Preparation method of thiamethoxam
A technology of thiamethoxam and oxadiazine, which is applied in the field of drug synthesis, can solve the problems of methanol solvent recrystallization, low content, and large amount of waste water, and achieve the effects of improving purity, stabilizing the reaction system, and promoting the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
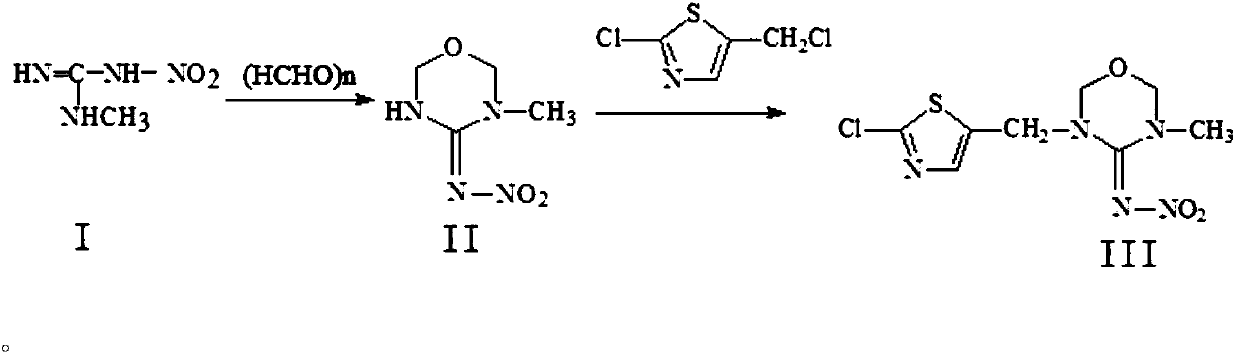
[0023] 1. Synthesis of compound II from compound I, the process is as follows:
[0024]
[0025] Add glacial acetic acid 140kg, sulfuric acid (concentration 98%) 20kg, water 40kg successively in reactor and start to stir, drop into paraformaldehyde 300kg then, be warming up to 40 ℃ after feeding, drop into methyl nitroguanidine 380kg, adjust temperature to 70 Insulate at ℃ for 10 hours, cool down to 40°C at the end of the heat preservation, add dropwise saturated sodium bicarbonate solution to adjust the pH to 6.8, add 200kg of water after the end, stir, cool down to 0°C, discharge, suction filter, centrifuge, and shake to obtain 3-methyl -4-nitroimine-1,3,5-oxadiazine, the content is more than 98%, and the yield is 82%. The filtrate and centrifuged mother liquor go to the three wastes for treatment. 1 HNMR (CDCl 3 ): δ9.74 (bs, NH), 5.00 (d, 2H, oxadiazine-6-H), 4.85 (s, 2H, oxadiazine-2-H), 3.01 (s, 3H, CH 3 ).
[0026] 2. Synthesis of compound III from compound II, t...
Embodiment 2
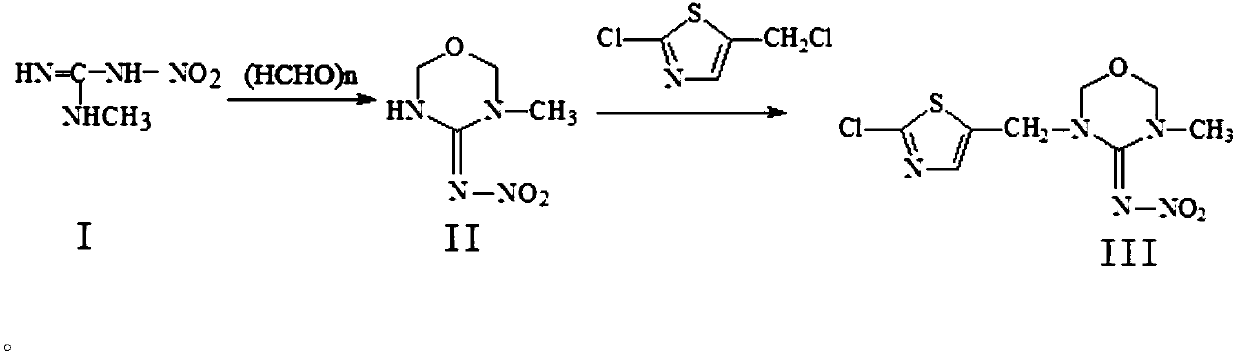
[0030] 1. Synthesis of compound II from compound I, the process is as follows:
[0031]
[0032] Add glacial acetic acid 120kg, sulfuric acid (concentration 98%) 20kg, water 50kg successively in reactor and start to stir, then drop into paraformaldehyde 300kg, be warming up to 43 ℃ after feeding, drop into methyl nitroguanidine 300kg, adjust temperature to 75 Insulate at ℃ for 12 hours, cool down to 46°C at the end of the insulation, add dropwise saturated sodium bicarbonate solution to adjust the pH to 7.0, add 200kg of water after the end, stir, cool down to 0°C, discharge, suction filter, centrifuge, and shake to obtain 3-methyl -4-nitroimine-1,3,5-oxadiazine, the content is more than 98%, and the yield is 83%. The filtrate and centrifuged mother liquor go to the three wastes for treatment. 1 HNMR (CDCl 3 ): δ9.75 (bs, NH), 5.00 (d, 2H, oxadiazine-6-H), 4.88 (s, 2H, oxadiazine-2-H), 3.02 (s, 3H, CH 3 ).
[0033] 2. Synthesis of compound III from compound II, the proc...
Embodiment 3
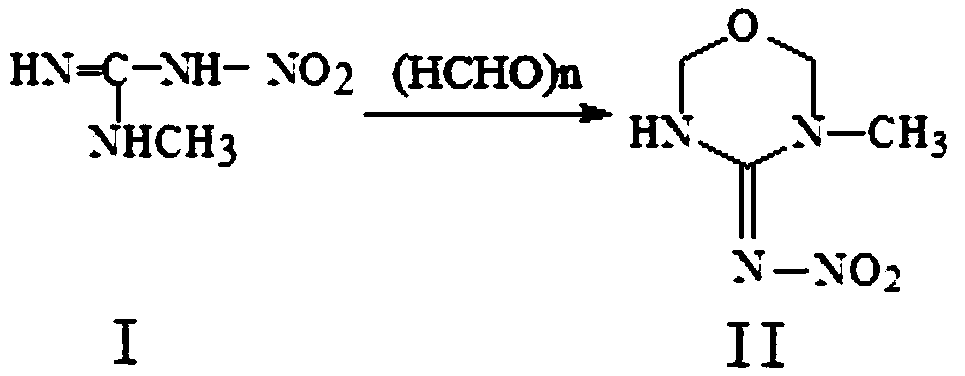
[0037] 1. Synthesis of compound II from compound I, the process is as follows:
[0038]
[0039]Add glacial acetic acid 140kg, sulfuric acid (concentration 98%) 20kg, water 60kg successively in reactor and start to stir, drop into paraformaldehyde 300kg then, be warming up to 46 ℃ after feeding, drop into methyl nitroguanidine 380kg, adjust temperature to 78 Keep warm at ℃ for 14 hours, cool down to 45°C after the heat preservation, add dropwise saturated sodium bicarbonate solution to adjust the pH to 6.6, add 220kg of water after the end, stir, cool down to 2°C, discharge, suction filter, centrifuge, and shake to obtain 3-methyl -4-nitroimine-1,3,5-oxadiazine, the content is more than 98%, and the yield is 85%. The filtrate and centrifuged mother liquor go to the three wastes for treatment. 1 HNMR (CDCl 3 ): δ9.75 (bs, NH), 5.02 (d, 2H, oxadiazine-6-H), 4.86 (s, 2H, oxadiazine-2-H), 3.01 (s, 3H, CH 3 ).
[0040] 2. Synthesis of compound III from compound II, the proce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com