Preparation method of S-4-chlorine-3-hydroxy butyric acid ethyl ester with optical purity
A technology of ethyl hydroxybutyrate and S-4-, which is applied in the field of preparation of chemical API intermediates, can solve the problems of expensive catalysts, short process flow, and high production costs, and achieve reduced dosage, high yield, and reduced production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
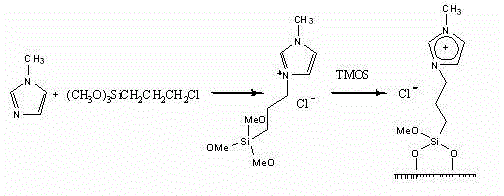
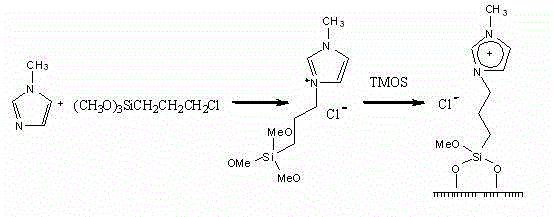
[0031] Preparation of nano-inorganic-organic hybrid ionic liquid materials: firstly synthesize siloxane-containing active ionic liquid, then use sodium dodecyl sulfate as a template, add sodium dodecyl sulfate in different weight ratios, and contain siloxane Based active ionic liquid, tetramethoxysilane (TMOS), reacted under different conditions to obtain nine kinds of hybrid ionic liquid materials, which can be used as immobilized materials for chiral catalysts. The reaction principle is as follows. Reaction conditions, the results are shown in Table 1.
[0032]
[0033]
[0034] Table 1
[0035]
Embodiment 2
[0037] Preparation of chiral catalyst:
[0038] (1) [RuCl 2 (COD)] n preparation of
[0039] Add 1g of ruthenium trichloride hydrate into 40 ml of absolute ethanol, stir until dissolved, and filter out the insoluble matter, then add 4.8 ml of COD into the ethanol solution, stir at room temperature for 25 h, filter with suction, wash the solid with cold acetone, Dry to give 1.22g [RuCl 2 (COD)] n, yield 89.7%.
[0040] (2) Ru 2 Cl 4 ((R)-BINAP) 2 NET 3 and Ru 2 Cl 4 ((R)-Segphos) 2 NET 3 preparation of
[0041] 0.75g [RuCl 2 (COD)] n , 2g (R)-BINAP or R-Segphos was added to 75 ml of toluene, then 1.13g triethylamine was added dropwise, refluxed for 10 h under nitrogen protection, the solvent was distilled off under reduced pressure, and the solvent was distilled off with CH 2 Cl 2 The product was washed out, filtered, and CH was distilled off under reduced pressure 2 Cl 2 , dried in vacuum to get Ru 2 Cl 4 ((R)-BINAP) 2 NET 33 2.65 grams, yield 98.0%, or...
Embodiment 3
[0043] Immobilization of Chiral Catalysts
[0044] 0.1 g Ru 2 Cl 4 ((R)-BINAP) 2 NET 3 Add it into 20ml of dichloromethane, add 1.0 g of nano-inorganic-organic hybrid ionic liquid material No. 1-9, stir for 3 hours, and spin dry to obtain immobilized chiral catalyst No. 1-9.
[0045] 0.1 g Ru 2 Cl 4 ((R)-Segphos) 2 NET 3 Add it into 20ml of dichloromethane, add 1.0 g of nano-inorganic-organic hybrid ionic liquid material No. 1-9, stir for 3 hours, and spin dry to obtain immobilized chiral catalyst No. 10-18.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com