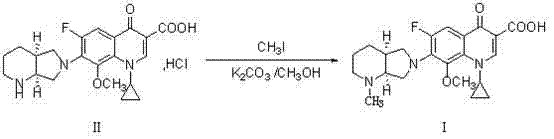
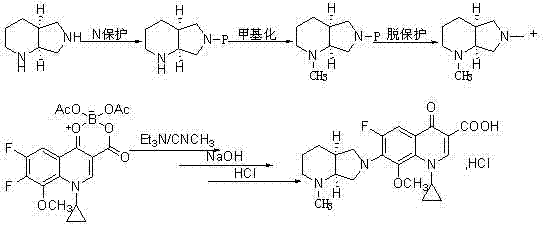
Novel method used for preparing N-methyl moxifloxacin
A technology of moxifloxacin methyl and moxifloxacin hydrochloride, which is applied in organic chemistry and other fields, can solve the problems of high price and achieve the effects of simple operation, cost reduction and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] At room temperature, mix and stir 43.8g of moxifloxacin hydrochloride and 150ml of formic acid, heat up to about 70°C to dissolve, add 40ml of formaldehyde solution with a concentration of 37%, and continue to heat up to 100°C to react until the TLC monitoring raw material point disappears. After the reaction solution was concentrated under reduced pressure, 120ml of 2N hydrochloric acid was added to dissolve it, extracted three times with 80ml of dichloromethane each time, the organic layer was discarded, and the pH value of the aqueous layer was adjusted to 10-11 with 40% sodium hydroxide solution. Cool to 0~5°C, keep warm and crystallize for 0.5h, then filter. The filter cake was dried under reduced pressure at 50-80°C to obtain the target compound. Yield is 86.7%, and purity is 99.1% (HPLC area normalization method), and mass spectrogram sees figure 1 .
Embodiment 2
[0039] At room temperature, mix and stir 43.8g of moxifloxacin hydrochloride and 87.6ml of formic acid, heat up to about 70°C to dissolve, then add 6g of paraformaldehyde, continue to heat up to 100°C to react until the TLC monitoring raw material point disappears. After the reaction solution was filtered, the filtrate was concentrated under reduced pressure and dissolved in 120ml of 2N hydrochloric acid, extracted three times with 80ml of dichloromethane each time, the organic layer was discarded, and the pH value of the aqueous layer was adjusted to 10 with 40% sodium hydroxide solution. ~11. Cool to 0~5°C, keep warm and crystallize for 0.5h, then filter. The filter cake was dried under reduced pressure at 50-80°C to obtain the target compound. The yield was 84.4%, and the purity was 98.9% (HPLC area normalization method).
Embodiment 3
[0041] At room temperature, mix and stir 43.8g of moxifloxacin hydrochloride, 7.5g of ammonium formate and 219ml of formic acid, heat up to about 70°C to dissolve, add 40ml of formaldehyde solution with a concentration of 37%, continue to heat up to 100°C for reaction, and react until TLC monitoring until the raw material point disappears. After the reaction solution was concentrated under reduced pressure, 120ml of 2N hydrochloric acid was added to dissolve it, extracted three times with 80ml of dichloromethane each time, the organic layer was discarded, and the pH value of the aqueous layer was adjusted to 10-11 with 40% sodium hydroxide solution. Cool to 0~5°C, keep warm and crystallize for 0.5h, then filter. The filter cake was dried under reduced pressure at 50-80°C to obtain the target compound. The yield was 88.5%, and the purity was 99.4% (HPLC area normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com