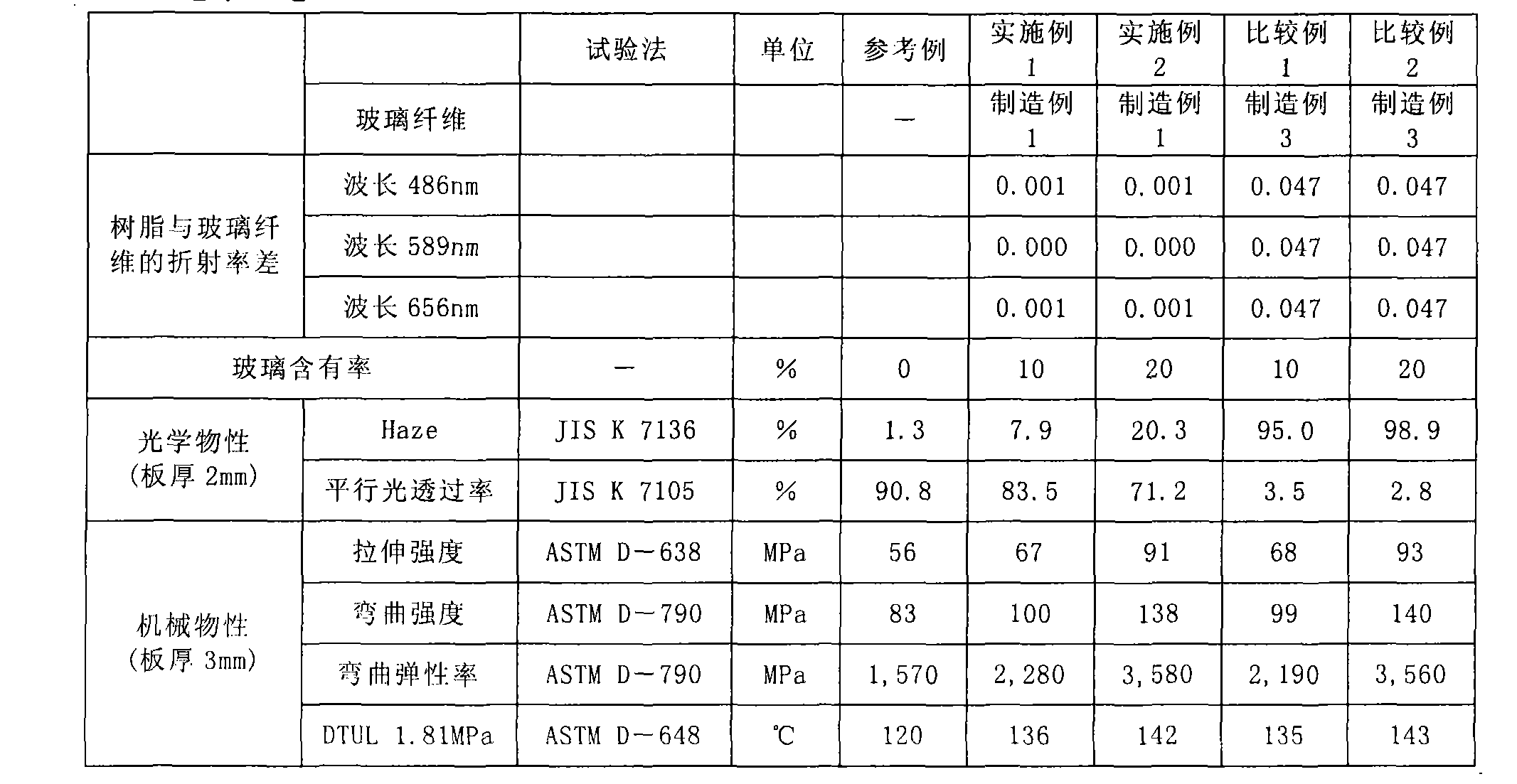
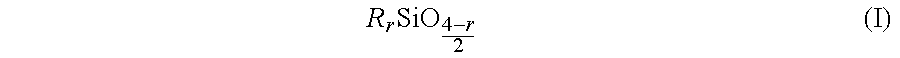Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1100 results about "Barium oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Barium oxide, BaO, is a white hygroscopic non-flammable compound. It has a cubic structure and is used in cathode ray tubes, crown glass, and catalysts. It is harmful to human skin and if swallowed in large quantity causes irritation. Excessive quantities of barium oxide may lead to death.
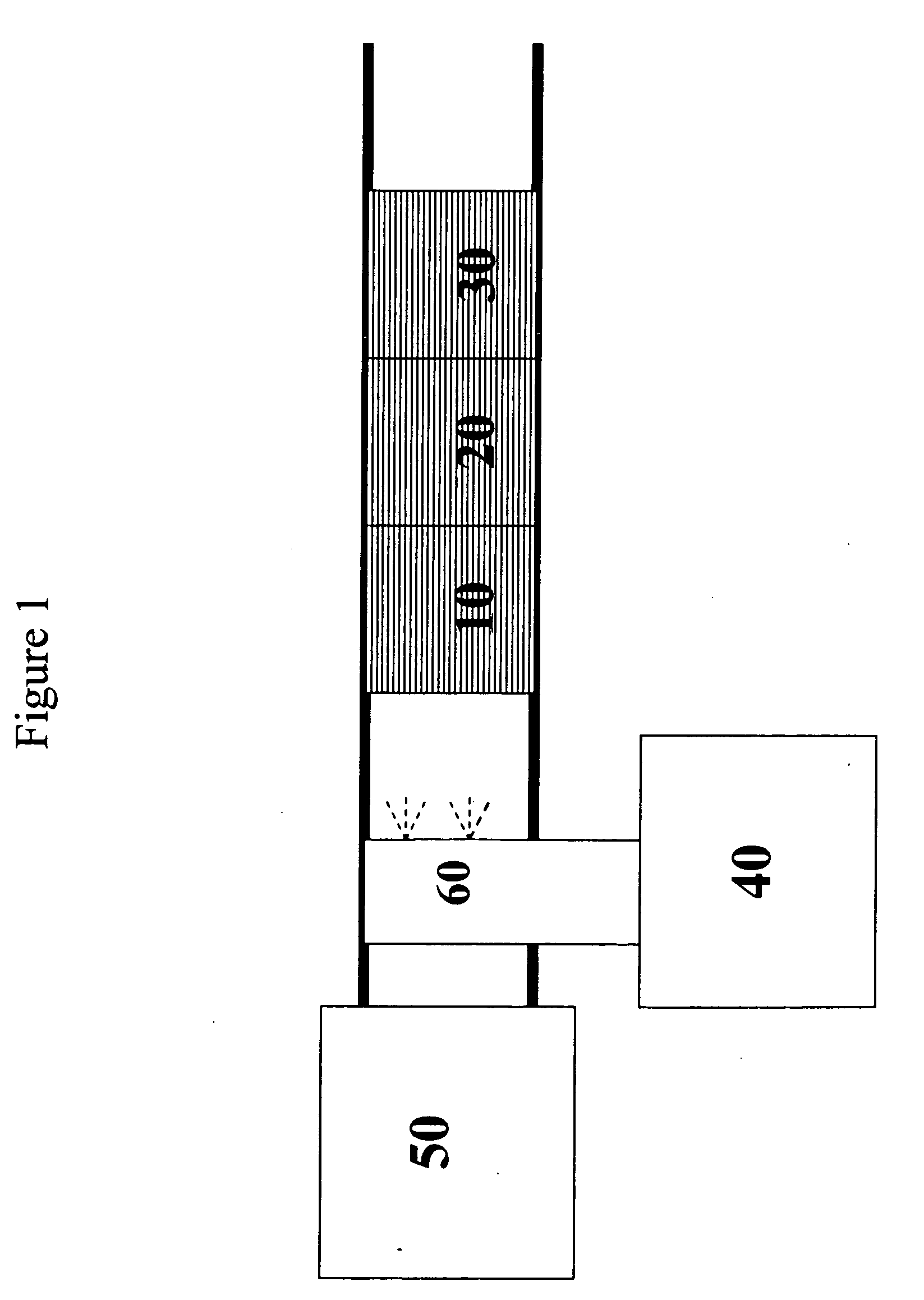
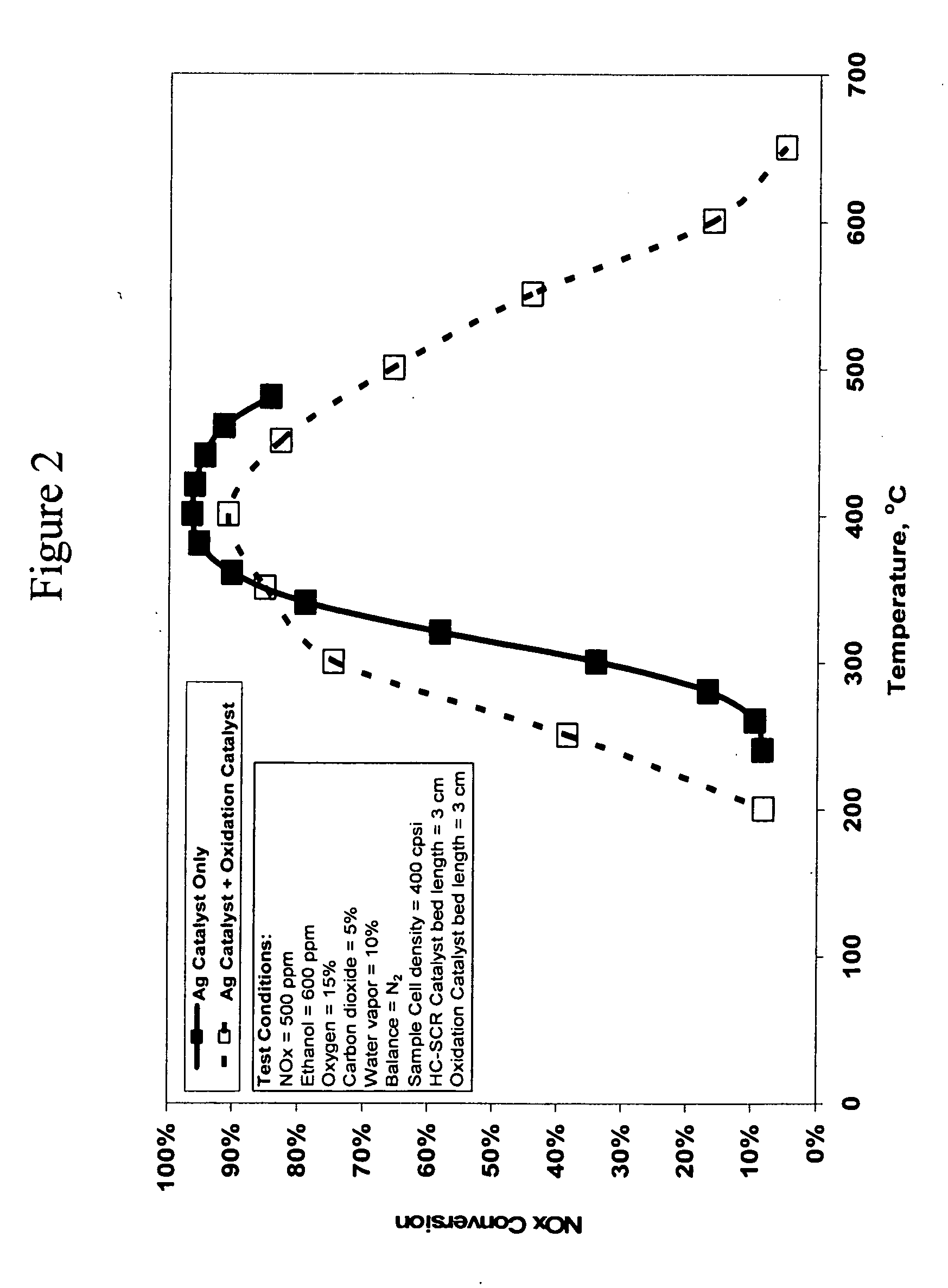
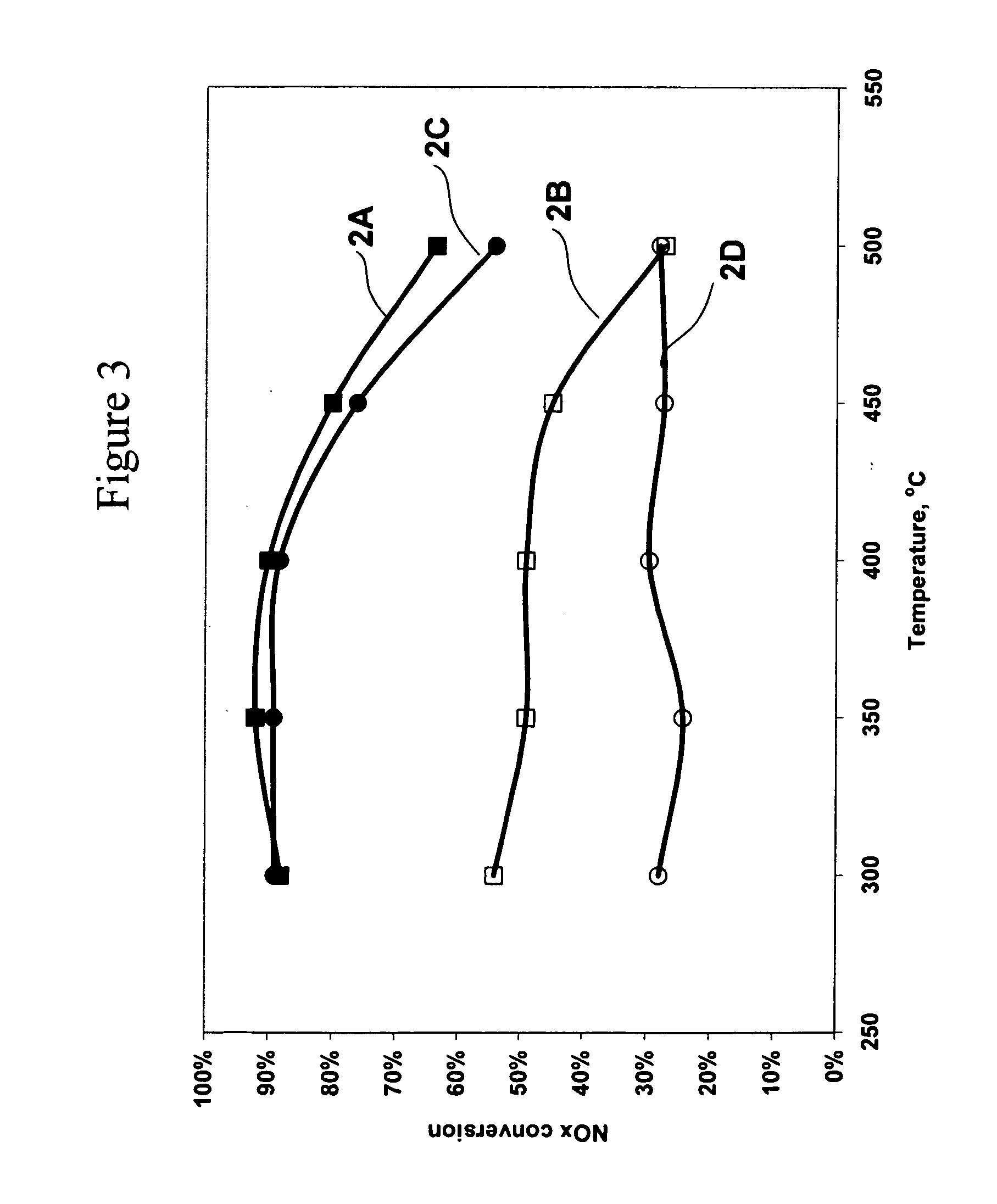
Catalyst and method for reducing nitrogen oxides in exhaust streams with hydrocarbons or alcohols
A catalyst system and a method for reducing nitrogen oxides in an exhaust gas by reduction with a hydrocarbon or oxygen-containing organic compound reducing agent are provided. The catalyst system contains a silver catalyst and a modifier catalyst, where the modifier catalyst contains a modifier oxide, where the modifier oxide is selected from the group consisting of iron oxide, cerium oxide, copper oxide, manganese oxide, chromium oxide, a lanthanide oxide, an actinide oxide, molybdenum oxide, tin oxide, indium oxide, rhenium oxide, tantalum oxide, osmium oxide, barium oxide, calcium oxide, strontium oxide, potassium oxide, vanadium oxide, nickel oxide, tungsten oxide, and mixtures thereof. The modifier oxide is supported on an inorganic oxide support or supports, where at least one of the inorganic oxide supports is an acidic support. The catalyst system of the silver catalyst and the modifier catalyst provides higher NOx conversion than either the silver catalyst or the modifier catalyst alone.
Owner:CATALYTIC SOLUTIONS INC
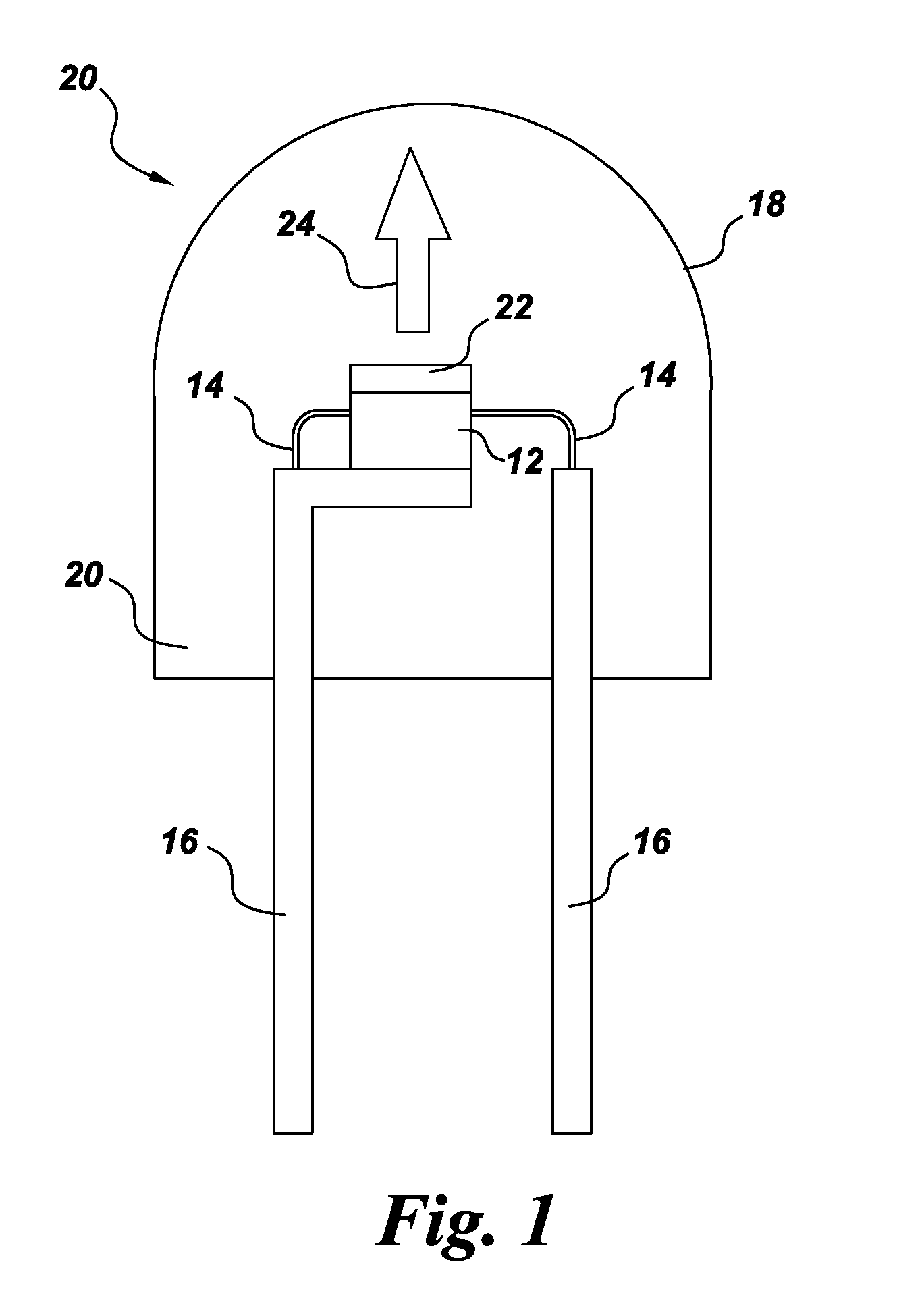
Chip antenna and mobile communication apparatus using same
InactiveUS6075491ASatisfactory antenna characteristicImprove reliabilitySimultaneous aerial operationsAntenna supports/mountingsCapacitanceElectrical conductor
A chip antenna 10 includes, inside a rectangular-parallelepiped base 11 having barium oxide, aluminum oxide, and silica as main constituents, a conductor 12 wound in a spiral form along the length direction of the base 11, and an LC parallel resonance circuit 13, which is inserted in the intermediate portion of the conductor 12 and which is connected electrically in series with the conductor 12, and includes, on the surface of the base 11, a power-feeding terminal 14 for applying a voltage to the conductor 12. The conductor 12 is separated into a first conductor 121 and a second conductor 122 by the LC parallel resonance circuit 13. The LC parallel resonance circuit 13 is formed of a coil L1, which is an inductance element, and a capacitor C1, which is a capacitance element, which are connected in parallel.
Owner:MURATA MFG CO LTD
Exhaust gas catalyst
InactiveUS6180075B1Favorable degree of conversionExceptional heat and aging resistanceNitrogen compoundsInternal combustion piston enginesZirconium hydrideCerium(IV) oxide
A single-layered three-way catalytic converter containing palladium as the only catalytically active noble metal, with high activity and heat resistance. The catalyst contains, in addition to finely divided, stabilized aluminum oxide, at least one finely divided cerium / zirconium mixed oxide and optionally finely divided nickel oxide as well as highly dispersed amounts of cerium oxide, zirconium oxide and barium oxide. The palladium is distributed largely uniformly throughout the entire catalyst.
Owner:DMC2 DEGUSSA METALS +1
Silicone rubber compositions for producing cables or profiles with retention of function in the event of fire
InactiveUS6387518B1Improve the level ofPropertyFireproof paintsAntifouling/underwater paintsZinc boratePlatinum complex
A composition which is useful for producing profiles and cable insulation which retain their function in the event of fire, comprise peroxidically crosslinking or condensation-crosslinking silicone rubber, metal oxides selected from magnesium oxide, aluminum oxide, tin oxide, calcium oxide and barium oxide and metal compounds of this class which produce oxides on heating, boric acid, zinc borate, and a platinum complex having at least one unsaturated group.
Owner:WACKER CHEM GMBH
Coefficient of thermal expansion filler for vanadium-based frit materials and/or methods of making and/or using the same
ActiveUS20120213954A1Improve sealingReduce sealClimate change adaptationWindows/door improvementMetal chlorideFrit
Certain example embodiments relate to seals for glass articles. Certain example embodiments relate to a composition used for sealing an insulted glass unit. In certain example embodiments the composition includes vanadium oxide, barium oxide, zinc oxide, and at least one additional additive. For instance, another additive that is a different metal oxide or different metal chloride may be provided. In certain example embodiments, a composition may be combined with a binder solution that substantially or completely burns out by the time the composition is melted. In certain example embodiments, a CTE filler is included with a frit material. In certain example embodiments, a vacuum insulated glass unit includes first and second glass substrates that are sealed together with a seal that includes the above-described composition.
Owner:GUARDIAN GLASS LLC
Vanadium-based frit materials, and/or methods of making the same
ActiveUS20120213952A1Little loss of strengthImprove sealingGlass blowing apparatusGlass reforming apparatusMetal chlorideFrit
Certain example embodiments relate to improved seals for glass articles. Certain example embodiments relate to a composition used for sealing an insulted glass unit. In certain example embodiments the composition includes vanadium oxide, barium oxide, zinc oxide, and at least one additional additive. For instance, another additive that is a different metal oxide or different metal chloride may be provided. In certain example embodiments, a vacuum insulated glass unit includes first and second glass substrates that are sealed together with a seal that includes the above-described composition.
Owner:GUARDIAN GLASS LLC
Method for preparing silicon-free or low-silicon acidproof ceramic fracturing propping agent
The invention discloses a method for preparing a silicon-free or low-silicon acidproof ceramic fracturing propping agent, which comprises the following steps of: (1) preparing the following raw materials in percentage by weight: 30 to 95 percent of alumina, 5 to 70 percent of barium carbonate and 0 to 10 percent of sintering aid, wherein the sintering aid is one or more of titanium dioxide, boric acid, calcium carbonate, magnesium oxide and dolomite; (2) performing ball milling on the raw materials in the step (1) in a ball mill for 24 to 48 hours; (3) preparing the raw materials obtained in the step (2) into semi-finished product particles; and (4) sintering the semi-finished product particles obtained in the step (3) at the temperature of between 1,500 and 1,800 DEG C for 1 to 3 hours, and cooling to the room temperature. The method has simple process; the barium carbonate is decomposed at high temperature to generate barium oxide, and the barium oxide is fully reacted with alumina to generate stable barium aluminate; and the barium aluminate has strong acidproof performance and protects other components in the finished product against corrosion of acid liquor, so the acidproof performance of the finished product is greatly improved.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
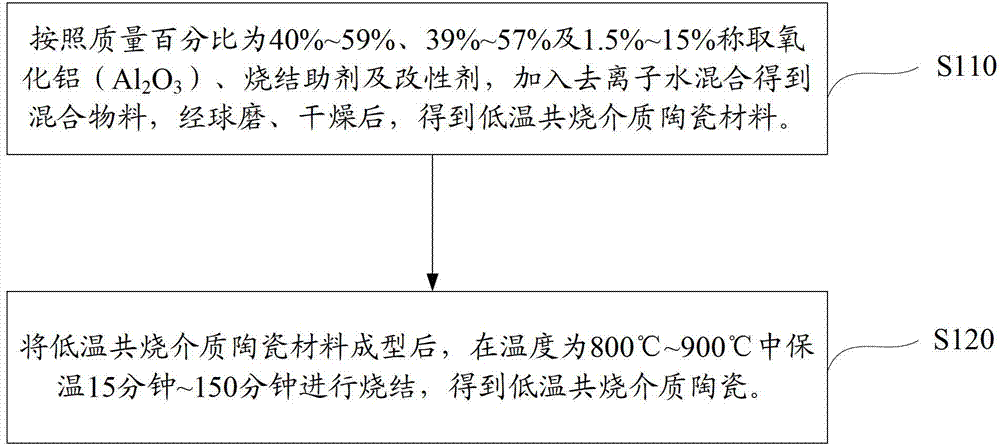
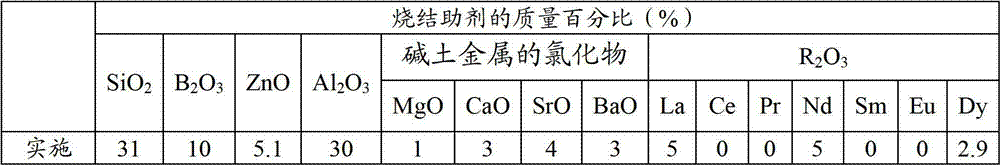
Method, sintering aid and materials for preparation of low-temperature cofired medium ceramic and application
A sintering aid for a low-temperature cofired medium ceramic material is composed of, by weight, 31%-45% of silicon dioxide, 1%-10% of boron oxide, 5.1%-10% of zinc oxide, 18%-30% of aluminum oxide, 11%-24% of alkaline earth metallic oxide and 5%-15% of oxide with the general formula of R2O3, wherein R refers to at least one of lanthanum, cerium, praseodymium, neodymium, samarium, europium and dysprosium, and the alkaline earth metallic oxide refers to one of magnesium oxide, calcium oxide, barium oxide and strontium oxide. Adding the sintering aid into the low-temperature cofired medium ceramic material enables the prepared low-temperature cofired medium ceramic to have excellent thermal mechanical performance and dielectric performance. In addition, the invention provides the low-temperature cofired medium ceramic material and application thereof and a method for preparing the low-temperature cofired medium ceramic.
Owner:GUANGDONG FENGHUA ADVANCED TECH HLDG
Process for producing polysiloxanes and use of the same
A process for the preparation of an organosilicon condensate which comprises reacting together a silicon containing compound having at least one silanol group and a silicon containing compound having at least one —OR group or at least one silanol group (or a compound having both groups) in the presence of strontium oxide, barium oxide, strontium hydroxide or barium hydroxide and optionally a solvent such as water, methanol, ethanol, 1-propanol, 2-propanol, 1-butanol and 2-butanol, acetone or toluene.
Owner:ZETTA RES & DEV LLC RPO SERIES
Organic luminescence device and its production method
InactiveUS20050122039A1Excellent gas barrier propertiesDischarge tube luminescnet screensLayered productsWater vaporOxygen
An organic luminescence device uses a substrate with a gas-barrier film in which a gas-barrier film containing an amorphous oxide and at least two kinds of oxides selected from the group consisting of boron oxide, phosphorus oxide, sodium oxide, potassium oxide, lead oxide, titanium oxide, magnesium oxide, and barium oxide is formed on a substrate. The selected two kinds of oxides are a combination of an oxide of an element having a large atomic radius and an oxide of an element having a small atomic radius. The substrate is made of glass or plastic. As a result, the organic luminescence device using a substrate excellent in gas-barrier capability to prevent the infiltration of oxygen, water vapor, etc. from outside is provided.
Owner:PANASONIC CORP
Packaging material used for a display device and method of forming thereof
InactiveUS20040084686A1Non-macromolecular adhesive additivesSemiconductor/solid-state device detailsZinc bromideAlkaline earth metal
A packaging material used for a display device which is a desiccative-containing adhesive agent. The desiccative-containing adhesive agent is composed of a liquid-state organic material selected from a group including epoxy resin, polyurethane, bakelite, polyamide, acrylic resin and polysiloxane, and a solid-state desiccative selected from a group including alkaline metal oxide, alkaline-earth metal oxide, metallic halide, barium oxide, calcium oxide, calcium sulfate, calcium chloride, lithium chloride, calcium bromide, potassium Carbonate, aluminum oxide, magnesium oxide, copper sulfate, zinc chloride, zinc bromide, cobalt chloride, silica gel, zeolite and molecular sieve.
Owner:DELTA OPTOELECTRONICS +1
Glass flake
InactiveUS20050049133A1Overcome lack of heat resistanceGood molding effectPigmenting treatmentOther chemical processesHeat resistanceBoron trioxide
The present invention provides a glass flake that is substantially free of boron trioxide (B2O3), barium oxide (BaO), zinc oxide (ZnO), and fluorine (F) and has sufficient heat resistance and good formability for glass flake. The glass flake of the present invention includes a glass composition that contains the following components, expressed in mol %; 50≦SiO2≦65; 4≦Al2O3<12; 5≦SrO≦25; 10<(MgO+SrO)≦30; 20≦(MgO+CaO+SrO)≦45; and 0<(Li2O+Na2O+K2O)<2; wherein the glass composition is substantially free of B2O3, BaO, ZnO, and F. This glass flake is useful for resin compositions, paints, cosmetics, ink compositions, and the like.
Owner:NIPPON SHEET GLASS CO LTD
Amorphous polyamide resin composition and molded product
Provided are an amorphous polyamide resin composition having high transparency, and is excellent in heat resistance and stiffness, and a molded product thereof. The glass filler contains, expressed in terms of oxides by mass %, 68 to 74% of silicon dioxide (SiO2), to 5% of aluminum oxide (Al2O3), 2 to 5% of boron oxide (B2O3), 2 to 10% of calcium oxide (CaO), 0 to 5% of zinc oxide (ZnO), 0 to 5% of strontium oxide (SrO), 0 to 1% of barium oxide (BaO), 1 to 5% of magnesium oxide (MgO), 0 to 5% of lithium oxide (Li2O), 5 to 12% of sodium oxide (Na2O), and 0 to 10% of potassium oxide (K2O), where a total amount of lithium oxide (Li2O), sodium oxide (Na2O), and potassium oxide (K2O) is 8 to 12%.
Owner:ASAHI FIBER GLASS CO LTD
Exhaust-gas purification catalyst to be used close to the engine and process for its production
InactiveUS6875725B2High activityOptimize dataNitrogen compoundsInternal combustion piston enginesTemperature stressCombustion
A starter catalyst for the purification of the exhaust gases from internal combustion engines, which include palladium on aluminum oxide and of barium oxide, as well as a process for its production. The barium oxide and palladium are together deposited in a finely divided state on the supporting material aluminum oxide and the average particle size of the palladium crystallites is between 3 and 7 nm. The small crystallite size of palladium and the barium oxide likewise deposited in finely divided state on the supporting material impart to the catalyst a high activity and long-term stability to high temperature stresses.
Owner:UMICORE AG & CO KG +1
Glass-ceramic composition, glass-ceramic sintered body, and monolithic ceramic electronic component
InactiveUS20060287184A1Improve electrical insulation reliabilityReduced insulation performanceCeramic layered productsPrinted circuit manufactureStrontium titanateLithium oxide
A glass-ceramic composition contains first ceramic particles principally containing forsterite; second ceramic particles principally containing at least one selected from the group consisting of calcium titanate, strontium titanate, and titanium oxide; and borosilicate glass particles containing about 3% to 15% lithium oxide, about 20% to 50% magnesium oxide, about 15% to 30% boron oxide, about 10% to 45% silicon oxide, about 6% to 20% zinc oxide, 0% to about 15% aluminum oxide, and at least one additive selected from the group consisting of calcium oxide, barium oxide, and strontium oxide on a weight basis. The content of the borosilicate glass particles is about 3% or more; the lower limit of the content of the additive is about 2%; and the upper limit of the additive content is about 15%, about 25%, or about 25% when the additive is calcium oxide, barium oxide, or strontium oxide, respectively, on a weight basis.
Owner:MURATA MFG CO LTD
Alkali-free aluminoborosilicate glass, and uses thereof
InactiveUS20020013210A1Improve heat resistanceFavorable processing rangeNon-linear opticsSemiconductor devicesAlkali freeSilicate glass
The invention relates to an alkali-free aluminoborosilicate glass having a coefficient of thermal expansion alpha20 / 300 of between 2.8.10-6 / K and 3.6.10-6 / K, which has the following composition (in % by weight, based on oxide): silicon dioxide (SiO2)>58-65, boric oxide (B2O3)>6-11.5, aluminum oxide (Al2O3)>20-25, magnesium oxide (MgO) 4-<6.5, calcium oxide (CaO)>4.5-8, strontium oxide (SrO) 0-<4, barium oxide (BaO) 0.5-<5, with strontium oxide (SrO)+barium oxide (BaO)>3, zinc oxide (ZnO) 0-<2, and which is highly suitable for use as a substrate glass both in display technology and in thin-film photovoltaics.
Owner:SCHOTT AG +1
Green body of red soil ceramic and formula of transmutation glaze of red soil ceramic
The invention relates to a green body of red soil ceramic. The green body of the red soil ceramic is prepared from the following raw materials: red soil, quartz, talc, limestone and dolomite; the transmutation glaze of the red soil ceramic is prepared from the following raw materials: potassium feldspar, albite, calcium oxide, barium oxide, kaolin, quartz powder, sodium tripolyphosphate, titaniumoxide, rutile, zinc oxide and a coloring agent. By addition of other mineral raw materials, chemical raw materials and the like, the physical performance and the chemical performance of the green body are changed and the technical problems that the green body glaze of the red soil product is difficult to combine and the product is loose and fragile are solved; the firing temperature of the glaze is reduced from 1,310 DEG C to 1,230 DEG C, so thatthe firing temperature is reduced by 80 DEG C, the using amount of the fuel and the discharge quantity of harmful gas are reduced, environmental protection is contributed and production cost is greatly saved; and by development of the lead-free transmutation glaze, the lead-free transmutation glaze is suitable for being combined with the green body of the red soil ceramic, so that the problem of pollution to the ceramic decorative environment is solved fundamentally.
Owner:FUJIAN JIAMEI GRP +1
Process for producing alkali free glass and alkali free glass plate
InactiveUS20080127679A1Small coefficient of thermal expansionLess bubbleGlass furnace apparatusGlass productionTO-18Platinum
To provide a process for producing and alkali free glass for effectively suppressing bubbles, and an alkali free glass produced by the process, which is suitable as a glass substrate for flat panel displays and has few bubbles.A process for producing an alkali free glass containing substantially no alkali metal oxide, which comprises melting a glass starting material having a matrix composition of the following composition, and subjecting the molten glass to a treatment process of removing bubbles under reduced pressure, stirring or transferring under a condition where the molten glass is in contact with a platinum member, wherein the starting material is prepared so as to contain SnO2 in an amount of from 0.01 to 2.0% per 100% of the total amount of the above matrix composition; the starting material is melted under heating at from 1,500 to 1,650° C.; then bubbles contained in the molten glass are permitted to rise to the surface of the molten glass, together with oxygen bubbles generated by a reduction reaction in which SnO2 in the molten glass is reduced to SnO; and in the above treatment process, the oxygen bubbles generated at the interface between the molten glass and the platinum member are permitted to be absorbed by an oxidation reaction in which SnO is oxidized to SnO2, under a condition where the molten glass is from 1,300 to 1,500° C.Composition as represented by the mass percentage:SiO258.4 to 66.0%,Al2O315.3 to 22.0%,B2O3 5.0 to 12.0%,MgO 0 to 6.5%,CaO 0 to 7.0%,SrO 4 to 12.5%,BaO 0 to 2.0%,MgO + CaO + SrO + BaO 9.0 to 18.0%.
Owner:ASAHI GLASS CO LTD
Electron beam device and electron beam application device using the same
ActiveUS20110240855A1Broadening energy widthIncrease brightnessMaterial analysis using wave/particle radiationElectric arc lampsElectron sourceAcceleration voltage
To obtain a SEM capable of both providing high resolution at low acceleration voltage and allowing high-speed elemental distribution measurement, a SE electron source including Zr—O as a diffusion source is shaped so that the radius r of curvature of the tip is more than 0.5 μm and less than 1 μm, and the cone angle α of a conical portion at a portion in the vicinity of the tip at a distance of 3r to 8r from the tip, is more than 5° and less than (8 / r)°. Another SE electron source uses Ba—O and includes a barium diffusion supply means composed of a sintered metal and a barium diffusion source containing barium oxide.
Owner:HITACHI HIGH-TECH CORP
Vanadium-based frit materials, binders, and/or solvents and methods of making the same
ActiveUS20120213953A1Improve sealingReduce sealLiquid surface applicatorsClimate change adaptationMetal chlorideFrit
Certain example embodiments relate to seals for glass articles. Certain example embodiments relate to a composition used for sealing an insulted glass unit. In certain example embodiments the composition includes vanadium oxide, barium oxide, zinc oxide, and at least one additional additive. For instance, another additive that is a different metal oxide or different metal chloride may be provided. In certain example embodiments, a composition may be combined with a binder solution that substantially or completely burns out by the time the composition is melted. In certain example embodiments, a vacuum insulated glass unit includes first and second glass substrates that are sealed together with a seal that includes the above-described composition.
Owner:GUARDIAN GLASS LLC
Coefficient of thermal expansion filler for vanadium-based frit materials and/or methods of making and/or using the same
ActiveUS20120308747A1Little loss of strengthImprove sealingWindows/door improvementGlass blowing apparatusMetal chlorideFrit
Certain example embodiments relate to seals for glass articles. Certain example embodiments relate to a composition used for sealing an insulted glass unit. In certain example embodiments the composition includes vanadium oxide, barium oxide, zinc oxide, and at least one additional additive. For instance, another additive that is a different metal oxide or different metal chloride may be provided. In certain example embodiments, a composition may be combined with a binder solution that substantially or completely burns out by the time the composition is melted. In certain example embodiments, a CTE filler is included with a frit material. In certain example embodiments, a vacuum insulated glass unit includes first and second glass substrates that are sealed together with a seal that includes the above-described composition.
Owner:GUARDIAN GLASS LLC
Color stable phosphors
ActiveUS20110279011A1Discharge tube luminescnet screensLamp detailsZinc hydroxideMagnesium phosphate
An LED lamp includes a light source configured to emit radiation with a peak intensity at a wavelength between about 250 nm and about 550 nm; and a phosphor composition configured to be radiationally coupled to the light source. The phosphor composition includes particles of a phosphor of formula I, said particles having a coating composition disposed on surfaces thereof;((Sr1−zMz)1−(x+w)AwCex)3(Al1−ySiy)O4+y+3(x−w)F1−y−3(x−w) Iwherein the coating composition comprises a material selected from aluminum oxide, magnesium oxide, calcium oxide, barium oxide, strontium oxide, zinc oxide, aluminum hydroxide, magnesium hydroxide, calcium hydroxide, barium hydroxide, strontium hydroxide, zinc hydroxide, aluminum phosphate, magnesium phosphate, calcium phosphate, barium phosphate, strontium phosphate, and combinations thereof; andA is Li, Na, K, or Rb, or a combination thereof;M is Ca, Ba, Mg, Zn, or a combination thereof; and0<x≦0.10, 0≦y≦0.5, 0≦z≦0.5, 0≦x≦x.
Owner:GE LIGHTING SOLUTIONS LLC
Preparation method of leadless glass powder for electronic slurry
A process for preparing lead-free glass powder used for electronic paste, which comprises: firstly uniformly mixing silicon dioxide or sodium silicate, barium oxide or barium carbonate, boron oxide or boric acid, bismuth oxide or bismuth sulfate, aluminum oxide, aluminum sulfate or aluminum silicate, zinc oxide or zinc carbonate, sodium oxide, sodium carbonate or sodium bicarbonate, potassium oxide or potassium carbonate and titanium dioxide, then heating the mixed raw materials to melt, cooling the melted mixture, then grinding into power by a ball grinder, sieving by a sieve of 100-400 eye and lastly drying glass powder to obtain the lead-free glass powder. Because the glass power of the invention does not contain lead oxide (PbO) or compounds of lead oxide (PbO), the invention is harmless in the manufacture process of paste, simultaneously is beneficial for environmental protection, and is convenient for recycling and reusing electronic product modules containing the electronic paste.
Owner:IRICO
Hydrorefining catalyst for preparing fuel oil from coal tar, preparation method and application method thereof
ActiveCN102068992AImprove operational stabilityImprove handling strengthCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsPtru catalystProcess engineering
The invention provides a hydrorefining catalyst special for new coal tar, a preparation method and an application method thereof, which are used for solving problems existing in the conventional hydrogenation catalyst for coal tar. The hydrorefining catalyst consists of active components, aids and carriers, wherein the active components refer to MoO3 and NiO or MoO3 and CoO; the aids refer to Bi2O3 and K2O; and the carriers refer to alumina and barium oxide. According to calculation by the form of an oxide, the active components, the aids and the carriers account for 15 to 35 percent, 2 to 5 percent and 60 to 83 percent of the total mass of the catalyst respectively. The catalyst is prepared by the following steps of: kneading, grinding, extruding into strips, molding, soaking, performing catalyst post-treatment and the like. New ultrasonic soaking technology is introduced, and the preparation condition of the catalyst is controlled strictly. Compared with the prior art, the catalyst has high surface physical and chemical properties, high purified atom degumming activity, high hydrogenation saturation capability, high stability and high mechanical intensity, and the quality and yield of hydrogenation product oil can be enhanced effectively.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
A method for recovering and preparing a lithium-manganese-iron phosphate positive-electrode material covered with carbon from waste lithium iron phosphate batteries
InactiveCN108923090AHigh recovery rateHigh purityCell electrodesNanotechnologyLithium iron phosphateFiltration
The invention provides a method of recovery and reutilization of waste lithium iron phosphate batteries. The method includes (1) separating positive electrode material mixture from the waste lithium iron phosphate batteries; (2) fully dissolving the positive electrode material mixture with sulfuric acid, performing filtration to obtain a first filtrate, adding ammonia water into the filtrate understirring until the pH value of the system is 1.0-1.9, stirring the mixture, and performing filtration to obtain a second filtrate and iron phosphate precipitate; (3) adding barium hydroxide or bariumnitrate into the second filtrate and performing filtration to obtain a third filtrate; (4) adding the third filtrate, the iron phosphate precipitate, a manganese source, a phosphorus source and a carbon source according to the element ratio of a product to be prepared that is lithium-manganese-iron phosphate LiFe<1-x>Mn<x>PO4 to obtain a solution mixture; and (5) subjecting the solution mixture to ball milling, drying and crushing, pre-sintering a product at a first temperature in an inert atmosphere, and sintering the product at a second temperature to obtain the lithium-manganese-iron phosphate positive-electrode material covered with carbon. Through the method, all elements in the waste lithium iron phosphate batteries are recovered and reutilized.
Owner:SHENZHEN DYNANONIC
Catalyst for preparing isophorone by acetone condensation method
InactiveCN101698147AImprove conversion rateHigh selectivityOrganic compound preparationCatalyst activation/preparationCeriumLanthanum
The invention provides a catalyst for preparing isophorone by acetone condensation method. The catalyst is sinter formed by the step that magnesium oxide or nitrate, calcium oxide or nitrate, barium oxide or nitrate, zirconium oxide or nitrate, rare earth metal oxide or nitrate and aluminium oxide are mixed and then sintered at 400-600 DEG C for 4-6 hours. The sinter contains 25.4-46.4% of aluminium element, 3-18% of magnesium element, 1.4-7.1% of calcium element, 0.8-4.2% of barium element, 0.7-1.5% of zirconium element and 0.5-6% of rare earth metal element; wherein the rare earth metal is one or two of lanthanum, cerium and yttrium. The catalyst is used for catalyzing acetone condensation reaction, can effectively improve acetone conversion and selectivity of isophorone and causes the acetone conversion and selectivity of isophorone to respectively reach more than 86% and 65%.
Owner:GUANGZHOU UNIVERSITY
EL device
InactiveUS6891329B2Improve performanceDischarge tube luminescnet screensElectroluminescent light sourcesBarium titanateSilicon oxide
The EL device of the present invention has a structure in which a first electrode, a first insulator layer, an electroluminescence-producing light emitting layer, a second insulator layer, and a second electrode layer are successively stacked on an electrical insulating substrate. At least one of the first insulator layer and the second insulator layer has as a main component barium titanate and in addition 0.1 to 3 mole % magnesium oxide, 0.05 to 1.0 mole % manganese oxide, no more than 1 mole % yttrium oxide, 2 to 12 mole % of barium oxide and calcium oxide, and 2-12 mole % silicon oxide.
Owner:IFIRE IP CORP
Catalyst for decomposing nitrous oxide and preparation method of catalyst
InactiveCN104437499AHigh activityIncreased reactive sitesNitrous oxide captureDispersed particle separationReaction temperatureAdipic acid
The invention discloses a catalyst for decomposing nitrous oxide and a preparation method of the catalyst. The catalyst is prepared by the deposition precipitation method. The method comprises the processes of preparing a deposit, washing, forming a deposit, activating through the catalyst, etc.; the catalyst comprises active ingredients, a carrier which is zirconium oxide, and additives, wherein the active ingredients include one or more than two of Co, Ni, Fe and Mn; the additives include one or more of cerium oxide, lanthanum oxide and barium oxide. A catalyst activity experiment shows that nitrous oxide can be completely catalytically decomposed into nitrogen and oxygen at the temperature ranging from 450 to 700 DEG C; therefore, the activation temperature is low, and the T50 (reaction temperature under conversion rate of 50%) is about 350 DEG C. The catalyst is high in activity and outstanding in heat resistance, has a wide operation window, and enables the concentration of nitrous oxide in industrial waste gas to be greatly reduced, and therefore, the environmental pollution is decreased; the catalyst is particularly suitable for N2O removal of a nitric plant and an adipic acid plant.
Owner:LANZHOU TIANYUE ENVIRONMENTAL PROTECTION TECH CO LTD
Non-crystalline polyamide resin composition and product thereof
The invention provides a non-crystalline polyamide resin composition with enough transparency and excellent rigidity, mechanical performance, physical performance and heat resistance, and a product thereof. The composition adopts glass filler which is represented by the mass percentage based on oxide, contains 68-72 percent of silicon dioxide (SiO2), 2-5 percent of aluminum oxide (Al2O3), 2-5 percent of boron oxide (B2O3), 2-10 percent of calcium oxide (CaO), 0-5 percent of zinc oxide (ZnO), 0-5 percent of strontium oxide (SrO), 0-1 percent of barium oxide (BaO), 1-5 percent of magnesium oxide (MgO), 0-5 percent of lithium oxide (Li2O), 5-12 percent of sodium oxide (Na2O) and 0-10 percent of potassium oxide (K2O), wherein the total amount of lithium oxide, sodium oxide and potassium oxideis 8-12 percent.
Owner:ASAHI FIBER GLASS CO LTD
Glaze composition for redware products
The invention provides a glaze composition for redware products and relates to the technical field of redware. The glaze composition is prepared from the following components in parts by weight: 1-3 parts of copper oxide, 5-9 parts of barium oxide, 4-6 parts of silicon carbide, 25-35 parts of quartz, 10-20 parts of borax, 0.1-0.5 part of graphene, 0.005-0.03 part of ferric oxide, 0.01-0.06 part of titanium carbide, 10-20 parts of dolomite, 5-15 parts of shell and 0.1-1 part of glass. According to the invention, lead material is removed and the quartz and glass are added, the quartz is used for increasing the surface strength of glazing color, the glass is used for increasing smoothness, and the hand feeling is good. The graphene and titanium carbide are added, so that the adsorption capacity among materials is great, and a layer of mesh structure can be formed on the surface of a green body and cannot fall off after firing.
Owner:卢群山
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com