Method for preparing chiral medicinal intermediate R-3-hydroxy butyric acid ethyl ester by microbial fermentation
A technology of ethyl hydroxybutyrate and microbial fermentation, which is applied in the direction of microbial-based methods, biochemical equipment and methods, and carboxylate preparation, and can solve the difficulties in the preparation of chiral catalysts, expensive coenzyme factors, and low stereoselectivity and other problems, to achieve the effect of breaking through low chiral purity, breaking through technical bottlenecks, and simplifying the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The present invention will be described in detail below by describing the preferred embodiments of the present invention. The following description does not limit the invention.
[0031] (1) Microbial fermentation
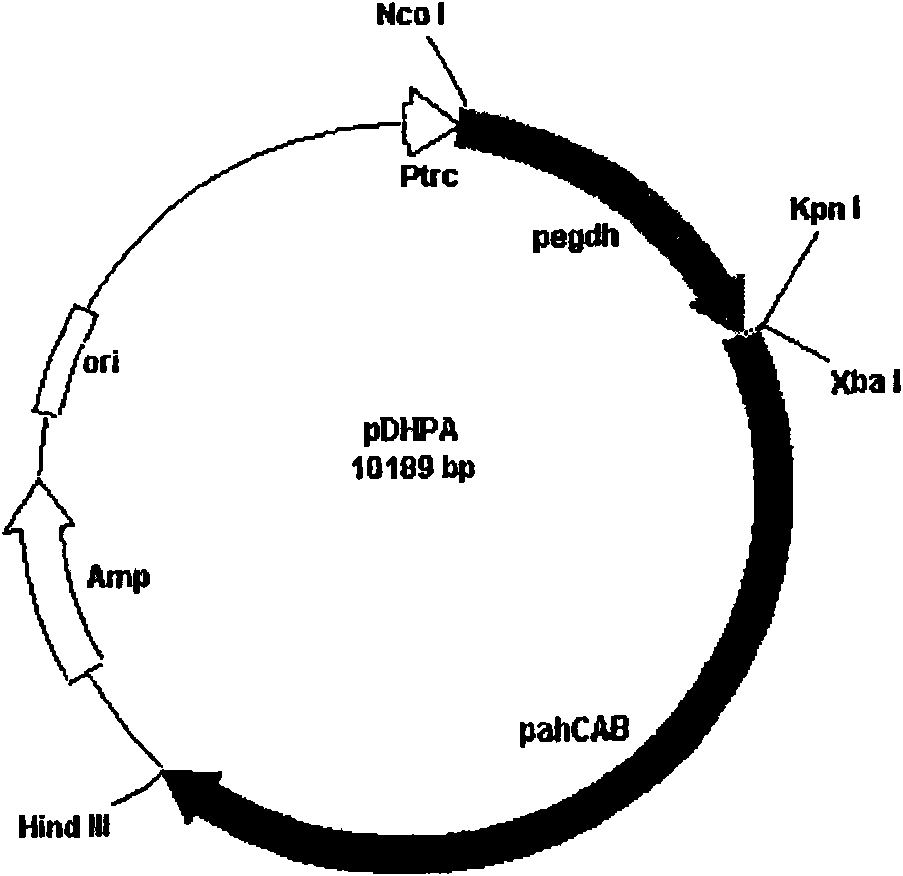
[0032] Recombinant strains: β-ketothiolase (phaA), acetoacetyl-CoA reductase (phaB) and PHA synthase (phaC) for the biosynthesis of R-3-hydroxybutyric acid were cloned by molecular biology methods; Engineering technology constructs expression vectors of the above biosynthetic enzymes and introduces them into microorganisms capable of synthesizing R-3-hydroxybutyric acid to obtain recombinant strains;
[0033] Bacterial growth medium (LB): yeast powder 5g / L, peptone 10g / L, NaCl 10g / L, pH7.2.
[0034] Fermentation medium: 4.5g / L KH 2 PO 3 , 3.6g / L (NH 4 ) 2 SO 4 , 1.23g / L MgSO 4 , 2.04g / LNH 4 CI, 1g / L citric acid, 100mg / L ampicillin and 1ml / L trace elements ((g) in 1mol / L HCl: 0.05FeSO 4 ·7H 2 O, 0.011 ZnSO 4 ·7H 2 O, 0.0025MnSO 2 4H 2 O, 0.005C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com