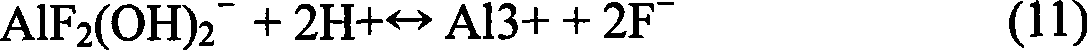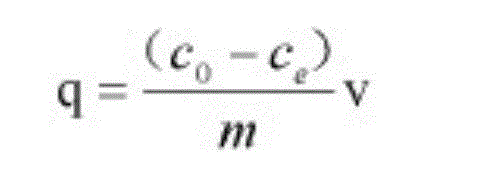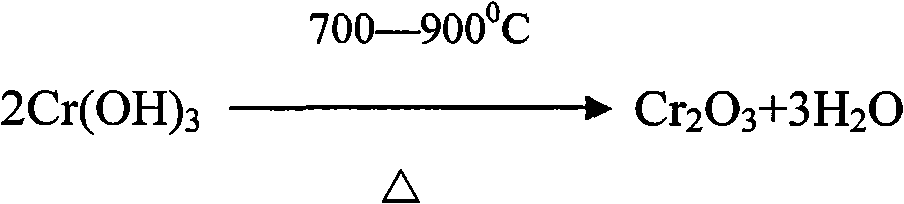Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
775results about How to "Solve the use problem" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for comprehensive treatment of oilfield waste
ActiveCN102849880ALarge amount of processingReduce governance costsWater/sewage treatment by centrifugal separationFatty/oily/floating substances removal devicesSolid phasesChemistry
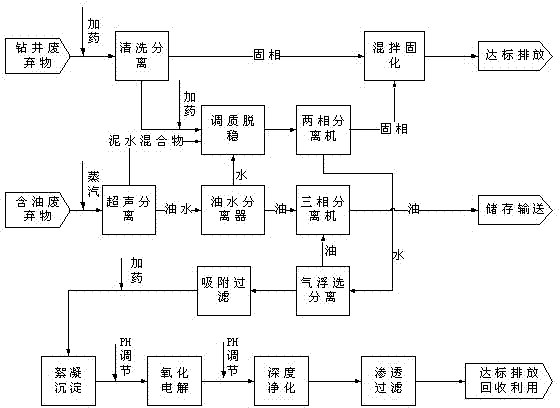
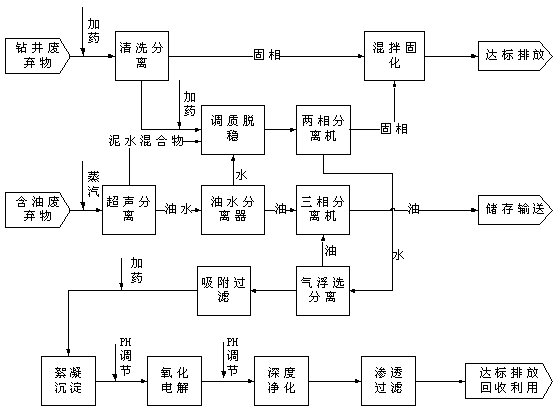
The invention discloses a method for comprehensive treatment of oilfield waste, which is characterized by comprising the process steps of cleaning and separation, ultrasonic separation, tempering and destabilization, oil-water separation, centrifugal separation, mixed curing, air floatation separation, adsorption filtration, flocculating settling, oxidization electrolysis, deep purification and permeable filtration. Thus, the oilfield waste is treated and utilized in a classified manner, so that sump oil is recovered, the solid phase achieves the discharge standard of oilfield sludge treatment design specifications (SY / T6851-2012) or is reutilized for construction material manufacturing, and the liquid phase achieves the sewage comprehensive discharge standard (GB8978-1996) after being subjected to water treatment.
Owner:RUIJIE ENVIRONMENTAL PROTECTION TECH CO LTD
Method for comprehensive treatment of oilfield waste
ActiveCN102849880BSolve the use problemMeet the actual needs of protectionWater/sewage treatment by centrifugal separationFatty/oily/floating substances removal devicesElectrolysisSludge
The invention discloses a method for comprehensive treatment of oilfield waste, which is characterized by comprising the process steps of cleaning and separation, ultrasonic separation, tempering and destabilization, oil-water separation, centrifugal separation, mixed curing, air floatation separation, adsorption filtration, flocculating settling, oxidization electrolysis, deep purification and permeable filtration. Thus, the oilfield waste is treated and utilized in a classified manner, so that sump oil is recovered, the solid phase achieves the discharge standard of oilfield sludge treatment design specifications (SY / T6851-2012) or is reutilized for construction material manufacturing, and the liquid phase achieves the sewage comprehensive discharge standard (GB8978-1996) after being subjected to water treatment.
Owner:RUIJIE ENVIRONMENTAL PROTECTION TECH CO LTD
Method for treating aluminum electrolysis waste cathode carbon block using acid and alkali combination method
A method for treating aluminum electrolytic waste cathode carbon blocks by an acid and alkali combination method is characterized by comprising the following steps: (1) aluminum electrolytic waste cathode is added to concentrated basic solution for reaction; a filtered solid phase is obtained by filtering and is added to concentrated acid for reaction; and carbon powder is obtained after filtration; (2) filtrates are mixed and dropwise added with the concentrated basic solution, the pH value thereof is adjusted to 9, the obtained mixture is kept standing and filtered to obtain cryolite; (3) the filtrate is added with saturated bleach solution, kept standing and filtered to obtain calcium fluoride; (4) the fourth filtrates is heated, evaporated and crystallized to obtain sodium chloride solid. With the purposes of waste utilization and environmental protection, the method successfully provides the process for treating aluminum electrolytic waste cathode by the acid and alkali method, which causes the waste cathode to not be the conventional waste and pollutant and to become artificial mineral resources. The method solves the problem of waste utilization in aluminum electrolysis industry, improves benefit, and improves the comprehensive level of the aluminum electrolysis industry of China.
Owner:NORTHEASTERN UNIV +1
Method for preparing high purity ferric phosphate using ferrous sulfate as by-product of white titanium pigment
InactiveCN101531355AEasy to oxidizeSolve the use problemPhosphorus compoundsLithium iron phosphateGlaze
The invention relates to a preparation method of pure ferric phosphate, characterized in that: iron vitriol, phosphorus source as by-product of white titanium pigment are used as reactant and purificant and oxidant are added into the reactant and the reaction temperature is controlled at 25-100 DEG C and the reaction time is 0.5-10 hours, thus white ferric phosphate powder (FePO[4]2-4H[2]O) with 2-4 crystal water is obtained. The technological process has features of simple process, low production cost, high product purity, increased added value of ferrous sulfate as by-product of white titanium pigment and the product can be used as ceramic metal glaze-color glaze material and raw material for producing lithium iron phosphate as lithium ionization cell anode material.
Owner:GUANGXI UNIV +1
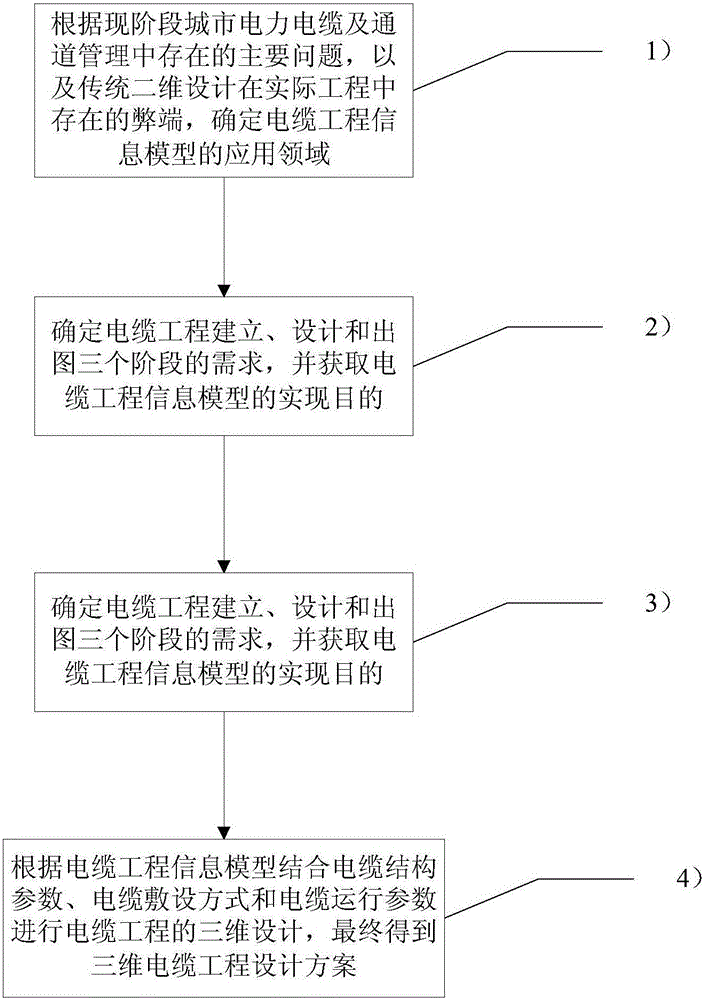
Three-dimension design method based on cable engineering information model
InactiveCN105005676ASolve the difficulty of quickly copyingSolve the problem of easy modificationSpecial data processing applicationsPower cableThree stage
The invention relates to a three-dimension design method based on a cable engineering information model. The three-dimension design method comprises the following steps: 1), according to main problems existing in city power cable and channel management at the present stage and defects of the conventional two-dimension design, which exist in actual engineering, determining an application field of the cable engineering information model; 2), determining demands in the three stages of cable engineering building, design and drawing and obtaining the achieving purpose of the cable engineering information model; 3), building the cable engineering information model by using a three-dimension design platform and according to a standard model base and regional digital model base; 4), carrying out three-dimension design on cable engineering according to the cable engineering information model and by combining cable structure parameters, a cable laying method and cable running parameters, and finally, obtaining a three-dimensional cable engineering designing scheme. Compared with the prior art, the three-dimension design method provided by the invention has the advantages of being intuitive and accurate, and advanced in platform and the like.
Owner:SHANGHAI MUNICIPAL ELECTRIC POWER CO +1
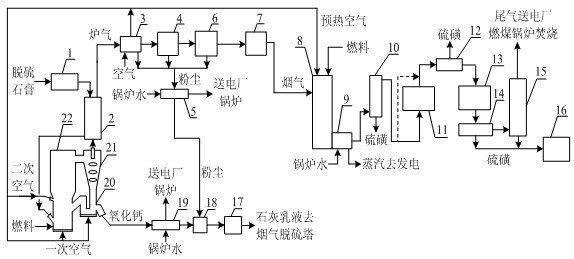
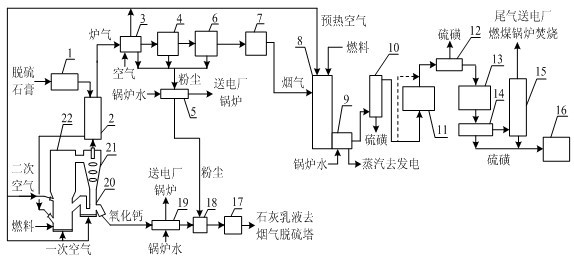
Method for preparing calcium oxide and sulfur by double-atmosphere fluidized roasting of desulfurated gypsum
InactiveCN102303883ASolve the use problemAbundant raw materialsEnergy inputSulfur preparation/purificationDust controlSulfur dioxide
The invention provides a method for preparing calcium oxide and sulfur by double-atmosphere fluidized roasting of desulfurated gypsum, belonging to the technical fields of solid pollutant treatment and in environmental protection chemical production. The method comprises the following steps: drying and dehydrating aged desulfurated gypsum, and then entering a multi-stage suspension heat exchangerfor pre-heating; entering hot-state gypsum into a circulating fluidized bed decomposition furnace, and introducing primary and secondary air for decomposition; cooling discharged hot-state calcium oxide, wherein cooled hot-state calcium oxide is used as a desulfurizer for later use; entering discharged smoke containing sulfur dioxide into the multi-stage suspension heat exchanger; removing calcium oxide dust in cooled hot smoke through multi-stage dust removing equipment; and feeding the smoke without the dust into a high-temperature-resistant high-efficiency filter for further removing the dust, and feeding clean smoke into a sulfur recovery system; and preparing the sulfur product by the processes of hot reduction, catalytic reduction, Clause reaction and the like, and feeding Clause tail gas into a coal burning boiler in a power plant for combustion. The method has the advantages of good environmental protection benefits and strong economic benefits.
Owner:CHINA PETROCHEMICAL CORP +1
Preparation method of modified bagasse cellulose based heavy metal adsorbent
InactiveCN102716728ASolve the use problemSolve secondary pollutionOther chemical processesWater/sewage treatment by sorptionWater bathsCellulose
The invention relates to a preparation method of a modified bagasse cellulose based heavy metal adsorbent. The preparation method comprises the technological steps of: defibering natural fibers, washing with distilled water and ethanol, stirring and activating in NaOH solution, putting the fibers into dimethyl sulfoxide for water-bath heating and gelatinization, adding deionized water and initiators, feeding in nitrogen for initiation and adding acrylic acid monomers for reaction to obtain graft copolymerization products; adding polyethylene polyamine monomers for reaction, washing and drying to obtain crude cellulose based heavy metal adsorbent; and washing with deionized water, soaking and rinsing in ethanol, rewashing with deionized water, extracting by using acetone and drying to obtain the refined heavy metal adsorbent. The preparation method provided by the invention has the advantages that the method is simple, the cost is low, the environmental friendliness is good, the adsorption effect is excellent, the method is compliant with the concept of circular economy and sustainable development, the problem of secondary pollution caused by bagasse combustion is solved, the water bodies which are polluted by heavy metals are purified and the economic advantage is remarkable.
Owner:GUANGXI UNIV
Planting method for interplanting Chinese angelica in orchard
ActiveCN103250610AImprove survival rateImprove qualityCultivating equipmentsHorticultureMedicinal herbsEcological environment
The invention relates to a planting method for interplanting Chinese angelica in an orchard and belongs to the technical field of traditional Chinese medicine materials and agricultural planting. The planting method for interplanting the Chinese angelica in the orchard comprises the steps that the Chinese angelica is interplanted in the orchard of peaches, pears, plums, apples and cherries, and field management can be carried out according to corresponding cultivation technologies. According to the planting method, spatial ecological niche complementary advantages of the Chinese angelica and fruit trees are fully utilized, a utilization rate of the orchard fields can be increased, a survival rate of seedlings of the Chinese angelica is increased, quality of the Chinese angelica medical materials is improved, incomes of farmers are increased, ecological environment of the orchard is improved, damage of weeds and diseases and pests is reduced, less pesticides are used, growth of the fruit trees is promoted, orchard management cost is lowered, and the planting method has good economic and ecological benefits.
Owner:INST OF MEDICINAL PLANTS YUNNAN ACAD OF AGRI SCI +1
Soil improvement agent and improvement method of heavy metal lead-contaminated soil
InactiveCN103484125AWeaken the poisonImprove water and fertilizer retention capacityContaminated soil reclamationOrganic fertilisersBiomassContaminated soils
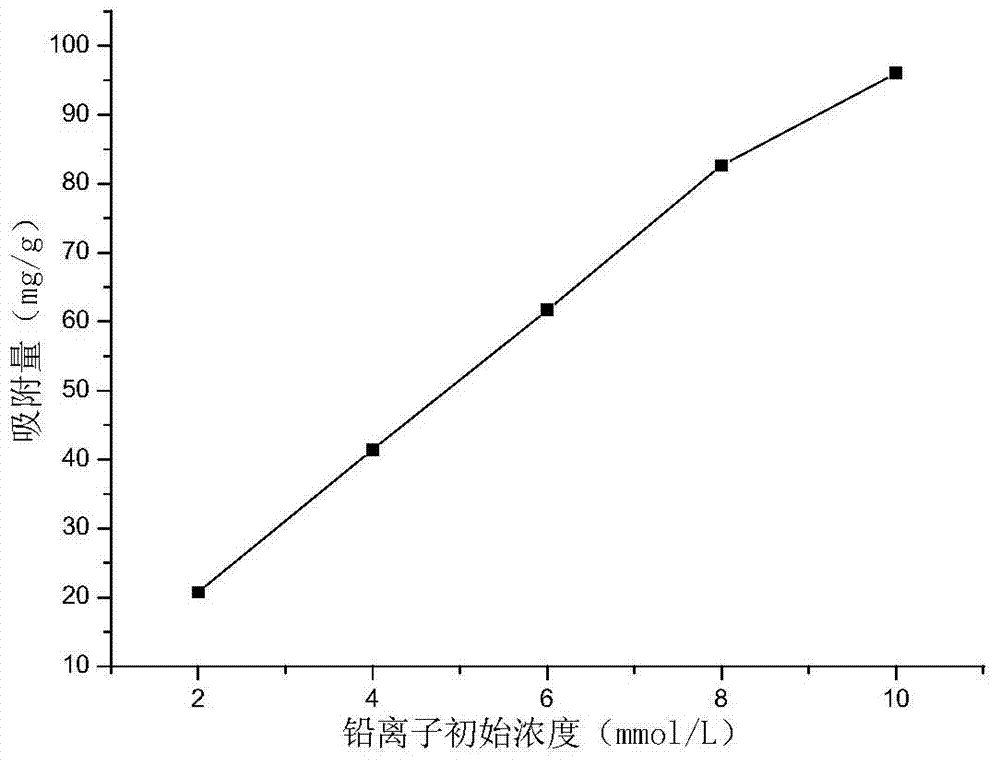
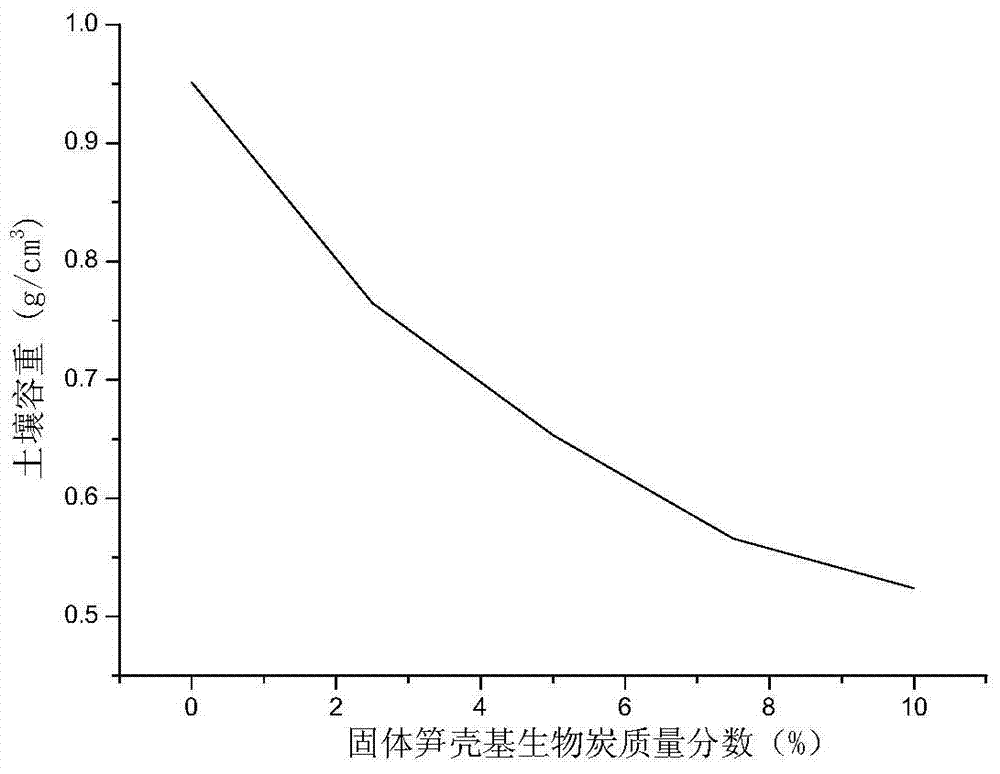
The invention discloses a soil improvement agent and improvement method of heavy metal lead-contaminated soil, relating to a soil improvement agent. The soil improvement agent is solid bamboo shoot shell-based biochar, and elements obtained by isolating oxygen at 400 DEG C and pyrolyzing for 30 minutes include C, H, N, K, P, Mg, Ca, Si and O. The soil improvement agent is prepared by the steps of cleaning, drying and grinding a bamboo shoot shell, and putting into a pyrolyzing furnace; isolating oxygen at 380-420 DEG C and pyrolyzing for 20-40 minutes; drying, pre-charring and charring the biomass bamboo shoot shell to obtain solid bamboo shoot shell-based biochar. The improvement method comprises the steps of adding 2.5-10% of the soil improvement agent by mass into the heavy metal lead-contaminated soil; adding water to 70% of the saturation moisture capacity of the field oil; covering with a film to prevent water loss; forming air ports in the film at an interval of 5cm; adding water every 2-3 days to supplement the lost water; culturing, taking out the product and performing air drying to obtain improved soil.
Owner:XIAMEN UNIV
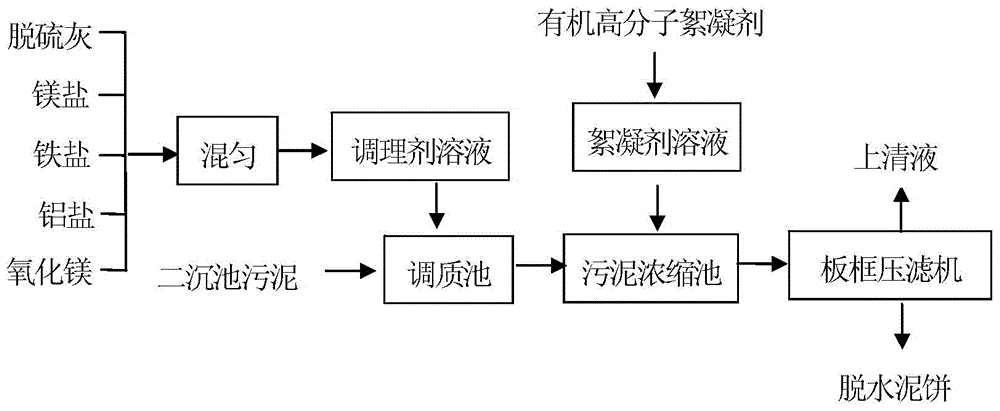
Sludge dewatering conditioning agent and dewatering method thereof
ActiveCN105314815AThe conditioning process is simpleReduce dosageSludge treatment by de-watering/drying/thickeningChemistryIron salts
The present invention discloses a sludge dewatering conditioning agent, which comprises, by weight, 30-70% of desulfurization ash, 5-15% of a magnesium salt, 5-30% of an iron salt, 2-20% of an aluminum salt, and 0.1-15% of magnesium oxide. The invention further provides a dewatering method of the conditioning agent, wherein the dewatering method comprises conditioning agent solution preparing, sludge conditioning, flocculant solution preparing, sludge dewatering and other steps. According to the present invention, the characteristics of wide used raw material source, and low raw material price, low preparation cost and good dewatering effect are provided, the method can be performed through the existing sludge dewatering facility, the implementation process is simple, the stability and the reliability of the sludge dewatering process can be effectively improved, and the sludge dewatering conditioning agent and the dewatering method can be widely used for treatments of various wastewater, sewage and sludge.
Owner:BAOSHAN IRON & STEEL CO LTD +1
Flyblow manure compound fertilizer and production method thereof
InactiveCN101081766AReduce dosageSolve the use problemSuperphosphatesClimate change adaptationPhosphoric acidWater content
The re-mixed maggot dung fertilizer is produced through regulating the water content of swine waste to about 70 % and setting inside a plastic basin, inoculating maggot and culturing at 25-30 deg.c temperature and 60-70 % relative humidity for 3-4 days and separating maggot to obtain maggot dung; crushing maggot dung in 1000 kg; crushing ammonium sulfate 260 kg, calcium superphosphate 180 kg, potassium chloride 58 kg and dicyan diamide 0.5 kg; mixing all the materials, pelletizing, drying and sieving to obtain the re-mixed maggot dung fertilizer with total amount of nitrogen fertilizer, phosphate fertilizer and potash fertilizer in 16 % and organic substances in 20 %. The present invention has the advantages of obvious fertilizer effect, capacity of reducing the amount of applied inorganic fertilizer, best utilization of maggot raising waste, low production cost, etc.
Owner:TIANJIN HANGU DISTRICT FUXIANG FERTILIZER PROCESSING FACTORY
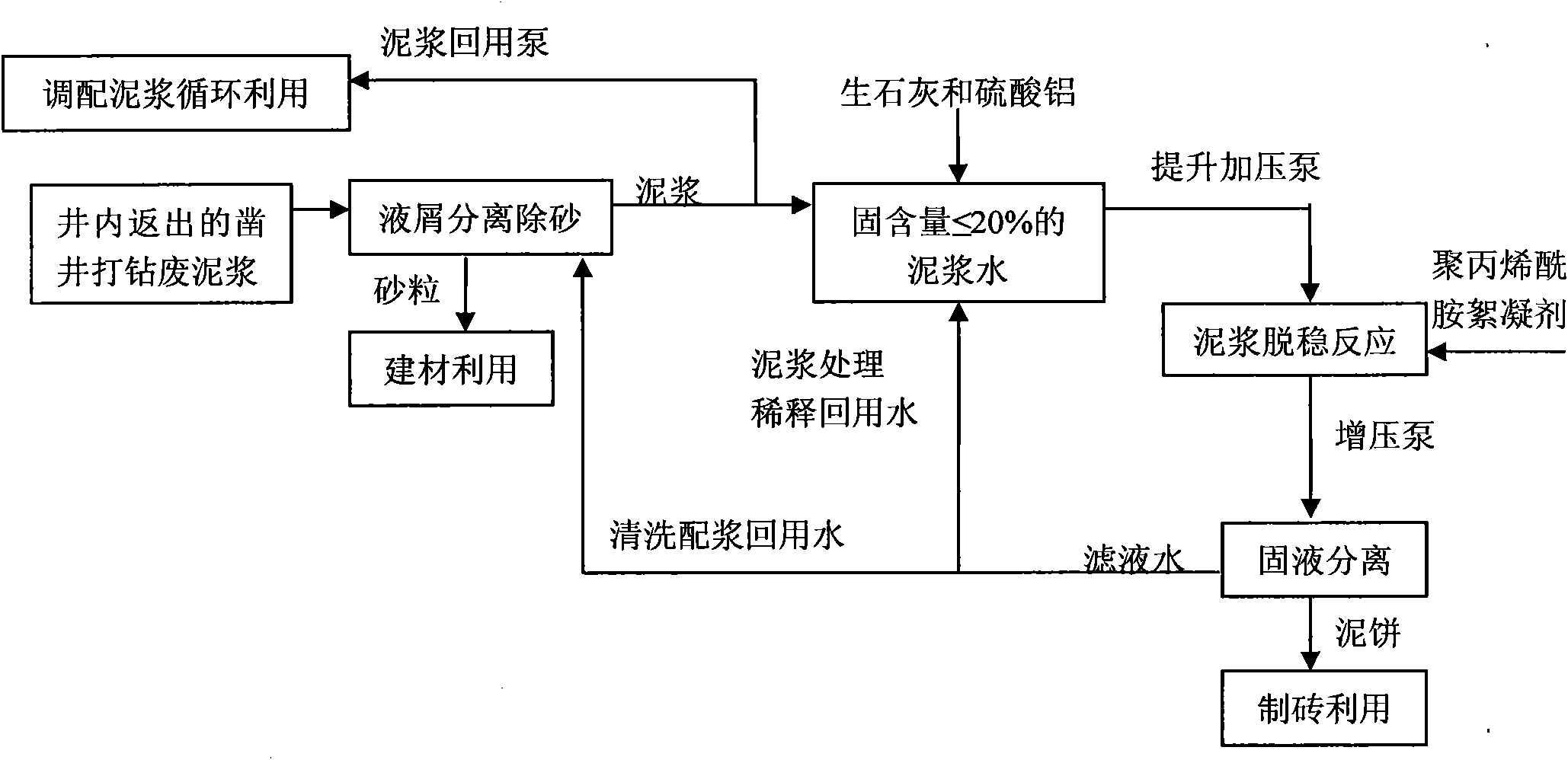
Treatment method of waste slurry generated by gigging well and drilling
ActiveCN101774746ASolve processingSolve the use problemSludge treatment by de-watering/drying/thickeningSlurryCement paste
The invention discloses a treatment method of waste slurry generated by gigging a well and drilling, which comprises the following steps of: separating liquid from scraps to remove sands in waste slurry which is generated by gigging a well and drilling and is reflowed from the well; adding inorganic flocculating agent and water in the sanded-removed waste slurry generated by gigging the well and drilling to dilute, stir and damage stability to obtain cement paste with solid content which is less than or equal to 20%; adding polyacrylamide flocculant into the obtained cement paste to stir and perform a cement paste destabilization reaction; after the cement paste destabilization reaction, separating solid from liquid in the cement paste; and returning filter water to the step of separating liquid from scraps to remove the sands as cleaning proportioned water to recycle and / or return to the step of diluting, stirring and damage stability as water for treating and diluting the cement paste. The method can solve the problem for treating and using the waste slurry generated by gigging the well and drilling, can separate sands from mud and water in the waste slurry generated by gigging the well and drilling, can reach the standard and be comprehensively utilized, has large treatment amount to reach 1000 m<3> / h, can treat the waste slurry on a large scale, thereby being capable of reducing the treatment cost of the system. The method can comprehensively utilize and recycle all the waste slurry generated by gigging the well and drilling.
Owner:PUYANG TIANDIREN ENVIRONMENTAL PROTECTION TECH CO LTD +1
Method for extracting ketene dimer by rectifying residues and pumped liquid
The invention relates to a method for extracting ketene dimer by rectifying residues and pumped liquid. The method comprises the following steps that firstly, hydrolysis is carried out, wherein primary residues and the pumped liquid are mixed in the proportion of 2:1 and heated to 65-75 DEG C through a membrane type evaporator, ketene dimer is evaporated out, crude ketene dimer obtained through cooling of a primary condenser and a secondary condenser enters a high-order storage tank and is mixed with crude ketene dimer generated in a polymerization section, and then mixed crude ketene dimer enters a rectifying section; secondly, secondary residues formed in the membrane type evaporator and acetic anhydride in the condensers enter a hydrolysis section for hydrolysis, and acetone, dilute acetic acid and solid waste residues are obtained; thirdly, tail gas at the hydrolysis section is fed to a water scrubber to be absorbed. The method has the advantages that crude ketene dimer is recycled through heating of the membrane evaporator, the yield of crude ketene dimer is increased inside the system, consumption of glacial acetic acid is reduced, waste gas and residues generated in the production process are digested and absorbed, solid waste is greatly reduced, and zero waste water emission is achieved.
Owner:ANHUI JINGHE IND
Zymolysis rice milk beverage and preparation method thereof
InactiveCN101181088AFully dispersedImprove product qualityFood preparationWater bathsCarboxymethyl cellulose
The invention discloses a fermentation rice milk drink and a preparation method thereof, which adopts fermentation rice as raw material that is processed through immersion, water bathing, cooking, fermentation, beating and pulp refining, and then the pH value of the obtained emulsion is adjusted; thermostable alpha-amylase Unikamyl HT is added according to a portion of 10 kilograms of the material being added with 3 to 5ml of thermostable alpha-amylase Unikamyl HT; then the obtained starch milk is sent into the ejector device through a flow meter; through flash cooking, the temperature of the obtained material should rise to 95 to 98 DEG C; then thermal insulation, enzyme stabilization, cooling and pH value regulation are done; efficient glucoamylase Unikase GA is added according to the portion of 10 kilograms of the material being added with 5 to 15ml of efficient glucoamylase Unikase GA; the temperature of the obtained material should rise to 100 DEG C, and the killing of enzyme is done; next, the obtained material are added with water, citric acid, xanthan and sodium carboxymethyl cellulose; the temperature raises by 55 to 60 DEG C so as to do secondary homogeneous; finally, the super-high-temperature sterilization and aseptic packing are done. The fermentation rice has specific flavor of rice wine, and is characterized by sweet and fragrant taste, light yellow or ivory color, being rich in nutrition and with the functions of health care and skin-maintaining and stable storage.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing carbon molecular sieve from apple dregs
InactiveCN1472134ALarge specific surface areaWide variety of sourcesOther chemical processesMolecular sieveBiomass
A process for preparing carbon molecular sieve from apple dregs includes such steps as pretreating apple dregs, and carbonizing in vacuum carbonizer or atmosphere protected carbonizer. Its advantages are large specific surface area up to 1000 sq.m / g, high micropore uniformity, low cost and easy control.
Owner:SHANGHAI JIAO TONG UNIV
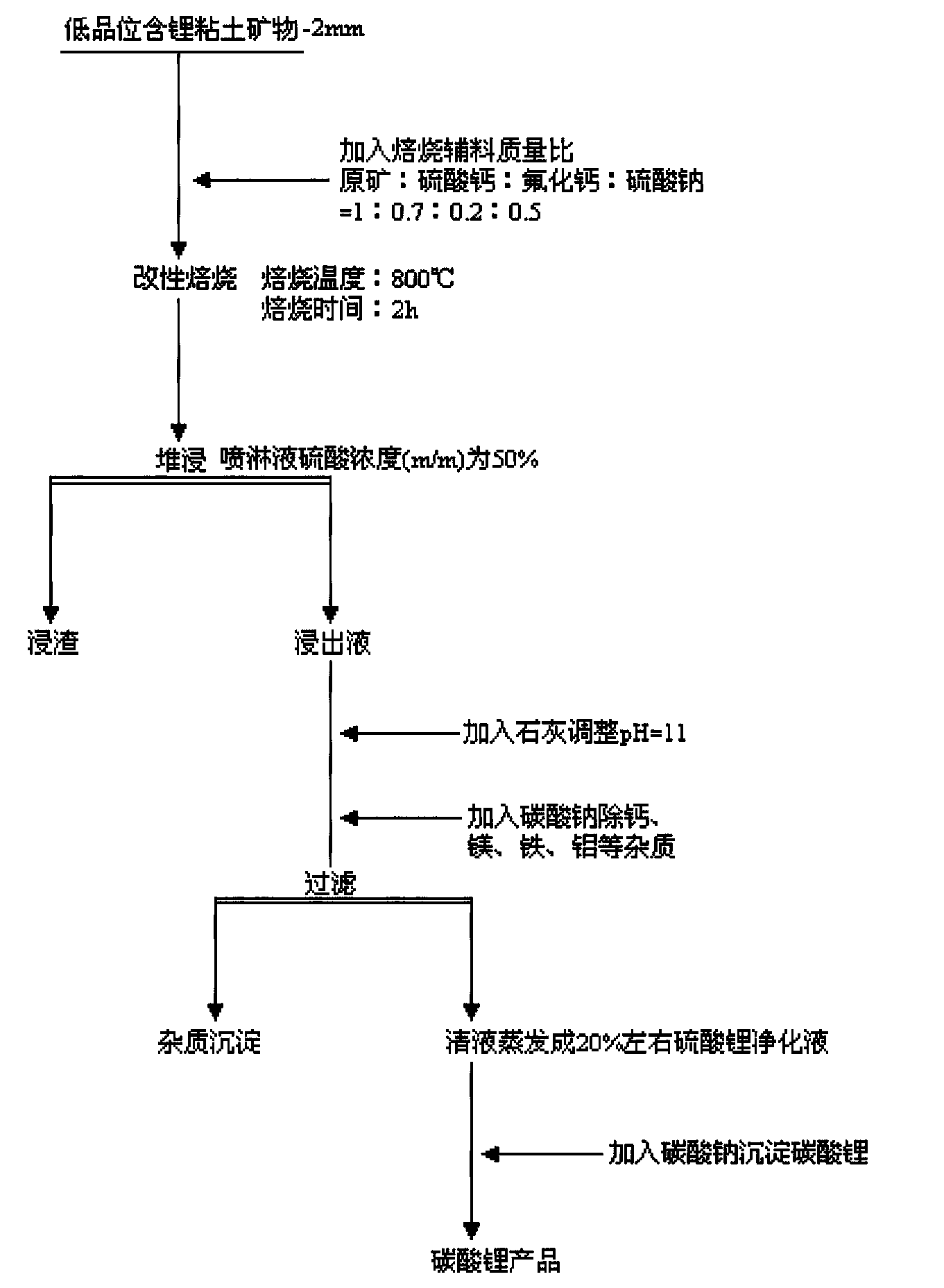
Method for extracting lithium from low grade lithium-containing clay mineral
The invention provides a new technology of 'modifying roasting-dump leaching' for large-scale low grade lithium-containing clay mineral in north of Henan province, solving the problem of utilization of low grade lithium-containing clay in the area. The new technology overcomes the defects of long technological process and high production cost of traditional lithium extraction technology, and the lithium leaching efficiency reaches 91%.
Owner:河南省岩石矿物测试中心
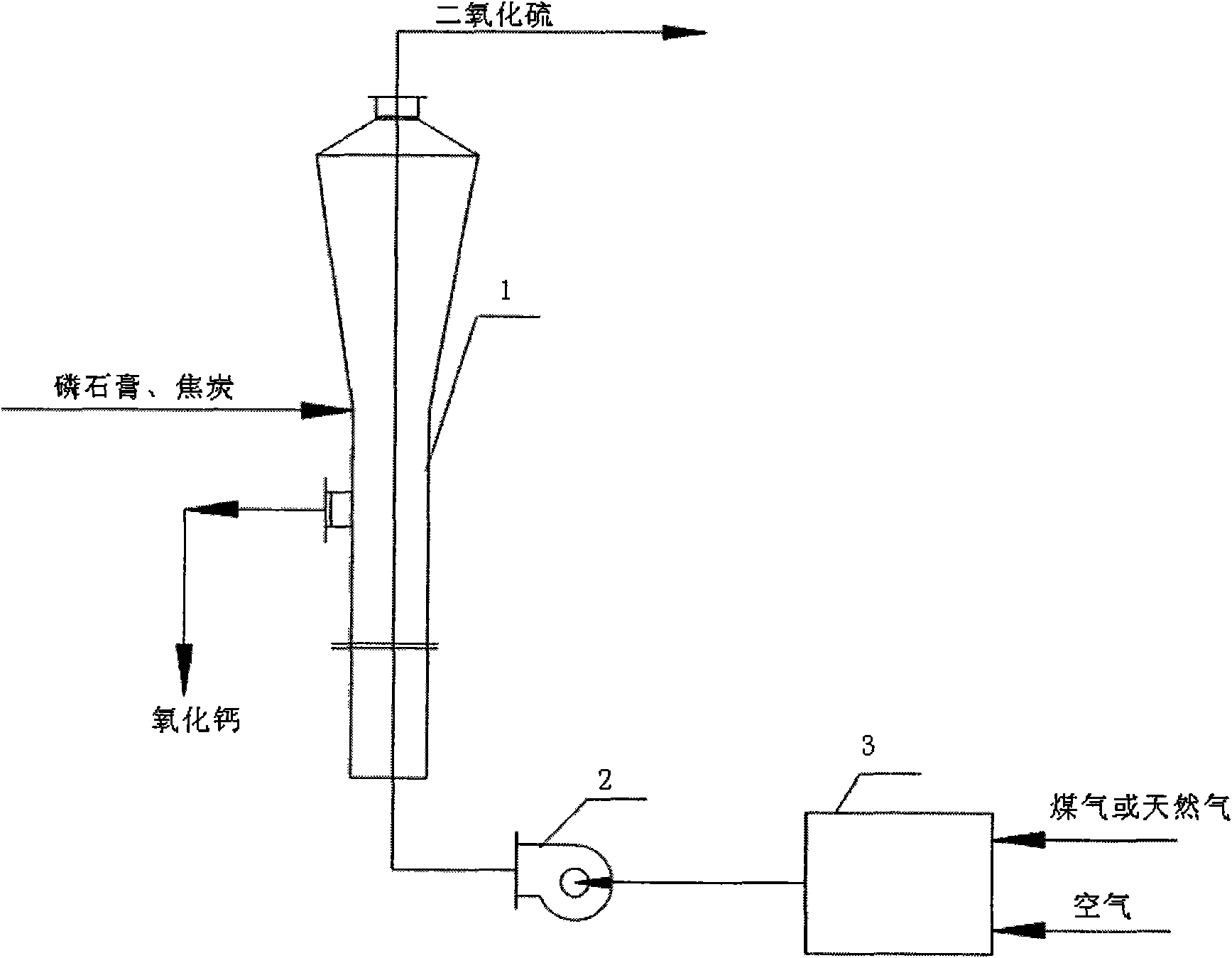
Production process for preparing calcium oxide and sulfur dioxide by decomposing ardealite
InactiveCN101602518ASolve the use problemReduce ecological damageSolid waste disposalSulfur compoundsDry weightReaction temperature
The invention belongs to the technical field of environmental protection treatment and chemical engineering production, and relates to a production process for preparing calcium oxide and sulfur dioxide by decomposing ardealite. The process comprises the following steps of: mixing the ardealite and charcoal according to a dry weight ratio of 10-20:1, granulating the mixture to obtain particles of which the particle size is between 1 and 4mm, drying and dewatering the particles, putting the particles in a fluidized bed decomposing furnace to be directly contacted with high-temperature furnace gas for reaction for 15 to 50 minutes at a temperature of between 1,000 and 1,300 DEG C, and generating the sulfur dioxide gas and the calcium oxide. The process can solve the problem of comprehensively utilizing waste residues of ardealite, the content of SO2 in the discharged tail gas is high enough to be used for producing sulfuric acid, and the calcium oxide which is a byproduct can be used in various industries such as construction, road, aquaculture and the like, so that the process meets the requirements on the scientific outlook on development; moreover, compared with the process for co-production of cement by sulfuric acid prepared by the ardealite, the process greatly saves investment and electric consumption.
Owner:SINOPEC NANJING ENG & CONSTR
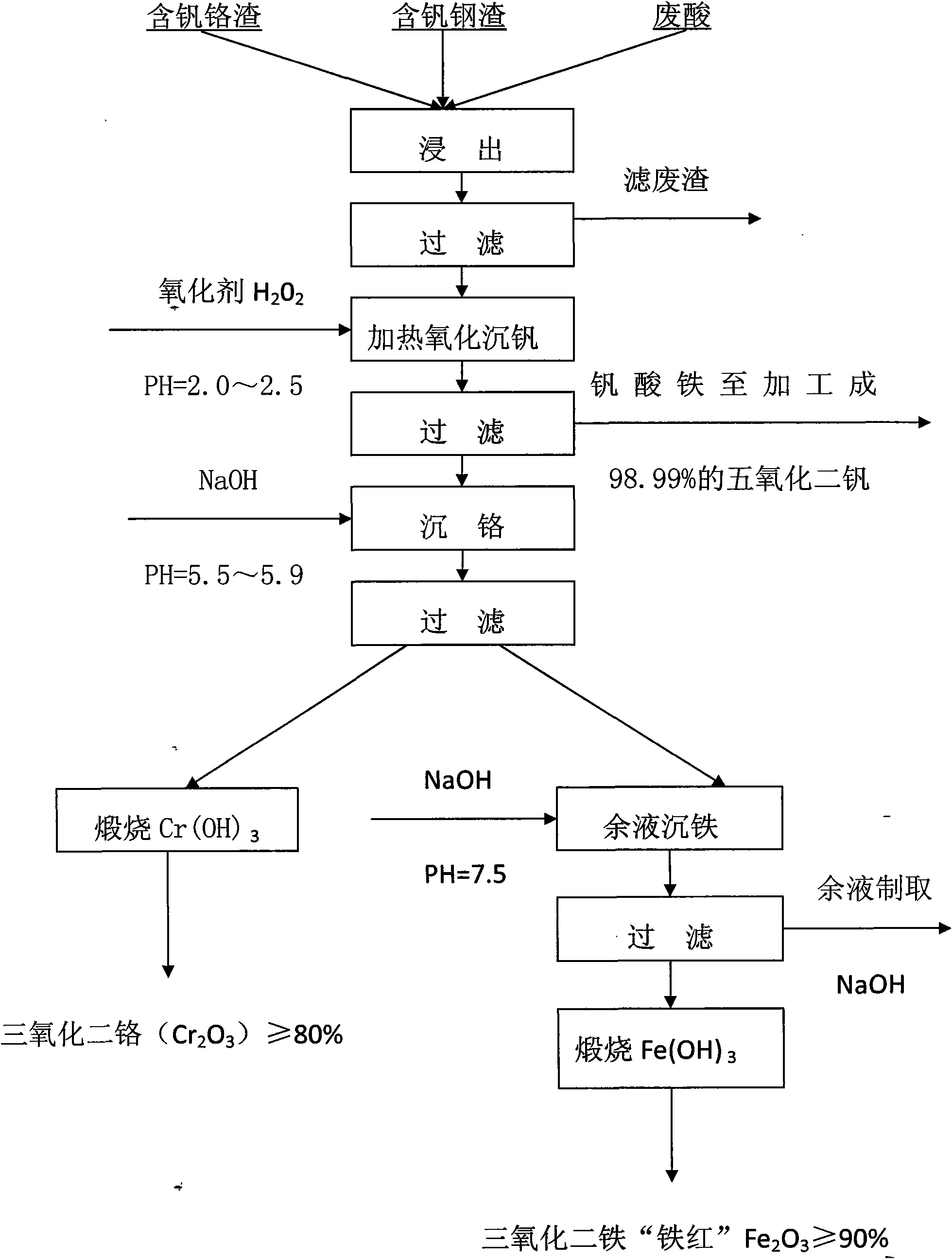
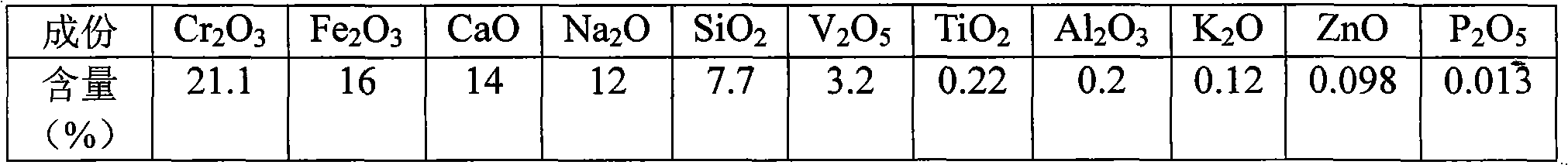
Process for extracting vanadium and chromium from chromic slag by using waste acid of titanium powder plant
InactiveCN101979683AFiltration process goes wellAchieve the purpose of separationProcess efficiency improvementChromium(III) hydroxideSlag
The invention discloses a method for separating and extracting vanadium and chromium. The method comprises the following steps of: (1) producing chromium fine sand (Cr2O3) of which the content is over 80 percent and ferric vandate of which the content is over 20 percent from two waste materials by taking waste acid of a titanium powder plant as a leaching agent and vanadium-chromium slag (containing 2.5 to 4.5 percent of vanadium and 14 to 25 percent of chromium) as a raw material; (2) putting the vanadium-chromium slag into the waste acid to allow the chromium and the vanadium in the slag to form chromium sulfate and vanadyl sulfate which can be dissolved in water very easily, wherein the leaching time is about 6 hours; (3) adding a certain amount of steel making steel slag during leaching to fulfill the aim of generating a great deal of calcium sulfate when a great deal of calcium oxide meets the acid during filtration, and wrapping, adsorbing or and stopping 'silica gel' formed by silicon dioxide in the chromium slag by the calcium sulfate which is used as a filter medium to ensure that the filtration is performed smoothly; (4) adjusting the pH value of the filtrate to be 2.5 by using sodium hydroxide, and then adding an oxidant and oxydol to ensure that the chromium in the solution is oxidized to be hexavalent, the iron is oxidized to be trivalent, and the vanadium is oxidized to be pentavalent; (5) heating the leaching solution to the temperature of between 70 and 90 DEG C to ensure that the vanadium and the iron is combined together to generate water-fast 'ferric vandate', wherein the time for thermal precipitation is about one hour, and the vanadium residual in the solution is not more than 0.4 g / L; (6) adding sodium hydroxide into the solution of which the ferric vandate is filtered out, and fully stirring the mixture until the pH value of the solution is between 5.5 and 5.9 to ensure that the chromium in the solution is completely converted into chromium.
Owner:PANZHIHUA SHUOSHENG IND & TRADING
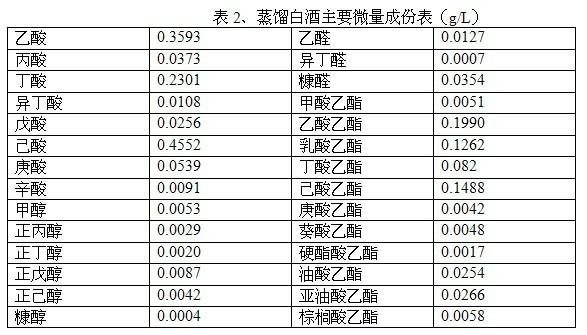
Method for making wine through grain of distiller of strong aromatic Chinese spirits by secondary fermentation and wine made by same
The invention discloses a method for making wine through grain of distiller of strong aromatic Chinese spirits by secondary fermentation, which comprises the following steps: utilizing strong aromatic Chinese grain of distiller as a substrate; adding yeast in the grain of distiller for piling fermentation; adding distillation tails, yellow water, esterase, acetic acid fermentation liquor and the like and stirring evenly; and then sealing by a plastic film for making the wine through the secondary fermentation. The invention fully utilizes a special culture medium, i.e. odour precursor and distilled grain remained in the grain of distiller to generate odour substances through the secondary fermentation of the grain of distiller. The grain of distiller is piled up after the yeast is added in, which aims to prompt the self-growth of the microorganism so as to lay a basis for esterification. The invention can also utilize the starch and the like in the grain of distiller so as to improve the utilization ratio of the distilled grain. The plastic film is used for sealed anaerobic fermentation after the piling fermentation so as to improve the production of the ester, and effectively avoid the discordant substance to body generated by microorganism in pit mud. The novel wine which is light on the palate and balance in fragrance is obtained by distillation under normal pressure after the fermentation.
Owner:LUZHOU LAOJIAO CO LTD +1
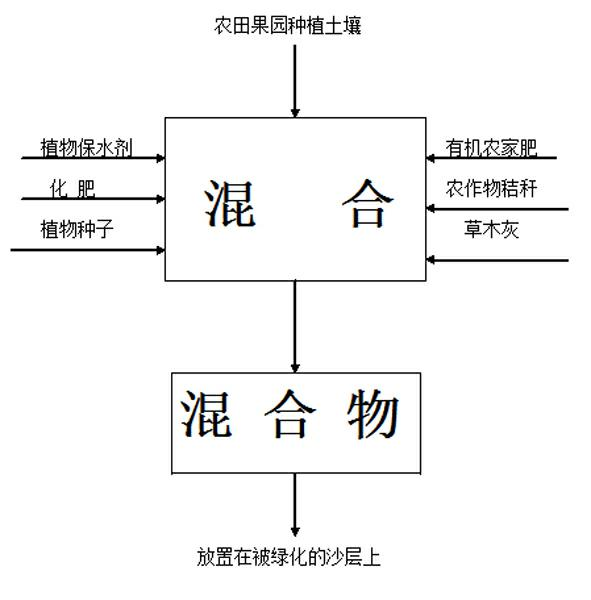
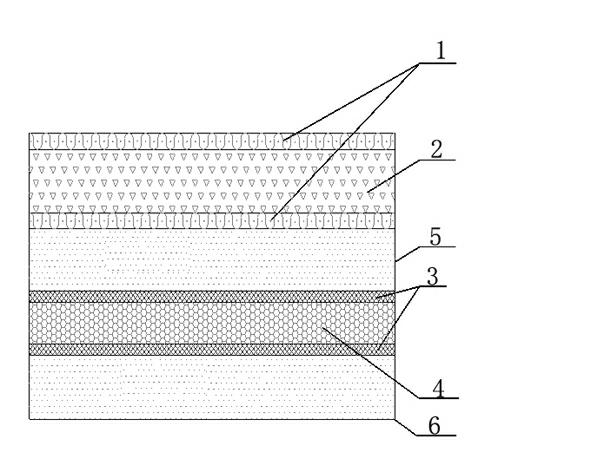
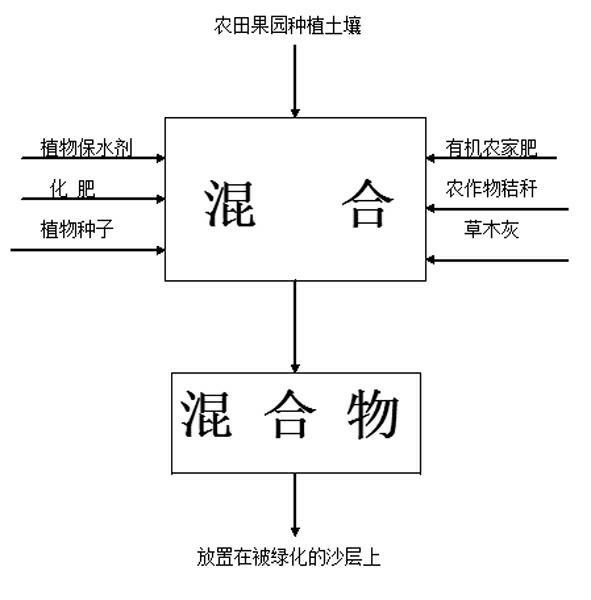
Method for afforesting desert and retaining water
InactiveCN102160483ABlock flowConserve waterClimate change adaptationAfforestationVegetationEcological environment
The invention relates to the field of ecological environment protection, in particular to a method used for afforesting a desert. The method used for afforesting the desert comprises the following steps of: 1, excavating a filling slot; 2, paving a waterproof impervious layer; 3, paving a sand layer; 4, paving a plant growing layer; and 5, paving a straw plaited grid. The waterproof impervious layer is arranged in the sand layer, the waterproof impervious layer stops flow direction of water, and water can not leak everywhere deep in the sand layer, thus the aim of saving water is achieved; the used material is environmentally friendly, and human and natural environment can not be polluted; water supply is reasonable, water can be uniformly supplied to plants used for afforesting, the plants can rapidly grow, and the problems of water for afforesting the desert and rainwater seepage control can be effectively solved. The method has a wide application field and is convenient for standardization construction. By applying the method to afforest the desert, a conditioned desert can be afforested, desert vegetation coverage rate can be improved, and a local ecological environment of the desert can be improved.
Owner:甘肃源岗农林开发有限公司
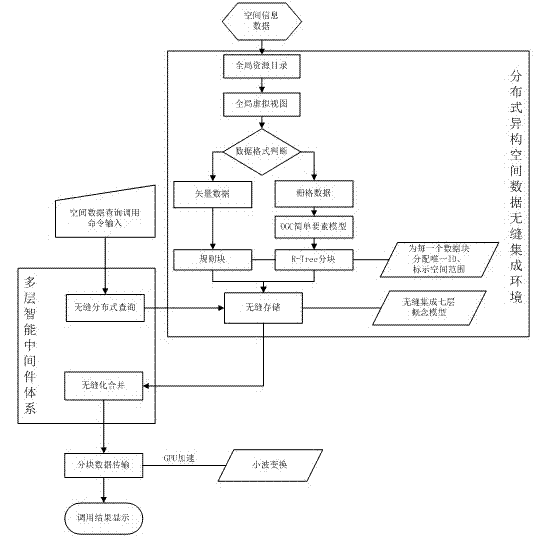
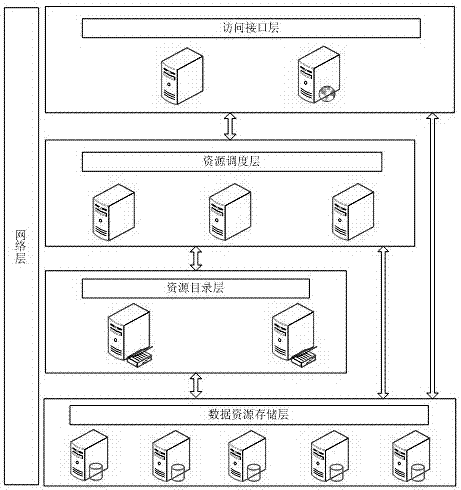
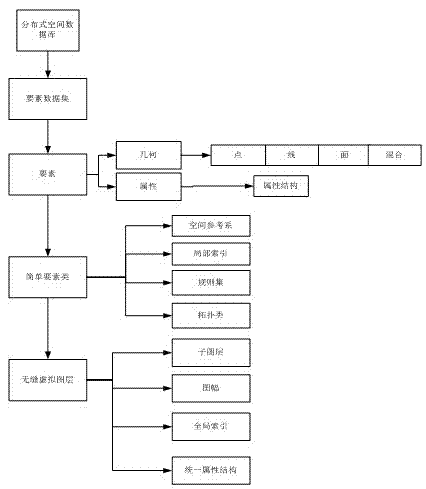
Mass multisource heterogeneous spatial information data seamless integration management method
InactiveCN102902782AEfficient integrationFacilitate geographic information sharingSpecial data processing applicationsGeographic information systemInformation resource
The invention discloses a mass multisource heterogeneous spatial information data seamless integration management method. The method comprises the steps of: building a distributed seamless integration conceptual model, and under the distributed heterogeneous spatial data seamless integration environment, using a multi-layer intelligent middleware system to build a cross-platform, exchangeable, safe and reliable spatial data management system which supports a heterogeneous database, the distributed computation and the cooperative services. According to the method provided by the invention, normalized sharing and low-level integration of the geographic information resource are realized, the data utilization rate is increased, and further the information timeliness and the working efficiency are improved.
Owner:ZHEJIANG UNIV +1
Balling method using medium- and low-grade phosphate rocks or ground phosphate rocks
ActiveCN103663396AHigh strengthHigh phosphorus contentPhosphorus compoundsPhosphoric acidCalciums magnesium
The invention relates to a balling method using medium- and low-grade phosphate rocks or ground phosphate rocks. The method comprises the following steps: S1, preparing materials, namely A, 85-100 parts of 60-120-mesh medium-grade and low-grade phosphate rocks or ground phosphate rocks, B, 1-10 parts of water or aqueous liquor of phosphoric acid, C, 2-8 parts of coke powder with the granularity of 60-120 meshes and D, 1-8 parts of one or more of phosphate fire clay, kaolin, sodium silicate, sodium carbonate, calcium carbonate and serpentine; S2, stirring, namely after uniformly mixing the raw materials, pressing the mixture into balls by use of a dry powder ball press machine, after pre-drying, delivering the mixture to a vertical calcining kiln, using purified yellow phosphorus tail gas, heating to 850-950 DEG C; starting roasting to obtain a finished product. The method is free from binders and low in cost, and the medium-grade and low-grade phosphate rocks are not hardened. The value of the medium-grade and low-grade phosphate rocks can be improved, and the ground phosphate rock of a mine can be fully utilized. The phosphate rock balls prepared are suitable for production of yellow phosphorus or calcium magnesium phosphate fertilizers.
Owner:刘静忠 +1
Method for preparing high-purity magnesium oxide with high boron salt lake brine
InactiveCN102491379ALarge particlesReduce extraction costsBoron-oxygen compoundsMagnesiaReaction temperatureIon-exchange resin
Provided is a method for preparing high-purity magnesium oxide with high boron salt lake brine. Salt lake brine is evaporated through a salt pan, concentrated to crystallize potassium sulfate, sodium chloride and potassium chloride and is drawn with lithium in adsorption mode so as to obtain master sauce brine containing magnesium and boron. Concentrated sulfuric acid is added into master sauce brine for reacting, and coarse boracic acid and acidized brine are obtained after cooling and filtering. Potential of hydrogen (pH) value of acidized brine is adjusted to be 5.5-6.5, and acidized brine passes through ion exchange resin adsorbing boron. When boron concentration in effluent liquid is higher than 5 mg / L, brine is not injected, boron-removed brine is obtained, then boron-removed brine and ammonium chloride solution are filled with ammonia for stirring and to produce magnesium sedimentation reaction, reaction temperature ranges from 60 DEG C to 80 DEG C, pH ranges from 7.5 to 8.0, the reaction is stopped when concentration of free ammonia reaches 1.8-2.2 mol / L, and magnesium hydroxide and magnesium sedimentation mother solution are obtained. Magnesium oxide is obtained by calcining magnesium hydroxide, content of magnesium oxide is larger than 99.8%, and magnesium extraction ratio is larger than 90%. Sedimentation mother solution adopts lime to steam ammonia, and generated ammonia circulates to magnesium sedimentation reaction. Mother solution after ammonia steaming is evaporated, concentrated and crystallized to obtain calcium chloride. Ion exchange resin adsorbing boron is washed, analyzed and regeneratively cycled for use. Boron-containing analysis solution is concentrated and cooled to pick up coarse boracic acid, and coarse boracic acid is recrystallized to obtain refined boracic acid with purity larger than 99%. High-purity magnesium oxide prepared by the method is high in purity, good in economic benefit, free of environment pollution, strong in operability and favorable for industrial production.
Owner:CENT SOUTH UNIV
Methods for preparing shredded mushroom and mushroom dried meat floss
The invention discloses methods for preparing shredded mushroom and mushroom dried meat floss by using stems of waste mushrooms generated during mushroom processing. The method for preparing shredded mushroom comprises the following steps of: sequentially carrying out softening process, acid soaking process, high-pressure saturated steam process and shredding process on the stems of mushrooms to prepare the shredded mushroom. The method for preparing mushroom dried meat floss comprises the following steps of: mixing the shredded mushroom with raw materials with different flavors, frying and twisting to be loose. Pyroligneous and high-pressure steam process in the method provided by the invention can soften and separate fibrous tissues of the stems of mushrooms furthest so that the fibers of the stems of the mushrooms are completely and thoroughly separated, and the diameter of the prepared shredded mushroom stem is between 0.4mm and 0.5mm. The mushroom dried meat floss prepared with the method provided by the invention is mellow and tasty, contains more dietary fibers, and has favorable practical value and popularization prospect.
Owner:健盛食品股份有限公司
Preparation method of hydrophobic nano-silica
ActiveCN102502663AAvoid reunionImprove hydrophobicitySilicaNanotechnologyPhotovoltaic industryHydrolysis
The invention belongs to the technical field of nanometer material, particularly relates to a preparation method of hydrophobic nano-silica. Silicon tetrachloride is added to alkaline solution to perform a hydrolysis reaction so as to prepare silica, and organic modifiers are added to liquid reactant for reaction; then hydrophobic nano-silica is obtained through water washing and drying; or the organic modifiers are added for reaction after the liquid reactant is processed through water washing, and then hydrophobic nano-silica is obtained through drying. The preparation method effectively solves the utilization problem of a by-product that is silicon tetrachloride in photovoltaic industry and other industrial productions, is simple, fast, safe and controllable, achieves lower cost, and can process silicon tetrachloride massively.
Owner:河南海博瑞硅材料科技有限公司
Method for removing heavy metal cadmium from rice by utilizing complex lactobacillus fermentation
ActiveCN104585563AHigh removal rateThe process is simple and reliableFood preparationFodderPEDIOCOCCUS PENTOSACEUS
The invention relates to a method for removing heavy metal cadmium from rice by utilizing complex lactobacillus fermentation. The method comprises the following steps: crushing a rice sample with the content of cadmium of more than 0.2mg / kg, sieving, and adding deionized water; inoculating a mixed zymocyte suspension containing a lactobacillus plantarum seed culture solution and a pediococcus pentosaceus seed culture solution, standing, fermenting at a constant temperature, washing fermented rice flour with water, and centrifugally dewatering to remove free heavy metal cadmium ions in the rice; and finally, drying centrifuged rice flour wet residues. By adopting the method provided by the invention, the removal rate of the cadmium in the rice is up to 70% above; the dewatered rice flour can be used as a feed or used for producing fermented rice flour after being dried by hot air. The content of the heavy metal cadmium in the rice can be effectively reduced, and the utilization problem of the rice with the excessive cadmium is effectively solved.
Owner:HUNAN AGRICULTURAL UNIV
Tobacco floating seed rearing culture medium reproduction process
InactiveCN101531551ASolve the use problemOrganic fertilisersFertilizer mixturesNicotiana tabacumDecomposition
The invention relates to a tobacco floating seed rearing culture medium reproduction process which uses crops straw to carry out tri-dimensional cultivation of oyster mushroom with raw culture medium, uses mushroom residue as raw material to carry out microorganism fermentation decomposition disinfection to prepare organic matter and further prepares culture medium for tobacco seed rearing to carry out floating seed rearing. The process includes steps of adding microorganism decomposing agent into the discarded mushroom residue, utilizing high temperature naturally generated in the decomposition process to kill edible fungus mycelium, sprinkling carbendazim to kill other competitor, mixing decomposed and sterilized mushroom residue, vermiculite and perlite according to volume ratio of 5:3:2, and uniformly stirring to obtain the tobacco floating seed rearing culture medium. The process synthetically utilizes crops straw, and can generate substantial economic benefit and social benefit.
Owner:谢德平
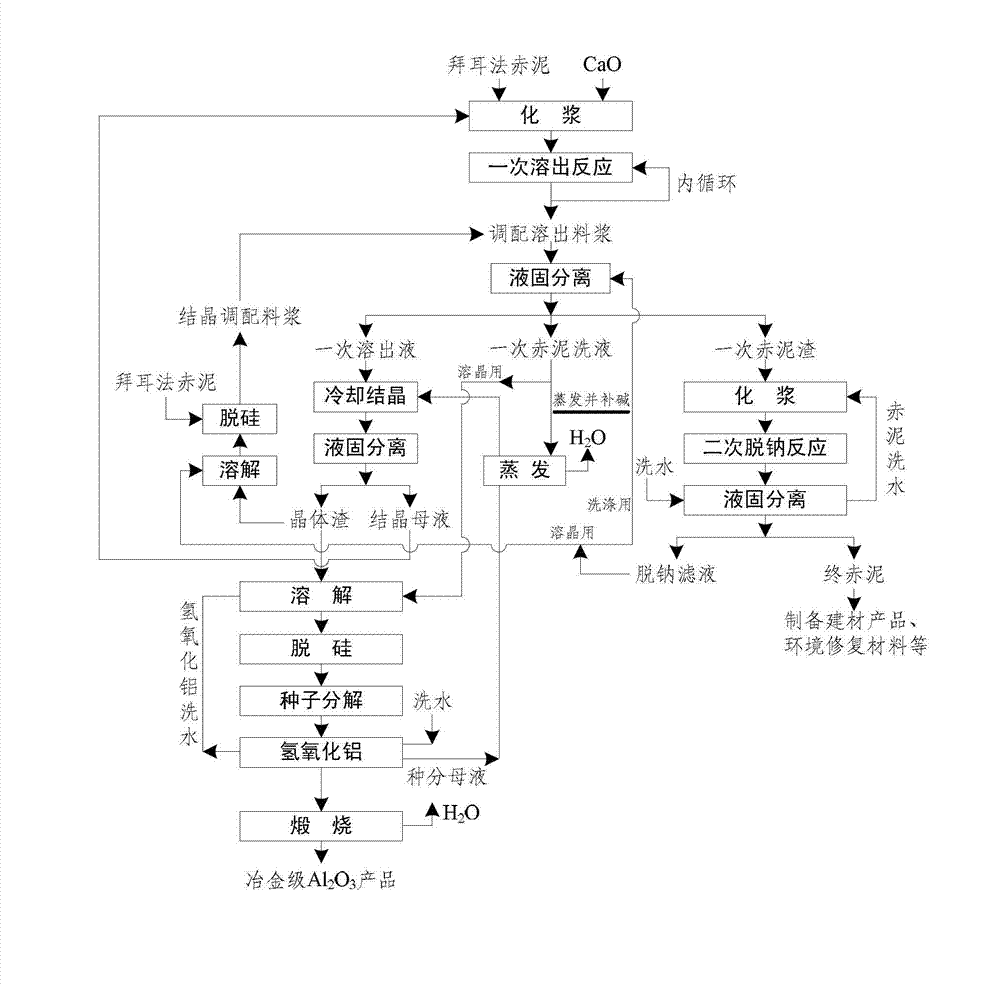
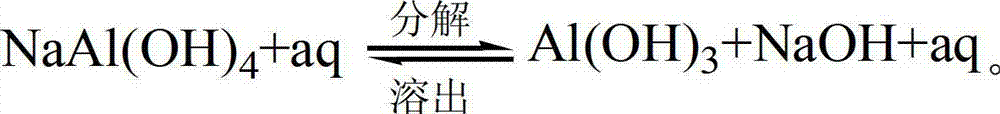
Method for recycling alumina and sodium oxide from bayer process red mud
ActiveCN103030160AHigh dissolution rateEfficient cycleAlkali metal oxidesAluminium oxides/hydroxidesBrickRed mud
The invention relates to a method for recycling alumina and sodium oxide from bayer process red mud. In the method, by adopting a high molecular ratio and high alkali concentration sodium aluminate solution, aluminum extracting reaction can be rapidly carried out under mild operating conditions, so that the recovery rate of alumina in the red mud reaches more than 85%, and the defects, such as equipment scabbing and the like can be effectively prevented and even eliminated; by implementing an efficient crystallization process of an intermediate product of hydrated sodium aluminate, the cycle efficiency of a dissolution medium is greatly improved; due to complete transformation of a phase in the aluminum extracting reaction, a reaction process of recycling sodium oxide can be performed at low temperature and normal pressure; and after secondary sodium removal reaction, the sodium oxide content in final red mud is not more than 1% and is far less than 6-8% of the bayer process red mud. Therefore, the red mud can be doped in large proportion for preparing cement, brick and roadbed materials, concrete additives, environmental remediation materials and other fillers, and the problems such as resource utilization of the red mud, potential environment hazard and the like are hopefully solved.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Enrichment and separation method of blue green algae phycocyanin
ActiveCN101560254AImprove adsorption capacityReduce manufacturing costDepsipeptidesPeptide preparation methodsChromatographic separationFreeze-drying
An enrichment and separation method of blue green algae phycocyanin belongs to the technical field of biochemical separation engineering. The invention comprises the following steps: blue green algae disrupted solution is prepared; JDN-3 type resin enriches phycocyanin in the blue green algae disrupted solution; phycocyanin is eluted and separated; chromatographic separation is carried out on the JDN-3 type resin; freeze drying is carried out on eluent after desalination to obtain phycocyanin with certain purity; the recovery rate of albumen is up to 85.5%. The method is simple, directly uses the JDN-3 type resin to enrich the blue green algae disrupted solution without using usual ammonium sulfate salt precipitation, organic solvents, isoelectric points and other similar methods, reduces the dosage of chemical agents in the preparation process, simplifies the steps of the purification process, avoids the loss of products; the phycocyanin obtained by the chromatography of the JDN-3 type resin has higher purity which is up to A620 / A280>2.0. The raw material uses fresh algae or dried algae powder, or even field blue-green algae in Taihu Lake, thereby providing a technical method for solving scale use of algal resource.
Owner:DONGTAI CITY SPIRULINA BIO ENG CO LTD
Aluminum-chromium-zirconium composite air bricks and manufacturing method thereof
ActiveCN101792323ASmall coefficient of thermal expansionImprove mechanical propertiesMelt-holding vesselsFurnace componentsBrickMullite
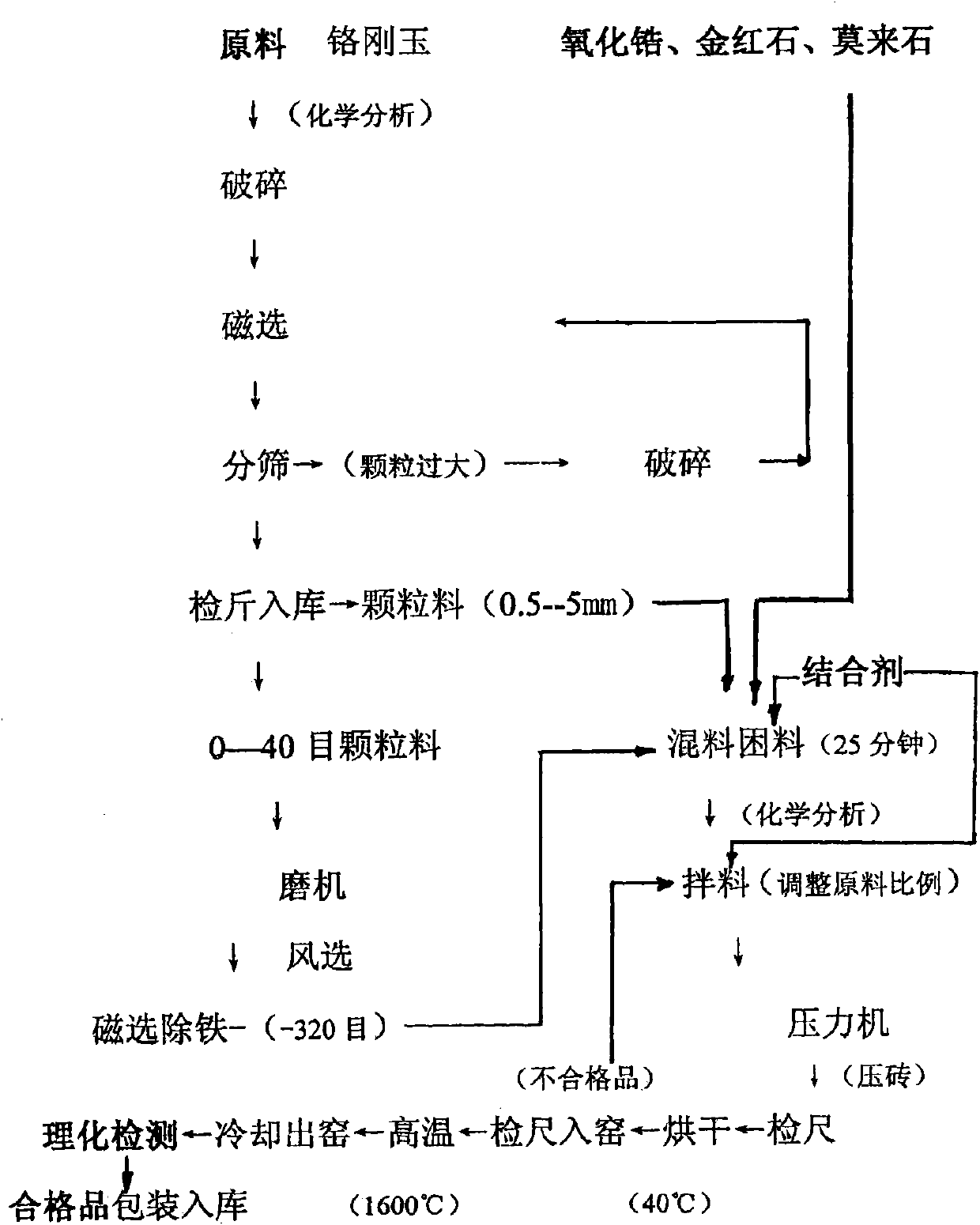
The invention discloses aluminum-chromium-zirconium composite air bricks and a manufacturing method thereof, and relates to industrial bricks and a manufacturing method thereof. Each brick comprises the following chemical components in percentage by weight: more than or equal to 72 percent of Al2O3, more than or equal to 15 percent of Cr2O3, more than or equal to 4 percent of ZrO2, more than or equal to 5 percent of mullite, more than or equal to 3 percent of TiO2, and more than or equal to 1 percent of Fe2O3. The aluminum-chromium-zirconium composite air brick comprises the following processsteps: crushing a raw material chrome corundum until the largest granule has the grain size of less than or equal to 5mm; performing deironization, magnetic separation and sieving, and feeding granules with the grain size of 0.5 to 5mm into a blender mixer to mix the granules and zirconium oxide, rutile, mullite and a binding agent; grinding granules with 0 to 40 meshes into granules of -320 meshes, performing magnetic separation and deferrization on the granules, mixing the granules and the zirconium oxide, rutile, mullite and the binding agent, standing for 25 minutes and performing brick-pressing operation; and drying the bricks at the temperature of 40 DEG C, and firing the bricks in a high-temperature kiln at the temperature of between 1,600 and 1,800 DEG C. The aluminum-chromium-zirconium composite air bricks are applied to an airheater in oil field, a scrap metal incinerator and a copper converter, and the service life of the furnaces is improved.
Owner:锦州大隆特种耐火材料有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com