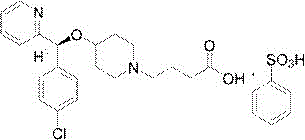
Improved preparation method of bepotastine besilate
A technology of bepotastine besylate and benzenesulfonic acid, which is applied in the field of drug synthesis, can solve the problems of complex preparation method, high cost, limited application and the like, and achieve the effects of shortening process time, improving yield and shortening process time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Step (1): Preparation of S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine N-acetyl-L-phenylalanine salt
[0057] Dissolve 4.542kg of 2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine in 182L of ethyl acetate, stir and heat up to 65°C, stir to dissolve, then add 1.522kg of N-acetyl- L-phenylalanine, react for 1 hour. Cool to 20°C, crystallize for 6h, filter, and redissolve the filter cake in 130L ethyl acetate at 80°C, cool to 20°C for 6h, filter to obtain S-2-[(4-chlorophenyl)(4- Piperidinyloxy)methyl]pyridine N-acetyl-L-phenylalanine salt 3.061kg, yield 80% (optical purity 99%).
[0058] Step (2): Preparation of S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine
[0059] Dissolve 2.856Kg of S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine N-acetyl-L-phenylalanine salt in 14.3L of water, add 2.2L of 5N hydrochloric acid solution, add 5.6L ethyl acetate to extract twice, discard the organic phase, add 4.5L 5N NaOH solution to the water phase, extrac...
Embodiment 2
[0085] Step (1): Preparation of S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine N-acetyl-L-phenylalanine salt
[0086] Dissolve 4.542kg of 2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine in 182L of ethyl acetate, stir and heat up to 50°C, stir to dissolve, then add 1.522kg of N-acetyl- L-phenylalanine, react for 1 hour. Cool to 20°C, crystallize for 6h, filter, and redissolve the filter cake in 130L ethyl acetate at 80°C, cool to 20°C for 6h, filter to obtain S-2-[(4-chlorophenyl)(4- Piperidinyloxy)methyl]pyridine N-acetyl-L-phenylalanine salt 2.831kg, yield 74% (scientific purity 99%).
[0087] (2) and (3) are the same as in Embodiment 1.
Embodiment 3
[0089] Step (1): Preparation of S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine N-acetyl-L-phenylalanine salt
[0090] Dissolve 4.542kg of 2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine in 182L of ethyl acetate, stir and heat up to 77°C, stir to dissolve, then add 1.522kg of N-acetyl- L-phenylalanine, react for 1 hour. Cool to 20°C, crystallize for 6h, filter, and redissolve the filter cake in 130L ethyl acetate at 80°C, cool to 20°C for 6h, filter to obtain S-2-[(4-chlorophenyl)(4- Piperidinyloxy)methyl]pyridine N-acetyl-L-phenylalanine salt 3.085kg, yield 81% (optical purity 99%).
[0091] (2) and (3) are the same as in Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com