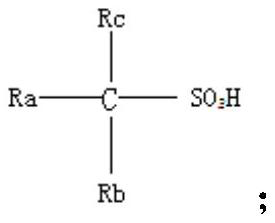
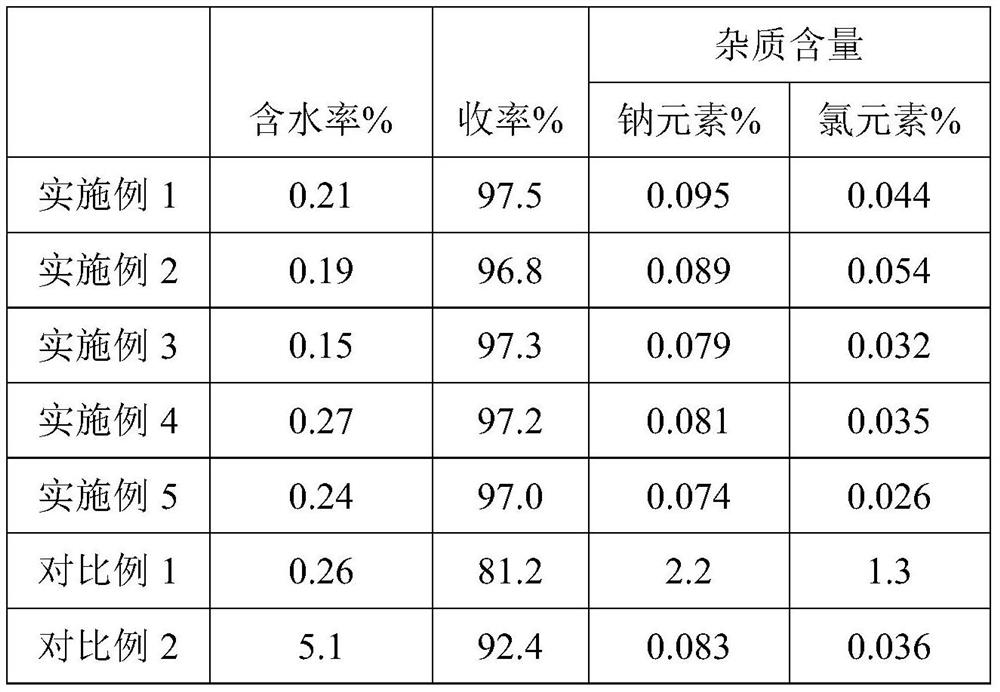
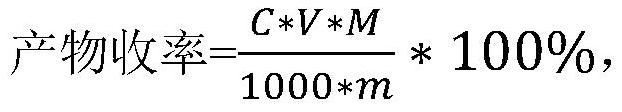Preparation method and application of organic sulfonic acid
A technology of organic sulfonic acid and organic sulfonate, which is applied in the field of fine chemicals, can solve the problems of complex preparation technology of polyhydroxy alkanesulfonic acid, high risk factor of operation, high water content of products, etc., and achieve technical practical operation Strong performance, high reaction yield, simple and reliable reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] This embodiment provides a method for preparing an organic sulfonic acid, the structural formula of the organic sulfonic acid is The concrete preparation steps of described organic sulfonic acid comprise:
[0065] S1. prepare organic sulfonate;
[0066] S2. reacting an organic sulfonic acid salt with a proton donor to obtain a crude organic sulfonic acid;
[0067] S3. purifying the crude organic sulfonic acid to obtain a finished product.
[0068] The preparation of organic sulfonate in the S1 step is specifically, adding sodium 1-chloro-2-hydroxy-3-propanesulfonate (CAS No. 126-83-0) and sodium carbonate into a three-necked flask, adding water, and Refluxing and stirring for 5 hours at 85° C. to obtain an organic sulfonate; the molar ratio of sodium 1-chloro-2-hydroxy-3-propanesulfonate, sodium carbonate and water is 2:1.2:36.
[0069] The proton donor is hydrogen chloride gas;
[0070] The step S2 specifically includes: introducing a proton donor into the organic...
Embodiment 2
[0076] This embodiment provides a method for preparing an organic sulfonic acid, the structural formula of the organic sulfonic acid is The concrete preparation steps of described organic sulfonic acid comprise:
[0077] S1. prepare organic sulfonate;
[0078] S2. reacting an organic sulfonic acid salt with a proton donor to obtain a crude organic sulfonic acid;
[0079] S3. purifying the crude organic sulfonic acid to obtain a finished product.
[0080] The preparation of the organic sulfonate in the S1 step is as follows: mixing sodium bisulfite and water, stirring to dissolve, adjusting the pH to 7 with aqueous sodium hydroxide solution, and then adding 1,4-butenediol, at 40°C The reaction was stirred for 3 hours. During the reaction, dilute hydrochloric acid was used to control the pH=7 to the end of the reaction to obtain an organic sulfonate; the molar ratio of sodium bisulfite, 1,4-butenediol, and water was 1.4:1: 12.4.
[0081] The proton donor is hydrogen chlorid...
Embodiment 3
[0088] This embodiment provides a method for preparing an organic sulfonic acid, the structural formula of the organic sulfonic acid is The concrete preparation steps of described organic sulfonic acid comprise:
[0089] S1. prepare organic sulfonate;
[0090] S2. reacting an organic sulfonic acid salt with a proton donor to obtain a crude organic sulfonic acid;
[0091] S3. purifying the crude organic sulfonic acid to obtain a finished product.
[0092] The preparation of organic sulfonate in the S1 step is specifically, adding sodium 4-chloro-1-hydroxy-butanesulfonate (CAS No. 54322-20-2) and sodium carbonate into a three-necked flask, adding water, and stirring at 85 Under the condition of reflux and stirring for 5 hours, an organic sulfonate was obtained; the molar ratio of sodium 4-chloro-1-hydroxy-butanesulfonate, sodium carbonate and water was 2:1.2:36.
[0093]The proton donor is hydrogen chloride gas.
[0094] The step S2 specifically includes: introducing a prot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com