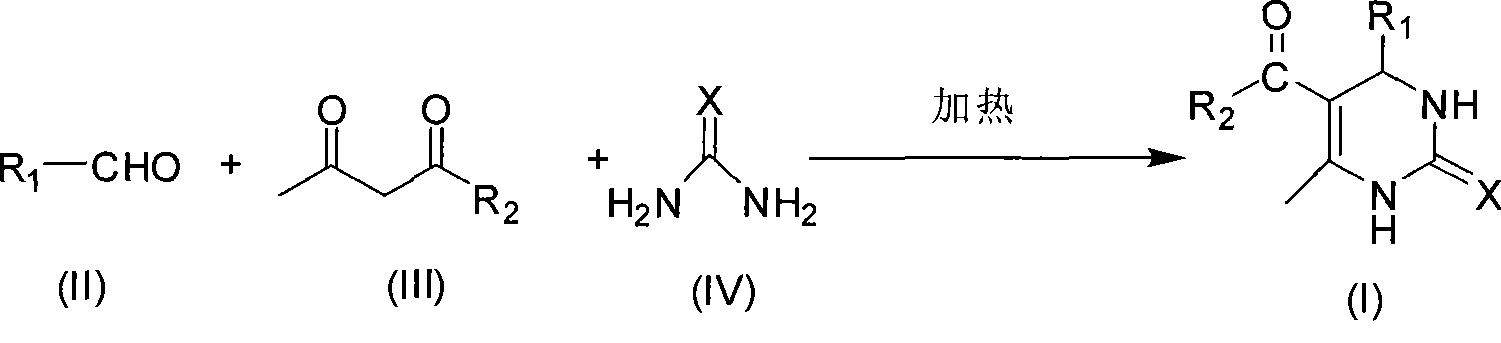Process for synthesizing 3,4-dihydropyrimidine-2-keto
A technology of dihydropyrimidine and synthesis method, applied in 3 fields, can solve the problems of long reaction time, expensive reagents, low yield and the like, and achieves the effects of low production cost, good product purity and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
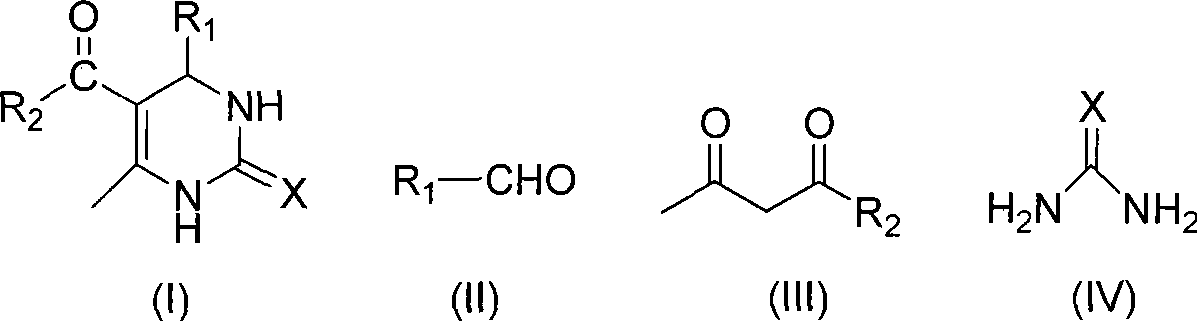
[0024] In a 100mL round bottom flask, add 21.22g (0.2mol) benzaldehyde, 20.03g (0.2mol) acetylacetone, 18.02g (0.3mol) urea, mix well, stir and heat at 110°C for 1.5 hours, cool, suction filter, filter The cake was washed with ice water, then washed with a small amount of 40% ethanol-water solution by volume, and then washed with water, and the solid was dried and weighed to obtain 44.89 g of the product, with a yield of 97.6%.
Embodiment 2
[0026] In a 100mL round bottom flask, add 21.22g (0.2mol) benzaldehyde, 20.03g (0.2mol) acetylacetone, 18.02g (0.3mol) urea, mix well, stir and heat at 80°C for 1.5 hours, cool, suction filter, filter The cake was washed with ice water, then with a small amount of 40% ethanol-water solution by volume, and then with water, and the solid was weighed dry to obtain 42.77 g of the product, with a yield of 93%.
Embodiment 3
[0028] In a 100mL round bottom flask, add 21.22g (0.2mol) benzaldehyde, 26.03g (0.2mol) ethyl acetoacetate, 18.02g (0.3mol) urea, mix well, stir and heat at 150°C for 1.5 hours, cool, and filter with suction , the filter cake was washed with ice water, then washed with a small amount of 40% volume concentration ethanol-water solution, and then washed with water, and the solid was weighed after drying to obtain 49.94 g of the product, with a yield of 96.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com