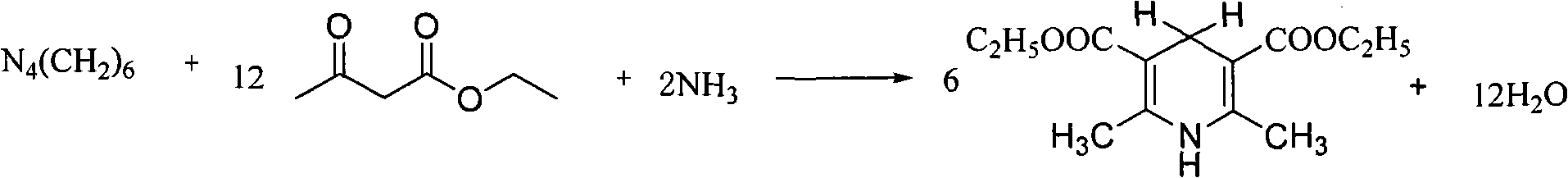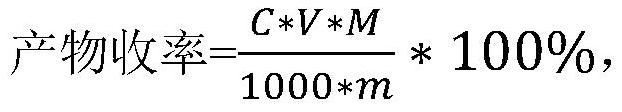Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89results about How to "No need for recrystallization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for synthesizing tolfenpyrad
The invention provides a method for synthesizing tolfenpyrad, relates to a preparation method of the tolfenpyrad, and the method is used for solving the problems such as tedious process and poor product purity of an existing tolfenpyrad synthesis method. The method comprises the following steps of: 1, synthesizing ethyl propionyl pyruvate; 2, synthesizing ethyl 3-ethyl-5-pyrazolecarboxylate; 3, synthesizing ethyl 1-methyl-3-ethyl-5-pyrazolecarboxylate; 4, synthesizing ethyl 1-methyl-3-ethyl-4-chloro-5-pyrazolecarboxylate; 5, synthesizing 4-(4-methyl phenoxy) cyanophenyl; 6, synthesizing 4-(4-methyl phenoxy) benzylamine; and 7, synthesizing the tolfenpyrad. Since sodium ethoxide is replaced by sodium hydroxide, the method provided by the invention has the characteristics of short reaction time, no generation of isomer and high purity of the product; and the obtained product has high purity and does not need re-crystallization.
Owner:HEILONGJIANG UNIV
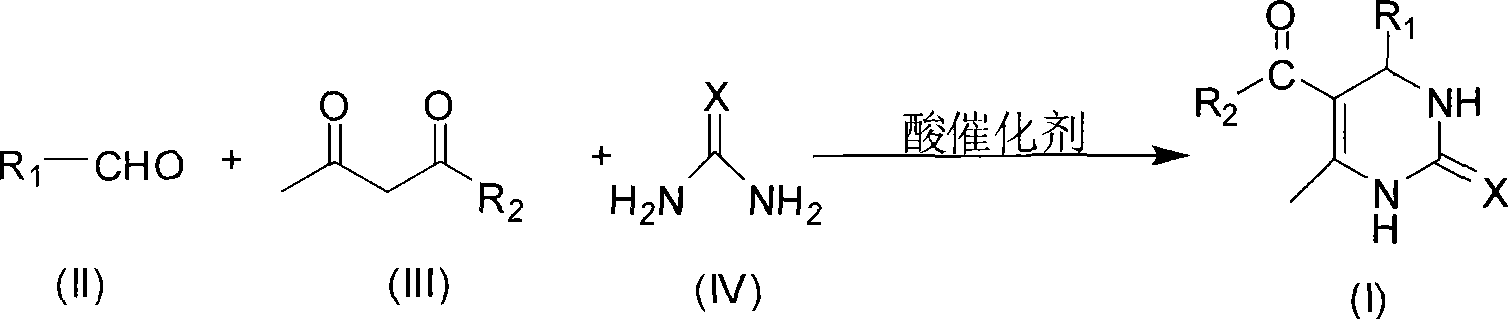
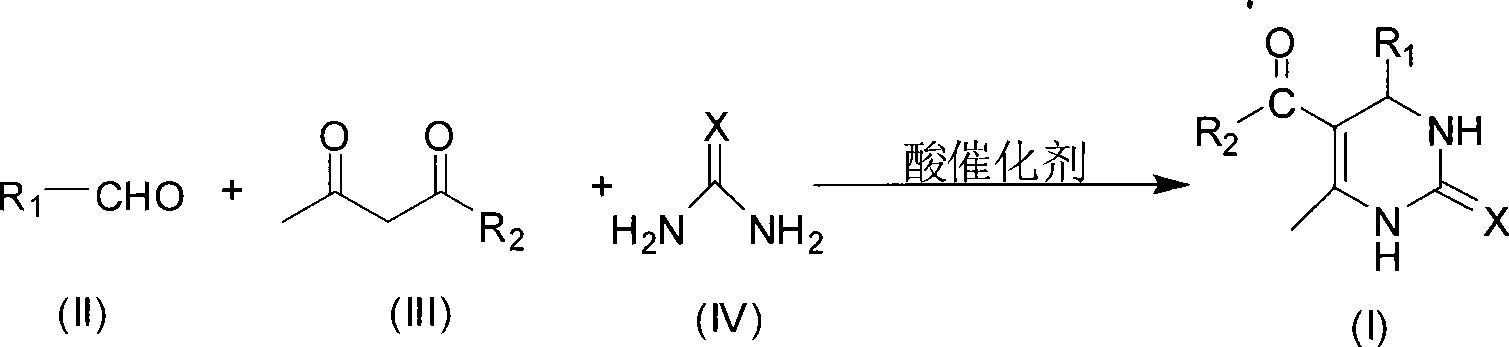
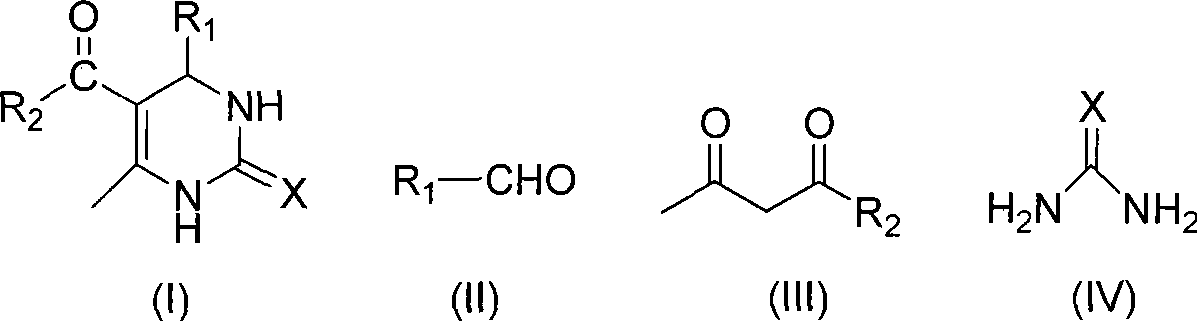
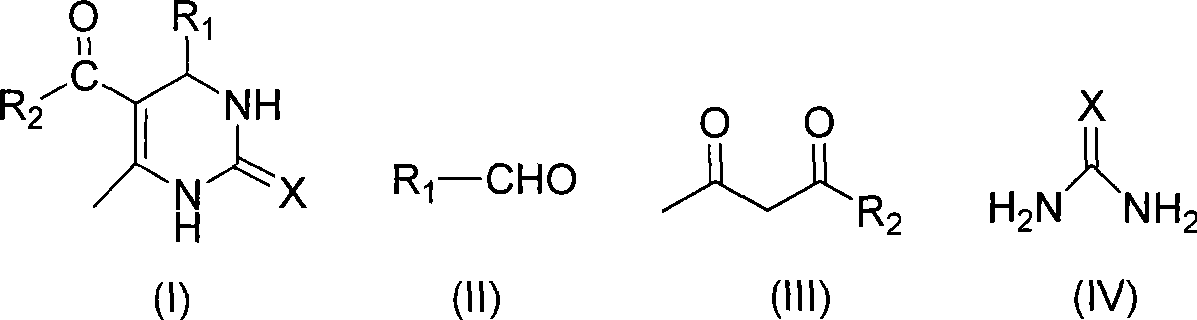
Room temperature solvent-free synthesis of 3,4-dihydropyrimidine-2-ketone
InactiveCN101367767AIn line with the idea of green chemistryReduce manufacturing costOrganic chemistryFiltrationSynthesis methods
The present invention discloses a synthesis method of 3, 4-dihydro pyrimidine-2-alkone at the room temperature without a solvent. The synthesis method comprises the following steps: under the condition without a solvent, an aldehyde compound as shown in Formula (II), beta-dicarbonyl compound as shown in Formula (III) and a compound as shown in Formula (IV) are mixed for reaction with an acid catalyst at the room temperature, a TLC is used for examining the end point of the reaction, the filtered residue can be acquired through filtration after the reaction is completed, and the product as shown in Formula (I) can be acquired after the filter residue is washed and dried. The present invention has the advantages that the operation is simple, the yield rate of the reaction is high, the purity of the product is high, the process is conducive to the environmental protection, the product cost is low, and the present invention is suitable for industrial production.
Owner:山东兴安智慧科技有限公司
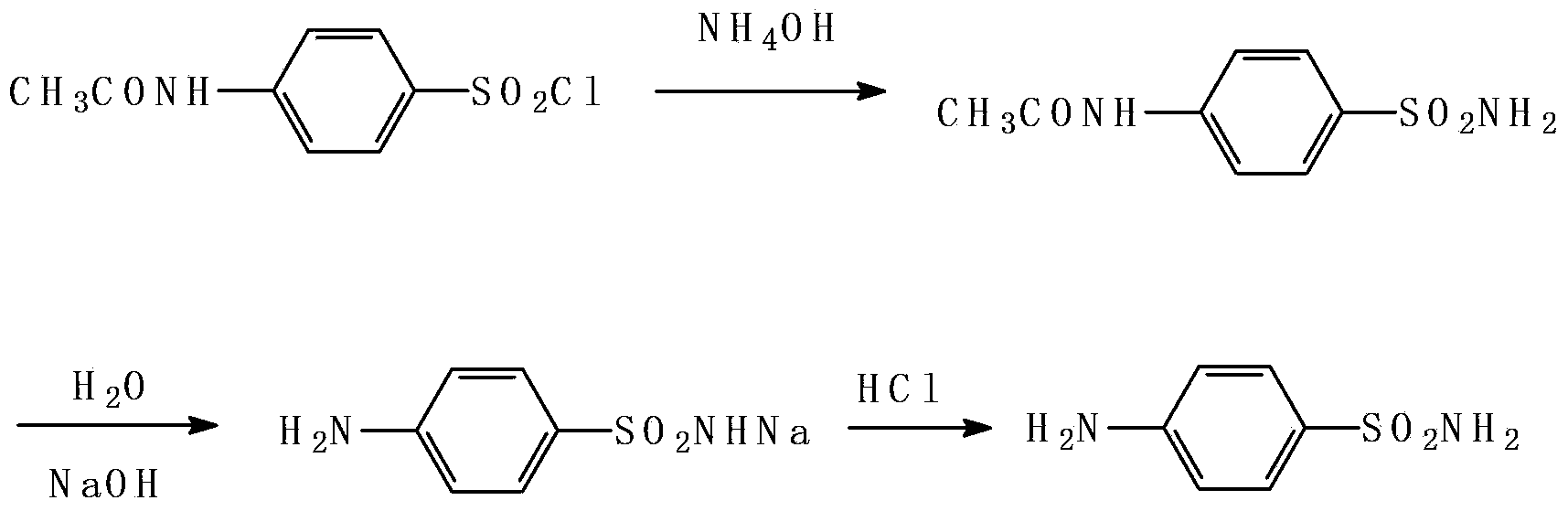
Preparation method of sulfanilamide
ActiveCN103483230AReduce volumeHigh yieldSulfonic acid amide preparationAmmonium hydroxideSodium hydroxide
The invention discloses a preparation method of sulfanilamide. The preparation method comprises the steps: adding n-acetylsulfanilyl chloride into ammonium hydroxide, reacting to obtain p-acetamidobenzene sulfamide, adding p-acetamidobenzene sulfamide into sodium hydroxide, hydrolyzing under the alkaline condition to obtain sodium sulfanilamide, reacting the sodium sulfanilamide with hydrochloric acid, directly concentrating under the non-separation condition to obtain the sulfanilamide. The purity of the finished sulfanilamide product obtained by adopting the preparation method can achieve more than 99.5% without the need of recrystallization; the product yield is high and can achieve more than 90%.
Owner:NANTONG BAISHENG CHEM
Process for synthesizing 3,4-dihydropyrimidine-2-keto
InactiveCN101463011AIn line with the idea of green chemistryReduce processingOrganic chemistryHigh energyReaction temperature
The invention discloses a synthetic method of 3,4-dihydropyrimidin-2-one. In a condition without solvent and catalyst, an aldehyde compound of formula (II), a beta-dicarbonyl compound of formula (III) and an urea compound of formula (IV) are taken as raw materials, stirred and heated for 0.5-10h reaction at the reaction temperature of 80-150 DEG C, reaction solution is separated to obtain the 3,4-dihydropyrimidin-2-one of formula (I). In the whole process, only a little ethanol aqueous solution is used for washing, catalyst is not needed, a 'one pot synthesis' is used for preparation, and the operation is simple, thus avoiding the disadvantages of high energy consumption, serious pollution, high toxicity, inflammable and explosive properties and the like which are caused by the use of the solvent, and the method is environmentally friendly and suitable for the though of the green chemistry, and helps reduces the production cost.
Owner:无棣鑫岳化工集团有限公司
Method for preparing high-purity p-chloro-m-nitroacetoacetanilide
ActiveCN101177404AImprove solubilityHigh purityOrganic compound preparationCarboxylic acid amides preparationEthyl acetateDiketene
The invention discloses a method for preparing high-purity p-chloro-m-nitroacetoacetanilide, in which adopts the technical proposal that p-chloro-o-nitroaniline and catalyst are added in mixed solvent at 15 to 100 DEG C, and then diketene is dripped within 0.5 to 4 hours for reaction for 0.5 to 6 hours to prepare the p-chloro-m-nitroacetoacetanilide, wherein the mixed solvent can be either the solvent composed of two or more materials among water, acetone, tetrahydrofuran, acetonitrile, ethanol, toluene, benzene, isopropyl alcohol, chloroform, dichloromethane, ethyl acetate, butyl acetate and methyl acetate or circulating mother liquor. The circulating mother liquor can be recycled for as many as 10 times and extra catalyst is not needed with the purity of products exceeding 99% and the yield reaching 97%. The invention has the advantages of simple and controllable process, no need of recrystallization and high purity.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
Method for preparing vinylene carbonate through micro channel reaction
ActiveCN106749155AFully contactedHigh reaction mass transfer and heat release efficiencyOrganic chemistrySecondary cellsEngineeringEthyl Chloride
The invention provides a method for preparing vinylene carbonate through micro channel reaction. The method comprises the following steps: (1) preparing equipment, including an enhanced mass transfer micro channel reactor, a metering pump I, a metering pump II and a micro filter, wherein the enhanced mass transfer micro channel reactor comprises a preheater I, a preheater II, an ultrasonic device and a micro channel module; (2) uniformly mixing chloroethylene carbonate with an ester solvent so as to obtain a mixed liquid, synchronously inputting the preheated mixed liquid and triethylamine into a micro channel, heating, mixing, performing reaction, discharging a product obtained after the reaction is completed from a discharge valve, filtering so as to obtain a crude product, and rectifying the crude product into a rectifying still, so as to obtain the vinylene carbonate, wherein the mole ratio of the chloroethylene carbonate to the triethylamine is 1:(1.5-2.0). The method is simple, convenient and safe to operate, relatively small in quantity of byproducts, small in single solvent consumption, low in moisture content, relatively high in product purity and yield, small in environment pollution and applicable to industrial large-scale production, and continuous production can be achieved.
Owner:江苏瀚康新材料有限公司
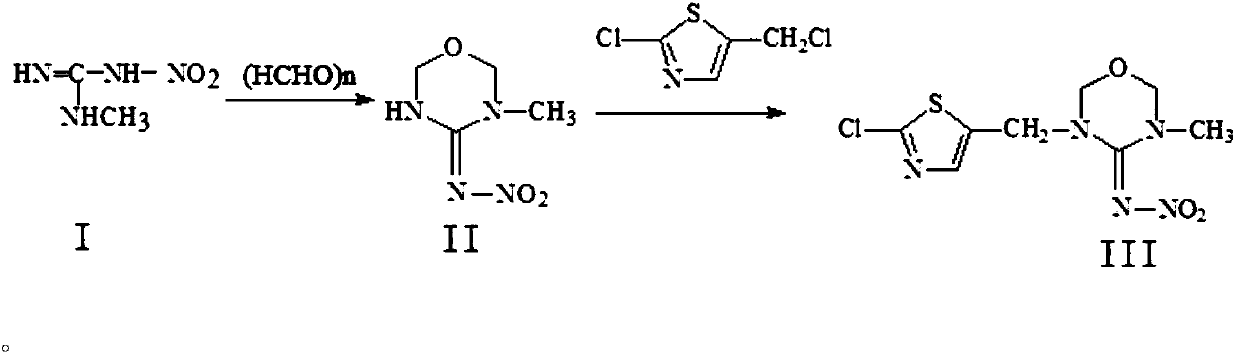
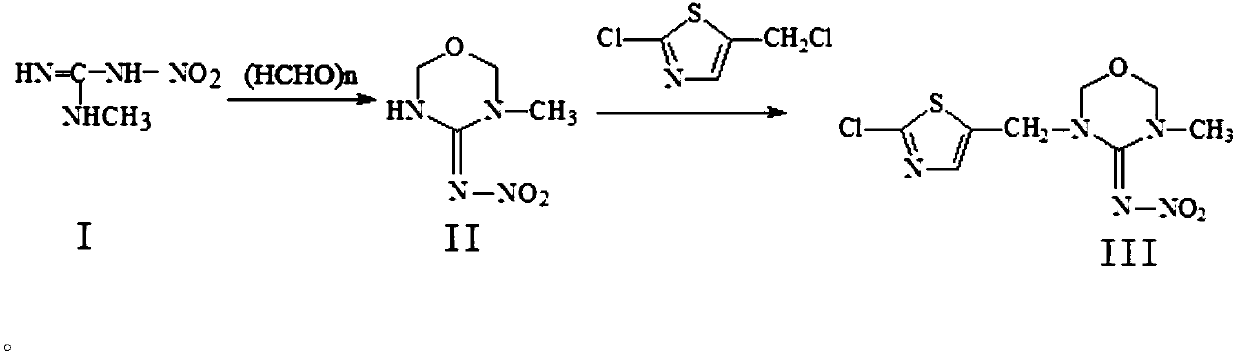
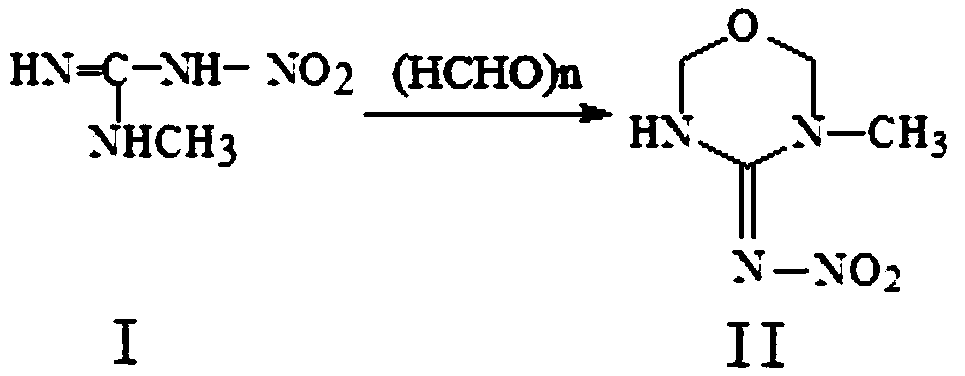
Preparation method of thiamethoxam
The invention discloses a preparation method of thiamethoxam. The preparation method of the thiamethoxam comprises the following steps: taking methylnitroguanidine as a starting material, preparing 3-methyl-4-nitroimine-1,3,5-oxadiazine (compound II), and then preparing the thiamethoxam. The method provided by the invention is simple in process, the finally synthesized thiamethoxam does not need recrystallization, the content of the final product is up to 98% or above, and the yield reaches 84% or above.
Owner:江苏绿叶农化有限公司
Preparation of 6-O-beta-D- glucosyl-3,6,16,25-tetrahydroxy cycloartane
InactiveCN101376669AEfficient removalHigh yieldMicroorganism based processesSteroidsOfficinalChinese traditional
The invention discloses a method for preparing 6-O-Beta-D-glucosyl-3,6,16,25-tetrahydroxycycloartane. The method comprises the following steps: inoculating pharmaceutical fungi in a solid culture medium containing Chinese traditional medicinal materials or residue thereof; fermenting under a certain fermentation condition; collecting the solid fermented material; and drying, pulverizing and extracting and separating to obtain the product. The method has the advantages that the process is simple, the waste reutilization is implemented, during the extracting and separating process, the macroporous resin method is not adopted to enrich the compound so that the repeated activation of the macroporous resin is obviated, the mixed n-butyl alcohol extraction solution is washed with sodium hydroxide solution to remove a great amount of phenolic acid impurities and color pigments, a neutral alumina chromatographic column is adopted for the purification so that the pigment impurities with the polarity similar to that of the target component is effectively removed so that the pure product can be directly obtained without need of the re-crystallization, the yield of the pure product is improved, and the method is worth extending and applying..
Owner:NANJING XIAOZHUANG UNIV
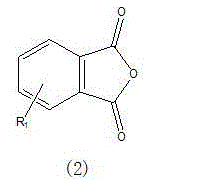
Method for preparing 2,2'-bis[4-(4-maleimidophenoxy)phenyl]propane
The invention provides a method for preparing 2,2'-bis[4-(4-maleimidophenoxy)phenyl]propane. The method comprises the steps of carrying out an amidation reaction and a dehydration close-loop reaction in a manner of taking maleic anhydride and 2,2'-bis[4-(4-aminophenoxy)phenyl]propane as main raw materials, taking a strong protonic acid as a catalyst, taking benzene or a derivative thereof and a high-boiling-point polar non-aprotic formamide di-substitutendum as solvents and taking phenols as a polymerization inhibitor, thereby preparing the 2,2'-bis[4-(4-maleimidophenoxy) phenyl]propane. According to the method provided by the invention, the process is simple, wastewater, obtained after water washing liquid separation, is subjected to distilling recovery and is not discharged as a pollutant, and residues, obtained after distillation, continue in service as a raw material of a next-batch reaction, so that the cost is reduced; the purity of the prepared product reaches up to 99.0% to 99.7%.
Owner:濮阳市高新技术创业服务中心
Preparation method of N-alkyl phthalimide compound
The invention relates to a preparation method of substituted-N-alkyl phthalimide, belonging to the technical field of compounds with a phthalimide structure. According to the preparation method, abundant substituted phthalic anhydride is utilized as a reaction raw material, a low-pollution single-alkyl amine water solution is utilized as an imidization reagent, low-grade carboxylic acid is utilized as a solvent, substituted-N-alkyl phthalimide is prepared by virtue of normal pressure reaction, and high-pollution methylamine and ethylamine gas are not used; the substituted phthalic anhydride is utilized as the raw material, industrial products of the compounds are complete, the source of the raw material is wide, the process is simple, and the cost is low; the reaction solvent can be recycled and reused, the treatment after the reaction is simple, the recrystallization is omitted, the yield can reach 83.t0% at most, and the purity is above 99%.
Owner:JINGZHOU TIANHE SCI TECH CHEM IND
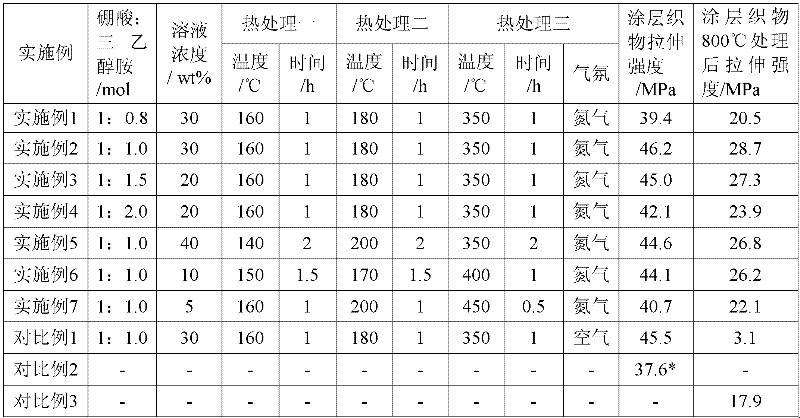
Coating method for reinforcing high silica glass fiber fabric
InactiveCN102505458AGood high temperature resistanceImprove high temperature oxidation resistanceFibre treatmentHeating/cooling textile fabricsAir atmosphereNitrogen atmosphere
The invention discloses a coating method for reinforcing high silica glass fiber fabric, which employs low-temperature firing process of ceramic materials using an organic precursor conversion method; a temperature-resistant coating containing boron nitride is coated on the surface of the high silica glass fiber fabric, thereby achieving the purposes of reinforcing the mechanical property and the temperature resistance of the high silica glass fiber fabric. Pyrolytic sintering is carried out in nitrogen atmosphere, thereby overcoming the defect of easy formation of boron oxide in air atmosphere, and effectively enhancing the mechanical property of the high silica glass fiber coating fabric. The coating method disclosed by the invention comprises the steps of: dipping and coating the surface of the high silica glass fiber fabric by using ethanol solution of boric acid and triethanolamine; and then carrying out programmed heating treatment to the high silica glass fiber coating fabric at a temperature in a range from 140 DEG C to 450 DEG C; and forming the temperature-resistant coating containing boron nitride in situ on the surface of the high silica glass fiber fabric.
Owner:南京彤天科技实业股份有限公司 +1
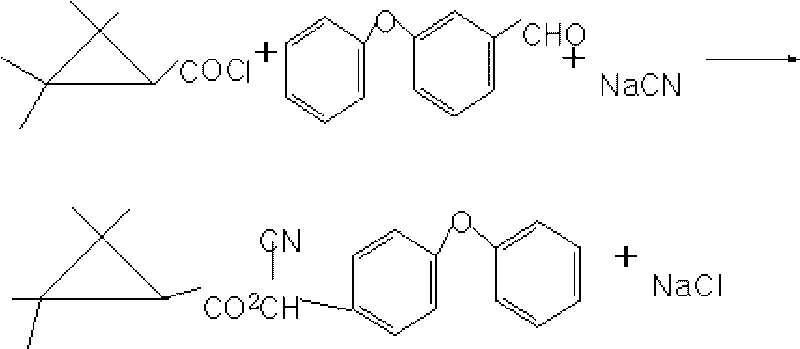
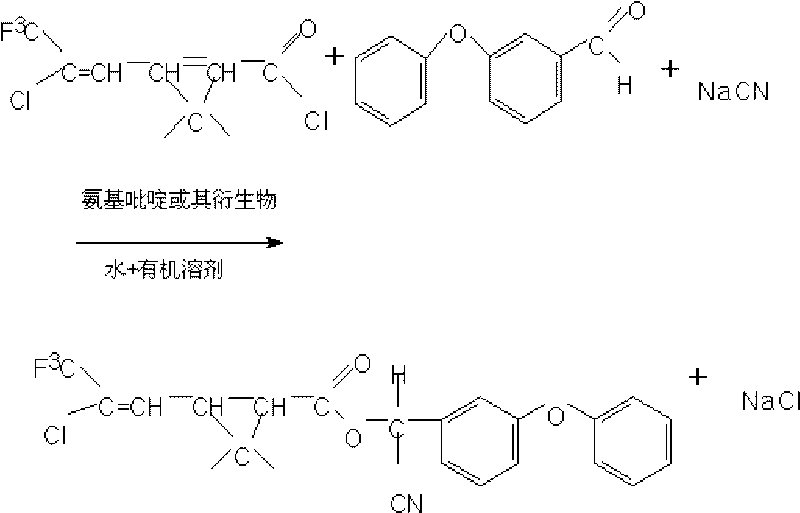
Method for preparing fenpropathrin
InactiveCN101709040AHigh purityGood colorPreparation by cyanide reactionThermal insulationSodium cyanide
The invention discloses a method for preparing fenpropathrin. The method comprises the following: (1) a step of preparing mixed solution, which is to mix ether aldehyde, sodium cyanide aqueous solution, organic solvent and aminopyridine or derivatives thereof serving as a phase transfer catalyst so as to obtain the mixed solution; (2) a step of preparing a fenpropathrin-containing crude product, which is to add methyl cyanide acyl chloride dropwise to the mixed solution obtained in a step (1) at 10 to 20 DEG C, perform thermal insulation reaction for 3 to 6 hours at 20 to 40 DEG C and obtain the fenpropathrin-containing crude product; and (3) a step of refining, which is to layer the fenpropathrin-containing crude product obtained in a step (2), remove cyanide-containing wastewater layers, use water to wash oil layers to be neutral, remove the organic solvent and obtain fenpropathrin crude oil. The method can obtain the fenpropathrin crude oil with high purity, excellent color and high yield, is simple in separation, needs no recrystallization, avoids the production of waste mother liquor, and reduces production cost.
Owner:JIANGSU HUANGMA AGROCHEM
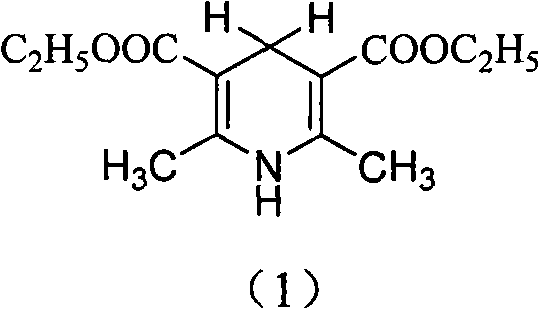
Synthesis method of 2,6-dimethyl-3,5-dicarbethoxy-1,4-dihydropyridine
ActiveCN101973929AReduce pollutionIn line with the idea of green chemistryOrganic chemistryAnimal feeding stuffAcetic acidDihydropyridine
Owner:山东润华兽药有限公司
Method for preparing cyhalothrin
InactiveCN101723856AHigh purityGood colorCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventEther
The invention discloses a method for preparing cyhalothrin, which comprises the following steps: (1) preparing a mixed solution as follows: mixing ether aldehyde, sodium cyanide water solution, organic solvent and aminopyridine or derivatives thereof as a catalyst to obtain the mixed solution; (2) preparing a crude product containing cyhalothrin: dropwise adding cyanuryl chloride to the mixed solution obtained in step (1) at the temperature of 0 DEG C-40 DEG C, and stirring after dropwise adding to obtain the crude product containing cyhalothrin; and (3) refining: layering the crude product containing cyhalothrin obtained in step (2), washing the oil layer with water, and removing the organic solvent to obtain the crude oil of the cyhalothrin. The method of the invention can obtain the crude oil of the cyhalothrin with high purity, good color and luster and high yield, has simple separation, does not need recrystallization, avoids the generation of discarded mother solution, and reduces the production cost.
Owner:JIANGSU HUANGMA AGROCHEM
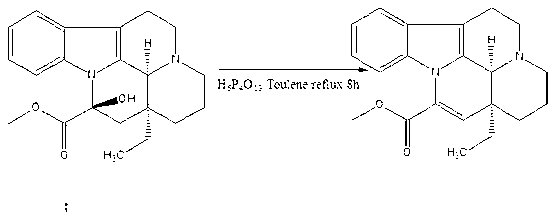
Synthesis method of vinpocetine
The invention discloses a synthesis method of vinpocetine. The synthesis method comprises adding vincamine into a three-neck flask, adding toluene into the three-neck bottle, stirring the mixture in ice water bath, dropwise adding polyphosphoric acid, placing the mixture into oil bath after half an hour, installing a water separator and a condensation tube, pumping to be vacuum, replacingnitrogen for two times, and performing reaction for 8 hours at the temperature of 120 DEG C; drying solvent by distillation after reaction is finished, adding ethanol and water, simultaneously dropwise adding caustic soda solution, utilizing saturated potassium carbonate solution to regulate pH to be 12 when the pH is 9, separating out solid, filtering and performing vacuum drying to obtain apovincamine; and adding absolute ethyl alcohol into the three-neck bottle, stirring the mixture in the ice water bath for one hour, then adding caustic alcohol into the mixture, adding the apovincamine after half an hour's stirring, placing a reaction bottle in the oil bath, performing reaction for 12 hours at the temperature of 80 DEG C, then steaming out solvent, adding the solvent into hydrochloric acid, extracting the mixture through ethyl acetate, adjusting pH value of the water phase to 12, separating out solid, filtering and drying to obtain the vinpocetine. The synthesis method is few in reaction step, low in energy consumption and less in environment pollution, recrystallization is not needed, and the purity of the vinpocetine can reach 99.5%.
Owner:JIANGSU QINGJIANG PHARMA
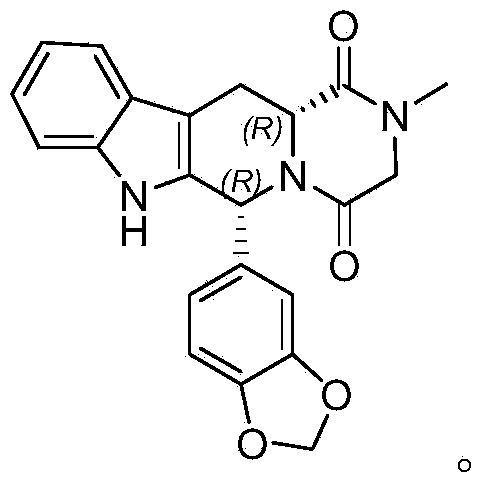
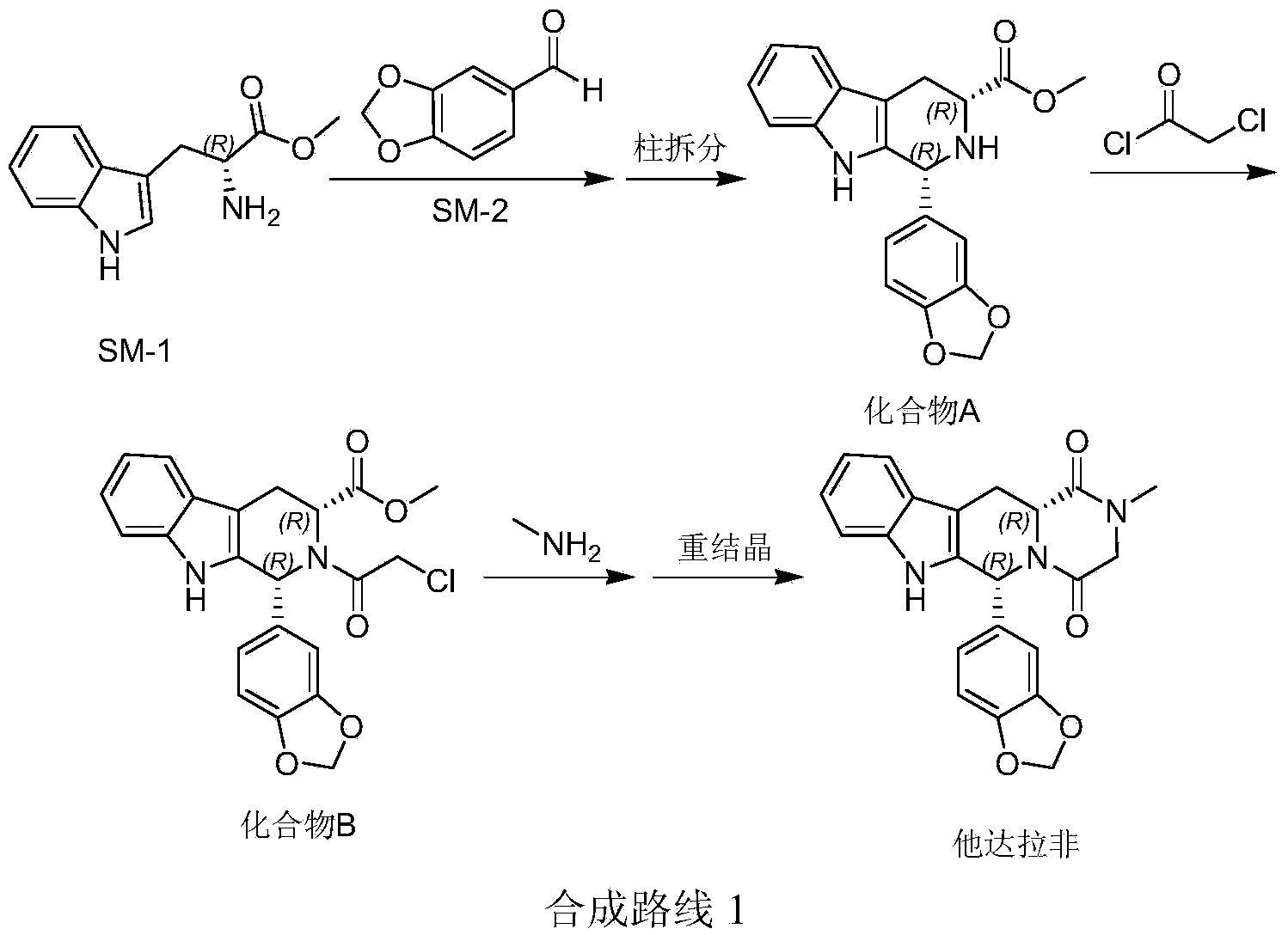
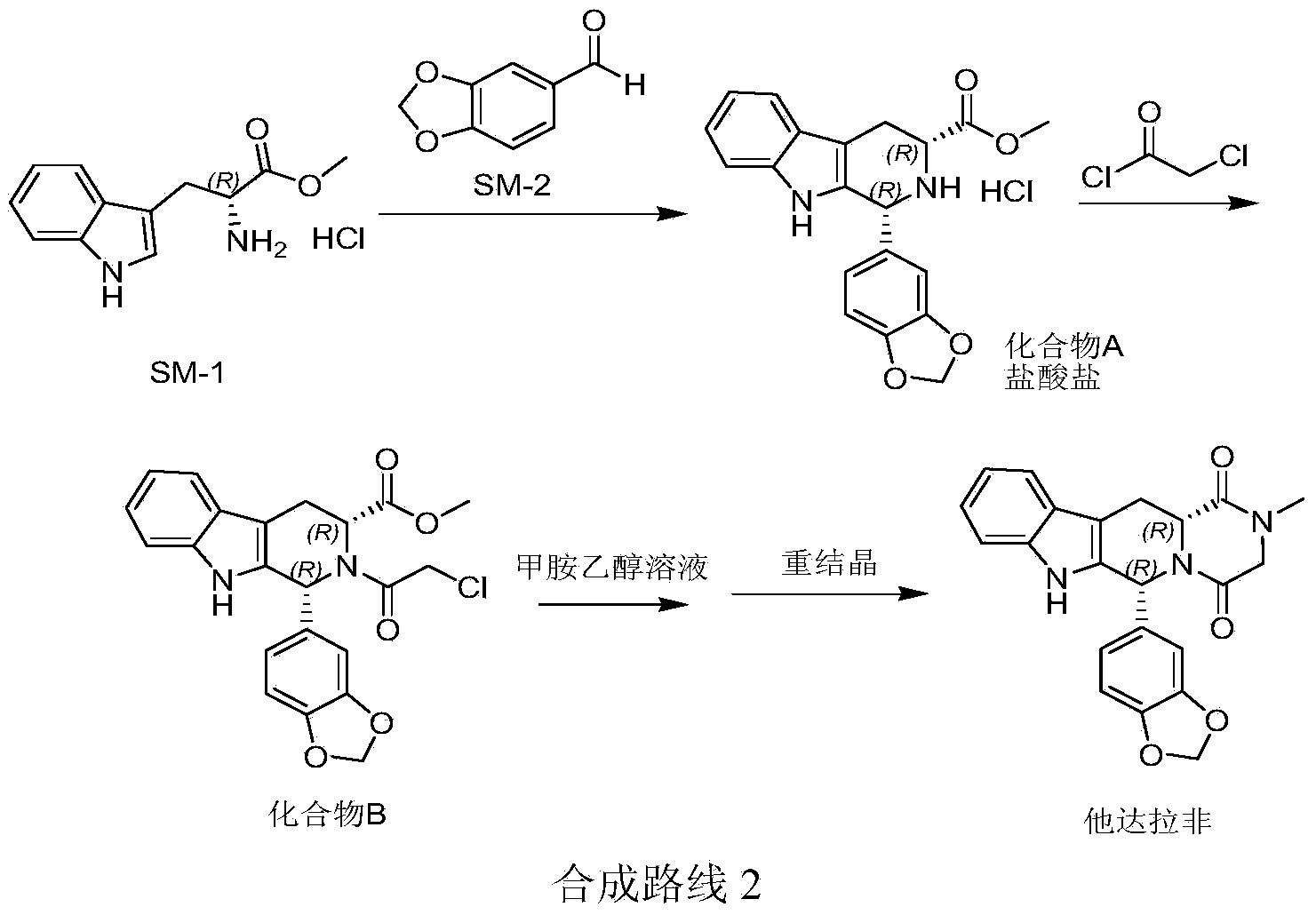
Method for preparing tadalafil
The invention discloses a method for preparing tadalafil. The method comprises the steps that water and catalyst tetrabutyl ammonium bromide are added to a compound B, a methylamine water solution is added to the compound B, and the temperature of the mixed solution is increased to the range from 60 DEG C to 90 DEG C to be kept for 6-10 hours for reaction; after reaction, the mixed solution is cooled to the temperature ranging from 20 DEG C to 35 DEG C, the temperature is kept, devitrifying is carried out for 3-6 hours, filtering is carried out, and filter cakes are washed and dried to obtain finished tadalafil. Compared with the prior art, the price of the adopted methylamine water solution is lower than that of a methylamine methylamine solution, water is adopted as reaction solvent, production cost is greatly reduced, and water is safe and friendly to the environment. The purity of the tadalafil prepared through the method is more than 99.90 percent, and recrystallization process is not needed. The method is easy to operate, low in production cost and pollution and easier to produce in an industrial mode.
Owner:山东安信制药有限公司
A kind of method that microchannel reaction prepares vinylene carbonate
ActiveCN106749155BFully contactedHigh reaction mass transfer and heat release efficiencyOrganic chemistrySecondary cellsEngineeringSolvent
The invention provides a method for preparing vinylene carbonate through micro channel reaction. The method comprises the following steps: (1) preparing equipment, including an enhanced mass transfer micro channel reactor, a metering pump I, a metering pump II and a micro filter, wherein the enhanced mass transfer micro channel reactor comprises a preheater I, a preheater II, an ultrasonic device and a micro channel module; (2) uniformly mixing chloroethylene carbonate with an ester solvent so as to obtain a mixed liquid, synchronously inputting the preheated mixed liquid and triethylamine into a micro channel, heating, mixing, performing reaction, discharging a product obtained after the reaction is completed from a discharge valve, filtering so as to obtain a crude product, and rectifying the crude product into a rectifying still, so as to obtain the vinylene carbonate, wherein the mole ratio of the chloroethylene carbonate to the triethylamine is 1:(1.5-2.0). The method is simple, convenient and safe to operate, relatively small in quantity of byproducts, small in single solvent consumption, low in moisture content, relatively high in product purity and yield, small in environment pollution and applicable to industrial large-scale production, and continuous production can be achieved.
Owner:江苏瀚康新材料有限公司
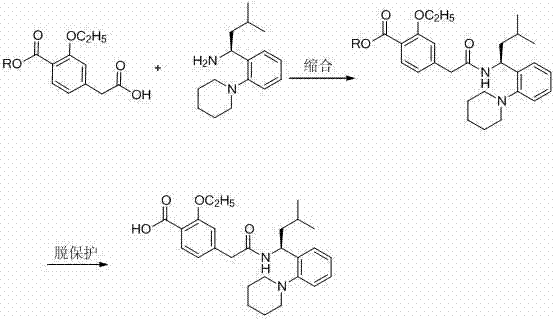
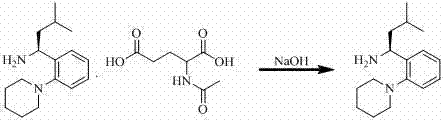
Synthetic method of repaglinide
The invention discloses a synthetic method of repaglinide, comprising the steps of dissolving N-acetyl glutamate of (S)-3-methyl-1-[2-(1-piperidyl)phenyl] butyl amine into dichloromethane, stirring, cooling, alkalifying, extracting and drying to obtain (S)-3-methyl-1-[2-(1-piperidyl)phenyl] butyl amine dichloromethane solution; cooling the solution, adding 3-ethyoxyl-4-carbethoxy phenylacetic acid, HOBT (N-hydroxybenzotriazole), DIEA (N,N-diisopropyl ethylamine) and EDC.HCl (1-ethyl-3-(3-dimethyllaminopropyl)carbodiimide hydrochloride) in turn and reacting; washing, drying and distilling to obtain repaglinide ethyl ester; adding ethanol, dropping alkali liquid, heating and reacting; and acidizing, growing crystals and performing suction filtration to obtain a product. The synthetic method has the advantages that EDC.HC1 is used as an acylation reagent, so that the reaction by-product urea is easy to elute; due to combination with HOBT, the condensation yield can be improved; the condensation condition is mild; the reaction time is short; the yield is 96%; the purity can reach more than 98%; recrystallization is not needed and the next hydrolytic reaction can be preformed directly; and the process is simple, so that the synthetic method is suitable for commercial production.
Owner:河北富格药业有限公司
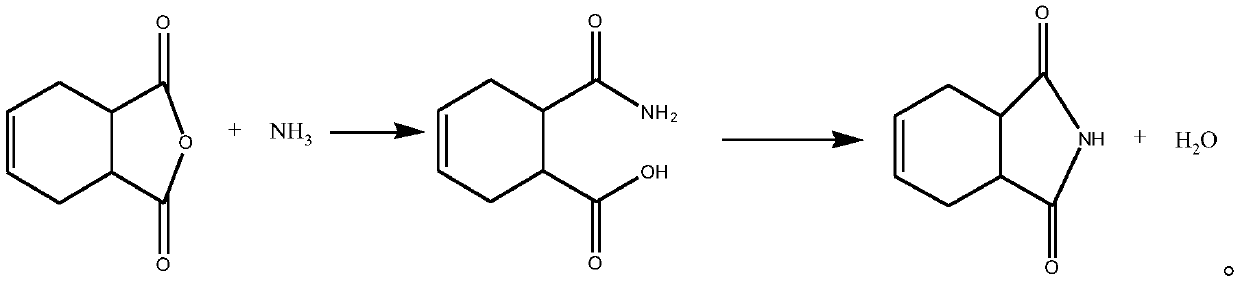
Production method of 1,2,3,6-tetrahydrophthalimide
InactiveCN110606819ASimple processReduce energy consumptionOrganic chemistryState of artTetrahydrophthalamic acid
The invention provides a production method of 1,2,3,6-tetrahydrophthalimide, which comprises the following steps: a) mixing 1,2,3,6-tetrahydrophthalimide with ammonia gas, and carrying out low-temperature reaction to obtain a reaction intermediate, wherein the temperature of the low-temperature reaction is 0-30 DEG C; and b) carrying out a dehydration ring-closure reaction on the reaction intermediate obtained in the step a) to obtain the 1,2,3,6-tetrahydrophthalimide. Compared with the prior art, with 1,2,3,6-tetrahydrophthalic anhydride and ammonia gas as raw materials, a stable intermediateis generated in a low-temperature anhydrous environment through a gas-solid method, then the intermediate is subjected to a dehydration ring-closure reaction, a product is obtained, no solvent is used in the whole process, the process is simple, a large amount of water or solvent does not need to be removed in the intermediate heating process, only water generated in the reaction needs to be distilled out, and energy consumption is greatly reduced; the comprehensive two-step yield can reach 97% or higher; moreover, gases such as carbon dioxide are not generated in the reaction process, and redundant ammonia gas can be recycled, so that the environmental protection cost is greatly saved.
Owner:NINGXIA G R FINE CHEM CO LTD
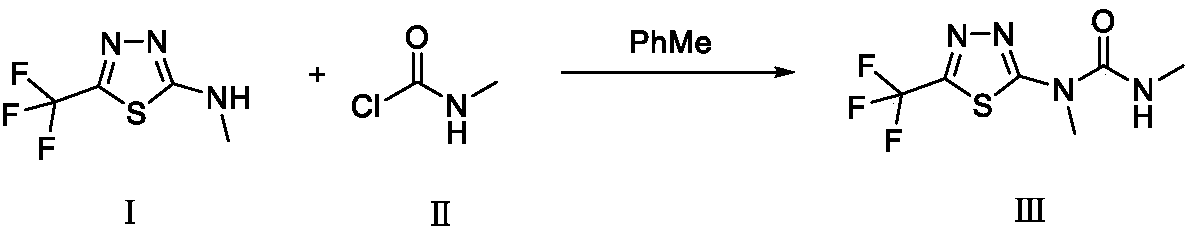
Preparation method of thiazafluron original medicine
The invention relates to a preparation method of a thiazafluron original medicine. The method comprises the following steps: dropwise adding an N-methylaminoformyl chloride solution into a 2-methyl ammonia-(5-trifluoromethyl)-1,3,4-thiadiazole solution; then introducing nitrogen after dropwise adding organic alkali; then after heating and cooling reactions, adding water to react; and filtering anddrying the solution to obtain a white solid thiazafluron. According to the preparation method, flammable and combustible methyl isocyanate which is unlikely to transport is not used, and a product which is high in yield and purity can be obtained without recrystallization.
Owner:HEBEI UNIV OF TECH
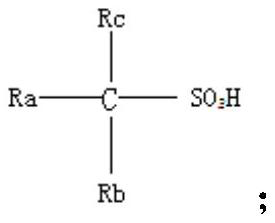
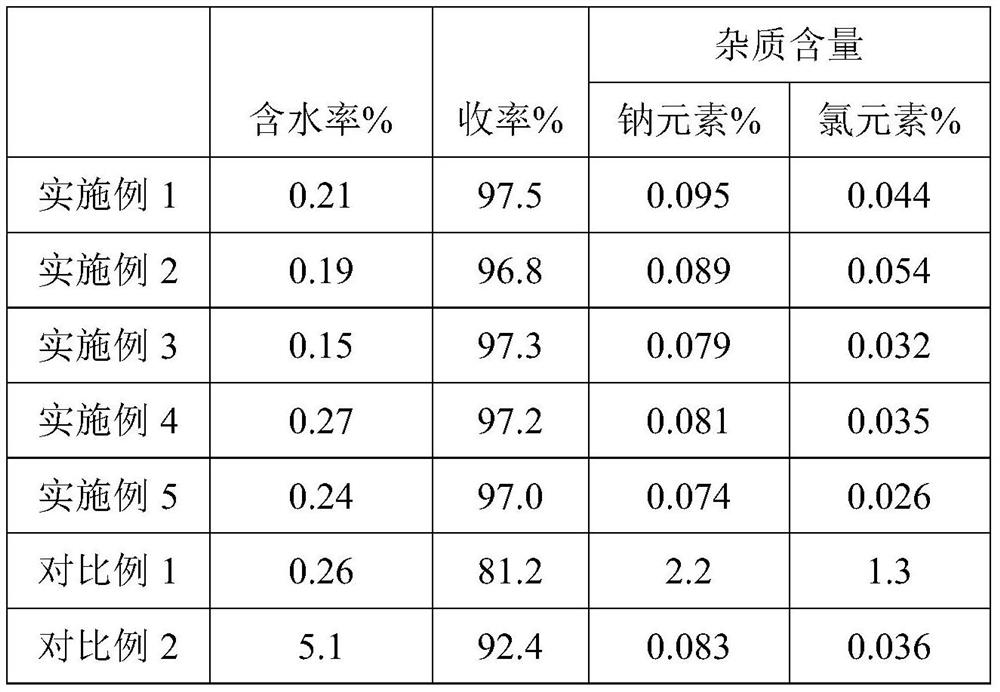
Preparation method and application of organic sulfonic acid
ActiveCN113666850AEfficient separationHigh reaction yieldSulfonic acids salts preparationSulfonic acid preparationAlkaneOrganic sulfonic acid
The invention relates to the technical field of fine chemicals, in particular to a preparation method and application of organic sulfonic acid. The preparation method of the organic sulfonic acid comprises the following specific steps: S1, preparing organic sulfonate; S2, reacting the organic sulfonate with a proton donor to obtain an organic sulfonic acid crude product; and S3, purifying the organic sulfonic acid crude product to obtain a finished product. The organic sulfonic acid obtained by the invention has the following advantages that (1) a gaseous proton provider is adopted, so that the production efficiency in the actual reaction process is improved while a polyhydroxy alkane sulfonic acid product with high reaction yield is obtained; (2) processing and purifying are performed by adopting enamel film evaporation and short-path molecular distillation equipment, and acidic byproducts and water in liquid components of reaction products are effectively separated to obtain a polyhydroxy alkane sulfonic acid finished product with the moisture content as low as 0.3%; and (3) recovery of hydrochloric acid can be realized while the polyhydroxy alkane sulfonic acid is obtained in the purification and separation process, and industrial mass production of the polyhydroxy alkane sulfonic acid is realized.
Owner:厦门威亮光学涂层技术有限公司
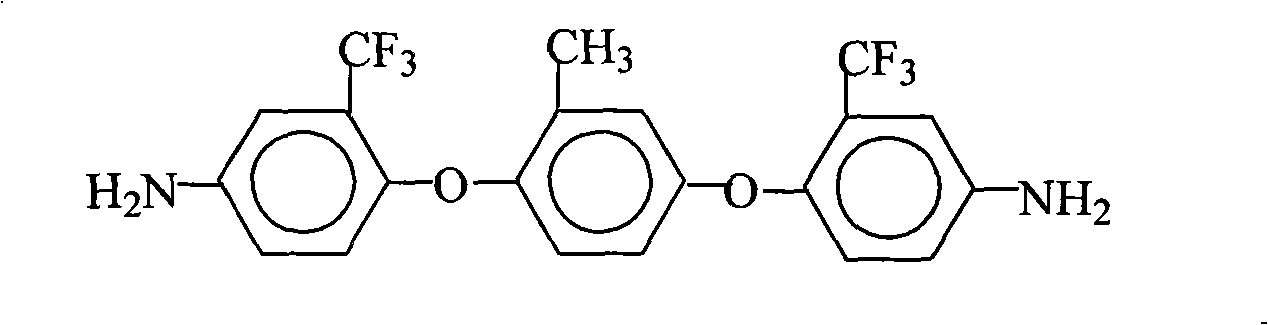
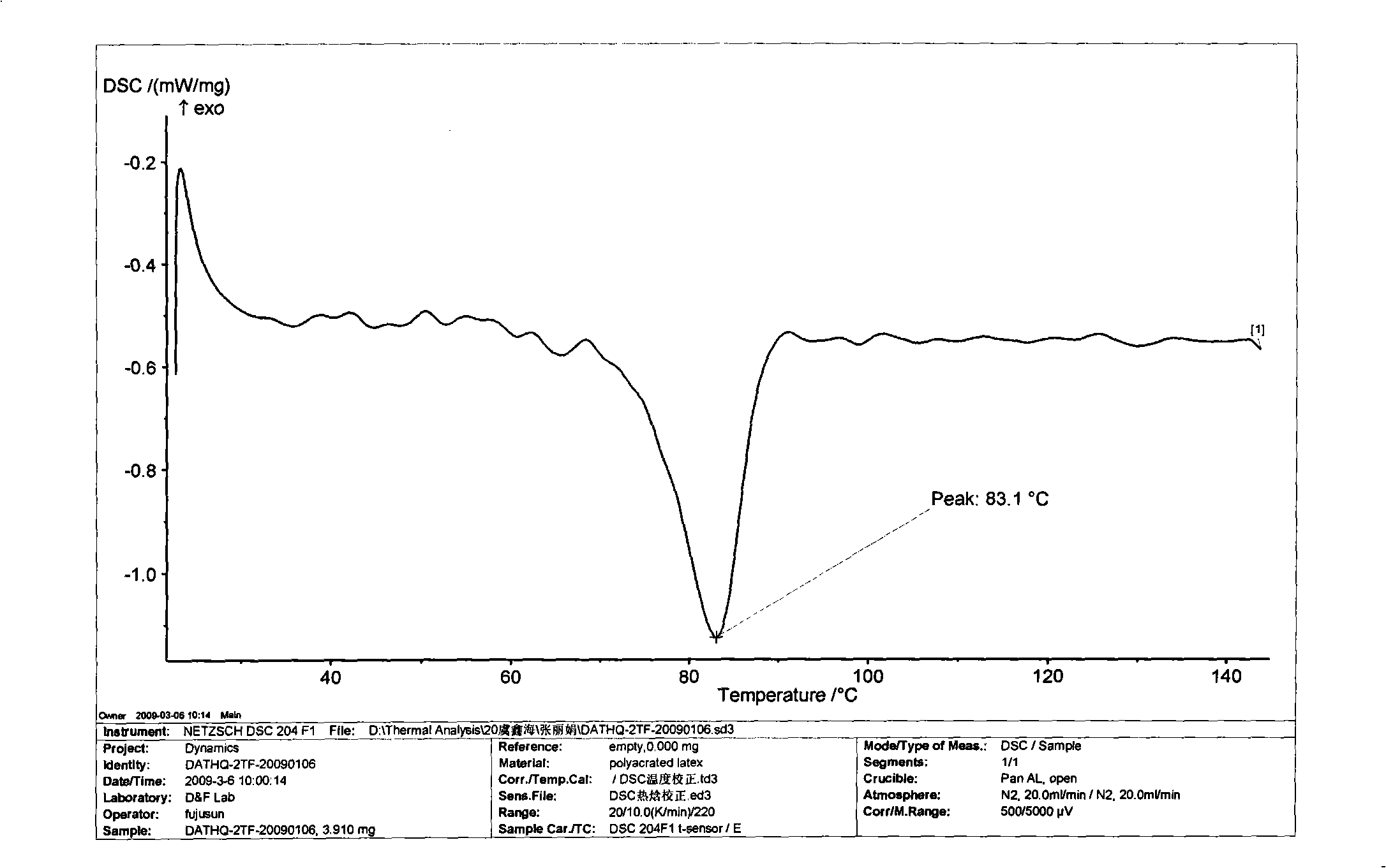
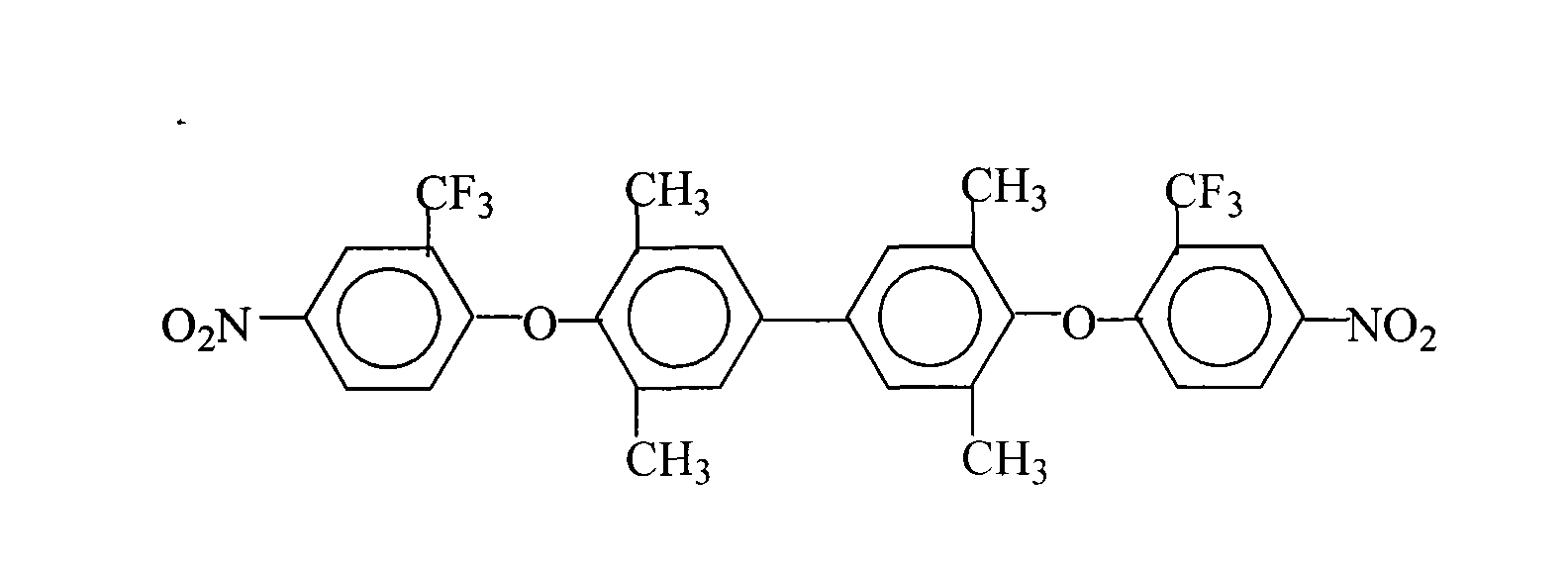
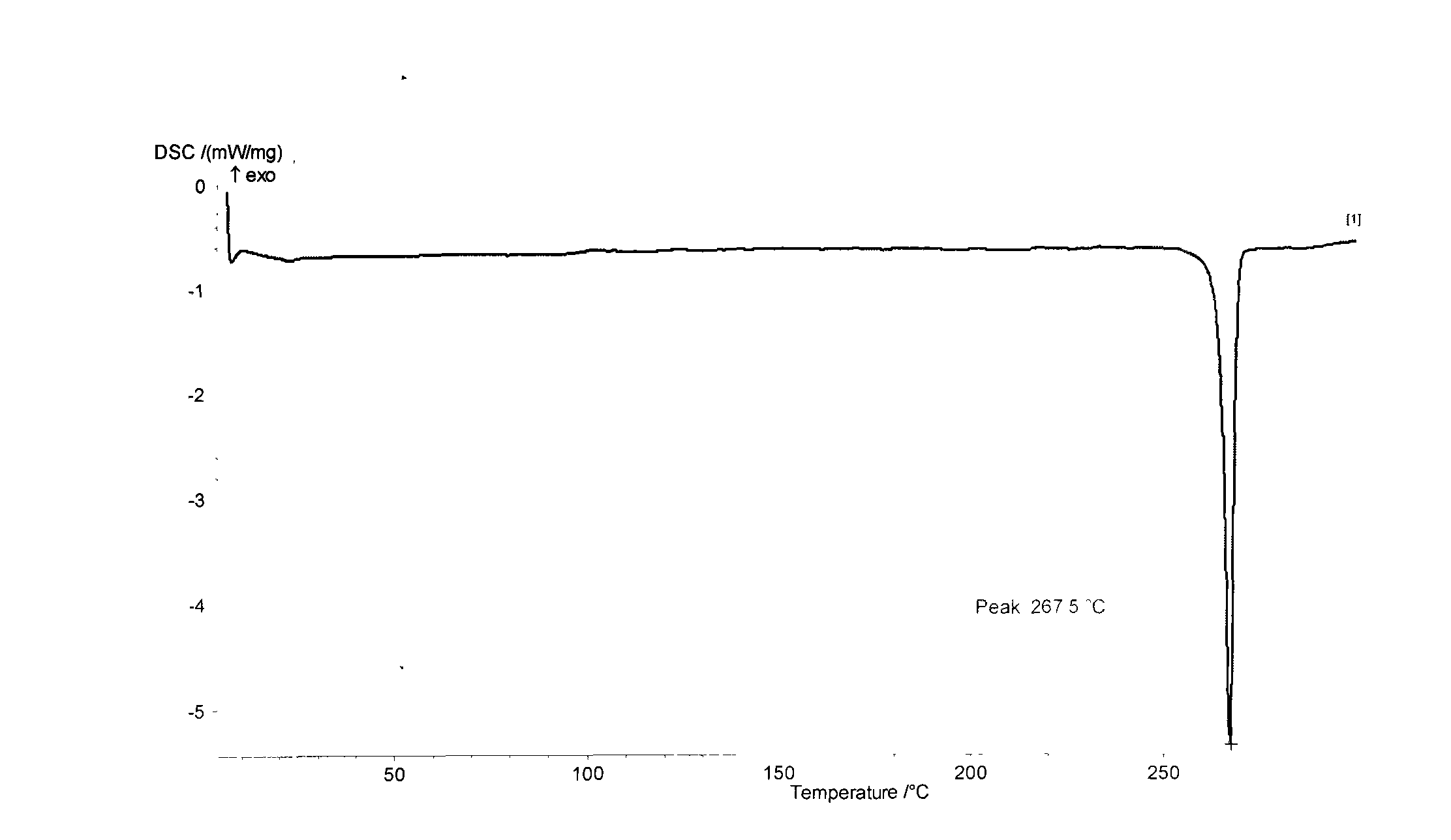
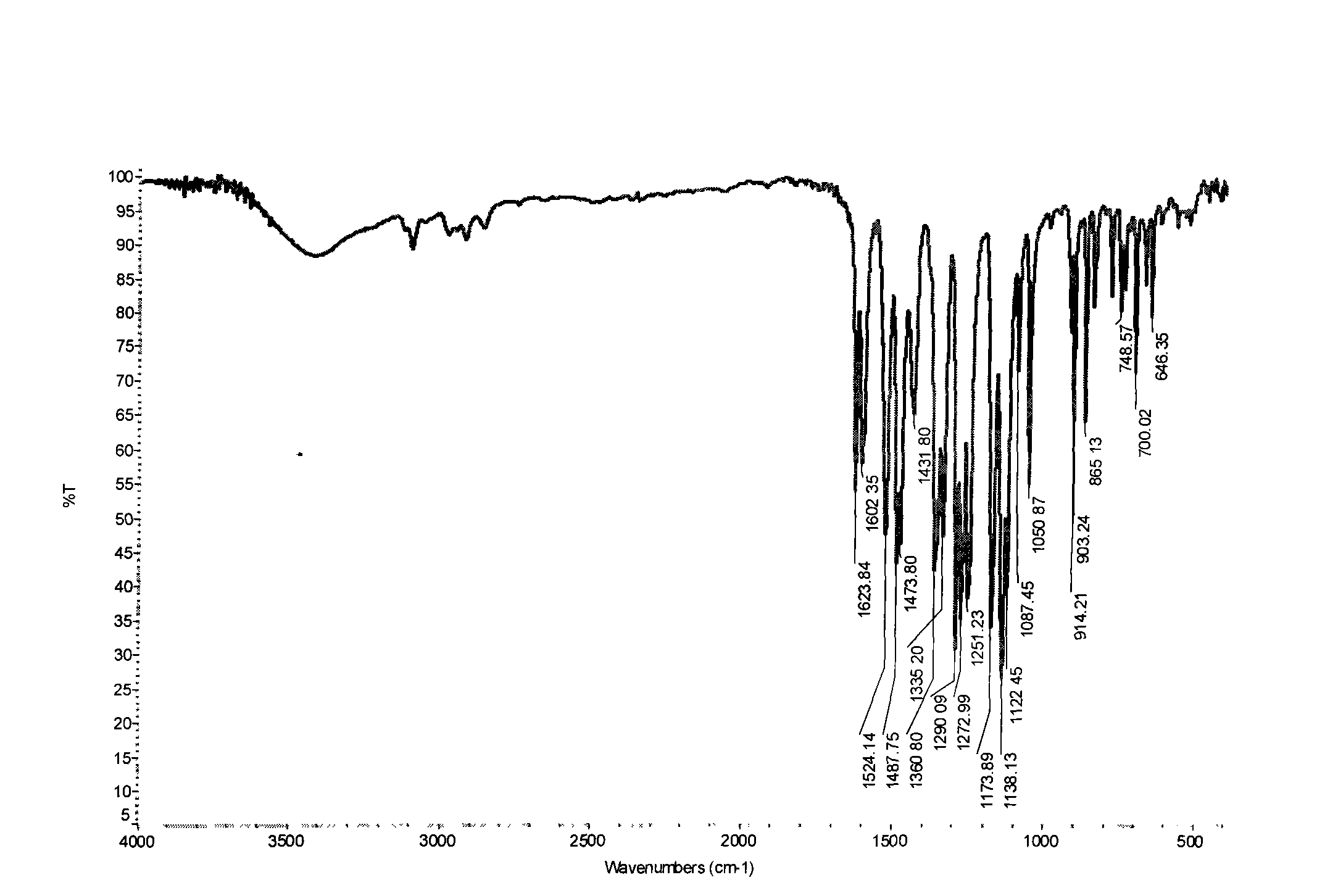
Preparation method of 4,4'-bi(2-trifluoromethyl-4-aminophenoxyl)biphenyl
InactiveCN101560163AConvenient sourceLow costOrganic compound preparationAmino-hyroxy compound preparationSolventHigh pressure
The invention relates to a preparation method of 4,4'-bi(2-trifluoromethyl-4-aminophenoxyl)biphenyl, comprising the steps of adding 4,4'-bi(2-trifluoromethyl-4-nitrophenoxyl)biphenyl, an organic mixed solvent containing alcohols, and a catalyst in a high pressure reaction kettle, infusing hydrogen, stirring, substituting residual air, continuously infusing hydrogen, heating and raising temperature, reacting for 2-4 hours, cooling the reaction system to room temperature, slowly discharging pressure, and infusing nitrogen to substitute all residual air so as to ensure that the high pressure reaction kettle is in inert atmosphere, filtering, recovering filter residue, maintaining the mother liquid, condensing the mother liquid such that the solid content of the mother liquid is in the range of 40-80%, cooling, putting the mother liquid aside, separating out white crystal product, filtering, washing and drying to obtain 4,4'-bi(2-trifluoromethyl-4-aminophenoxyl)biphenyl solid. The method has a simple technology, low cost, high purity and yield, and is environment-friendly and suitable for commercial production.
Owner:DONGHUA UNIV
Method for preparing cyhalothrin
InactiveCN101723856BHigh purityGood colorCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventEther
The invention discloses a method for preparing cyhalothrin, which comprises the following steps: (1) preparing a mixed solution as follows: mixing ether aldehyde, sodium cyanide water solution, organic solvent and aminopyridine or derivatives thereof as a catalyst to obtain the mixed solution; (2) preparing a crude product containing cyhalothrin: dropwise adding cyanuryl chloride to the mixed solution obtained in step (1) at the temperature of 0 DEG C-40 DEG C, and stirring after dropwise adding to obtain the crude product containing cyhalothrin; and (3) refining: layering the crude product containing cyhalothrin obtained in step (2), washing the oil layer with water, and removing the organic solvent to obtain the crude oil of the cyhalothrin. The method of the invention can obtain the crude oil of the cyhalothrin with high purity, good color and luster and high yield, has simple separation, does not need recrystallization, avoids the generation of discarded mother solution, and reduces the production cost.
Owner:JIANGSU HUANGMA AGROCHEM
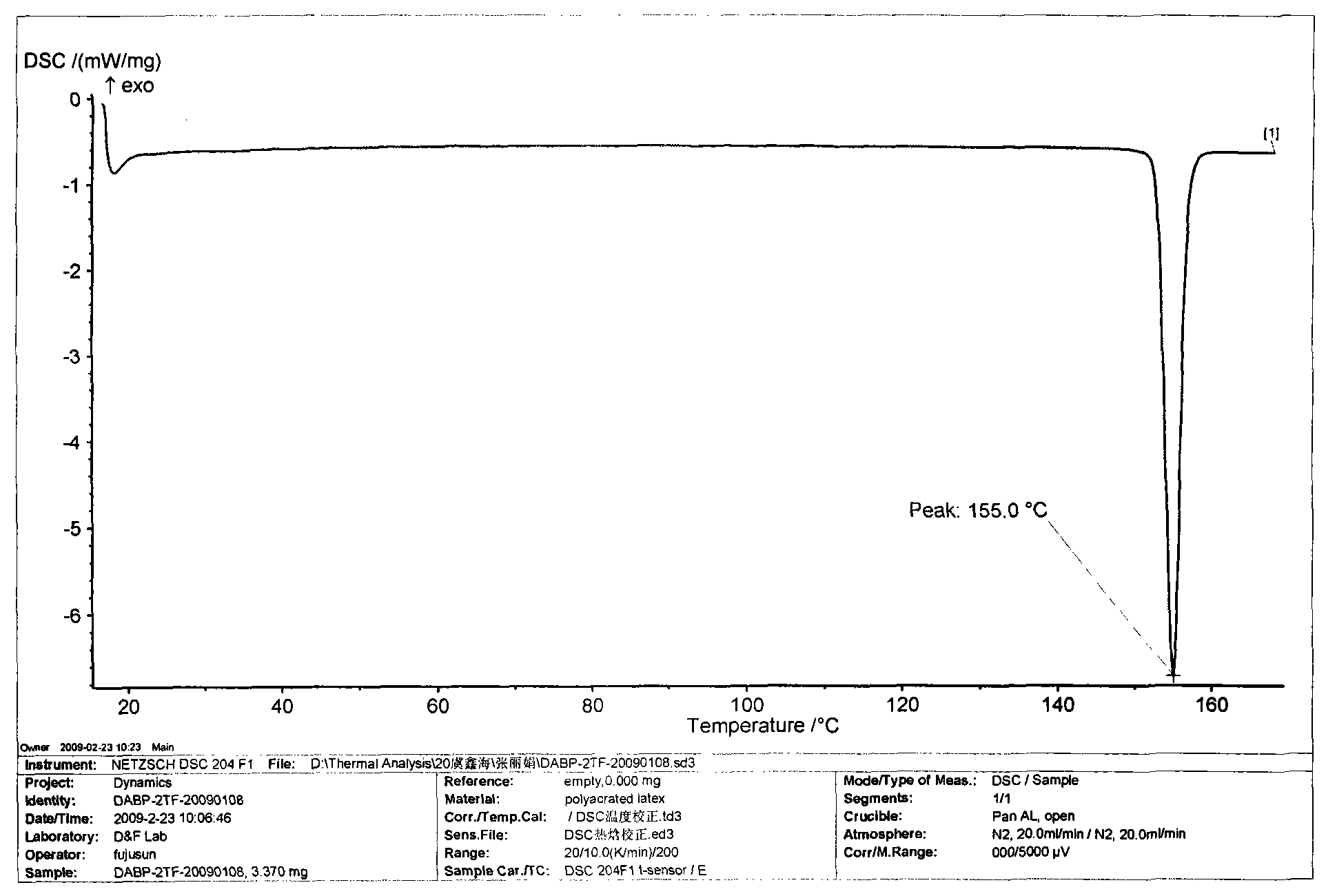
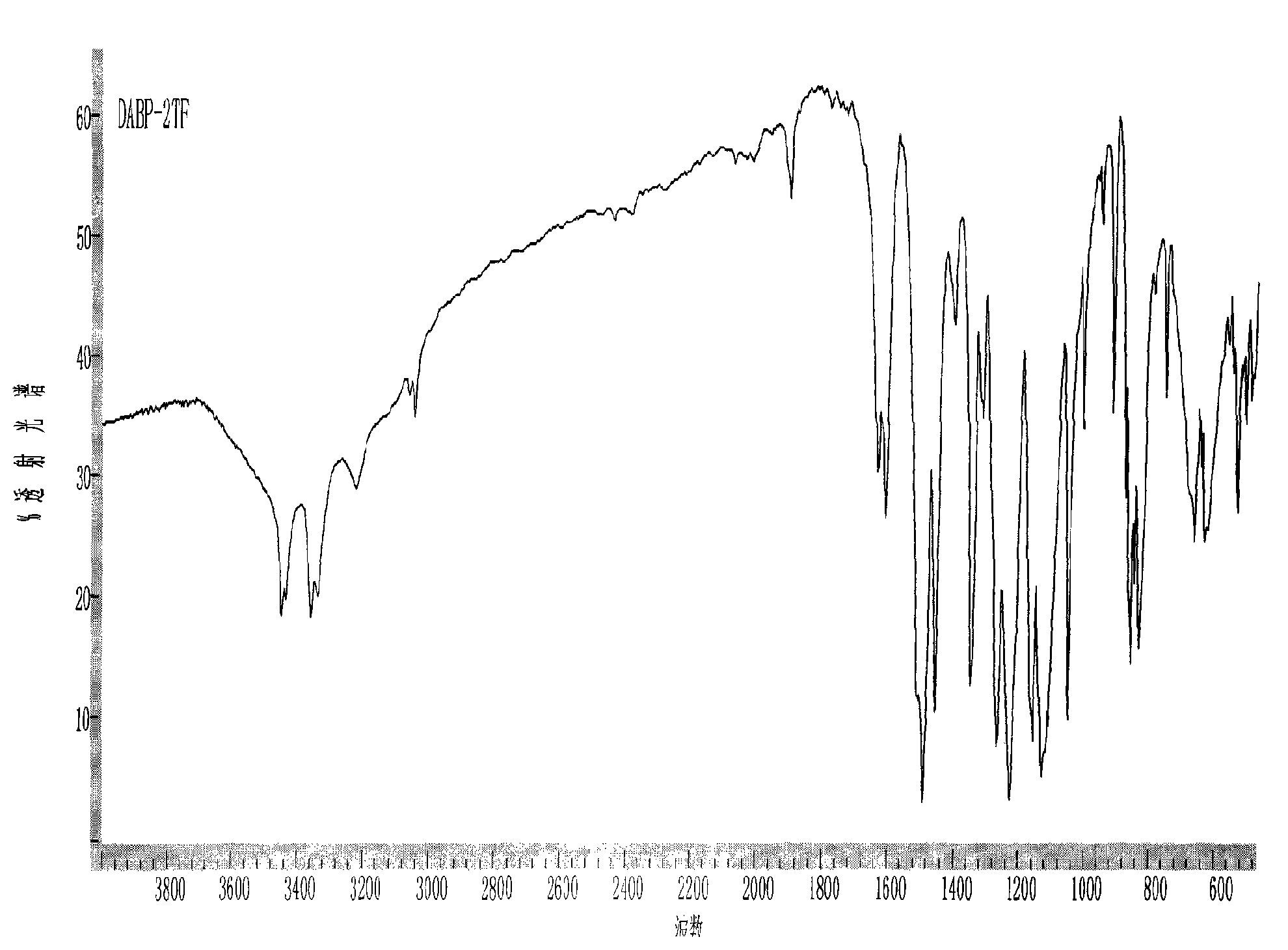
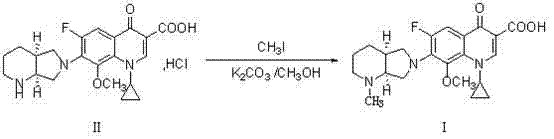
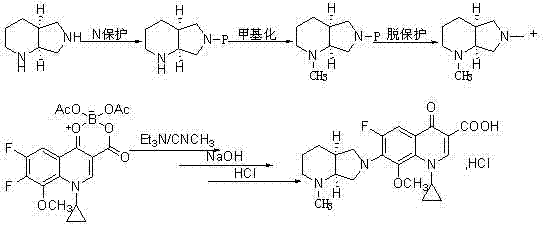
Novel method used for preparing N-methyl moxifloxacin
InactiveCN104513237AHigh purityNo need for recrystallizationOrganic chemistryCinoxacineMoxifloxacin hydrochloride
The invention relates to a novel method used for preparing N-methyl moxifloxacin. According to the novel method, N-methyl moxifloxacin is obtained via direct methylation of moxifloxacin hydrochloride II. Starting materials of the novel method are cheap and easily available; reaction conditions are mild; and the object product can be obtained via one-step reaction. The novel method is used for preparation of moxifloxacin hydrochloride impurity comparison products, is capable of realizing effective quantification control of the impurities, and is beneficial for increasing of quality of moxifloxacin hydrochloride bulk drug and preparations.
Owner:YAOPHARMA CO LTD +1
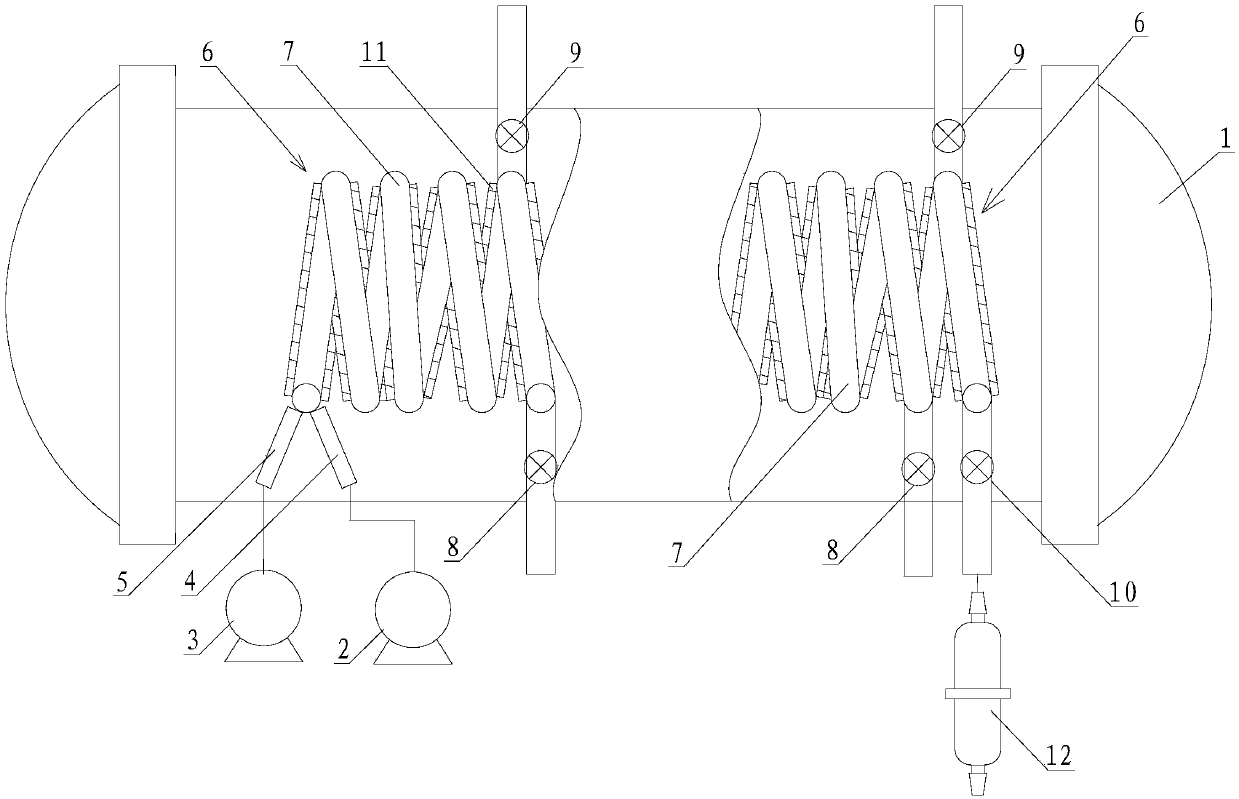
A high-efficiency purification device for plant extracts and a method for purifying plant extracts
ActiveCN105214333BSimple structureEasy to useSolution crystallizationOrganic solventPurification methods
The invention discloses an efficient purification device of plant extracts and a purification method of the plant extracts. The device comprises a purification tank and a drying cabinet, wherein the purification tank comprises a purification tank body, a purification tank cover, a solvent chamber and a filter plate, the solvent chamber is positioned on the periphery of the purification tank body and is fixed on the purification tank body, the purification tank body is separated from the solvent chamber by the filter plate, a liquid inlet tube and a liquid outlet tube are respectively arranged on the solvent chamber, the drying cabinet comprises a drying cabinet body and a cabinet door, a gas outlet is formed in the top of the drying cabinet body, a gas inlet tube is arranged at the bottom of the drying cabinet body, a sieve-shaped support plate is arranged on the lower part of the drying cabinet body, the drying cabinet body is divided into a purification tank placing chamber and a heating chamber by the sieve-shaped support plate, and a heat exchanger tube is installed in the heating chamber. According to the method, the purification device is utilized to purify the plant extracts by using chromatography, and a good effect is achieved. The purification device has the advantages of simple structure, convenience in use and low manufacturing cost. The method has the advantages of simple process flow and high purification efficiency, and the consumption of organic solvents is greatly reduced.
Owner:十堰赟天生物科技发展有限公司
Method for purifying and refining glycyrrhetic acid from liquorice by microwave auxiliary cloud point extraction
InactiveCN101260137BFastImprove extraction efficiencySteroidsPlant ingredientsHigh concentrationDissolution
The invention relates to the medicine chemical engineering field, in particular to a method for purifying and refining glycyrrhetic acids from liquorices by microwave assistant cloud point extraction. The method is as follows: firstly, by the microwave assistant extraction method, glycyrrhetic acids are extracted from liquorices quickly and efficiently; secondly, cloud point extraction is made bya glue ball solution formed by nonionic surfactants, and the pH value is adjusted to between 1.5 and 3.0, and after the glue ball solution is heated to the cloud point temperature, the glue ball solution is divided into two phases of a water phase and a glue ball phase, and the glycyrrhetic acids are mainly in the glue ball phase; by taking water as a solution, the pH value of the mixed solution is adjusted to between 6.0 and 8.0, and the cloud point back washing is made again, finally the water phase solution containing abundant glycyrrhetic acids is obtained, and coarse glycyrrhetic acids are obtained after the acidification and precipitation of the water phase solution, and the ethanol with high concentration is added to coarse glycyrrhetic acids, after heat dissolution and filtering, the glycyrrhetic acid tripotassium salt is obtained; the glycyrrhetic acid tripotassium salt is added with and dissolved in glacial acetic acids, and is crystallized and separated out, and thus the white glycyrrhetic acid monopotassium salt with high purity is obtained, the purity of the glycyrrhetic acid monopotassium salt is more than 90 percent. The method has the advantages of simple operation, no volatile organic solvent in the process of extraction and high enrichment and purification efficiency.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Preparation method of 2,5-bi(2-trifluoromethyl-4-aminophenoxyl)toluene
InactiveCN101560164BConvenient sourceLow costOrganic compound preparationAmino-hyroxy compound preparationOrganic solventToluene
The invention relates to a preparation method of 2,5-bi(2-trifluoromethyl-4-aminophenoxyl)toluene, comprising the steps of adding 2,5-bi(2-trifluoromethyl-4-nitrophenoxyl)toluene, an organic solvent, and a palladium / carbon catalyst in a reaction kettle, heating the reaction kettle, stirring, raising temperature to 70-80 DEG C, dripping a hydrazine hydrate aqueous solution, strictly controlling thedripping time within 1-1.5 hours, reacting for 2-4 hours at 70-80 DEG C after finishing dripping the aqueous solution, filtering when the solution is hot, removing filter residues, maintaining the mother liquid, dripping deionised water into the mother liquid while stirring, putting the mother liquid aside for 5-8 hours, separating out white solid product, filtering, washing and drying to obtain2,5-bi(2-trifluoromethyl-4-aminophenoxyl)toluene solid. The method has a simple technology, low cost, high purity and yield, and is environment-friendly and suitable for commercial production.
Owner:DONGHUA UNIV
Process for producing 3,3',5,5'-tetramethyl-4,4'-di(2-trifluoromethyl-4-nitrophenoxy)biphenyl
InactiveCN101492376AHigh purityHigh yieldOrganic chemistryOrganic compound preparationBenzeneOrganic solvent
The invention relates to a preparation method of 3, 3', 5, 5'- tetramethyl -4, 4'-double (2-trifluoromethane-4-nitrobenzene oxy) diphenyl. The invention comprises the following steps: (1) mixing 3, 3', 5, 5'-tetramethyl-4, 4'-dihydroxy diphenyl and 2-chlorine-5-nitryl trifluoromethyl benzene by the mol ratio of 1.0:2.0-2.6, and then adding the mixture into a mixed solvent of salifying agent, soluble organic solvent and non-soluble organic solvent for reaction for 4 to 15 hours at 100 to 180 DEG C; (2) filtering when the solvent is hot, eliminating filter residue, condensing mother liquid, cooling and stewing to precipitate a butter yellow solid product, and then filtering, washing and drying the product to obtain the butter yellow solid powder of 3, 3', 5, 5'-tetramethyl-4, 4'-double(2- trifluoromethyl-4-nitrobenzene oxy) diphenyl. The method with simple process, low cost, environment-friendliness and high purity and yield is applicable to the industrial production.
Owner:DONGHUA UNIV
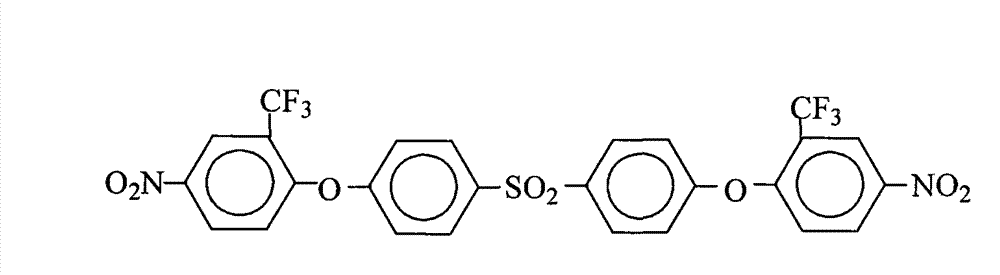
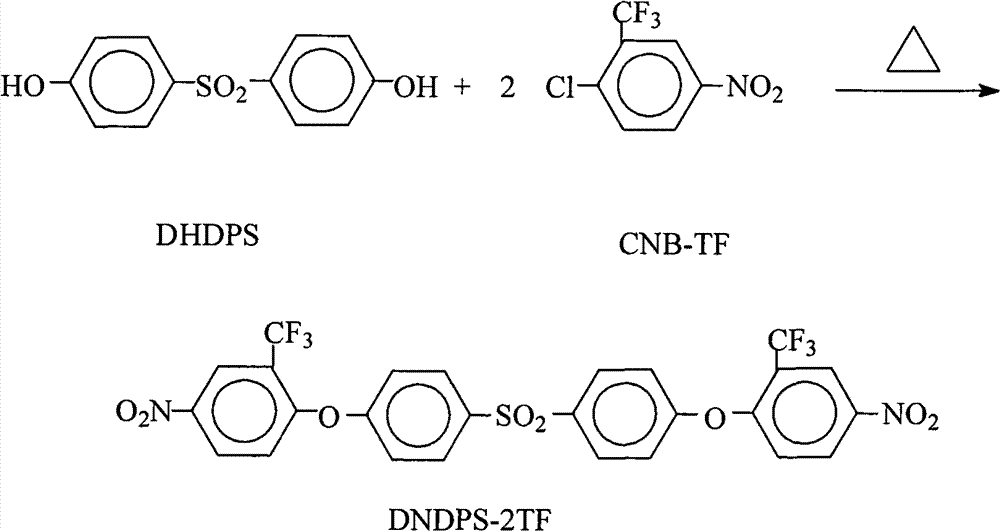
Method for preparing 4,4'-bis(4-nitro-2-trifluoromethylphenoxy)diphenylsulfone
InactiveCN101591278BConvenient sourceLow costOrganic chemistryOrganic compound preparationOrganic solventRoom temperature
The invention relates to a method for preparing 4,4'-bis(4-nitro-2-trifluoromethylphenoxy)diphenylsulfone, which comprises the following steps: adding 4,4'-dihydroxydiphenylsulfone and 2-chloro-5-nitrotrifluoromethylbenzene into a reaction kettle; adding a salt forming agent, strong polar aprotic organic solvent and an organic entrainer, stirring the mixture in the reaction kettle at room temperature for 30 minutes, and heating the mixture for azeotropic water separation reaction for 12 to 15 hours; and filtering the hot solution, removing filter residue, concentrating mother solution, cooling and standing the mother solution to precipitate a milky crystal product, filtering the solution, and washing and drying the milky crystal product to obtain the 4,4'-bis(4-nitro-2-trifluoromethylphenoxy)diphenylsulfone. The preparation method has the advantages that: the operation is simple, the raw material resource is readily available, the cost is low, and no corrosive substance is related to or generated; few kinds of organic solvents are used, and the organic solvents can be used repeatedly and circularly and are environmentally-friendly; and the product has a high yield up to more than 98 percent, a purity up to 99.8 percent and a melting point of from 198.4 to 199.2 DEG C and is suitable for industrial production.
Owner:DONGHUA UNIV
Preparation of 6-O-beta-D- glucosyl-3,6,16,25-tetrahydroxy cycloartane
InactiveCN101376669BEfficient removalHigh yieldMicroorganism based processesSteroidsChinese traditionalBiological activation
The invention discloses a method for preparing 6-O-Beta-D-glucosyl-3,6,16,25-tetrahydroxycycloartane. The method comprises the following steps: inoculating pharmaceutical fungi in a solid culture medium containing Chinese traditional medicinal materials or residue thereof; fermenting under a certain fermentation condition; collecting the solid fermented material; and drying, pulverizing and extracting and separating to obtain the product. The method has the advantages that the process is simple, the waste reutilization is implemented, during the extracting and separating process, the macro porous resin method is not adopted to enrich the compound so that the repeated activation of the macro porous resin is obviated, the mixed n-butyl alcohol extraction solution is washed with sodium hydroxide solution to remove a great amount of phenolic acid impurities and color pigments, a neutral alumina chromatographic column is adopted for the purification so that the pigment impurities with the polarity similar to that of the target component is effectively removed so that the pure product can be directly obtained without need of the re-crystallization, the yield of the pure product is improved, and the method is worth extending and applying..
Owner:NANJING XIAOZHUANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
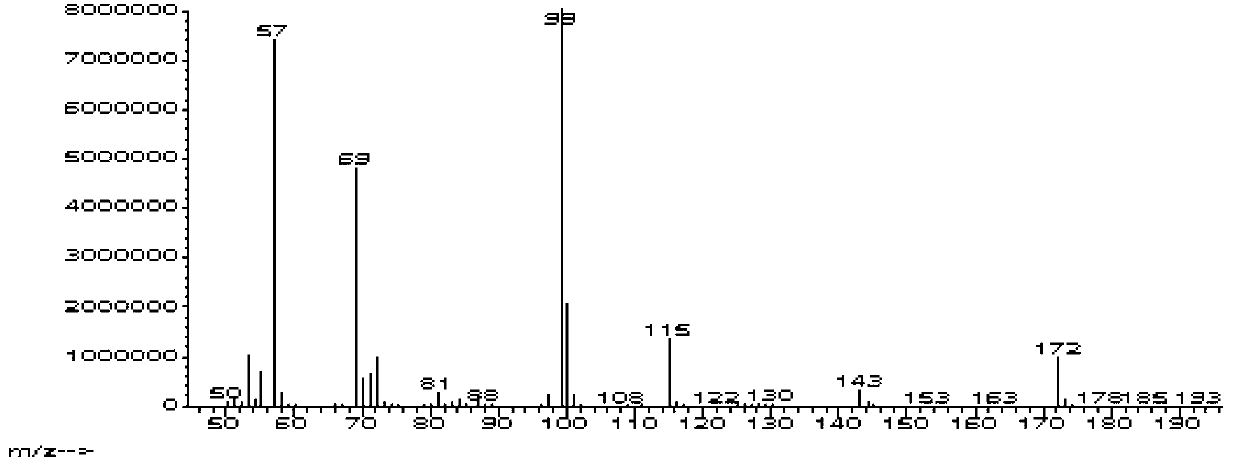
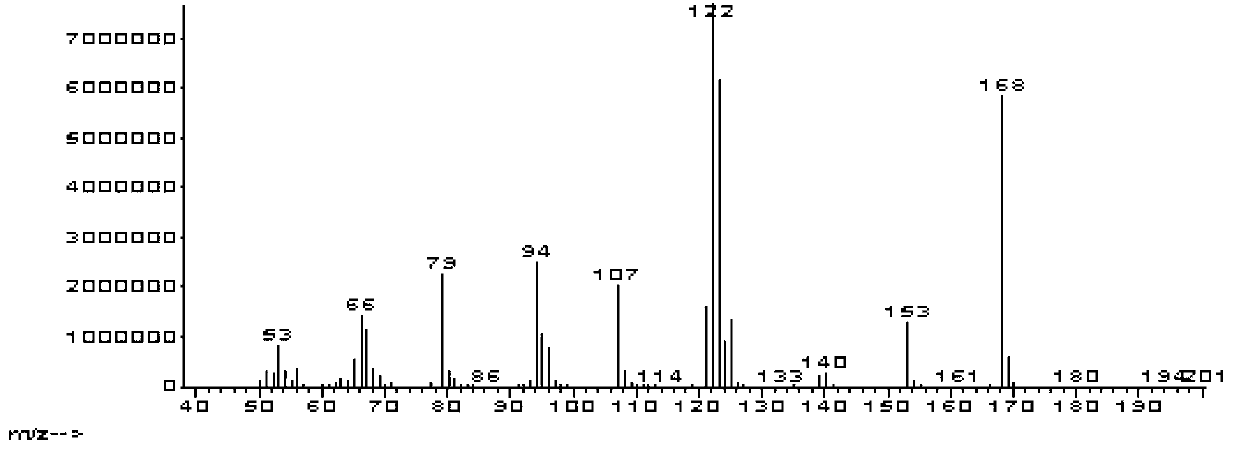
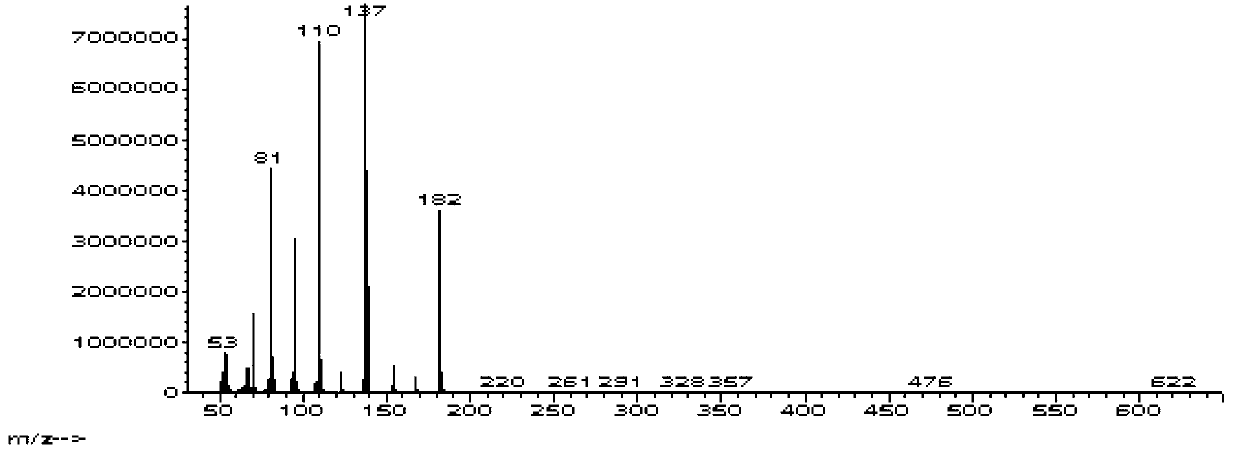
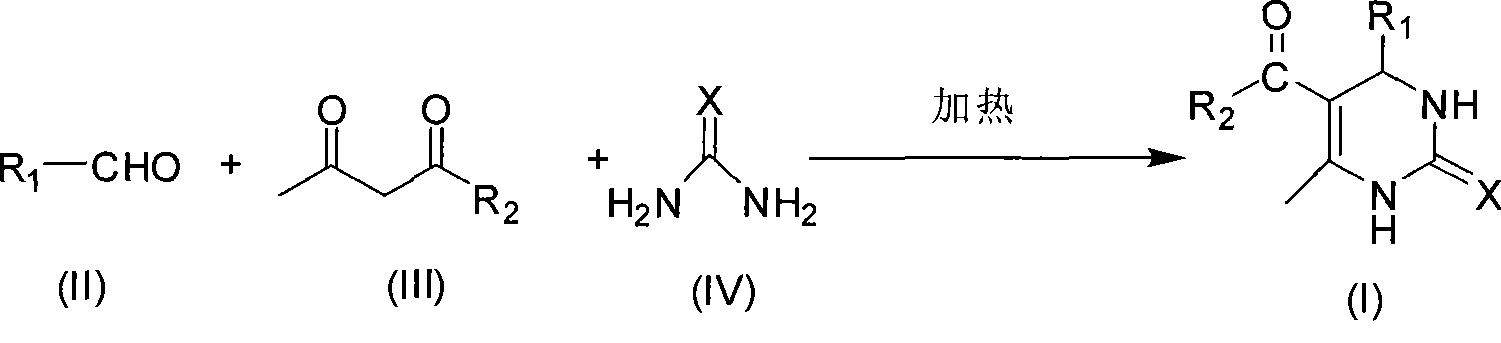


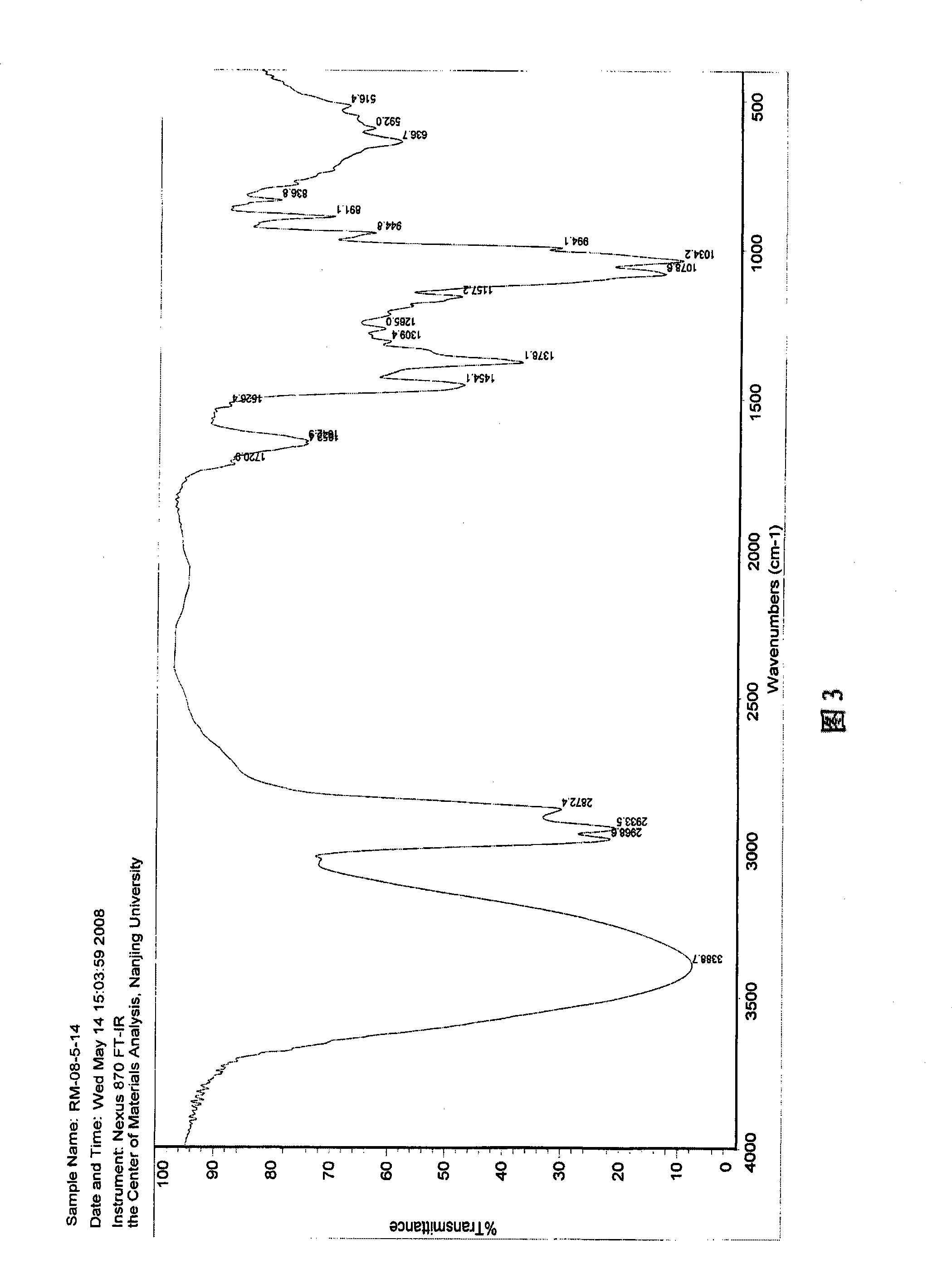
![Method for preparing 2,2'-bis[4-(4-maleimidophenoxy)phenyl]propane Method for preparing 2,2'-bis[4-(4-maleimidophenoxy)phenyl]propane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/00050c0b-295e-487a-8563-c602e16c3618/HDA0001230330910000011.png)
![Method for preparing 2,2'-bis[4-(4-maleimidophenoxy)phenyl]propane Method for preparing 2,2'-bis[4-(4-maleimidophenoxy)phenyl]propane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/00050c0b-295e-487a-8563-c602e16c3618/BDA0001230330900000031.png)