Preparation method of thiazafluron original medicine
A technology of tifluron technical and thiadiazole, which is applied in the field of synthesis of tifluron technical, can solve the problems of strict production equipment, inability to transport, and unreported synthesis process of tifluron, and achieve high purity and high yield rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
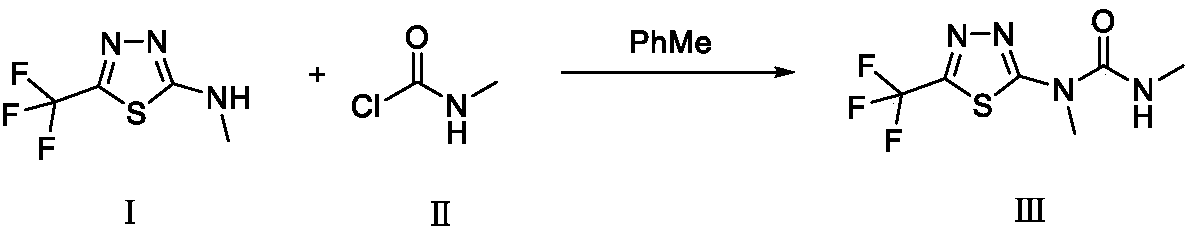
[0026] Example 1: Add 2-methylamino-(5-trifluoromethyl)-1,3,4-thiadiazole (I) 128g (0.7mol) and 700ml toluene to a 2L reaction kettle, at 30°C 500ml of toluene containing 80g (0.84mol) of N-methylcarbamoyl chloride (II) was added dropwise, and the addition was completed after 2 hours. At this temperature, 81 g (0.8 mol) of anhydrous triethylamine was added dropwise, and the addition was completed within 1.5 hours. The reaction was incubated at this temperature for 1 h. Afterwards, the temperature was raised to 70° C., and the reaction was kept at this temperature for 4 hours. Lower the temperature and add 150ml of water at 60°C, and continue to cool down to 10°C. , stirred and incubated for 0.5h, filtered, dried, and dried to obtain 168g of 97% white product tifluron. Yield 97%, melting point 135-137 ℃ (HPLC, H 2 O:CH 3 OH=20:80).
Embodiment 2
[0027] Embodiment 2: Add 2-methylammonia-(5-trifluoromethyl)-1,3,4-thiadiazole (I) 18.3g (0.1mol) and 100ml toluene in 500mL reactor, at 30 °C, 100 ml of toluene containing 13 g (0.14 mol) of N-methylcarbamoyl chloride (II) was added dropwise, and the addition was completed within 2 hours. At this temperature, 11.2 g (0.11 mol) of anhydrous triethylamine was added dropwise, and the dropwise addition was completed in about 1.5 hours. The reaction was incubated at this temperature for 1 h. Afterwards, the temperature was raised to 75° C., and the reaction was kept at this temperature for 4 hours. Cool down at 60°C, add 50ml of water, continue to cool down to 10°C, keep warm for 0.5h, filter, dry, and dry to obtain 24.5g of 95% white product Teflon. Yield 97.5%, melting point 135-137 ℃ (HPLC, H 2 O:CH 3 OH=20:80).
Embodiment 3
[0028] Embodiment 3: Add 2-methylammonia-(5-trifluoromethyl)-1,3,4-thiadiazole (I) 36.6g (0.2mol) and 200ml toluene in 1000mL reactor, at 30 °C, 150 ml of toluene containing 22.5 g (0.24 mol) of N-methylcarbamoyl chloride (II) was added dropwise. , into N 2 The temperature was raised to 75°C, and the reaction was kept at this temperature for 4h. Cool down to below 60°C, slowly add 200ml of water, continue to cool down to 10-15°C, keep warm for 0.5h, filter, dry, and dry to obtain 44g of 97% white product Teflon. Yield 89%, melting point 135-137 ℃ (HPLC, H 2 O:CH 3 OH=20:80).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com