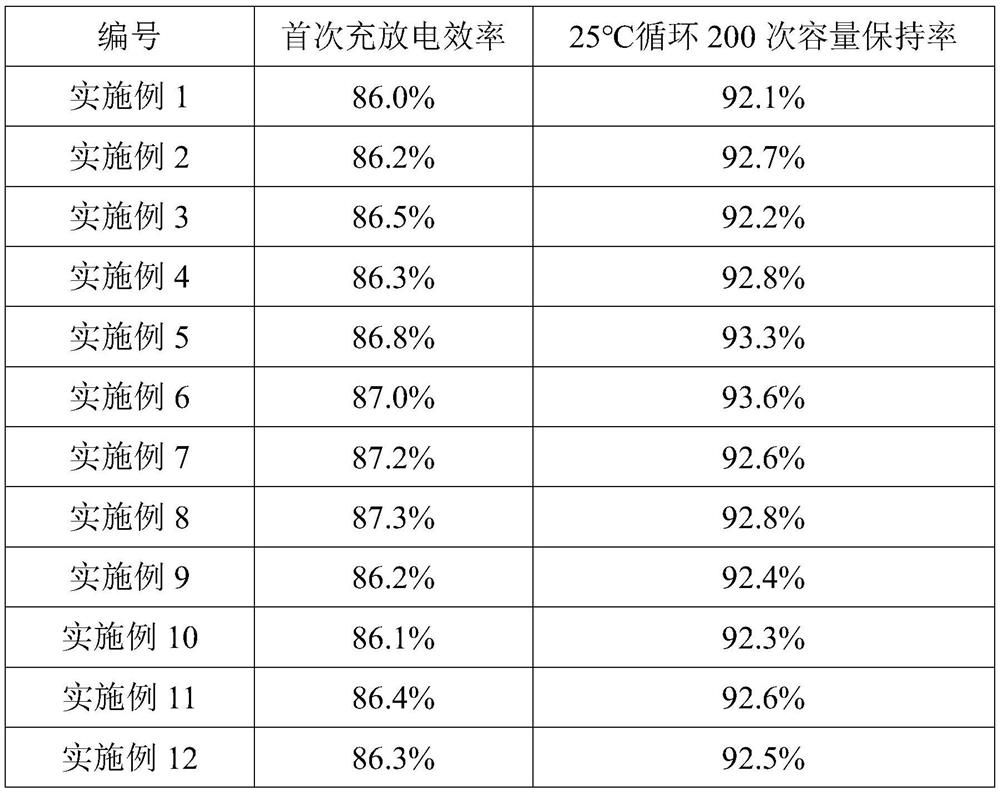
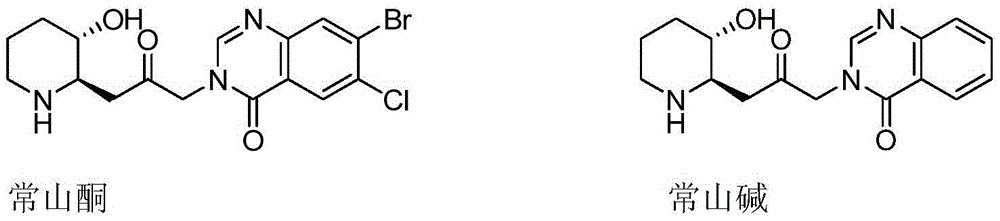Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
126 results about "Butyric acid ethyl ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Flavouring ingredient Ethyl butyrate, also known as ethyl butanoate, or butyric ether, is an ester with the chemical formula CH3CH2CH2COOCH2CH3, with one oxygen having a double bond. It is soluble in propylene glycol, paraffin oil and kerosene.; It can be synthesized by reacting ethanol and butyric acid.
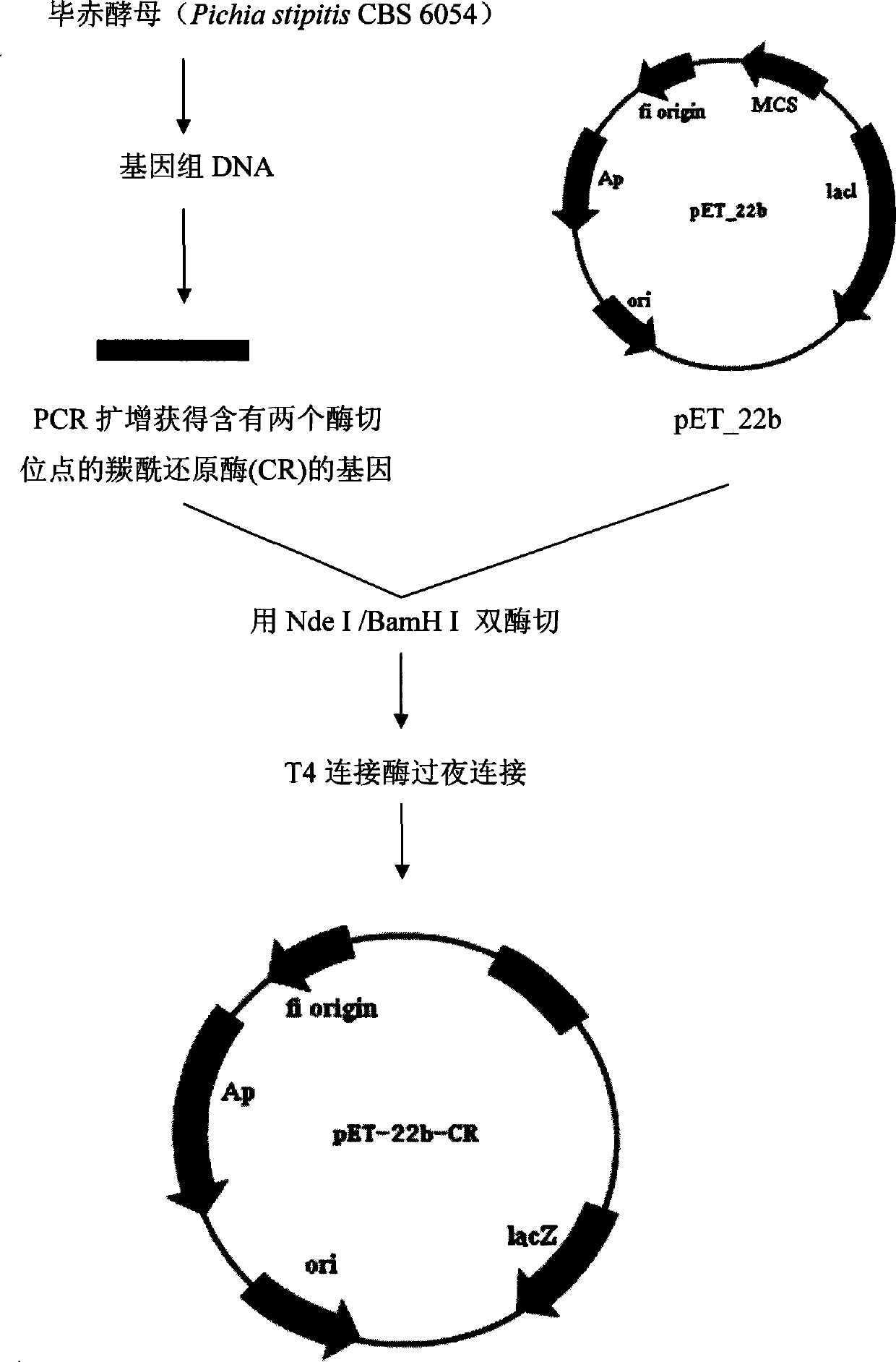
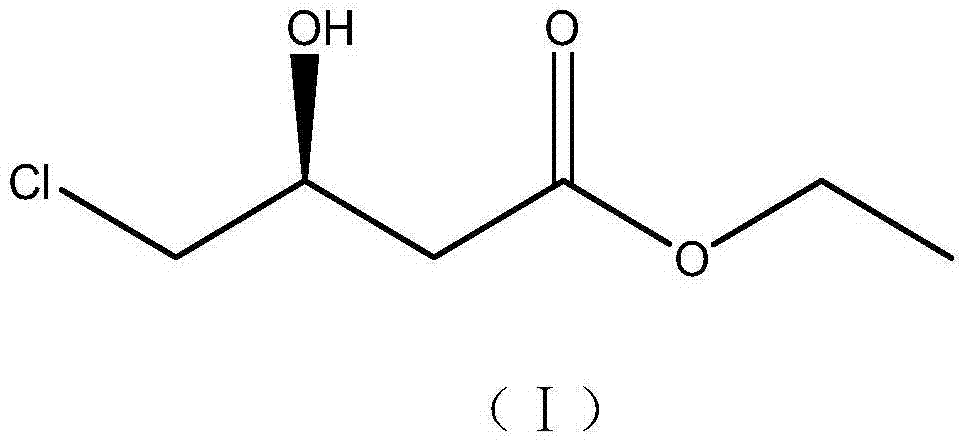
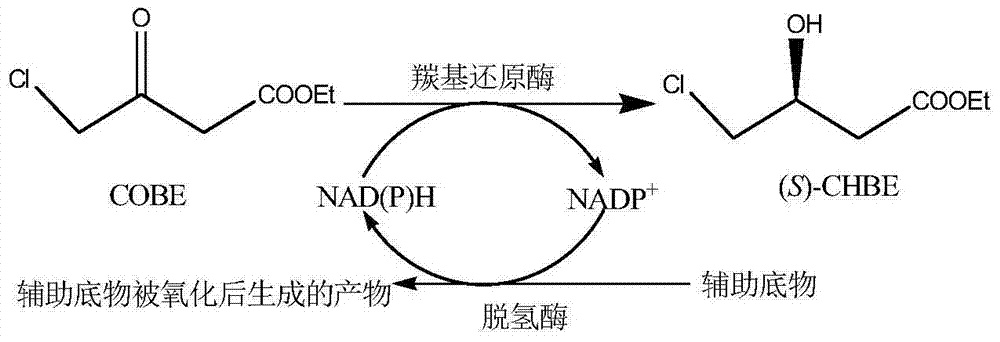
Use of carbonyl reductase in (S)-4-chloro-3 hydroxy butyric ether production
The invention discloses an application of carbonyl reductase with the amino acid sequence as shown in SEQ ID NO:2 to prepare (S)-4-chloro-3-hydroxybutanoic ethyl ester by asymmetric reduction of 4-chloracetyl ethyl acetate. The carbonyl reductase with the amino acid sequence as shown in SEQ ID NO:2 is taken as a catalyst, the ethyl 4-chloracetyl ethyl acetate is taken as a substrate, and NADPH is taken as a cofactor, and the (S)-4-chloro-3-hydroxybutanoic ethyl ester is prepared by the asymmetric reduction. The carbonyl reductase with the amino acid sequence as shown in SEQ ID NO:2 is applied to preparing the (S)-4-chloro-3-hydroxybutanoic ethyl ester by the asymmertric reduction of the 4-chloroacetyl ethyl acetate for the first time, which produces good effect; the yield of the substrate is up to 95%, and the enantiomer excess value of the product is 100%, the yield is high, and the production cost is greatly reduced.
Owner:NANJING UNIV OF TECH
Biological catalysis method for preparing statin medicinal intermediate
The invention provides a method for preparing and synthesizing a statin medicinal intermediate, namely, an (R)-3-hydroxy-glutaric acid ethyl ester or a hydroxy substituted derivative of the (R)-3-hydroxy-glutaric acid ethyl ester with high optical activity by performing chiral separation on a racemic 4-cyano-group-3-hydroxy butyric acid ethyl ester or a hydroxy substituted derivative of the 4-cyano-group-3-hydroxy butyric acid ethyl ester and a strain. The method comprises the following steps: performing a conversion reaction in a reaction system in which a compound shown in a formula (11) is used as a substrate and an enzyme obtained by fermenting and culturing rhodococcus erythropolis CCTCC No:M 209244 or CCTCC No:M 209194 is used as a catalyst at the temperature of between 10 and 50 DEG C; and after the reaction, separating and purifying reaction liquid to obtain the compound shown in formula (I). The method realizes obtaining the (R)-3-hydroxy-glutaric acid ethyl ester or the hydroxy substituted derivative of the (R)-3-hydroxy-glutaric acid ethyl ester with high optical activity by catalyzing and hydrolyzing the racemic 4-cyano-group-3-hydroxy butyric acid ethyl ester or the hydroxy substituted derivative of the 4-cyano-group-3-hydroxy butyric acid ethyl ester in one step under the condition of not hydrolyzing an ester group in the substrate or producing any byproduct, and overcomes the defects of more byproducts, low target product yield, low optical purity and the like brought by a conventional synthetic method in a hydrolyzing process, such as a chemical method.
Owner:ZHEJIANG UNIV OF TECH
Activity enhanced ketoreductase mutant, coding sequence and preparation method thereof
ActiveCN104342411ASignificantly high specific enzyme activityMild reaction conditionsBacteriaMicroorganism based processesStatineButyric acid ethyl ester
The invention relates to an activity enhanced ketoreductase mutant, which is derived from wild-type ketoreductase of Candida magnoliae, can transform 4-chloroacetoacetic acid ethyl ester into (S)-4-chloro-3-hydroxy butyric acid ethyl ester, and has one or more mutations of E9R, S42Q, T190L and E234M. The ketoreductase mutant provided by the invention has obvious high specific enzyme activity, which is increased by 2-20 times than wild-type ketoreductase. According to the invention, the reaction conditions are mild, the requirement for equipment is low, the production process has need for high temperature or cooling, the energy consumption is low, and as enzyme catalysis has high efficiency and specific selectivity, production of the statin drug key intermediate (S)-4-chloro-3-hydroxy butyric acid ethyl ester by the method generates no by-product, and purification can be convenient. In addition, most solvents involved in the reaction of the invention are water, thus having low discharge of three wastes, and being green and environment-friendly.
Owner:NANJING NUOYUN BIOLOGICAL TECH CO LTD
Ketoreductase mutant for producing (S)-4-chloro-3-hydroxy ethyl butyrate
ActiveCN104342412ASignificantly high specific enzyme activityMild reaction conditionsBacteriaMicroorganism based processesEthyl butyrateLactobacillus kefir
The invention relates to a ketoreductase mutant deriving from wild type ketoreductase of lactobacillus kefir. The ketoreductase mutant is capable of converting ethyl-4-chloroacetoacetate to generate (S)-4-chloro-3-hydroxy ethyl butyrate and has one or multiple of mutations in A94S, F147V, L199P and A202V. The ketoreductase mutant provided by the invention has obvious high specific enzyme activity which is improved by 2-20 times in comparison with the wild type ketoreductase; the enzyme can be used for biologically catalyzing to convert the ethyl-4-chloroacetoacetate to generate (S)-4-chloro-3-hydroxy ethyl butyrate; the reaction condition is mild, and the requirement on equipment is low, the production process is free from high temperature or cooling, and the energy consumption is low; since the enzyme catalysis is efficient and unique in selectivity, the method can be used for producing statins key intermediate (S)-4-chloro-3-hydroxy ethyl butyrate without producing byproduct, and the purification is convenient; in addition, the vast majority solvent in the reaction is water, the three-waste emission is low, and the ketoreductase mutant is environmental friendly.
Owner:NANJING LANGEN BIOLOGICAL SCI & TECH
Method for biologically preparing (S)-4-chloro-3-hydroxy butyric acid ethyl ester with recombinant escherichia coli expressed ketoreductase
InactiveCN103173503AHigh optical purityGenerate efficientlyMicroorganism based processesFermentationHydroxybutyric acidEthyl acetate
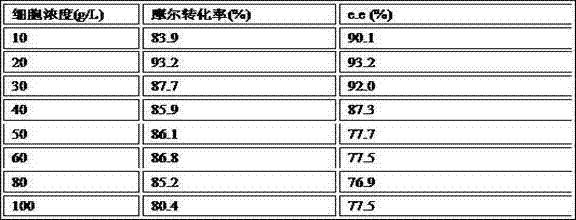
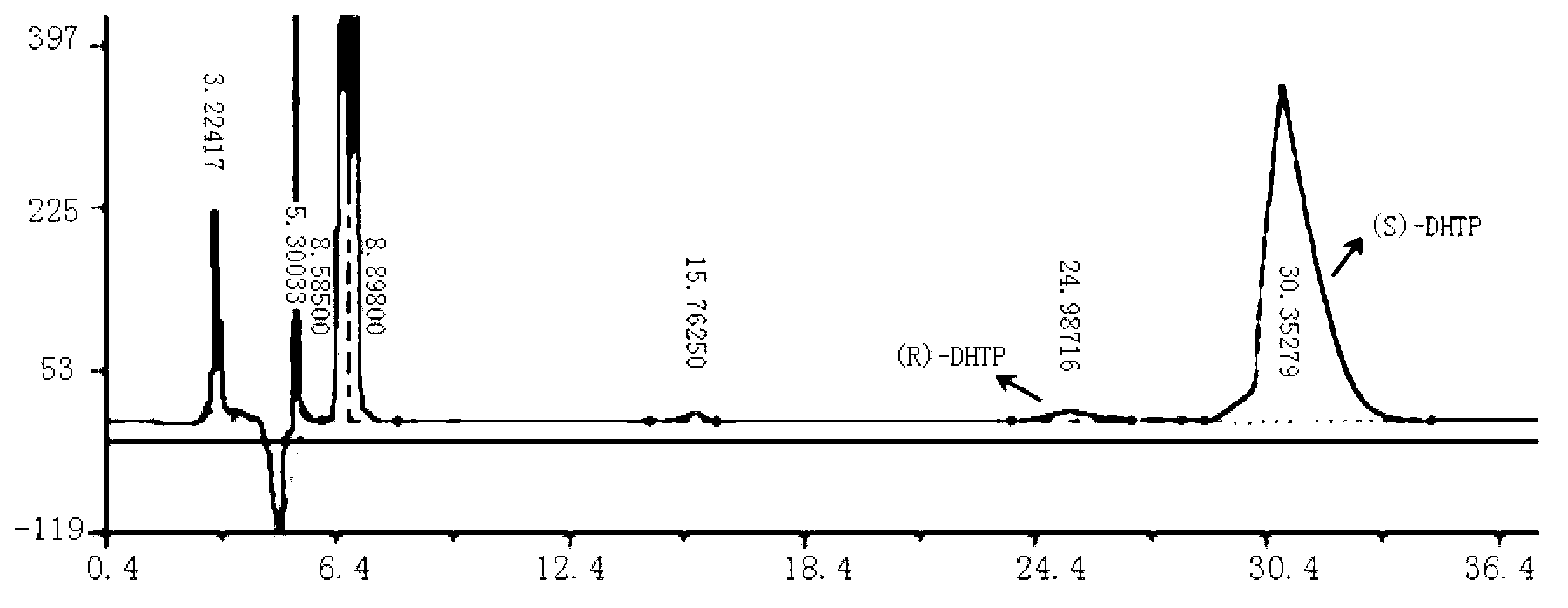
The invention discloses a method for biologically preparing (S)-4-chloro-3-hydroxy butyric acid ethyl ester with recombinant escherichia coli expressed ketoreductase and application thereof in preparing (S)-4-chloro-3-hydroxy butyric acid ethyl ester through asymmetric reduction of 4-chloroacetoacetic acid ethyl ester. The recombinant escherichia coli for expressing the gene of the ketoreductase (KRED) is coupled with glucose dehydrogenase (GDH) so that the escherichia coli cell is recombined into a catalyst; efficient preparation of (S)-4-chloro-3-hydroxy butyric acid ethyl ester through asymmetric reduction of 4-chloroacetoacetic acid ethyl ester in a single water phase is realized no matter in the presence of or in the absence of coenzyme NAD(P)H; and the molar transformation rate is higher than 92% and the enantiomer excess (e.e.) of the product is 100%.
Owner:JIANGXI NORMAL UNIV +1
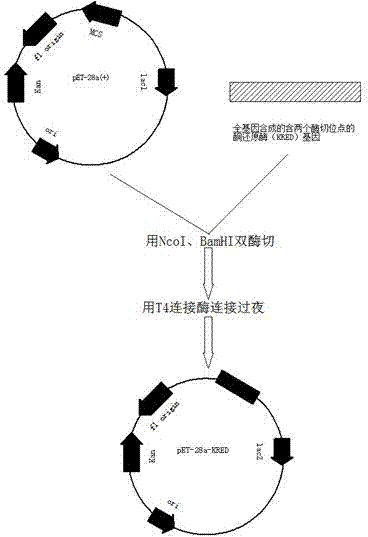
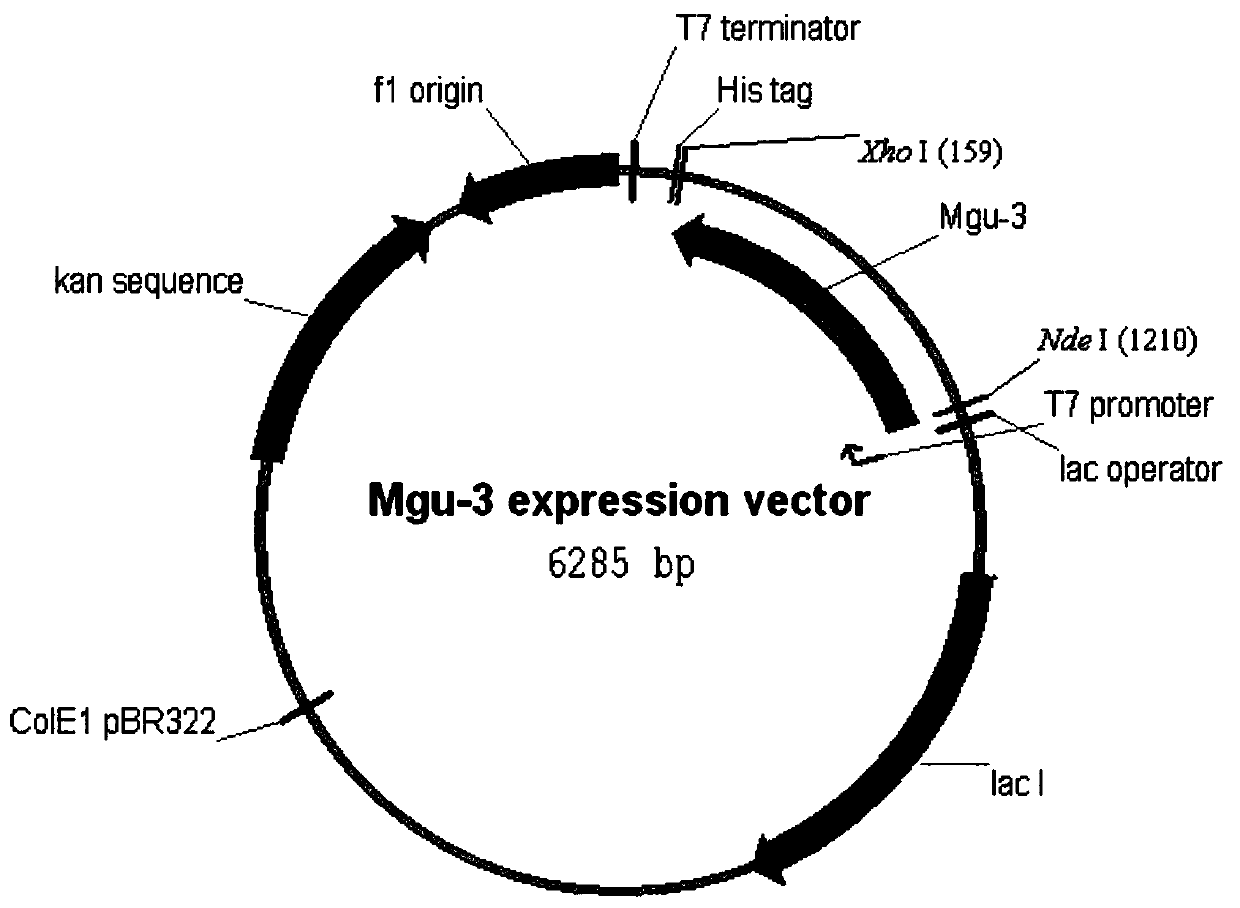
Carbonyl reductase expressed recombination engineering bacterium and application thereof
The invention discloses a carbonyl reductase expressed recombination engineering bacterium and application thereof. The engineering bacterium comprises a host cell and a recombinant vector transferred into the host cell, wherein the recombinant vector consists of an original vector and a target gene connected into the original vector, the host cell is Escherichiacoli Rosetta (DE3), the target gene is a carbonyl reductase gene, and the base sequence is shown in SEQ ID NO.1. According to the recombination engineering bacterium, the expression level of a carbonyl reductase is high, bacterial cells which are prepared through induced culture are used for converting N,N-dimethyl-3-oxy-3-(2-thienyl)-l-propylamine hydrochloride into (S)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)-l-propylamine or converting 4-chloroethyl acetoacetate into (S)-4-chloro-3-hydroxyethyl butyrate, and the two products are higher in both optical purity and conversion rate.
Owner:NINGBO MENOVO PHARMA +1
Application of aldo-keto reductase in catalytic generation of (R)-4-chlorine-3-hydroxy ethyl butyrate
InactiveCN104726507ADisinhibition effectImprove conversion abilityBacteriaOxidoreductasesHydroxybutyric acidPtru catalyst
The invention discloses an application of aldo-keto reductase in catalytic generation of (R)-4-chlorine-3-hydroxy ethyl butyrate. With aldo-keto reductase of which an amino acid sequence is as shown in SEQ ID NO:2 as a catalyst, with 4-ethyl acetoacetate as a substrate and NADPH as a cofactor, the (R)-4-chlorine-3-hydroxy ethyl butyrate is prepared by asymmetric reduction. The aldo-keto reductase of which the amino acid sequence is as shown in SEQ ID NO:2 is applied to the 4-ethyl acetoacetate to prepare the (R)-4-chlorine-3-hydroxy ethyl butyrate in an asymmetric reduction manner for the first time, so that a good effect is obtained; the enzyme activity can be up to 8.1U / mg; the conversion ratio of the aldo-keto reductase on the substrate can be up to 99.8%; the enantiomer excess value of the product is greater than 995; the yield is high; and the production cost is greatly reduced.
Owner:NANJING UNIV OF TECH
Novel esterase as well as coding gene and application of esterase
InactiveCN104140959AImprove qualityMild reaction conditionsHydrolasesMicroorganism based processesPropanoic acidNucleotide
The invention discloses a novel esterase as well as a coding gene and an application of esterase. The esterase EST04211 has an amino acid sequence shown in SEQ ID NO. 2. The amino acid sequence of the gene of the esterase EST04211 is shown in SEQ ID NO. 1. The novel esterase disclosed by the invention is obtained by cloning Bacillus SCSIO15121 derived from deep sea of the Indian Ocean. The esterase has the largest characteristic that the esterification rate is 97% in catalytic synthesis of ethyl isobutyrate, and under similar conditions, the esterase can also catalyze propionic acid, butyric acid, valeric acid, caproic acid and ethanol, propanol, butanol, pentanol and hexanol to synthesize corresponding short-chain aromatic ester flavors, such as ethyl propionate, propyl propionate and butyl propionate, the esterification rate mostly reaches 85%-95%. The esterase can be applied in the industrial bio-manufacturing of the short-chain aromatic flavors, such as ethyl isobutyrate and the ester flavors are high in quality, belongs to natural products and can be applied in production industries, such as foods, cigarettes and daily chemical products.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Synthetic method of atorvastatin calcium intermediate
InactiveCN103614430AFew stepsLow costOrganic chemistryMicroorganism based processesEthyl groupNitromethane
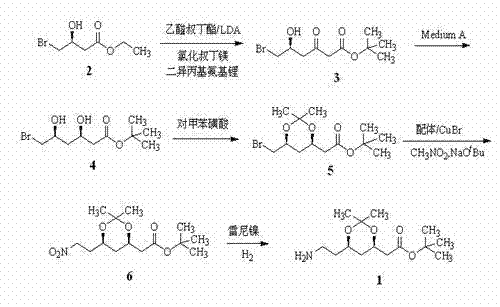
The invention discloses a synthetic method of an atorvastatin calcium intermediate. The synthetic method comprises the following steps: preparing [(4R, 6S)-6- brooethyl-2,2-diemthyl-1,3-dioxane-4-group] tert-butyl acetate (5) by (3S)-4-bromine-3-hydroxyl ethyl butyrate through condensation, asymmetric biological catalytic reduction and hydroxyl protection; and preparing an atorvastatin calcium intermediate [(4R, 6S)-6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxane-4-group] tert-butyl acetate through catalytic hydrogenation reduction of raney nickel after 5 is condensed with nitromethane under action of cuprous bromide and a nitrogen-containing compound ligand. According to the synthetic method disclosed by the invention, steps are shortened, cost is lowered, and therefore, the synthetic method is more suitable for large-scale preparation.
Owner:SUZHOU HEALTH COLLEGE
Leifsonia xyli HSO904-based short chain dehydrogenase, and encoding gene, carrier, engineering bacteria and application thereof
The invention discloses a leifsonia xyli HSO904-based short chain dehydrogenase, and an encoding gene, a carrier, engineering bacteria and application thereof. A gene of the leifsonia xyli HSO904-based short chain dehydrogenase has more than 90% of homology of a nucleotide sequence shown in SEQ ID NO. 1. A colon bacillus BL21 / pET28a (+)-SDR prepared by the recombination of the short chain dehydrogenase is used as an enzyme source, 3, 5-bis-trifluoro methyl acetophenone, trifluoromethyl acetophenone, 4-hydroxyl-2-butanone, acetoacetic ester, 4-chloro ethyl acetoacetate, acetoacetic acid tert-butyl acetate and the like are used as substrates to prepare corresponding chiral compounds such as (R)-3, 5-bis-trifluoromethyl phenethyl alcohol, trifluoromethyl benzaldehyde ethanol, 2-hydroxyl-butyl alcohol, 3-hydroxy ethyl butyrate, 4-chloro-3-hydroxy ethyl butyrate and 3-hydroxy butyric acid tert-butyl acetate through a catalytic asymmetric reduction reaction.
Owner:艾吉泰康(嘉兴)生物科技有限公司
Asymmetric syntheses method of ophthalmologic drug bepotastine besilate
The invention relates to an asymmetric synthesis method of an ophthalmologic drug pylistramine besylate. The method concretely comprises the following steps: oxidizing (4-chlorophenyl)(2-pyridyl)methanone to obtain (4-chlorophenyl)(2-pyridyl)ketone-N-oxide; carrying out asymmetric transfer hydrogenation reduction on the (4-chlorophenyl)(2-pyridyl)ketone-N-oxide through using a complex of monosulfonyl chiral diamine and metallic ruthenium, rhodium and iridium as a catalyst and a sodium formate or formic acid and triethylamine mixture or isopropanol as a hydrogen source in order to prepare (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide; reducing the (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide to prepare (S)-(4-chlorophenyl)(2-pyridyl)methanol; and condensing the (S)-(4-chlorophenyl)(2-pyridyl)methanol and ethyl 4-(4-bromopiperidine-1-yl)butyrate to obtain the ophthalmologic drug pylistramine besylate. The total yield is 61.6%.
Owner:YICHANG HUMANWELL PHARMA +1
Novel piracetam synthetic method
InactiveCN102718691AThe reaction steps are simpleMild reaction conditionsOrganic chemistryChemical synthesisOrganosolv
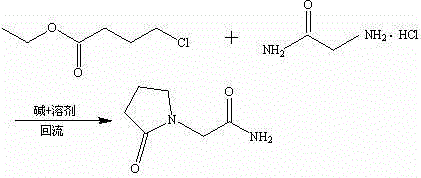
The invention belongs to the field of chemical synthetic techniques, in particular relates to a novel piracetam synthetic method. The method comprises the following steps of: mixing ethyl-4-chloro-n-butanoate, glycinamide hydrochloride, alkaline and an alcohol organic solvent, and heating and reflowing for 20 to 30 hours, and thermally filtering, evaporating to remove the solvent under the reduced pressure, and then recrystallizing, filtering and drying the residues to obtain white or almost white crystal which is piracetam, wherein the mole ratio of ethyl-4-chloro-n-butanoate to glycinamide hydrochloride to alkaline is 1: (1.1-1.8): (1.0-2.0). The method is simple and short in reaction step, and mild in reaction condition, is convenient to operate for production; and moreover, the method is environment-friendly, and the synthetic raw materials and solvents are cheap and easy to obtain, thus the method is suitable for industrial production.
Owner:SHANGHAI MODERN HASEN SHANGQIU PHARMA
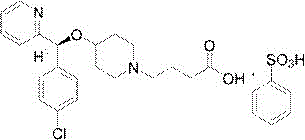
Improved preparation method of bepotastine besilate
The invention relates to an improved preparation method of bepotastine besilate.The improved preparation method of bepotastine besilate includes the following steps that firstly, 2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine and a resolving agent are reacted, and S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine N-acetyl-L-phenylalaninate is obtained; secondly, S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine N-acetyl-L-phenylalaninate is added with acid to be extracted, and S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine is obtained; thirdly, S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine and ethyl 4-bromobutyrate are subjected to a coupling reaction, a hydrolysis reaction and a salt-forming reaction in sequence in acetonitrile, and bepotastine besilate is obtained.According to the technical scheme, the yield is greatly increased, the process time is shortened, and the obtained product is high in purity, safe and stable.
Owner:SHIJIAZHUANG GERUI PHARMA CO LTD
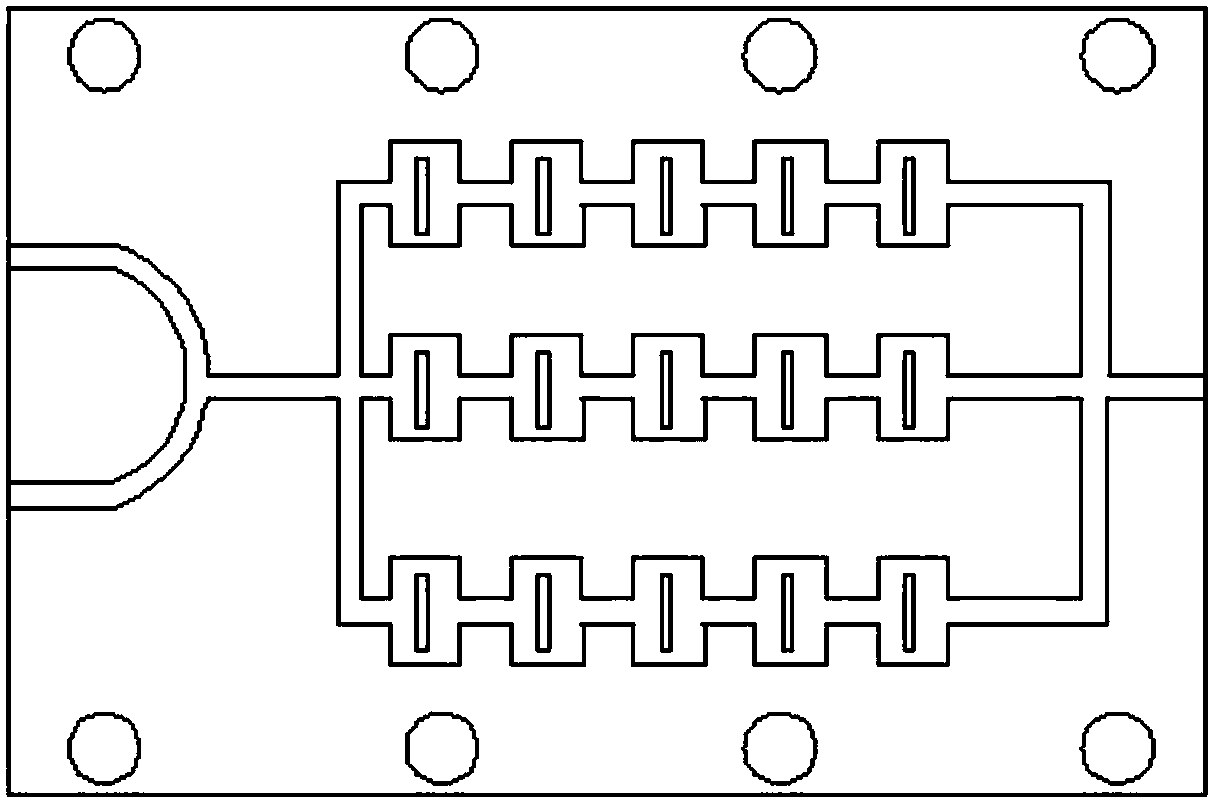
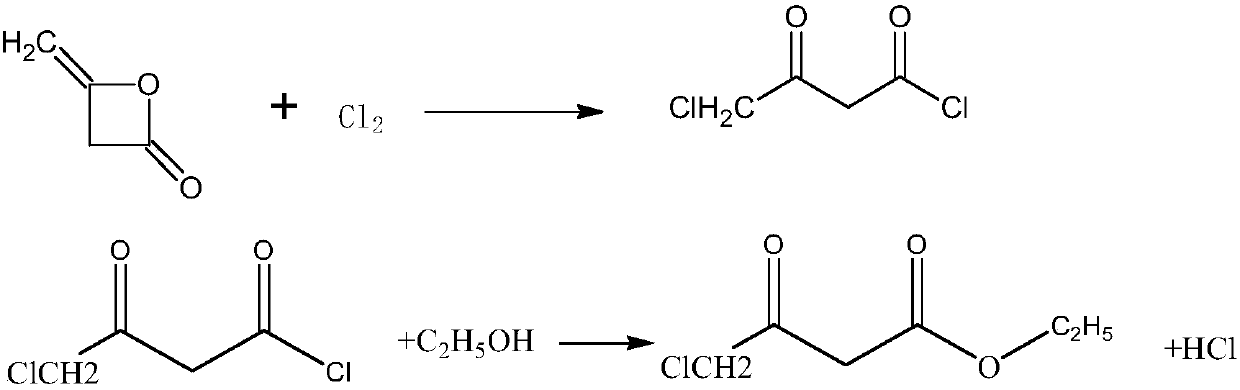
Continuous flow synthesizing method and system for 4-chloro-3-oxo-ethyl butyrate without solvent
InactiveCN107915622AProcess environmental protectionReduce manufacturing costPreparation from carboxylic acid halidesOrganic compound preparationAlcoholContinuous flow
The invention belongs to the field of chemical engineering, and particularly relates to a continuous flow synthesizing method and a system for 4-chloro-3-oxo-ethyl butyrate without solvent. The methodcomprises the following steps of in the reaction process, firstly, performing chlorination reaction on diketene and liquid chloride through a micro-channel reactor module 8, and then performing esterification reaction on the chlorinating liquid and ethyl alcohol through a reaction module 13, so as to obtain a crude product of the 4-chloro-3-oxo-ethyl butyrate; performing pressure-relief rectification on the crude product, so as to obtain a finished product of the 4-chloro-3-oxo-ethyl butyrate. The method can solve the problem that because the micro-channel reactor has certain drag, the liquidchloride cannot be fed by a conventional feeding device; in the micro-channel reactor, the mixing effect of gas and liquid reaction is poorer than the mixing effect of liquid and liquid reaction, themetering accuracy of gas is poor, the amount of reaction byproducts is increased, the yield rate is reduced, and the like.
Owner:NANTONG ACETIC ACID CHEM +1
Method for preparing chiral medicinal intermediate R-3-hydroxy butyric acid ethyl ester by microbial fermentation
ActiveCN101864457AReduce manufacturing costBreakthrough costOrganic compound preparationCarboxylic acid esters preparationButyric acid ethyl esterButyric acid
The invention discloses a method for preparing chiral medicinal intermediate R-3-hydroxy butyric acid ethyl ester. By using the method, a regenerative carbon source such as starch and the like can be efficiently and stably converted into (R)-3-hydroxy butyric acid by constructing multiple enzymes such as pha, phb and phc required for synthesizing the (R)-3-hydroxy butyric acid ethyl ester into genes of E.Coli, and the (R)-3-hydroxy butyric acid ethyl ester can be obtained by means of an auxiliary substrate ethanol at the same time. The preparation method of the invention can greatly lower theproduction cost so as to break through the technical bottlenecks of high industrialized cost and low chiral purity of the (R)-3-hydroxy butyric acid ethyl ester.
Owner:SHENZHEN ECOMANN BIOTECH
Environmental protection preparation method of (R)-(-)-4-cyano-3-hydroxy ethyl butyrate
PendingCN109628511AReduce manufacturing costAtom economy is highFermentationChemical industryEthyl butyrate
The invention discloses an environmental protection preparation method of (R)-(-)-4-cyano-3-hydroxy ethyl butyrate and belongs to the technical field of medicine and chemical industry. The method comprises the following steps of: using (S)- epichlorohydrin as a start material, carrying out nucleophilic addition reaction on the epichlorohydrin and liquid hydrocyanic acid under the catalysis of alkali to obtain (S)-(-)-4-chlorine-3-hydroxybutyronitrile; carrying out Pinner reaction on the (S)-(-)-4-chlorine-3-hydroxybutyronitrile and hydrogen chloride-ethanol to obtain a hydrochloride intermediate of urethane, and then hydrolyzing the intermediate to obtain (S)-(-)-4-chlorine-3-ethyl hydroxybutyrate; finally, reacting (S)-(-)-4-chlorine-3-ethyl hydroxybutyrate and hydrocyanic acid and ammonia water under the analysis of biological enzyme to prepare the (R)-(-)-4-cyano-3-hydroxy ethyl butyrate. In the whole synthesis process of the method, other organic solvents other than water are not used, the reaction condition is mild, the process is simple, the chemical purity and optical purity of the product are higher, no waste water, waste salt or waste gas is generated in the whole process,the method achieves win-win of economic benefit and environmental benefit and is favorable for achieving large-scale industrial production.
Owner:抚顺顺能化工有限公司
Preparation method for 1-benzyl-3-piperidone hydrochloride
ActiveCN105622444ALow costHigh purityOrganic compound preparationAmino-carboxyl compound preparationAcetic acidOrganic solvent
The invention discloses a preparation method for N-benzyl glycine ethyl ester. The method comprises the following steps: dissolving benzylamine in an organic solvent I, then adding 2-halogenated ethyl acetate, alkali and quaternary ammonium salt, and performing reaction to obtain the N-benzyl glycine ethyl ester. The invention also discloses a preparation method for 1-benzyl-3-piperidone hydrochloride. The method comprises the following specific steps: (1) preparing an intermediate IV (N-benzyl glycine ethyl ester); (2) dissolving the intermediate IV in an organic solvent II, then adding 4-halogenated ethyl acetate and alkali, and performing reaction to obtain an intermediate III; (3) performing reaction between the intermediate III and the alkali, reversely regulating a pH value to 6-8, performing concentration under reduced pressure, extracting by using ethyl acetate, performing washing and drying, and then performing concentration under reduced pressure to obtain an intermediate II; (4) performing reaction on the intermediate II and acid, performing rotary evaporation concentration, and adding a crystal solvent for crystallization to obtain a product. The route synthesis steps are short, the process is novel, the intermediates are high in purity, the product yield is high, and the cost is low.
Owner:CHONGQING WEIPENG PHARMA
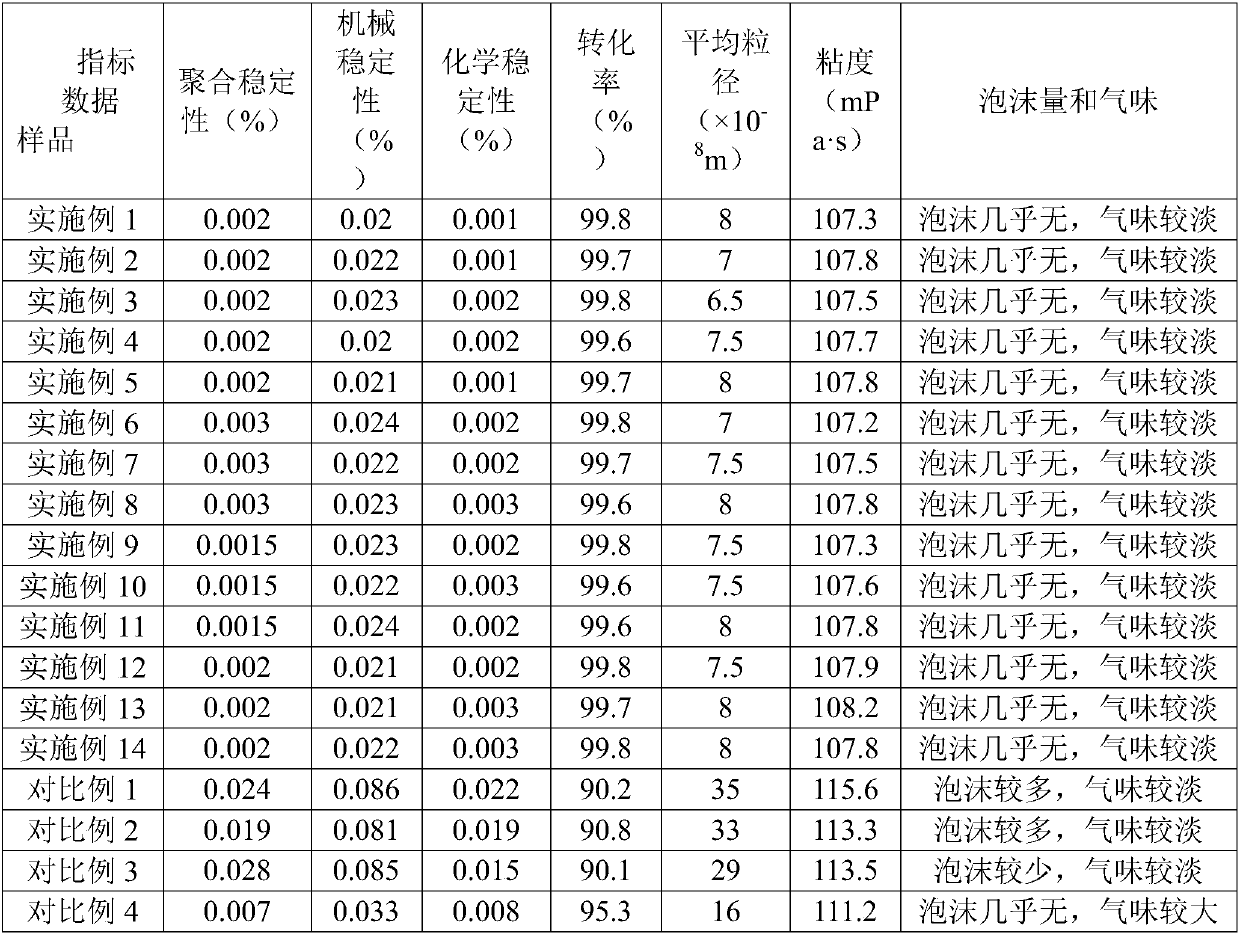
Carboxylic butadiene-styrene latex and preparation technology thereof
InactiveCN107936165AImprove polymerization stabilityImprove mechanical stabilityPolymer scienceMeth-
The invention discloses carboxylic butadiene-styrene latex and a preparation technology thereof. The carboxylic butadiene-styrene latex is prepared from water phase materials, oil phase materials, priming materials, and other materials, wherein the water phase materials comprise a first solvent, a first emulsifier, acrylic acid, N-hydroxymethyl acrylamide, acrylamide and a pH (potential of hydrogen) regulator; the oil phase materials comprise styrene, butadiene and ethyl 3-mercaptobutyrate; the priming materials comprise mbs seed latex, sodium dodecyl benzene sulfonate, and a liquid alkaline mixture; the other materials comprise a fourth solvent, ethylenediaminetetraacetic acid disodium salt, an initiator mixture, a water-based defoamer, liquid alkaline, and a second emulsifier; the firstemulsifier selects an anion emulsifier; the second emulsifier selects a non-ionic emulsifier; the first solvent and the fourth solvent are respectively soft water. The prepared carboxylic butadiene-styrene latex has the advantages that the conversion rate is high, and the polymerizing stability, mechanical stability and chemical stability are good.
Owner:杭州龙驹合成材料有限公司
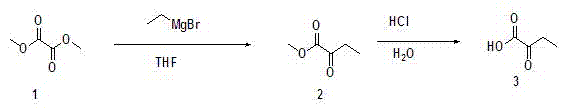
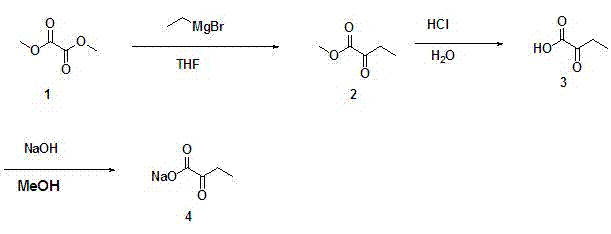
Ketobutyric acid and preparation method for ketobutyric acid salt
ActiveCN103073411AHigh yieldHigh purityOrganic compound preparationCarboxylic acid esters preparationDistillationEthyl group
The invention provides an Alpha-ketobutyric acid preparation method which comprises the following steps: A), taking dimethyl oxalate or diethyl oxalate as raw materials to solve in anhydrous furanidine, cooling, adding ethylmagnesium bromide solution, stirring, removing solvent through distillation under reduced pressure after reaction termination, allowing residual mixture to be subjected to rectification under reduced pressure, and collecting intermediate product 2-oxo methyl butyrate or 2-oxo ethyl butyrate; B), solving the intermediate product in a hydrochloric acid, heating, reflowing, stirring, performing extraction by vinegar naphtha after the completion of the reaction, collecting organic phase, drying, filtering, and removing the solvent through allowing the filtrate to be subjected to distillation under reduced pressure, so as to obtain a coarse product Alpha-ketobutyric acid; and c), allowing the coarse product Alpha-ketobutyric acid to be subjected to rectification under the reduced pressure, and collecting the Alpha-ketobutyric acid. The method provided by the invention has the advantages of inexpensive and easy-to-get raw materials, lower toxicity, simple preparation process, easiness in control, no complex purification process, high product purity, high productivity, and greatly reduced manufacturing cost, and is suitable for large scale industrialized application.
Owner:广东派特埃尔生物科技有限公司
Rhodococcus qingshengii and application thereof in preparation of ethyl (S)-4-chloro-3-hydroxy butyrate
ActiveCN103589665AImprove stabilityStrict stereoselectivityBacteriaMicroorganism based processesHydroxybutyric acidMicroorganism
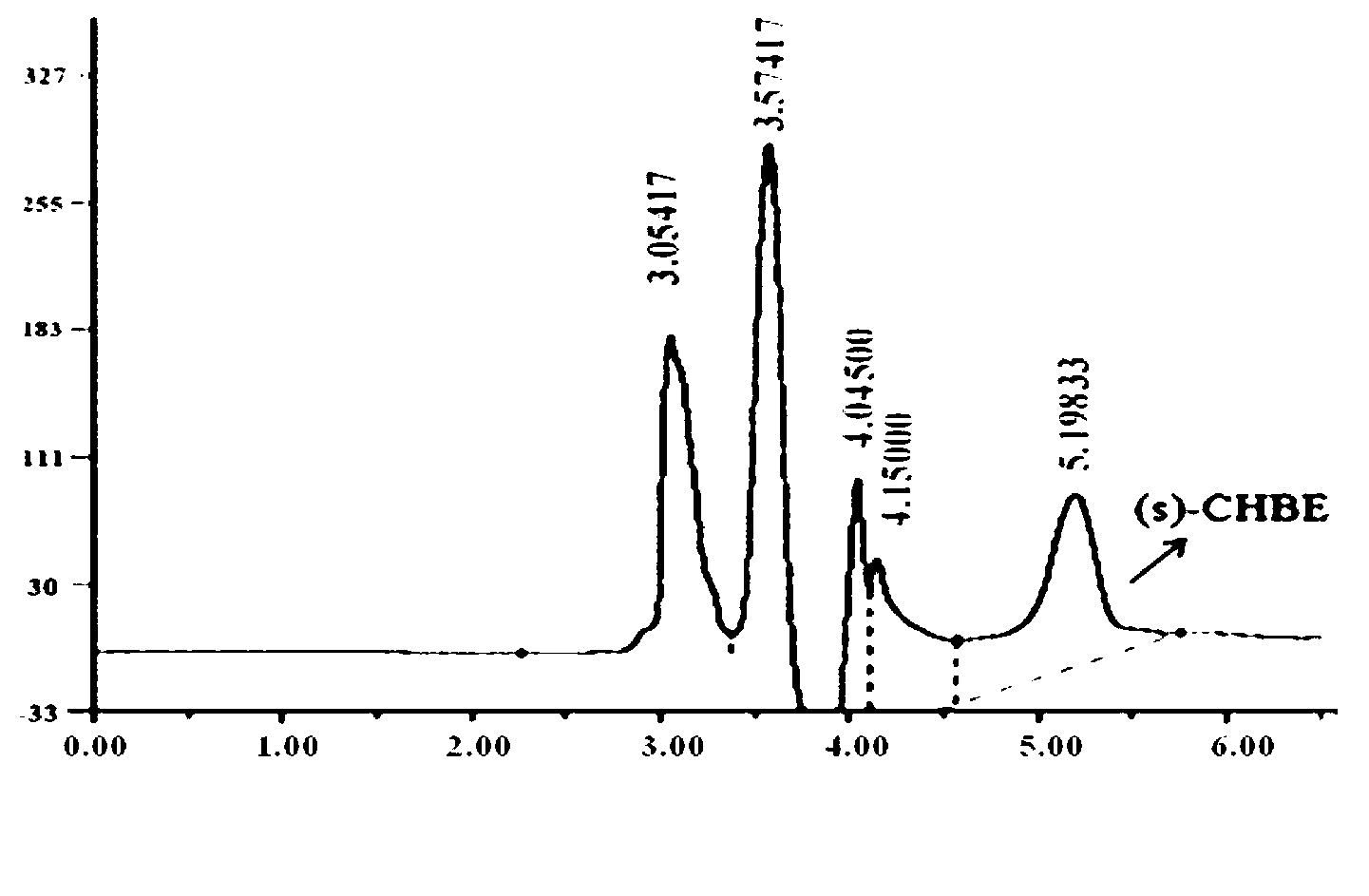
The invention provides a new strain-Rhodococcus qingshengii ZJB-12028 and an application of the strain in preparation of an atorvastatin key chiral intermediate ethyl (S)-4-chloro-3-hydroxy butyrate ((S)-CHBE) through microbial asymmetric reduction of ethyl-4-chloroacetoacetate. The strain is preserved in China Center for Type Culture Collection, wherein the address is Wuhan University, Wuhan, China 430072, the preservation date is September 4, 2013, and the preservation number is CCTCC NO: M2013390. The strain has good stability, strict stereoselectivity and high optical purity of product and has the advantages of mild reaction conditions, environmental friendliness and the like when being used for preparing (S)-CHBE, so that the strain has a high industrial application potential.
Owner:ZHEJIANG UNIV OF TECH
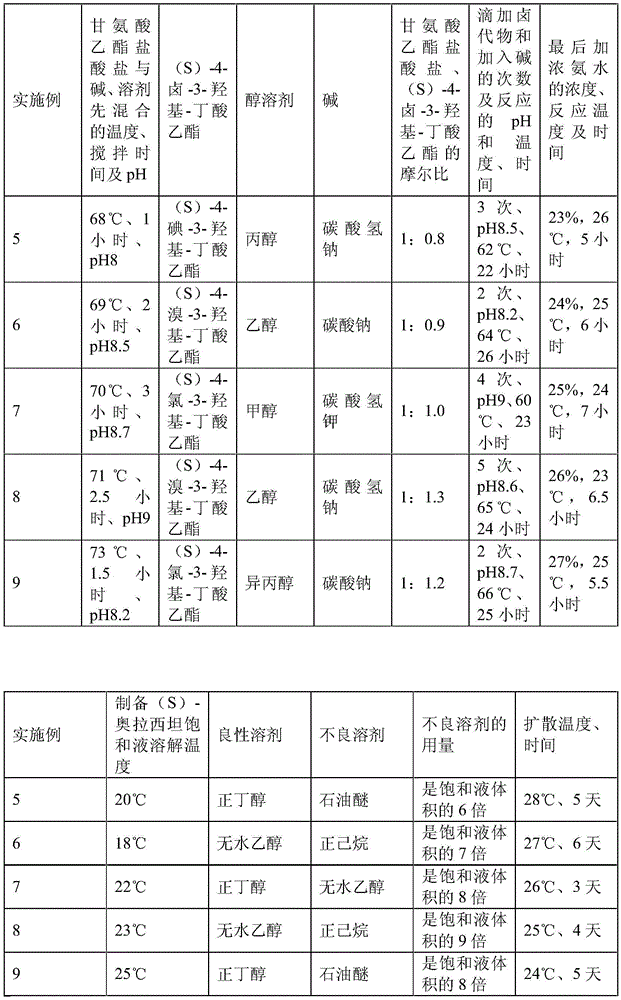
A preparation method of (s)-oxiracetam
The invention relates to a preparation method of (S)-oxiracetam, which comprises the following steps: reacting raw materials glycine ethyl ester hydrochloride and ethyl (S)-4-halo-hydroxy-butyrate in an alcohol solvent under alkaline conditions, filtering, washing with inorganic alcohol, concentrating, extracting, concentrating the water phase, introducing stronger ammonia water to react to obtain a (S)-oxiracetam crude product, and carrying out purification treatment on the crude product. The glycine ethyl ester hydrochloride is firstly mixed with the alkali and alcohol solvent, and the raw material ethyl (S)-4-halo-hydroxy-butyrate is dropwisely added and the alkali is added in different batches to control the pH value of the reaction at 8-9. The purification treatment comprises the following steps: dissolving the (S)-oxiracetam crude product in a benign solvent to prepare a saturated solution at room temperature, and dispersing with a poor solvent in a closed environment. The HPLC (high performance liquid chromatography) purity of the prepared (S)-oxiracetam is up to 99.0% %, and the yield can be up to 33%; and the method is mild in reaction conditions, simple to operate and beneficial to industrial large-scale production.
Owner:CHONGQING RUNZE PHARM CO LTD
Ketoreductase mutant with enhanced enzyme activity and applications thereof
ActiveCN111454921AIncrease the number of cyclesExpand the range of downstream applicationsBacteriaMicroorganism based processesWild typeMeyerozyma guilliermondii
The invention discloses a ketoreductase mutant with enhanced enzyme activity and applications thereof, and belongs to the technical field of biology. The mutant is derived from the wild-type ketoreductase of Meyerozyma guilliermondii and can convert ethyl 4-chloroacetoacetate to generate ethyl (R)-4-chloro-3-hydroxybutyrate; and compared with wild-type sequences, the mutant has higher alcohol dehydrogenase activity and is more than 90% similar to SEQ ID NO. 8. The mutant has obvious high-specific enzyme activity which is enhanced 2-10 times than the wild-type ketoreductase; the mutant is mildin reaction condition and low in equipment requirement, and high temperature or cooling is not needed during production, so that low energy consumption can be realized; as enzyme catalysis has efficient and exclusive selection, so that convenient purification can be achieved; and in addition, the vast majority of solvents in reaction is water, and the solvents such as butyl acetate are not neededto be added to form a two-phase reaction system, so that the mutant is low in discharging of waste gas, waste water and industrial residues, green and environment-friendly in preparation process.
Owner:NANJING LANGEN BIOLOGICAL SCI & TECH
System for producing ethyl butyrate continuously
InactiveCN103058860AEasy to separateReduce pollutionOrganic compound preparationCarboxylic acid esters preparationPtru catalystButyrate
The invention discloses a system for producing ethyl butyrate continuously. The system comprises a feeding system, a reaction system and a separating system; the feeding system comprises a butyric acid raw material tank, an ethanol raw material tank and a metering pump; the reaction system comprises a mixer, a reactor, solid catalyst, a crude product tank and a metering pump; and the separating system comprises a primary classification tower, an oil-water separator, a secondary dealcoholization tower, a tertiary finished product tower and a metering pump. The system has the advantage that continuous production of ethyl butyrate is realized through continuous mixing, feeding reaction and three-stage rectification separation.
Owner:YIXING HENGXING FINE CHEM
Preparation method of S-4-chlorine-3-hydroxy butyric acid ethyl ester with optical purity
ActiveCN103145557AReduce dosageNo pollution in the processOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydroxybutyric acidPtru catalyst
The invention discloses a preparation method of S-4-chlorine-3-hydroxy butyric acid ethyl ester with optical purity. The preparation method taking ethyl 4-chloroacetoacetate as a raw material comprises the following steps of: placing ethyl 4-chloroacetoacetate and a Ru2C14 (R-BINAP or Segphos) 2NEt3 immobilized catalyst in a high-pressure kettle, wherein an alcohol solvent is adopted, the temperature is controlled at 20-100 DEG C, and the hydrogen pressure is 10-100atm, and preparing the S-4-chlorine-3-hydroxy butyric acid ethyl ester by adopting an asymmetrical hydrogenation method; and steaming out alcohol, and then steaming out the S-4-chlorine-3-hydroxy butyric acid ethyl ester, wherein the immobilized chiral catalyst can be continuously and mechanically applied. The preparation method has the advantages of low production cost, no generation of three wastes (waste water, waste gas and industrial reside) and no pollution to environments. The raw material ethyl 4-chloroacetoacetate is cheap and easily available; the yield of the S-4-chlorine-3-hydroxy butyric acid ethyl ester is high; and the product can be almost quantificationally obtained and has good optical purity and good industrialization prospect.
Owner:SHIJIAZHUANG CHIRALS CHEM CO LTD
(R)-tert-butyl dimethyl siloxy-glutaric acid monoester preparation method
ActiveCN104370953AChiral naturalGood chiralityGroup 4/14 element organic compoundsHydroxybutyric acidGlutaric acid
The invention discloses a rosuvastatin intermediate (R)-tert butyl dimethyl siloxy-glutaric acid monoester preparation method, which belongs to the field of medicine. The method is as follows: compound (S)-4-halogen-3-hydroxy butyric acid ethyl ester is taken as a raw material for ester exchange to obtain other (S)-4-halogen-3-hydroxy butyric ester, (S)-4-halogen-3-tert butyl dimethyl siloxy benzyl butyrate is obtained by protection of the (S)-4-halogen-3-hydroxy butyric ester, and by condensation of the (S)-4-halogen-3-tert butyl dimethyl siloxy benzyl butyrate and chloroformate in the presence of magnesium, tert butyl lithium and n-butyl lithium, (R)-tert butyl dimethyl siloxy-glutaric acid ester benzyl ester is obtained, and the (R)-tert butyl dimethyl siloxy-glutaric acid monoester is obtained by catalytic hydrogenation deprotection. Compared with the original process, the raw material of the process is chiral, natural, needs no resolution and induction and no enzyme hydrolysis to produce the chiral center, so that the product chirality is better. Compared with the original process, enzymes are already-industrialized enzymes, the raw material is easily obtained, reaction conditions are relatively mild, and the process is easy to industrialize. Compared with the original process, the process is shorter and less in three wastes.
Owner:ZHEJIANG LEPU PHARMA CO LTD
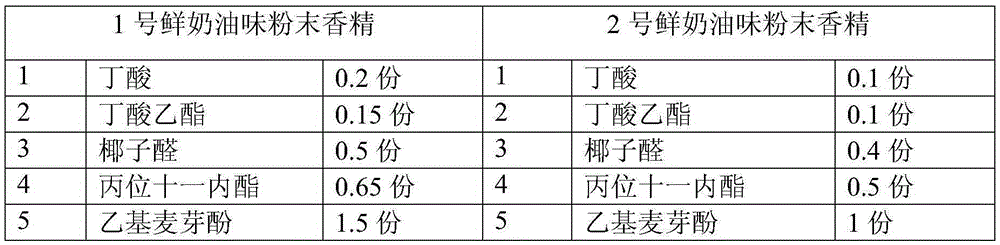
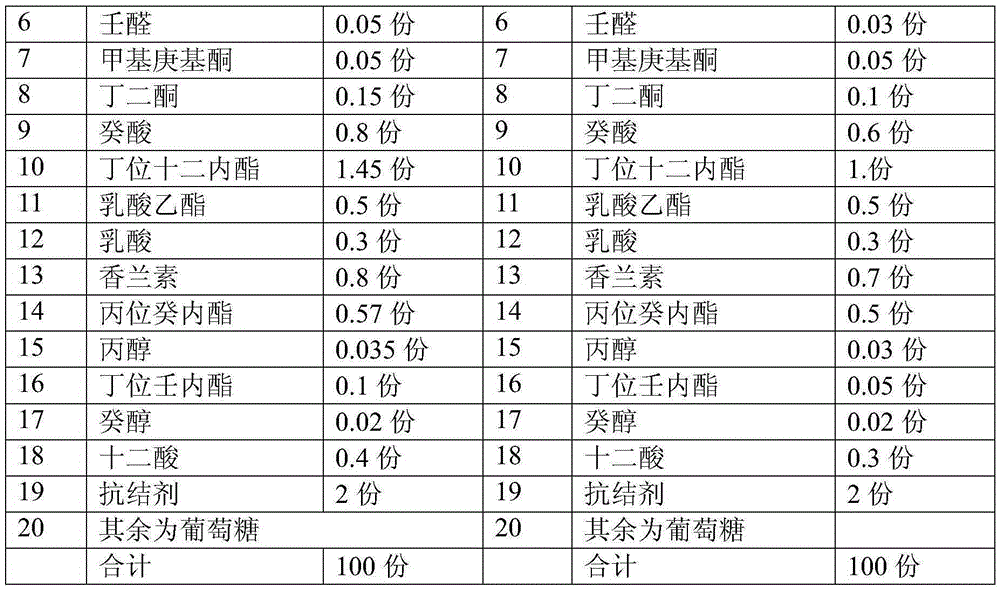
Whipped cream powder essence
The invention relates to whipped cream powder essence. The whipped cream powder essence comprises the following components in parts by weight: 0.1-0.3 part of butyric acid, 0.1-0.2 part of ethyl butyrate, 0.4-0.5 part of coconut aldehyde, 0.5-0.7 parts of gamma-unsecalactone, 1-1.5 parts of ethyl maltol, 0.03-0.06 part of nonanal, 0.05-0.1 part of methyl heptyl ketone, 0.1-0.15 part of diacetyl, 0.6-0.8 part of decanoic acid, 1-1.5 parts of delta-dodecalactone, 0.5-0.6 part of ethyl lactate, 0.3-0.4 part of lactic acid, 0.7-0.8 part of vanillin, 0.5-0.6 part of p gamma-decalactone, 0.03-0.05 part of propanol, 0.05-0.1 part of delta-nonalactone, 0.02-0.03 part of decanol, 0.3-0.4 part of dodecanoic acid, 0.5-2 parts of an anti-tackiness agent, and the balance of glucose, wherein the total part number is 100. The whole flavor balance of acid, ester, aldehyde, lactone and the like of the essence is taken into consideration seriously, so that the aroma of whipped cream can be more coordinated and perfect, the flavor is very rich, and the taste is smooth and delicious.
Owner:宁波威龙香精香料有限公司
Time resolution fluorescent immunochromatographic test strip for detecting pendimethalin, and preparation method and application thereof
ActiveCN109061156ALarge capacity adsorptionRelease fullyBiological testingFluorescence/phosphorescenceCelluloseNitrocellulose
The invention discloses a time resolution fluorescent immunochromatographic test strip for detecting pendimethalin, and a preparation method and application thereof. The time resolution fluorescent immunochromatographic test strip comprises a bottom plate, a sample absorbent pad, a combination absorbent pad, a nitrocellulose membrane and a water absorbent pad. A pendimethalin monoclonal antibody labelled by fluorescent microspheres is embedded into the combination absorbent pad, and a detecting area and a quality control area are fixed to the nitrocellulose membrane. A pendimethalin hapten-carrier protein conjugate is sprayed in the detecting area, and an anti-sheep and anti-mouse antibody is sprayed in the quality control area. A pendimethalin hapten is obtained in the modes that N-(1-ethyl propyl ether)-3,4-methyl toluidine and 4-bromo-butyric acid ethyl ester react to generate hydrocarbylation N-(1-ethyl propyl ether)-3,4-methyl toluidine, then the hydrocarbylation N-(1-ethyl propylether)-3,4-methyl toluidine reacts with concentrated nitric acid to generate nitro hydrocarbonylation N-(1-ethyl propyl ether)-3,4-methyl toluidine, and then the nitro hydrocarbonylation N-(1-ethyl propyl ether)-3,4-methyl toluidine reacts with KOH. The time resolution fluorescent immunochromatographic test strip and a detecting method provided by the invention have the advantages that operationis easy, sensitivity is high, the detecting speed is high, and the cost is low.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC +1
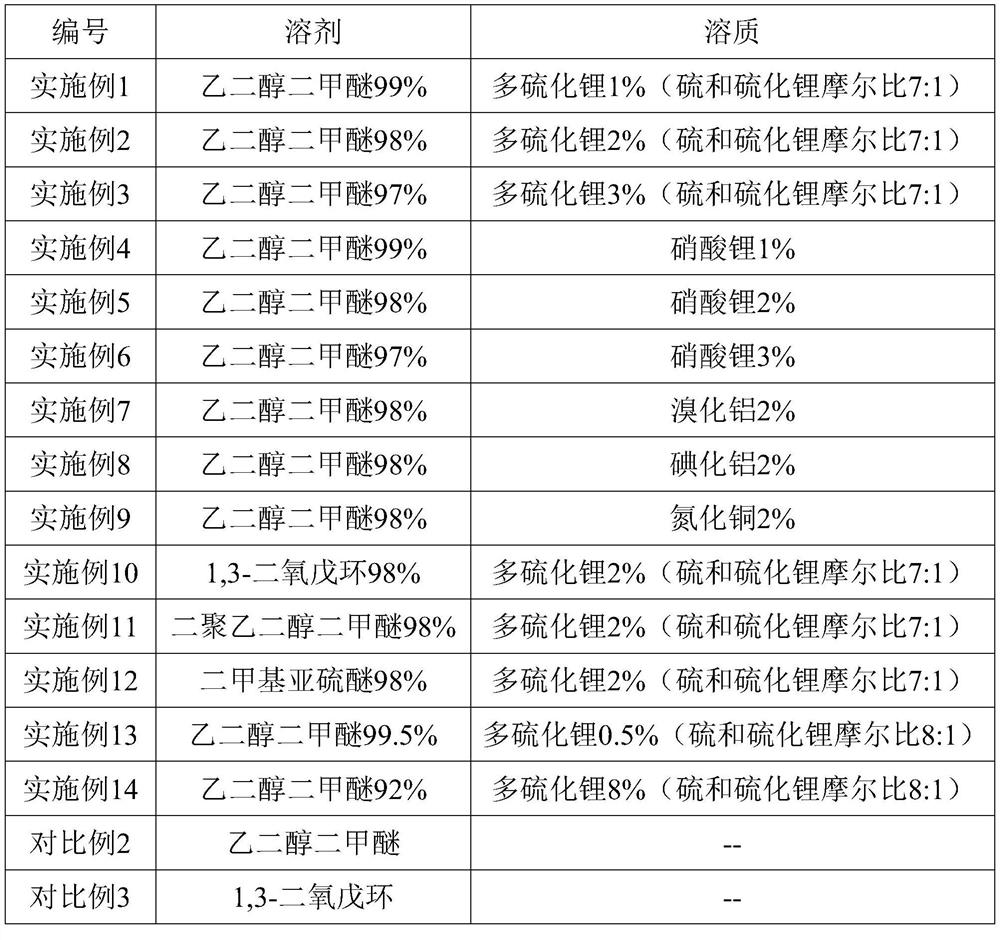
Interface pretreatment liquid for silicon-oxygen pre-lithiation negative electrode as well as preparation method and application of interface pretreatment liquid
InactiveCN113193174AGood chemical stabilityImprove solubilityCell electrodesFinal product manufacturePropanoic acidPolyethylene glycol
The invention relates to an interface pretreatment liquid for a silicon-oxygen pre-lithiation negative electrode as well as a preparation method and application of the interface pretreatment liquid. The interface pretreatment liquid comprises 0.1%-10% of solute and 90%-99.9% of solvent, the solvent is one or more selected from N-methyl pyrrolidone, ethylene carbonate, fluoroethylene carbonate, propylene carbonate, butylene carbonate, dimethyl carbonate, diethyl carbonate, ethyl methyl carbonate, gamma-butyrolactone, methyl formate, ethyl formate, methyl acetate, ethyl acetate, ethyl propionate, propyl propionate, ethyl butyrate, propyl butyrate, dimethyl sulfoxide, ethylene glycol dimethyl ether, 1, 3-dioxolane, dipolyethylene glycol dimethyl ether and dimethyl thionyl ether; and the solute is selected from one or more of lithium polysulfide, aluminum iodide, aluminum bromide, lithium nitrate and copper nitride. The solvent can fully infiltrate the internal pores of the lithium-rich negative plate and the surface of the negative active material, and the solute can react with the negative material, so that the stability of the SEI membrane is improved, and the first charge-discharge efficiency and long-term cycle performance of the battery are synergistically improved.
Owner:KUNSHAN BAOTRON NEW ENERGY TECH CO LTD
Process for preparing biodiesel and nervonic acid by using purpleblow maple oil as raw material
ActiveCN101285000BQuality improvementReduce manufacturing costPhysical/chemical process catalystsOrganic chemistryNervonic acidGlycerol
Owner:云南金枫生物科技有限公司
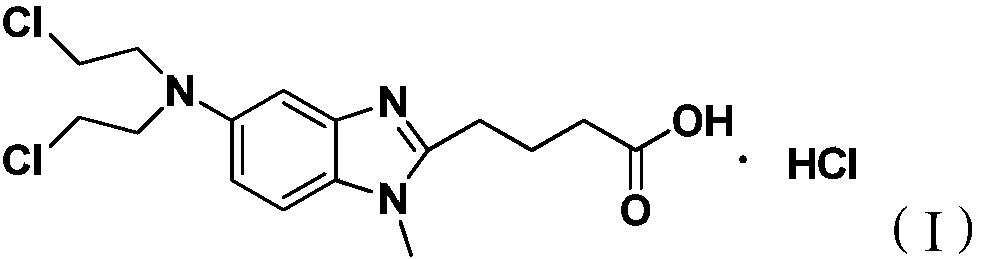
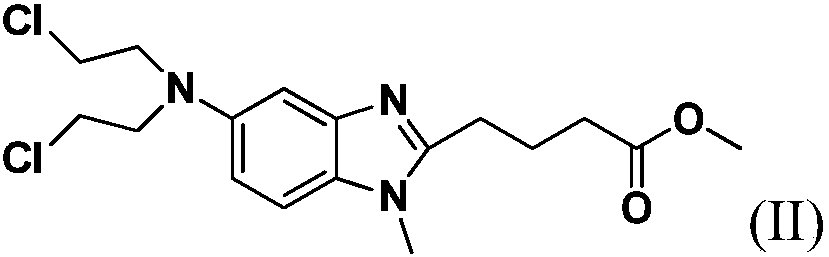
Preparation method for bendamustine hydrochloride crude product
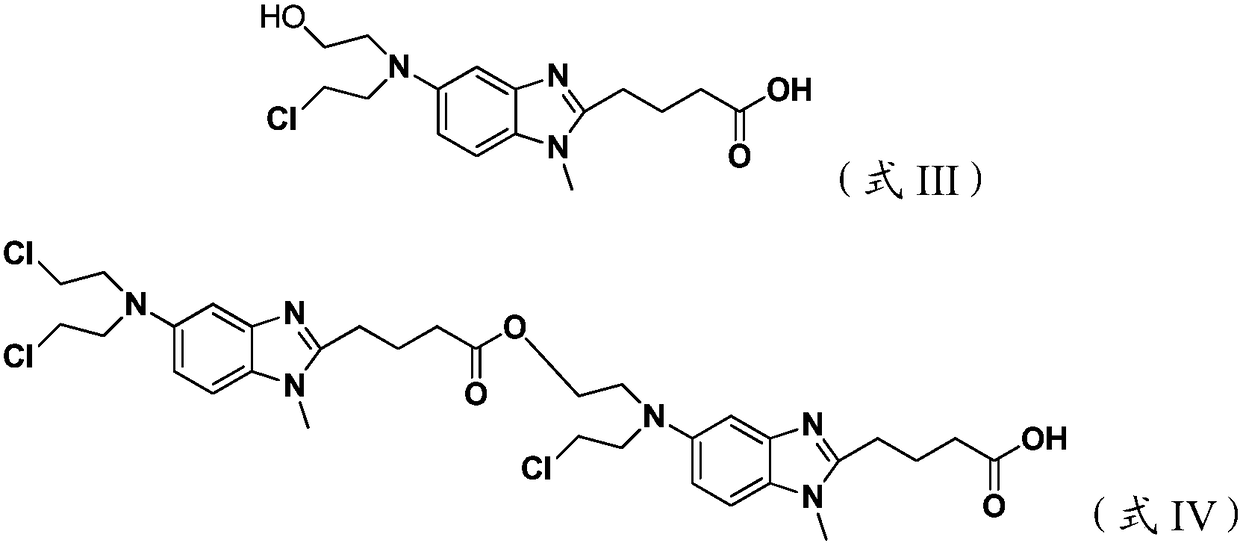
ActiveCN109422695AReduce pollutionStrengthen labor protectionOrganic chemistryBendamustine hydrochlorideVacuum drying
The invention discloses a preparation method for a bendamustine hydrochlorichloride crude product. The preparation method comprises the steps of dissolving methyl 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}butyrate or ethyl 4-{5-[bis-(2-chloro-ethyl)-amino]-1-methyl-1H-benzimidazol-2-yl}butyrate in concentrated hydrochloric acid, adding activated carbon, carrying out a reaction, performing cooling to 15-30 DEG C, and performing filtering; and adding purified water or a solution of alkali metal salts containing chloride ions to the obtained filtrate, performing cooling under stirring to -5 DEG C to 5 DEG C, continuing performing stirring for 0.5-2h, performing filtering, separately washing the obtained filtrate cake with icy purified water and icy acetone once, and performing vacuum drying at 40-50 DEG C to obtain the bendamustine hydrochlorichloride crude product. The crude product obtained by the preparation method of the invention has a purity of 99.2% or above and a yield of 75% or above. In addition, the preparation method has mild conditions, low pollution, and high yield and product purity, and is more suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com