Preparation method of N-alkyl phthalimide compound
A technology of alkyl phthalimide and phthalic anhydride, applied in the field of preparation of N-alkyl phthalimide compounds, can solve the problem of high cost of methyl iodide and dimethyl carbonate, etc. problem, to achieve the effect of low cost, easy processing and wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 30.0 g (0.203 mol) of phthalic anhydride, 60 g of glacial acetic acid and 2 g of acetic anhydride into the reaction flask, stir and heat to 60°C, keep warm for 20 minutes to partially dissolve the phthalic anhydride, then add 25.0 g (0.242 mol) of 30% methylamine aqueous solution at one time , heated to 130°C for 15 minutes and refluxed for 5 hours. Phthalic anhydride gradually dissolved during the process, and finally a colorless transparent solution was obtained. The product solution was concentrated to 60 g by distillation under reduced pressure, cooled to room temperature and allowed to stand overnight, and a large amount of needle-shaped white solid was precipitated. Suction filtration under reduced pressure and wash the filter cake with a small amount of acetic acid, and dry under reduced pressure at 100°C to obtain 26.7 g of white needle-like N-methylphthalimide, with a yield (calculated as phthalic anhydride) of 81.8%.
[0025] Melting point: 129.5-130.4°C....
Embodiment 2
[0030] Add 91.2 grams (0.5 mol) of 4-chlorophthalic anhydride and 200 grams of glacial acetic acid into a three-necked flask, stir and heat to 50°C for 15 minutes, and the 4-chlorophthalic anhydride is completely dissolved. Then, 67.1 g (0.65 mol) of 30% methylamine aqueous solution was added directly, and the temperature was raised to 120°C for 20 minutes to react for 5 hours. After the reaction was completed, it was naturally cooled to room temperature, and a large number of needle-like white crystals were precipitated overnight, filtered under reduced pressure and rinsed with a small amount of glacial acetic acid, and the filter cake was dried under reduced pressure to obtain white granular solid N-methyl-4-chloro-o-phthalic acid Dicarboximide (4-CPI) 78.2 grams, yield 80.2% (calculated as 4-chlorophthalic anhydride)
[0031] Melting point: 135.5-136.2°C
[0032] 1 H NMR (400MHZ DMSO-D 6 ) δ=7.92-7.91(t, J=1.2 Hz, 1H, Ph-H) 7.86(d, J=1.2 Hz, 2H, Ph-H) 3.05(s, 3H, CH 3 ) ...
Embodiment 3
[0038] Add 70.0 g of 3-nitrophthalic anhydride (0.752 mol) into 350 g of glacial acetic acid, stir and heat up to 50°C for 30 minutes, and the 3-nitrophthalic anhydride is partially dissolved. Add 93.2 g (0.902 mol) of 30% methylamine aqueous solution, then gradually dissolve the solid, heat up to 120°C in 15 minutes, and then reflux for 3 hours. The solution was concentrated under reduced pressure to 100 g, cooled naturally, and a large number of needle-shaped yellow solids were precipitated overnight, filtered under reduced pressure and washed with a small amount of glacial acetic acid, dried under reduced pressure to obtain light yellow blocky solid N-methyl-3-nitrate 55.6 grams of phthalimide (3-NPI), yield 74.6% (based on 3-nitrophthalic anhydride).
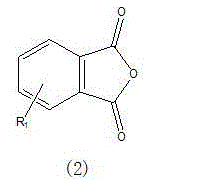
[0039] The general structural formula of this compound is expression (6)
[0040]
[0041] Melting point: 111.9-113.2°C
[0042] 1 H NMR (400MHZ DMSO-D 6 ): δ= 8.27-8.25(d, J=8 Hz, 1H, Ph-H) 8.16-8.14(d, J= 8 Hz, 1H, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com