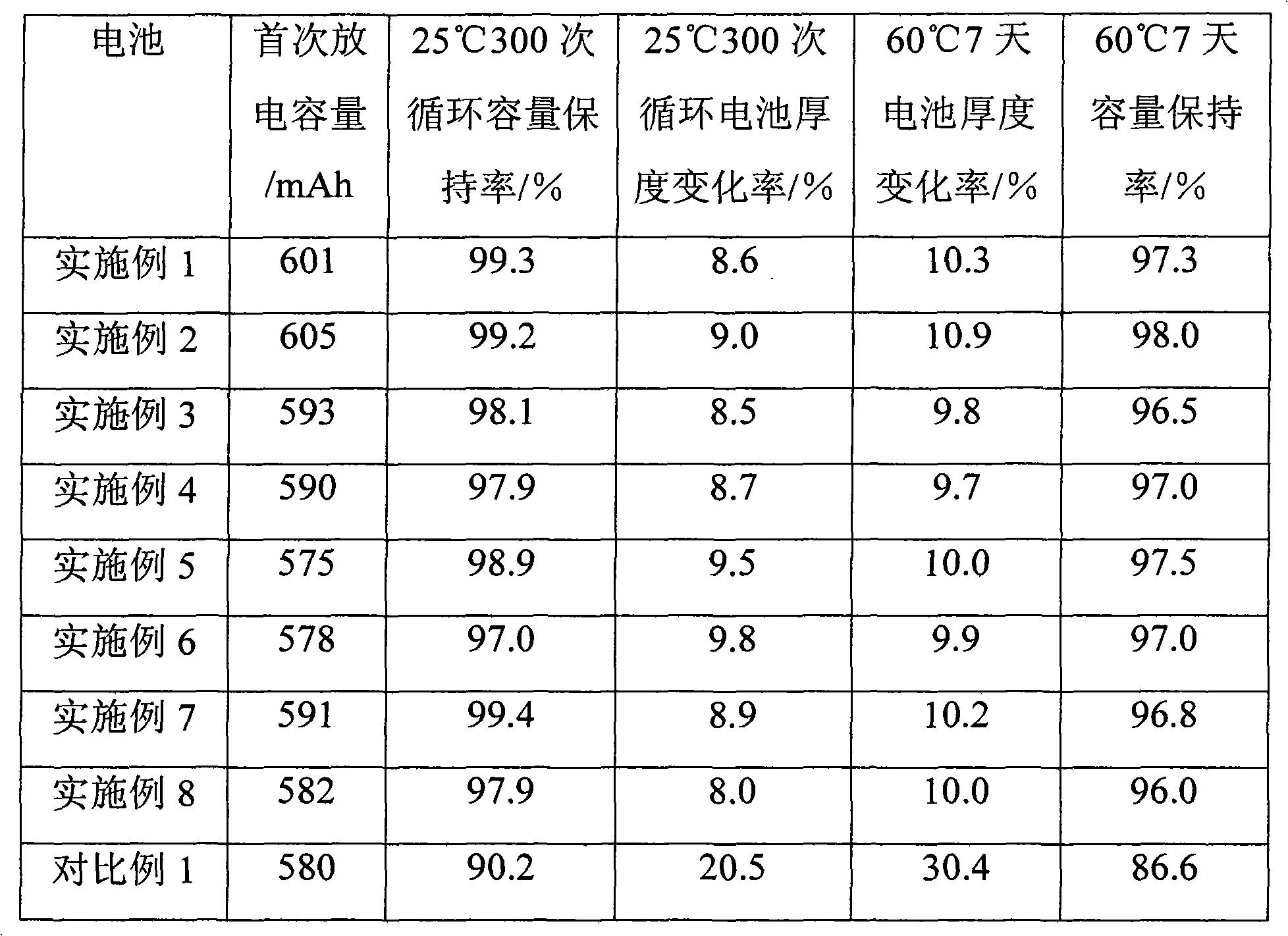
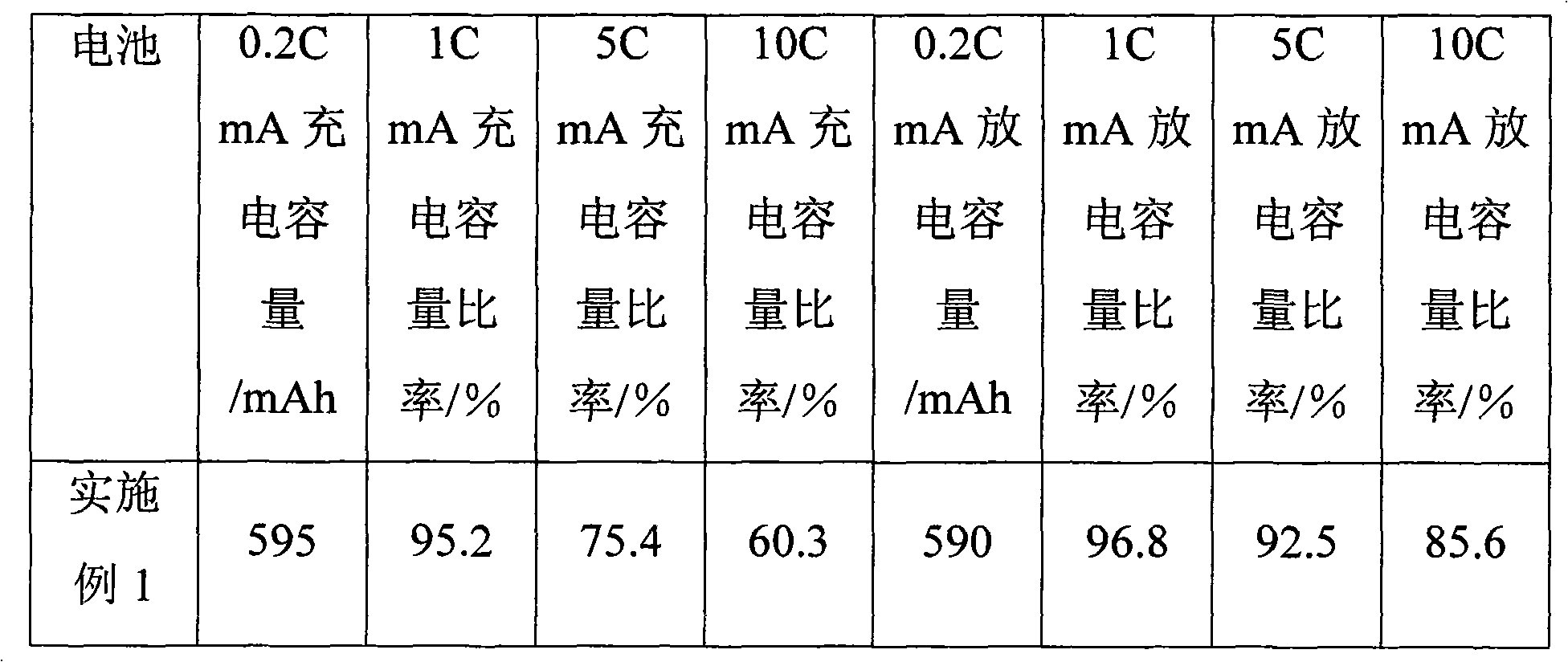
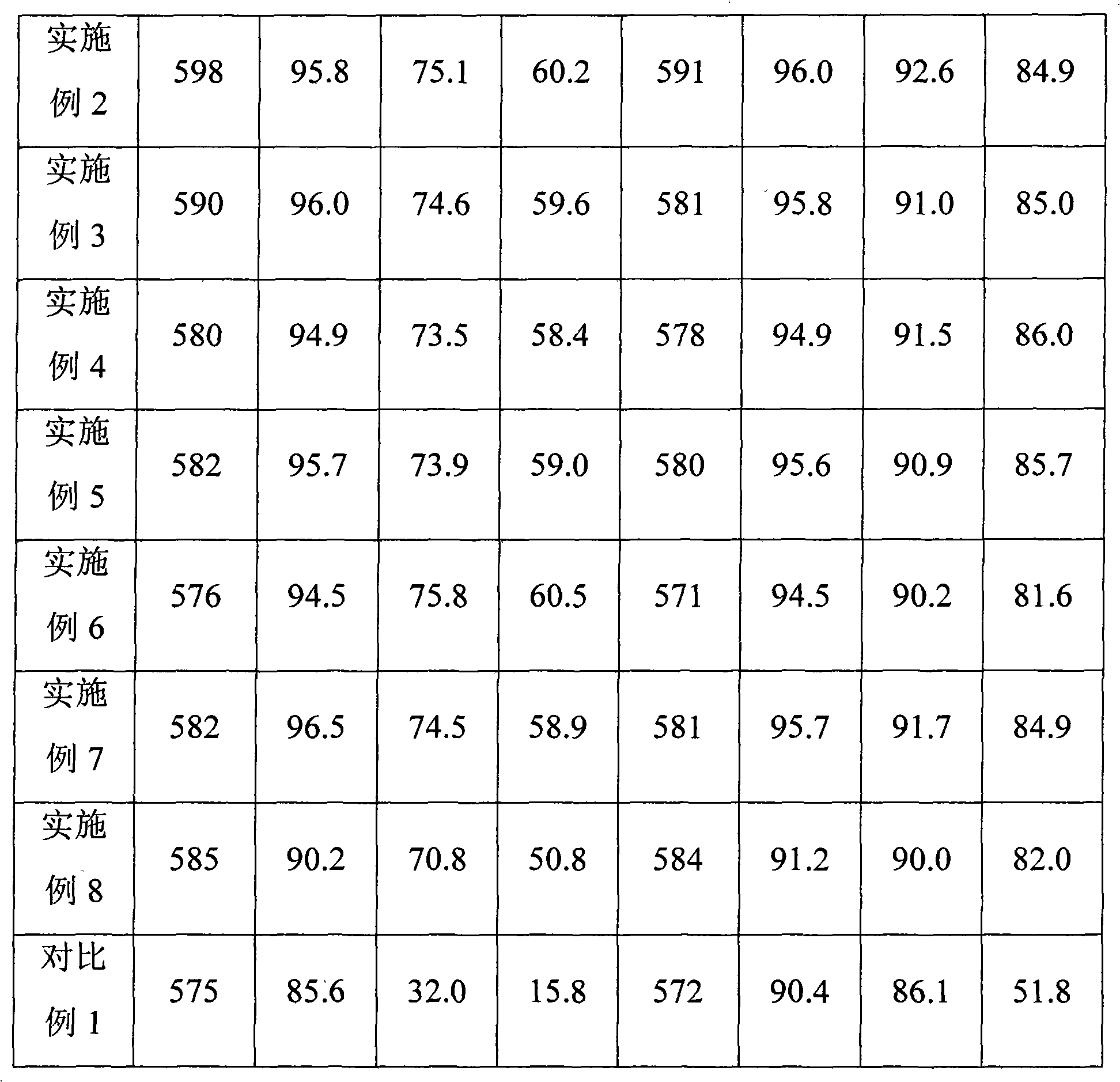
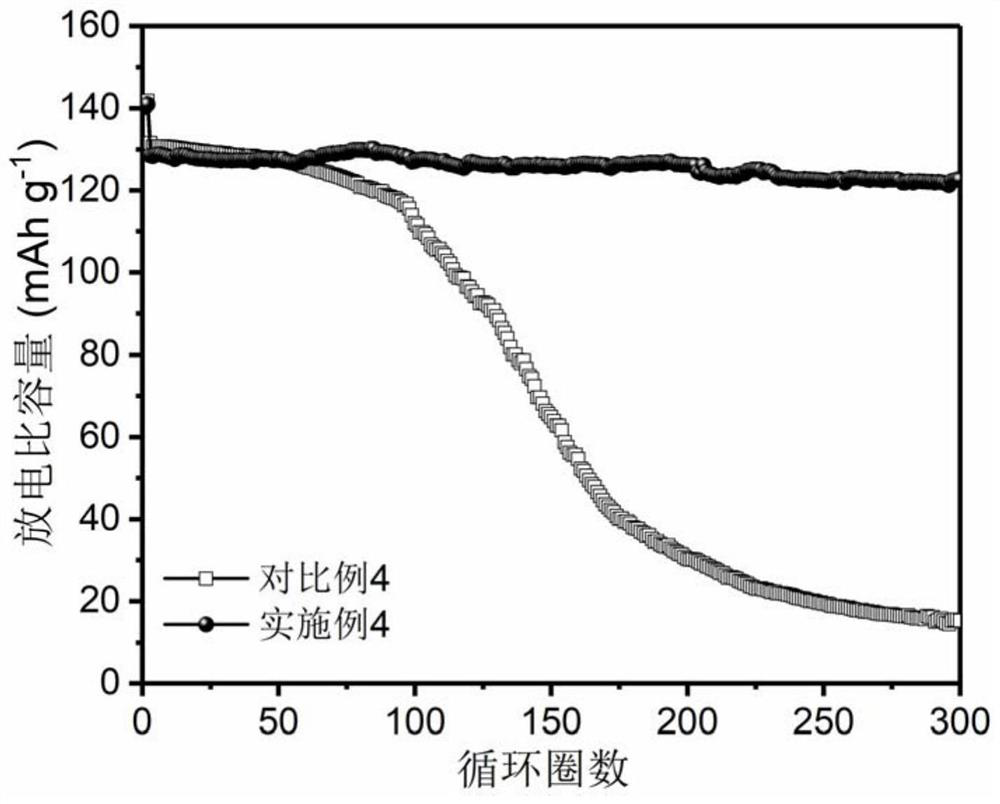
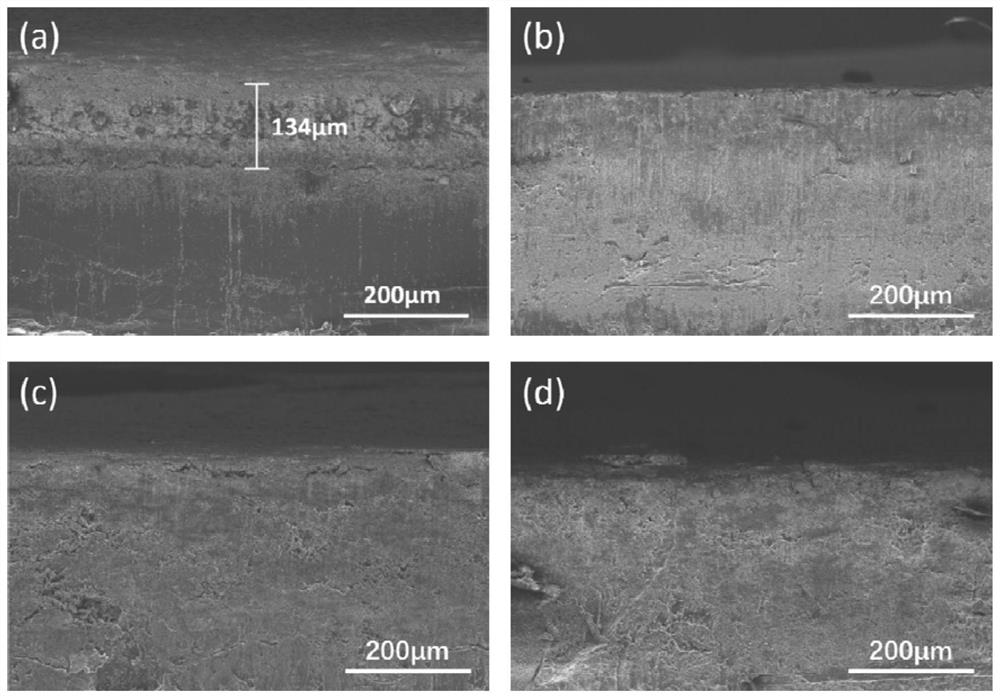
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Lithium chlorate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
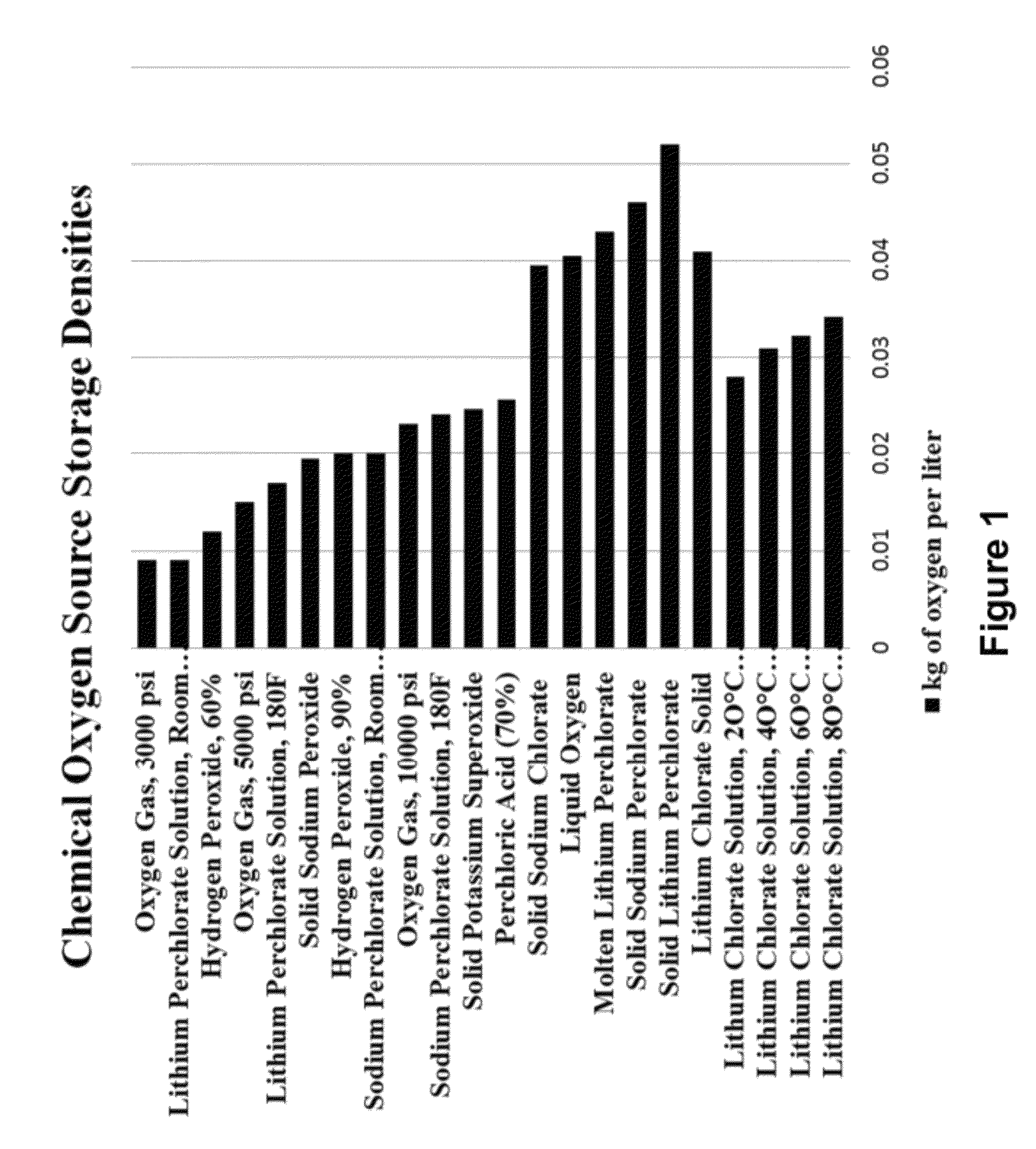
Lithium chlorate is the inorganic chemical compound with the formula LiClO₃. Like all chlorates, it is an oxidizer and may become unstable and possibly explosive if mixed with organic materials, reactive metal powders, or sulfur.
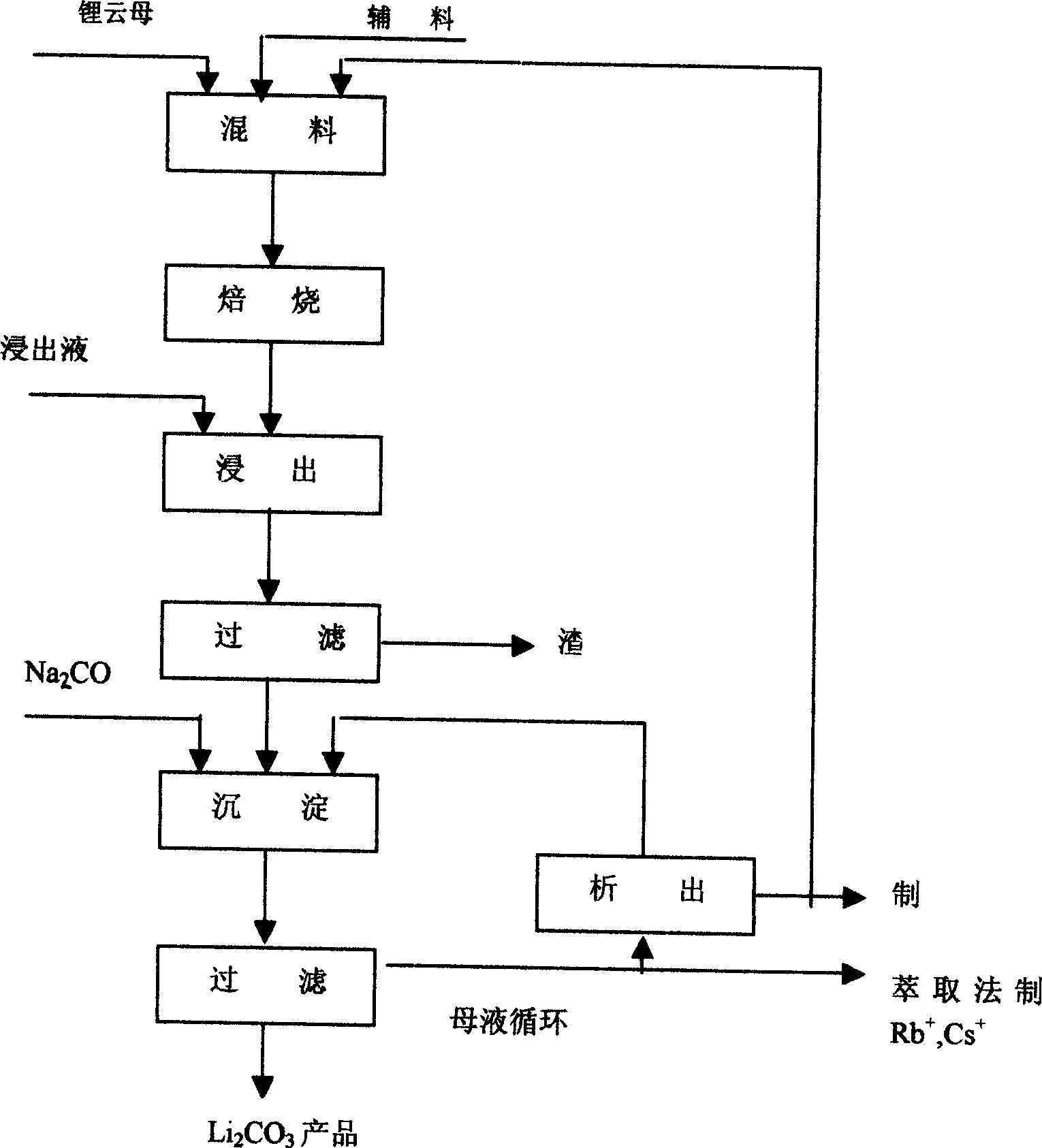
Process for preparing lithium chlorate by lithium extracted from lepidolite
InactiveCN1827527AReduce energy consumptionReduce investmentLithium carbonates/bicarbonatesLithium carbonateSulfate
The invention relates to a method for preparation of lithium carbonate extracting lithium from lithionite. The invention consists of milling, leaching and filtering after modifying by broasting lithonite ore together with the additive findings such as calcium fluoride, calcium sulfate and sodium sulfate at a certain temperature, adding sodium carbonate into the leachate to deposite lithium ion forming the deposition of lithium carbonate, and washing and drying the solid gained by filtering to get the product of lithium carbonate. The filtered mother liquid returns to the circulation to deposit lithium ion. After secondary circulation, we get the mixed salts of potassium sulfate and sodium sulfate as the educts by washing and drying the aforementioned filtered mother liquid. Some of the mixed salts return as findings roasted with lithonite ore for circulation use, while others can be used as raw material for preparation of potassium sulfate.
Owner:钟辉
Titanium composite, preparation method thereof and application thereof
ActiveCN101901905AImprove performanceEasy to preparePigmenting treatmentAlkali titanatesLithium chlorideHigh rate
Owner:BYD CO LTD
Solid-layered bleach compositions
ActiveUS8287755B2Inorganic/elemental detergent compounding agentsCosmetic preparationsPotassium hypochloriteBleach
The present invention provides a solid-layered composition having at least two parts. The first part comprises a) calcium hypochlorite, magnesium hypochlorite and mixtures thereof, b) a builder, c) a water-soluble polymer, d) an acid, and e) wherein the first part does not contain sodium hypochlorite, lithium hypochlorite, potassium hypochlorite and mixtures thereof. The second part comprises a) a surfactant, b) a builder, c) an acid, and d) wherein the second part does not contain any oxidant.
Owner:THE CLOROX CO
Solid-layered bleach compositions and methods of use
ActiveUS20110052726A1Inorganic/elemental detergent compounding agentsCosmetic preparationsPotassium hypochloriteBleach
The present invention provides a solid-layered composition having at least two parts. The first part comprises a) calcium hypochlorite, magnesium hypochlorite and mixtures thereof, b) a builder, c) a water-soluble polymer, d) an acid, and e) wherein the first part does not contain sodium hypochlorite, lithium hypochlorite, potassium hypochlorite and mixtures thereof. The second part comprises a) a functional ingredient, b) a builder or filler, and c) wherein the second part does not contain any oxidant.
Owner:THE CLOROX CO
Chemical carbon dioxide gas generator
A chemical carbon dioxide gas generator comprising: - a charge housing; - a carbon dioxide gas penetrable charge, contained in the said housing, the charge comprising a) 40-60 wt. % of a substance which upon decomposition generates carbon dioxide, which substance is selected from the group of magnesium carbonate, other carbonates, magnesium oxalate and other oxalates, b) 20-50 wt. % of an oxidiser selected from the group of sodium chlorate, potassium chlorate, lithium chlorate, other metal chlorates, sodium perchlorate, potassium perchlorate, lithium perchlorate, and other metal perchlorates, c) 1-20 wt. % of carbon or another fuel, d) 1-10 wt. % binder, said components a), b), c) and d) together forming 90-100 wt. % of the total weight of the charge; - an ignition device for igniting the charge; - a carbon dioxide gas treatment unit for reducing the content of one or more side-products - which may have been formed by the charge - in the generated carbon dioxide, and / or for cooling carbon dioxide gas generated by the charge; and - an outlet for carbon dioxide gas generated by the charge.
Owner:荷兰应用自然科技研究组织TNO
Method for preparing active lithium cobalt oxide
InactiveCN1595681AGood chemistryStable physical propertiesElectrode manufacturing processesLithium compoundsChemical reactionFiltration
Disclosed is a manufacturing method for active lithium cobalt, whose characteristics includes the following steps: make highly pure cobalt salt solution with level of purification, by using primitive cobalt ore as raw material; make cobalt salt solution with high level of purification whose density is 40~70g / L have chemical reaction with precipitator whose density is 60~200g / L, under the temperature of 40~80deg.C, with reaction time of 5~60 minutes, and its PH value is 7.2~9.5 after reaction, cobalt salt solution for battery is created by filtration, washing and dry; calcine cobalt salt solution for 2~7 hours under the temperature of 400~830deg.C, micronsized or millimicronsized cobaltosic oxide is made by shattering; mix the shattered micronsized cell lithium chlorate and micronsized or millimicronsized cobaltosic oxide according to micronsized or millimicronsized cobaltosic oxide of 1.00~1.04:1, and calcine for 10~20 hours under the temperature of 450~950deg.C, then the final product is formed by comminution and classification. The material made by the method has excellent electrochemical property except chemical and physical properties.
Owner:太阳集团高科技发展有限责任公司
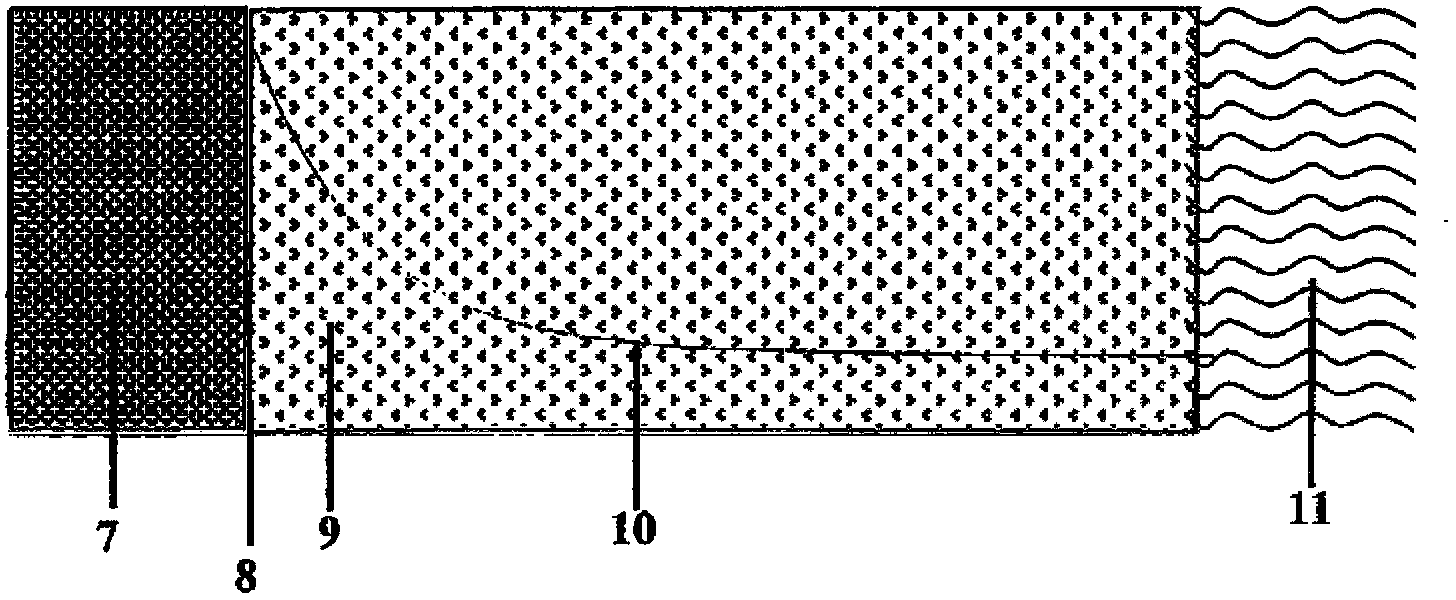
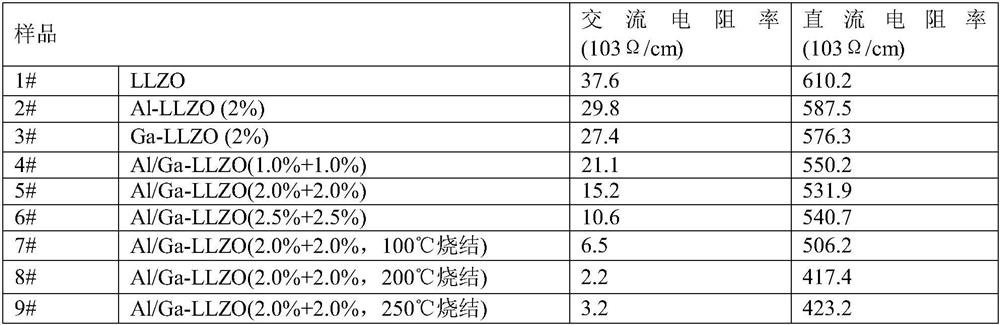
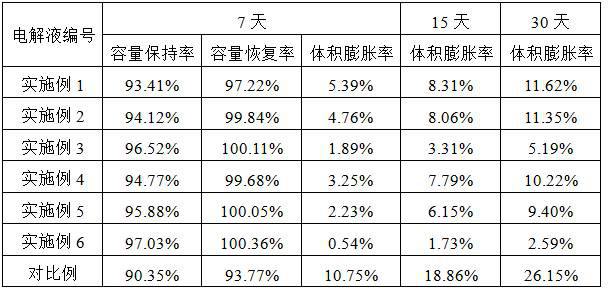
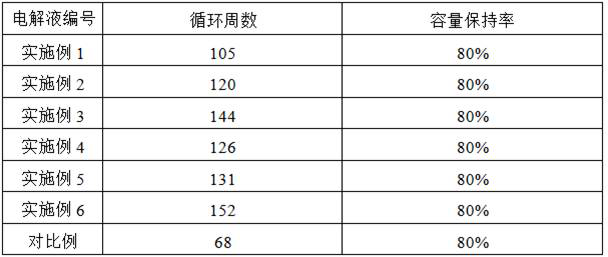
Preparation method of low-resistivity garnet type modified LLZO solid electrolyte
ActiveCN113363562AImprove mixing efficiencyImprove grain boundary conductivitySecondary cellsElectrolytesPhysical chemistryPolypropylene
The invention relates to the field of lithium batteries, and discloses a preparation method of a low-resistivity garnet type modified LLZO solid electrolyte. The method comprises the following steps: 1) adding raw materials into a three-dimensional high-energy vibration ball mill for ball milling after batching, sintering in air at 300-400 DEG C after ball milling, cooling, continuing ball milling, and performing anaerobic sintering under 300-500 Mpa and 900-1100 DEG C to prepare an Al / Ga doped modified LLZO solid electrolyte; and 2) uniformly mixing polypropylene carbonate and acetone, adding lithium perchlorate for fully dissolving, adding the Al / Ga doped modified LLZO solid electrolyte, continuously performing ultrasonic mixing uniformly, and sintering a finished product at a low temperature of 100-250 DEG C under 300-400 Mpa. The method disclosed by the invention can be used for remarkably reducing the actual resistivity of the LLZO solid electrolyte and is beneficial to application of the LLZO solid electrolyte in a solid battery.
Owner:WANXIANG 123 CO LTD
High-temperature-resistant high-voltage electrolyte of high-nickel lithium ion battery
PendingCN112216870AReduce exposureAvoid decompositionSecondary cellsOrganic electrolytesDifluorophosphateElectrolytic agent
A high-temperature-resistant high-voltage electrolyte of a high-nickel lithium ion battery is composed of a composite electrolyte lithium salt, an organic multi-component solvent and an additive, andthe composite electrolyte lithium salt is at least two of lithium hexafluorophosphate, lithium perchlorate, lithium difluorophosphate, lithium bis (fluorosulfonyl) imide, lithium bis (oxalato) borate,lithium difluoro (oxalato) borate, lithium difluoro (oxalato) phosphate and the like. The electrolyte lithium salt and the additive in the electrolyte participate in positive electrode film formationat the same time to hinder contact between the electrolyte and an electrode, so that decomposition reaction between the electrolyte in a high-potential region and an active substance is inhibited, and the storage and cycle performance of the lithium ion battery at high temperature and high voltage is improved.
Owner:HUNAN AEROSPACE MAGNET & MAGNETO
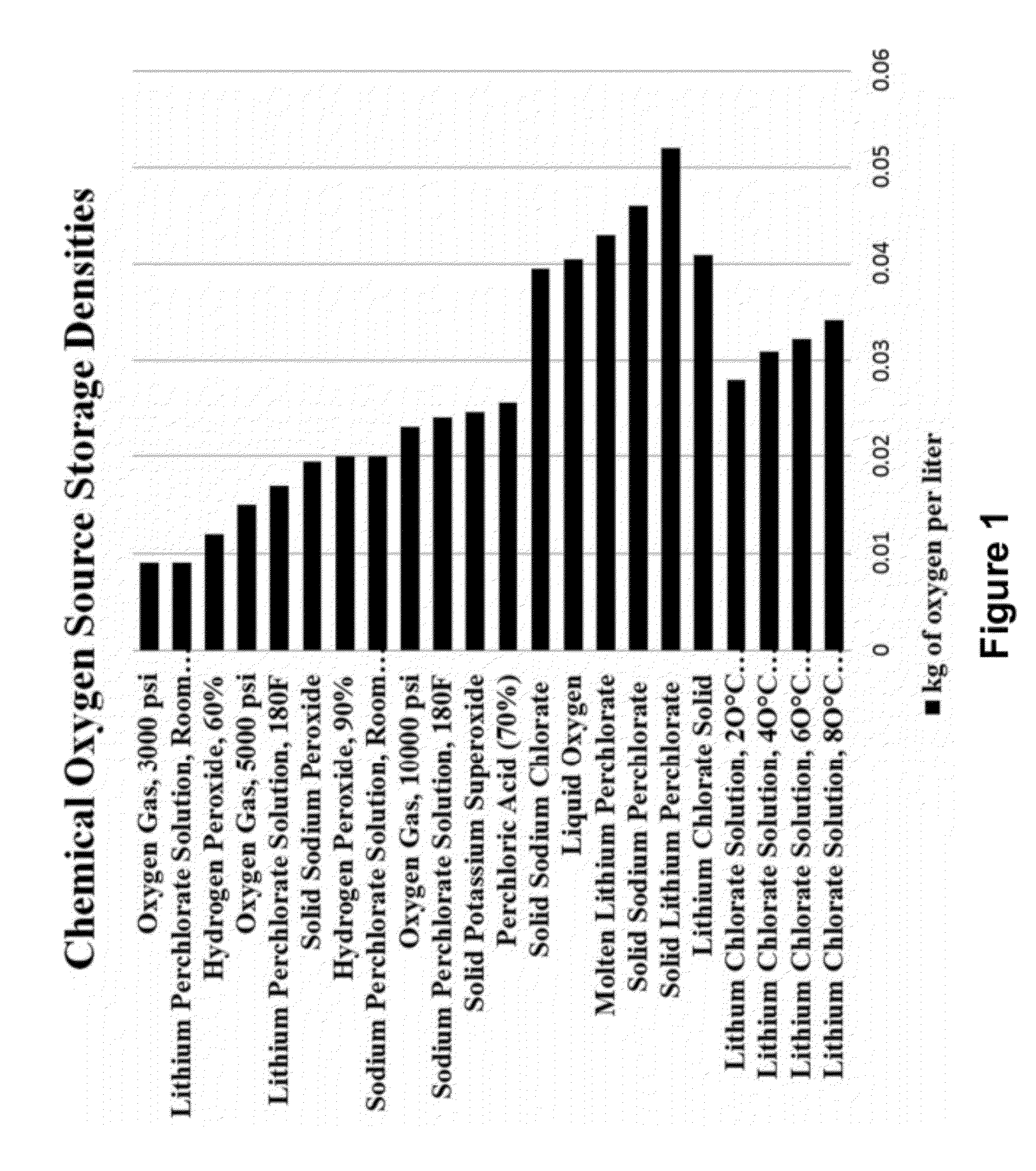
Oxygen-generating liquid composition
InactiveUS20110140038A1Increase contentEffective oxygen generating compoundChloratesOther chemical processesOxygenLiquid composition
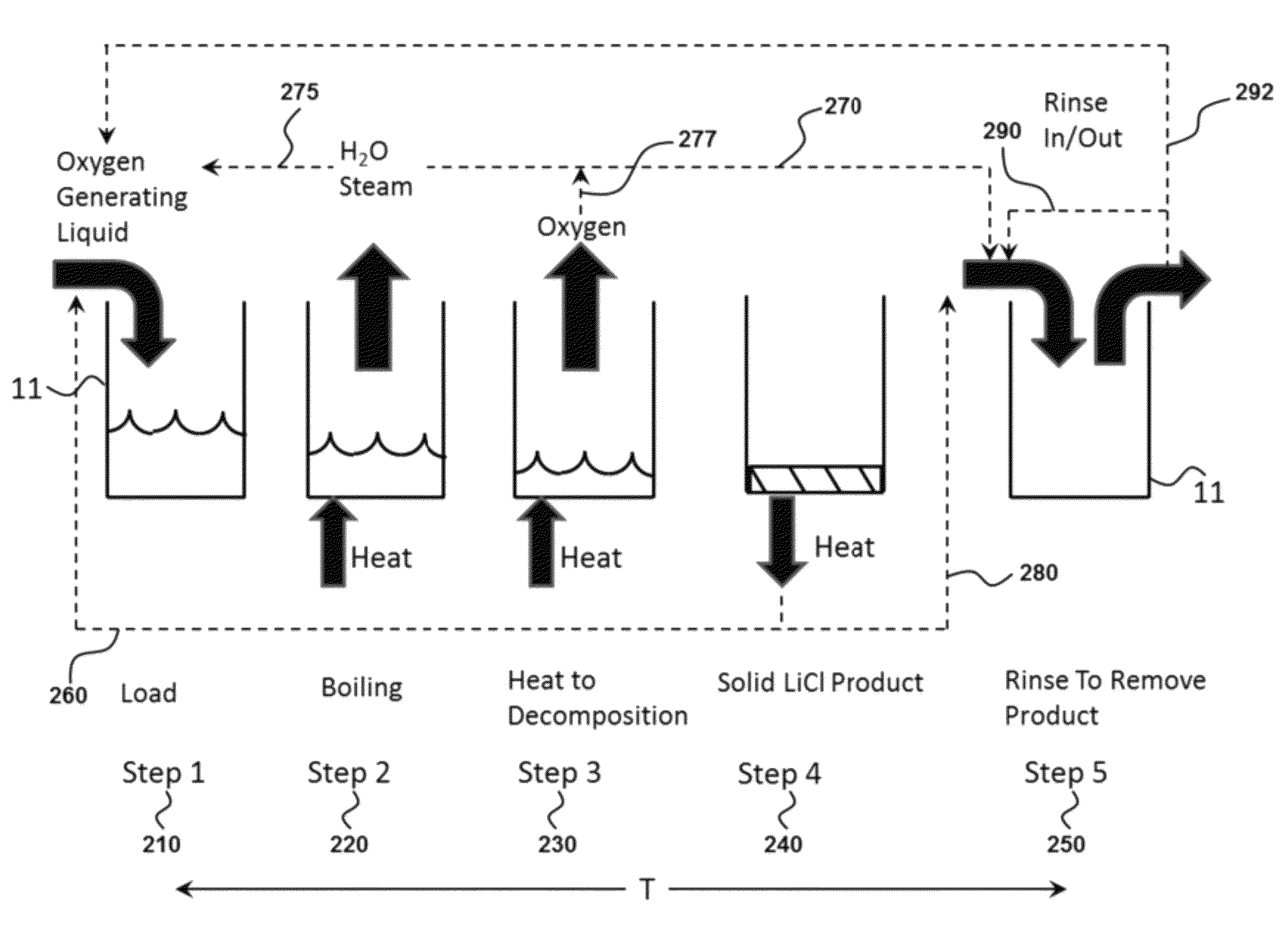
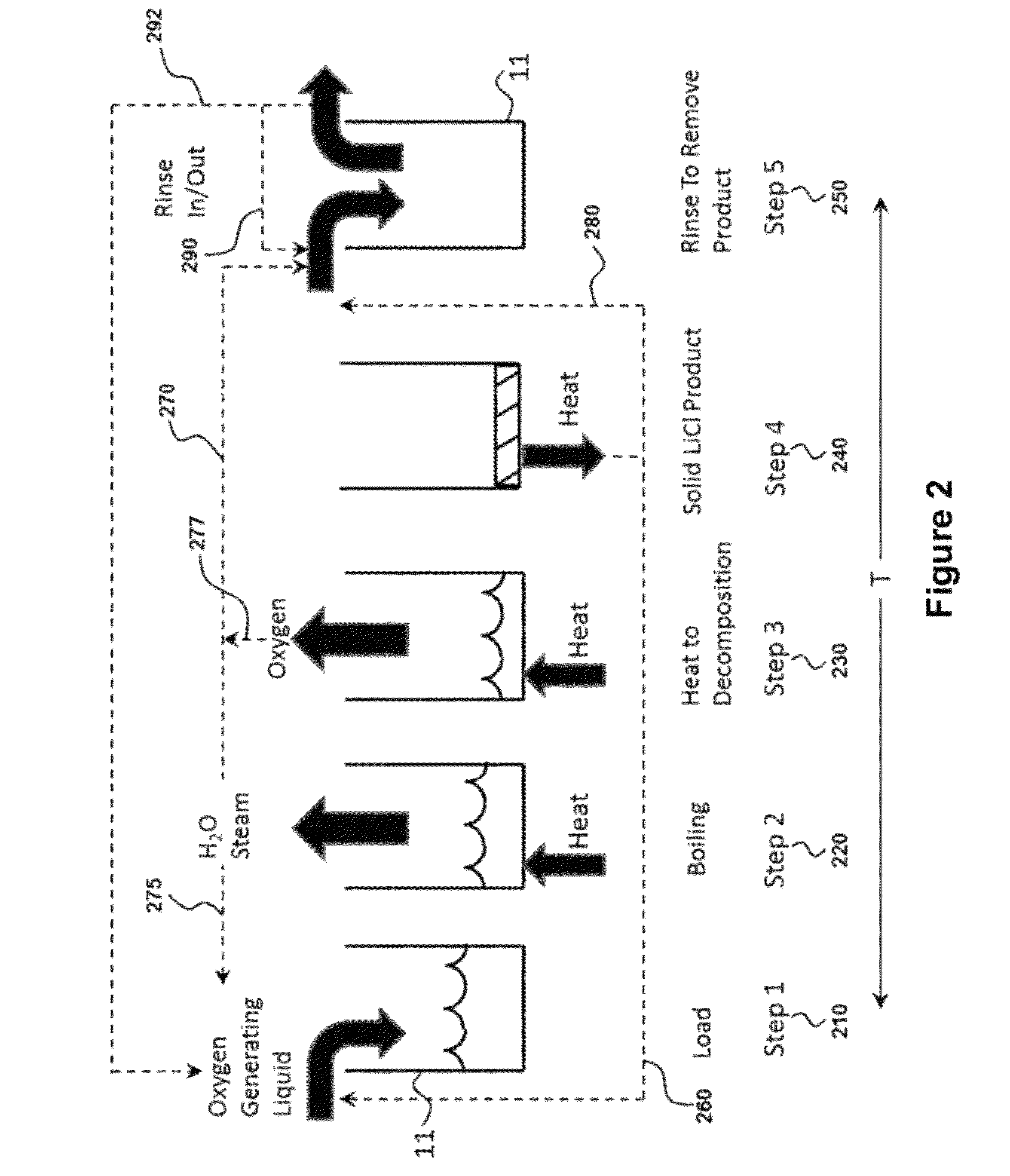
An oxygen-generating liquid composition is described comprising compositions of water plus lithium chlorate as a saturated solution and as a mixture of saturated solution plus precipitated solids. The composition further comprises catalysts. The oxygen is produced via thermal decomposition. Uses of the composition include generation of oxygen for power production or for breathable air. A principal benefit is that the composition is an easily handled liquid stored in un-pressurized tank.
Owner:API ENG
Hypochlorite denture compositions and methods of use
ActiveUS8361942B2Minimizes and eliminates formation of residueDissolve fastInorganic/elemental detergent compounding agentsCosmetic preparationsDicarbonateAlkaline earth metal
Owner:THE CLOROX CO
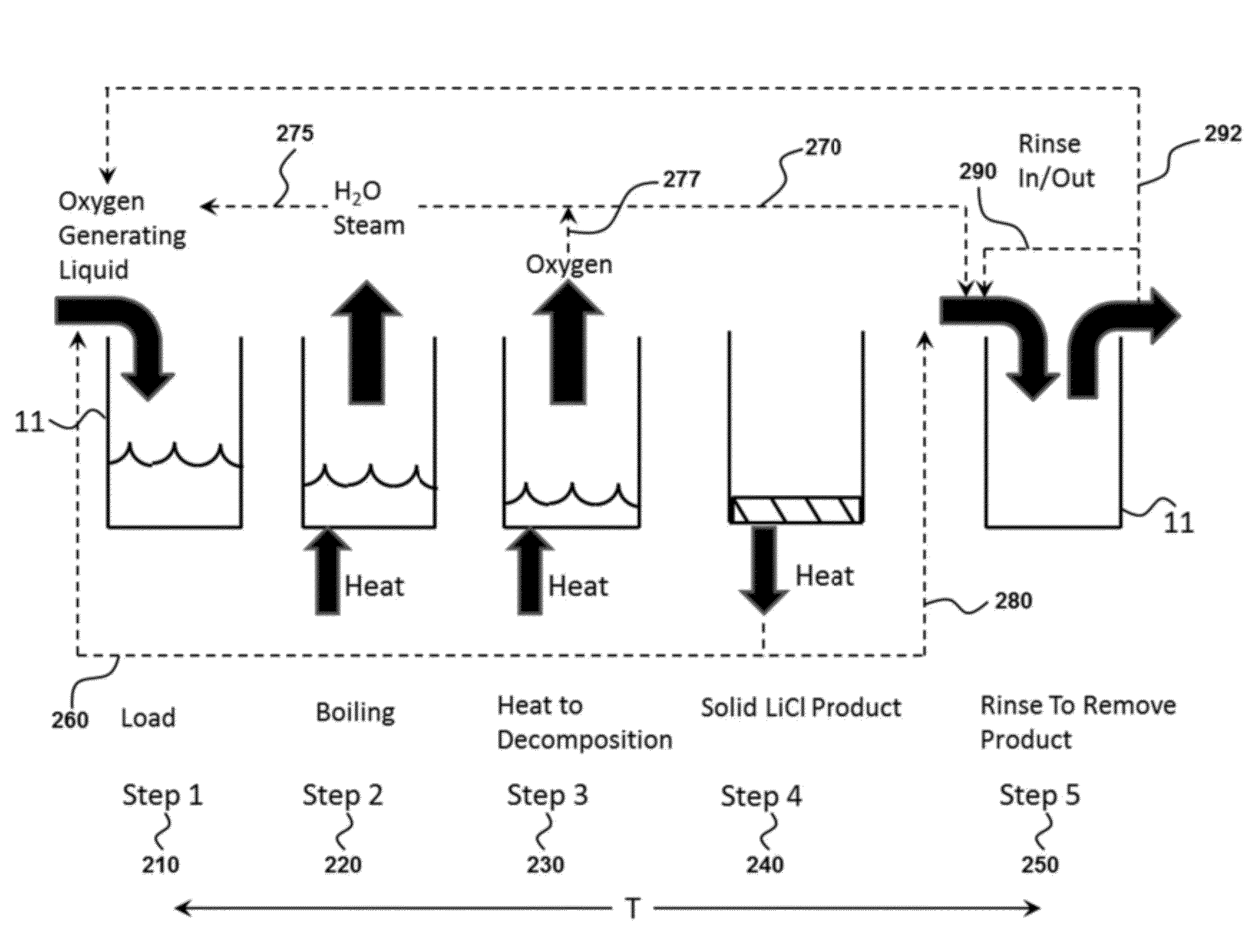
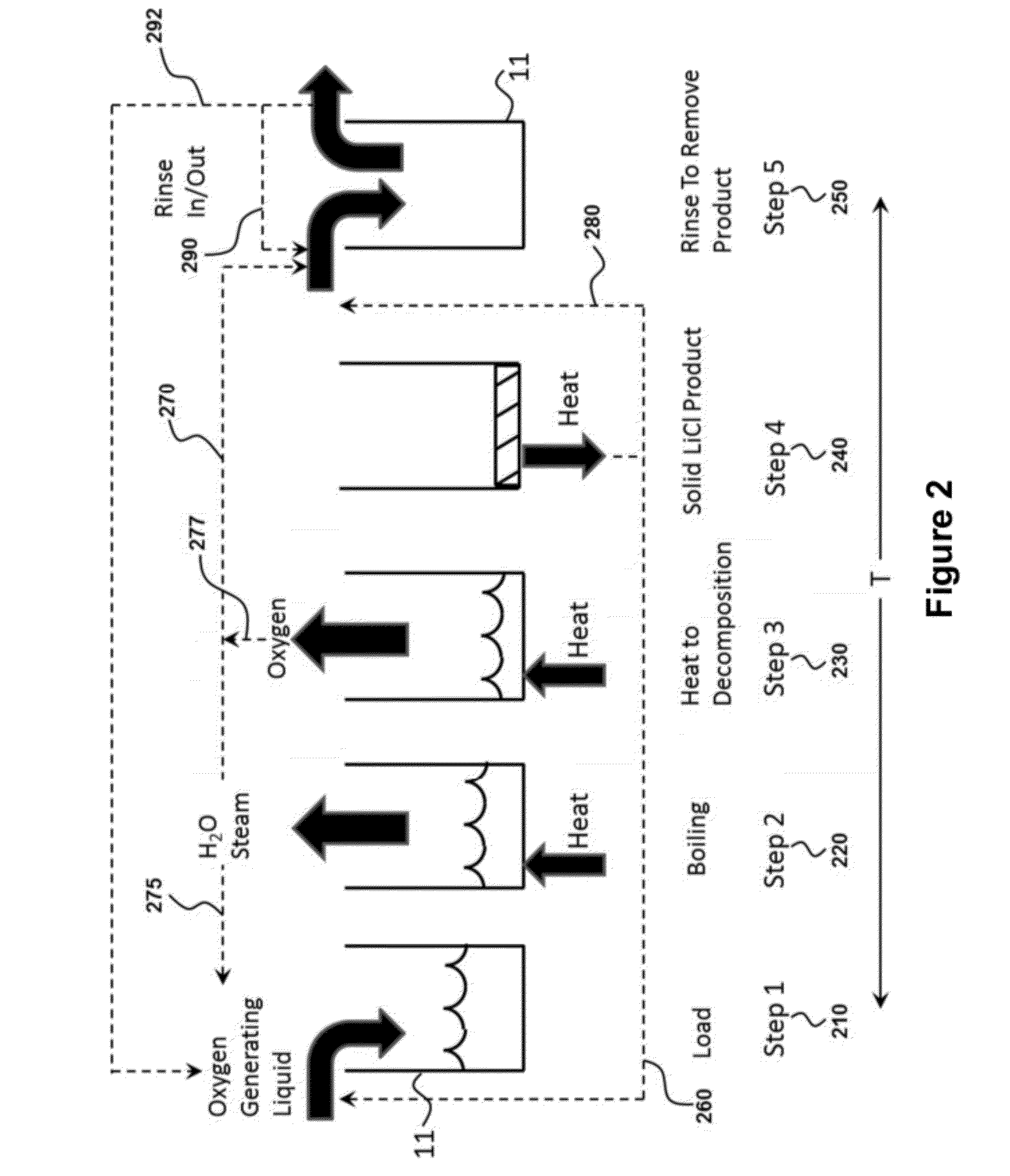
Oxygen storage and generation using an oxygen generating liquid
InactiveUS9090466B2Increase contentEffective oxygen generatingOxygen respiratorsEnergy inputContinuous flowApplication areas
Provided are methods and devices for storing and generating oxygen from a low temperature oxygen generating liquid. The oxygen storage method may use lithium chlorate plus water to store oxygen wherein all solids that may be present enter solution for delivery as a liquid to a reaction vessel. The oxygen production method may be a batch process with steps to heat the liquid, boil out the water, thermally decompose the lithium chlorate and then rinse out the remaining product. The apparatus for oxygen generation may use multiple reaction vessels operating sequentially to produce a continuous flow of oxygen with a rinse step in a separate area from the heat application area to remove end product solid. The device for oxygen storage includes a storage vessel and is configured to heat the oxygen generating liquid using waste heat present in the rinse liquid.
Owner:API ENG
A kind of graphene-based flexible conductive film material and preparation method thereof
ActiveCN105869708BReduce sheet resistanceMeet application needsNon-conductive material with dispersed conductive materialCable/conductor manufacturePolyvinyl alcoholPolyethylene glycol
Owner:深圳市宝康电子材料有限公司
Aqueous lithium ion electrolyte and battery
PendingCN112366367AImprove ionic conductivityLow viscositySecondary cells servicing/maintenanceAqueous electrolytesElectrolytic agentDimethyl Sulphone
The invention discloses an aqueous lithium ion electrolyte, which is characterized in that the electrolyte is formed by compounding dimethyl sulfone, lithium perchlorate, urea and water according to amolar ratio of (0.5-2): (0.5-1.6): (0.5-2): (0.3-2), and is a transparent homogeneous solution obtained by mixing and grinding four raw materials and then placing the mixture in a water bath for ultrasonic treatment. According to the invention, cheap non-flammable organic small molecules, lithium salt and water are creatively adopted to act together to broaden an electrochemical stability window,the prepared electrolyte has high ionic conductivity, low viscosity and good high and low temperature phase stability retentivity, the whole preparation process is simple and easy to implement, the preparation cost is reduced, and the electrolyte is very beneficial to realize industrial production.
Owner:JIANGSU UNIV OF TECH
Lithium iron phosphate long-life battery electrolyte for energy storage
InactiveCN111048832ALong cycle lifeLess side effectsSecondary cellsOrganic electrolytesElectrolytic agentLithium hexafluorophosphate
The invention discloses a lithium iron phosphate long-life battery electrolyte for energy storage. The main components of the electrolyte are as follows: a lithium salt, an organic solvent and a composite synergistic film-forming additive, wherein the lithium salt of the electrolyte is lithium hexafluorophosphate, lithium difluorooxalate borate, lithium perchlorate, lithium tetrafluoroborate and the like, and the organic solvent comprises cyclic carbonate and chain carbonate. On the basis of a conventional negative electrode film-forming additive, a positive electrode CEI film-forming additiveand a reinforced CEI film and SEI film passivation additive are added, so that the SEI film can stably exist under the conditions of normal temperature and high temperature, the reaction between theelectrolyte and a pole piece is reduced, the structures of the positive and negative electrode materials are more stable, and the cycle life under the conditions of normal temperature and high temperature is prolonged. When the electrolyte is applied to a lithium ion battery, the normal-temperature and high-temperature cycle life of the battery can be prolonged, and the national standard electrical performance and safety performance are completely met.
Owner:JIANGXI GANFENG BATTERY TECH
Light-metal-doped straw based carbon electrode material and preparation method thereof
InactiveCN104867695AHigh activityLarge specific surface areaHybrid capacitor electrodesHybrid/EDL manufactureNickel phosphatePhosphoric acid
The invention discloses a light-metal-doped straw based carbon electrode material which is characterized in that the material is prepared by the following parts of raw materials by weight: modified straw composite material 110-120, zeolite powder 2-3, light metal 4-5, ruthenium dioxide 1-2, calcium stearate 1-2, lithium perchlorate 2-3, nickel hypophosphite 1-2, sodium dodecyl sulfate 1-2, binders LA1321-2 and proper amount of deionized water. Carbonization, activation and doped modification are performed on crop straw to prepare high-activity and high specific surface area carbon. The additionally arranged light metal can accelerate charging speed of a capacitor so that time is saved and charging capacity is enhanced. The electrode material has excellent characteristics of high capacity, high multiplying power and long cycle.
Owner:ANHUI JIANGWEI PRECISION IND
All-weather high-rate lithium battery electrolyte and lithium ion battery
ActiveCN113346133AImprove conductivityImprove oxidation resistanceFinal product manufactureSecondary cells servicing/maintenanceElectrolytic agentElectrochemical window
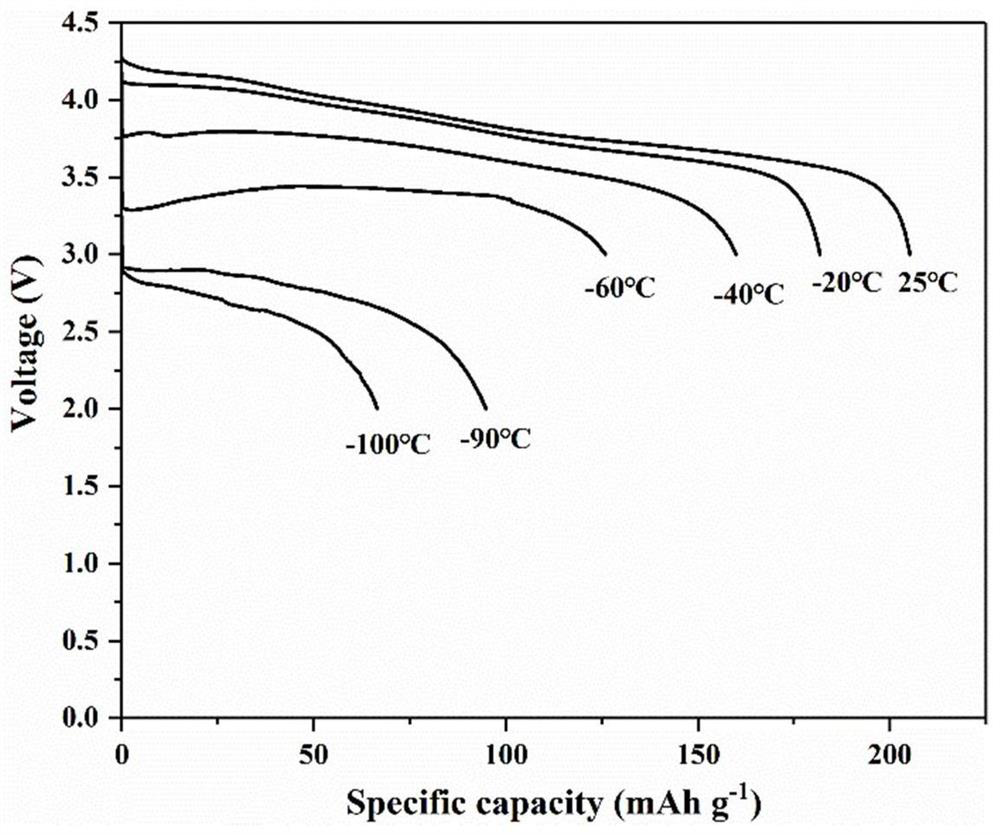
The invention provides an all-weather high-rate lithium battery electrolyte and a lithium ion battery. The lithium battery electrolyte comprises a lithium salt electrolyte, an organic solvent and an additive, wherein the freezing point of the organic solvent is-150--20 DEG C; and the additive is one or more of lithium nitrate, lithium perchlorate, lithium sulfate and lithium carbonate, and the mass content of the additive in the lithium battery electrolyte is 0.1-10%. The electrolyte provided by the invention is low in melting point of each component, small in viscosity and high in conductivity, and still keeps relatively high conductivity at a low temperature of -100 DEG C to -60 DEG C, so that the cycle performance of the ternary lithium battery under an extremely low temperature condition is greatly improved. In addition, the oxidation resistance of the electrolyte can be greatly improved, the electrochemical window of the electrolyte is widened, and a stable SEI film is formed, so that the electrolyte can adapt to efficient operation of a ternary positive electrode material at a high voltage of 4.2-4.5 V.
Owner:新源清材科技(北京)有限公司
Composite gel solid electrolyte, preparation method and all-solid-state lithium ion battery
ActiveCN111430792AImprove electrochemical stabilityMaintain mechanical propertiesFinal product manufactureElectrolyte accumulators manufactureSolid state electrolyteAcetic anhydride
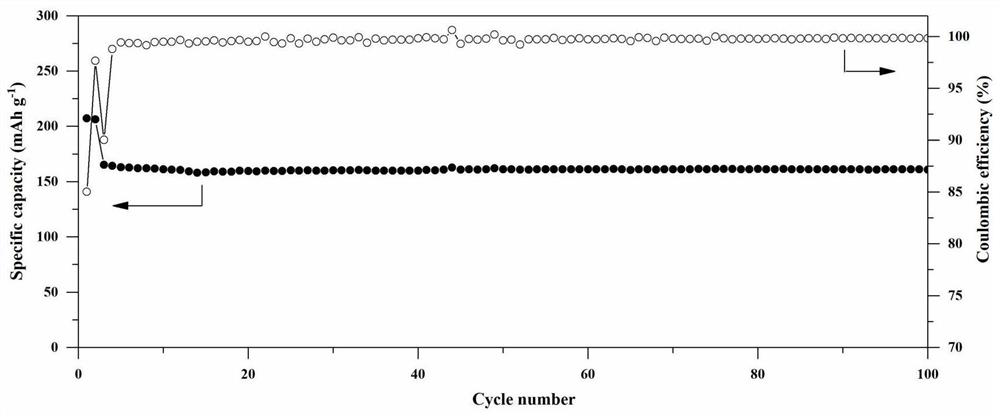
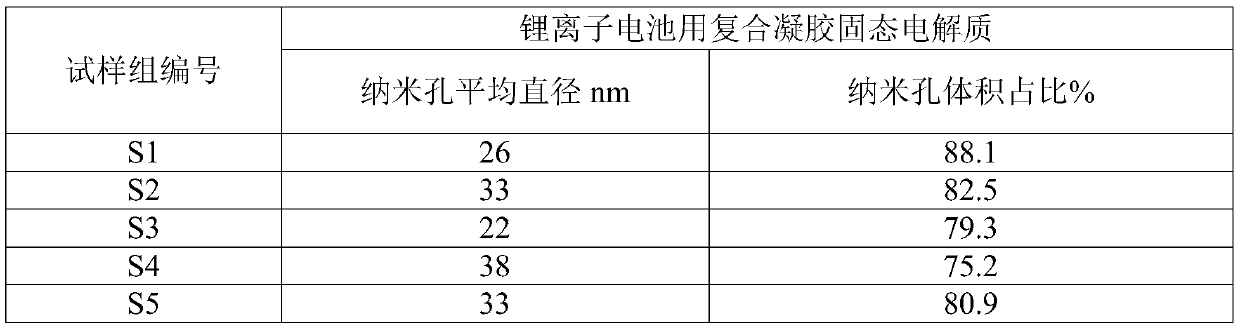
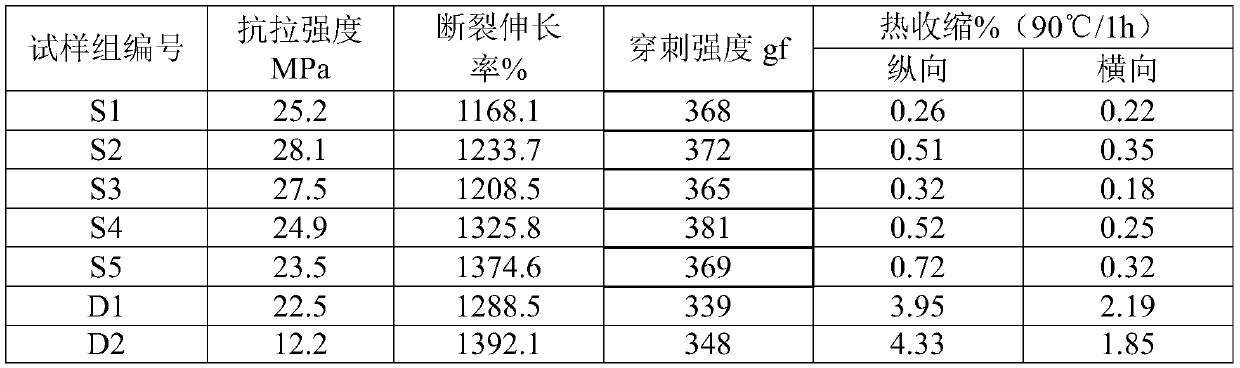
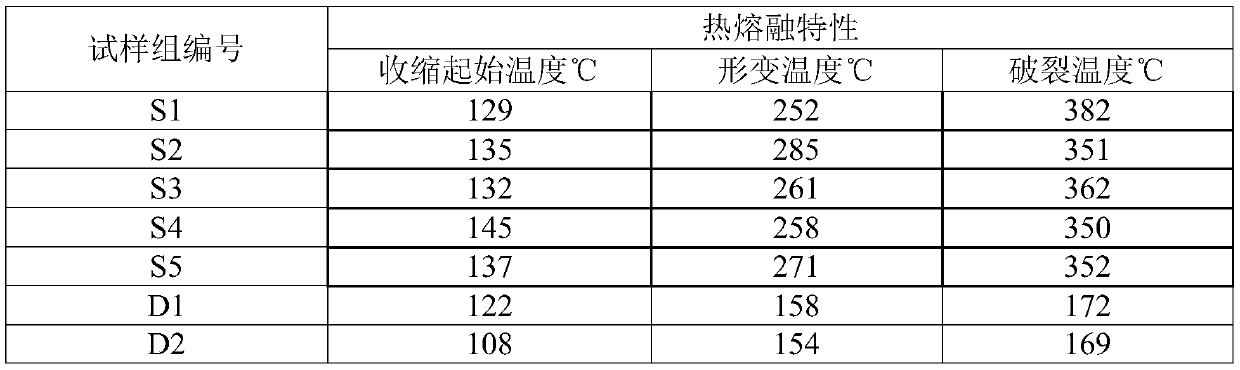
The invention relates to a composite gel solid electrolyte for a lithium ion battery and a preparation method thereof. The composite gel solid electrolyte for the lithium ion battery is prepared by compounding a lithium bis (trifluoromethylsulfonyl) imide composite polyimide gel precursor and a lithium-based gel precursor, wherein the lithium bis (trifluoromethanesulfonyl) imide composite polyimide gel precursor is prepared from the following components: 2, 2 '-dimethyl benzidine, 3, 3', 4, 4 '-biphenyl tetracarboxylic dianhydride, N-methyl-2 pyrrolidone, 3-aminopropyltrihexyloxysilane, lithium bis (trifluoromethanesulfonyl) imide and acetic anhydride; the lithium-based gel precursor is prepared from the following components: lithium perchlorate, glycerol, deionized water, polyacrylic acidand formamide. According to the composite gel solid electrolyte for the lithium ion battery, the longitudinal thermal shrinkage and the transverse thermal shrinkage can be reduced while the mechanical property and the puncture strength are maintained; the initial shrinkage temperature, the deformation temperature and the rupture temperature are effectively increased; meanwhile, the ionic conductivity and the lithium ion transference number are improved.
Owner:深圳市奥能动力科技有限公司
Method for detecting purity of lithium perchlorate
PendingCN112033922AImprove stabilityEasy to observeChemical analysis using titrationColor/spectral properties measurementsIon exchangePerchloric acid
The invention discloses a method for detecting the purity of lithium perchlorate, and belongs to the technical field of chemical analysis. The method for detecting the purity of lithium perchlorate comprises the following steps: injecting a strongly acidic ion exchange column into a lithium perchlorate solution to obtain a first exchange solution; washing the strongly acidic ion exchange column with water until the dropping solution is neutral, and collecting a second exchange solution and a washing solution; adding the second exchange solution and the washing solution into the first exchangesolution to obtain a mixed solution; adding a methyl red indicating liquid into the mixed solution to enable the mixed solution to be red; and measuring the lithium content of the mixed solution on anatomic absorption spectrometer, and obtaining the lithium perchlorate content according to the consumption of the sodium hydroxide standard solution and the molar concentration of the sodium hydroxide standard solution. The method for detecting the purity of the lithium perchlorate has the advantages of few reagents, cost saving, simple and convenient detection process and high stability of detection data.
Owner:百杰瑞(荆门)新材料有限公司
Bionic aqueous electrolyte, preparation method and application in supercapacitor
PendingCN112053857AEasy to prepareEasy to manufactureHybrid capacitor electrolytesHybrid/EDL manufactureElectrolytic agentImide
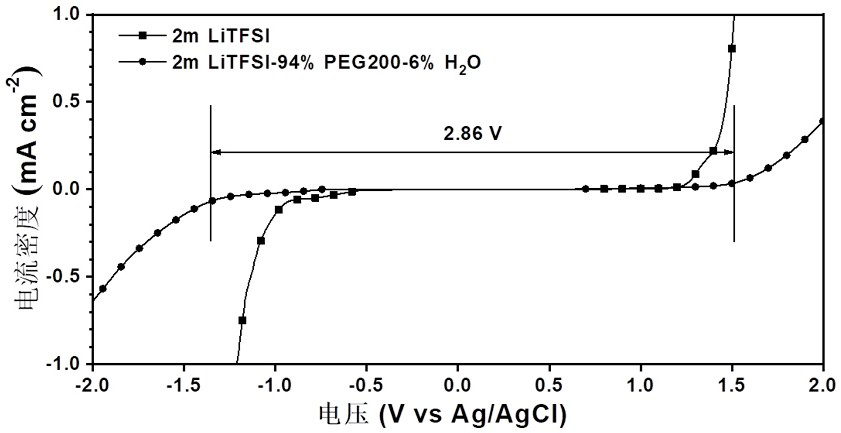
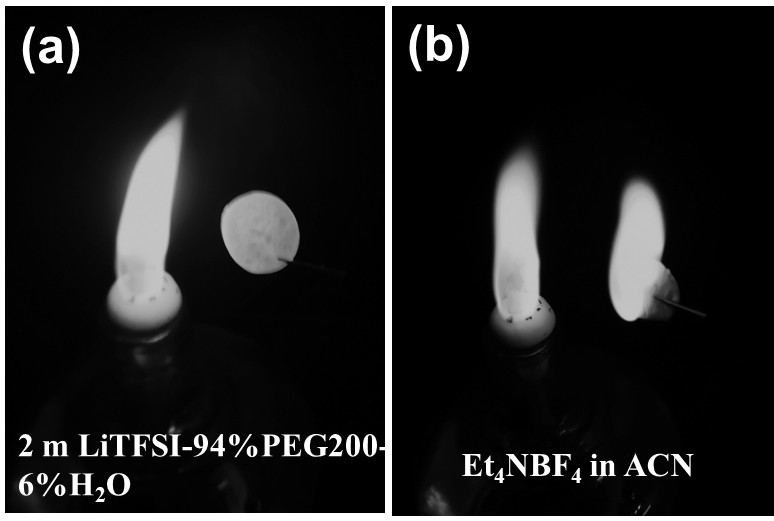
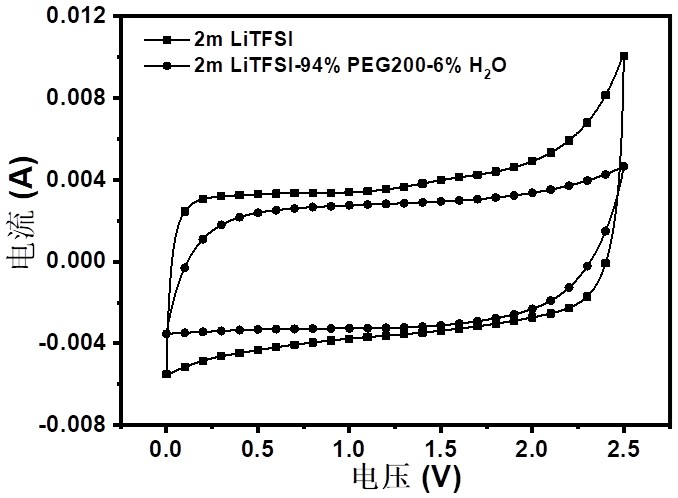
The invention relates to a bionic aqueous electrolyte, a preparation method and application of the bionic aqueous electrolyte in a supercapacitor. The bionic aqueous electrolyte is prepared from an electrolyte aqueous solution and small molecular weight polyethylene glycol(PEG); the electrolyte is one of lithium bis(trifluoromethanesulfonyl) imide, sodium perchlorate, lithium perchlorate or sodiumtrifluoromethanesulfonate; the small molecular weight PEG is one of the number-average molecular weight Mn of 200-600. The bionic aqueous electrolyte provided by the invention has a high electrochemical stability window, and is safe, non-combustible and resistant to high temperature; the preparation method of the bionic aqueous electrolyte is simple, so that the bionic aqueous electrolyte is easyto prepare, and large-scale production is facilitated. The voltage window of the water system super capacitor assembled based on the bionic water system electrolyte is up to 2.5 V, and the water system super capacitor can be safely and effectively applied in a normal-temperature environment and a high-temperature environment and has good capacitance behavior and rate capability.
Owner:NANCHANG UNIV
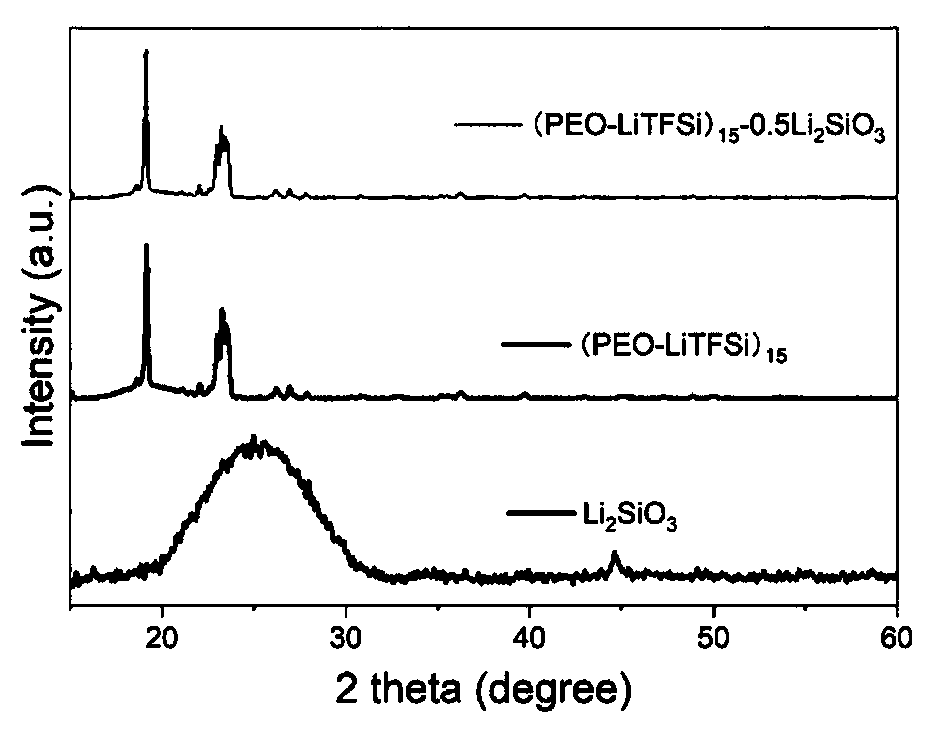
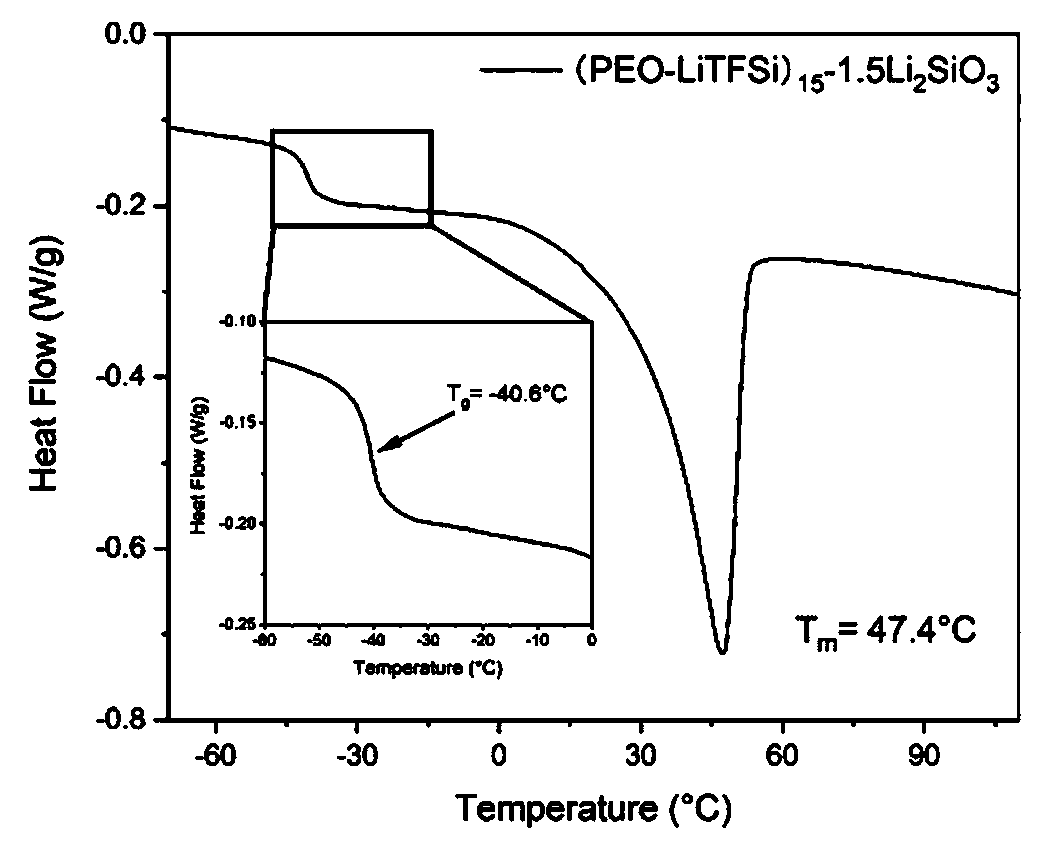
Lithium silicate modified solid polymer electrolyte material and preparation method thereof
PendingCN110783625AImprove electrolyte performanceImprove the overall performance of the electrolyteSolid electrolytesLi-accumulatorsLithium oxideLithium-ion battery
The invention relates to a lithium silicate modified solid polymer electrolyte material and a preparation method thereof. The lithium silicate modified solid polymer electrolyte is prepared by selecting lithium silicate as modified filler of the polymer solid electrolyte, polyepoxyethylene as a matrix and lithium perchlorate or lithium bis(trifluoromethanesulfonyl)imide as a lithium salt through asolution casting method. The introduced lithium silicate is decomposed to form amorphous silicon dioxide and lithium oxide which can be used as the electrolyte modified filler, and thereby great contribution to improvement of performance of the electrolyte is achieved. The modified solid polymer electrolyte prepared by the method has good ionic conductivity, electrochemical stability and thermalstability, is suitable for a lithium ion battery under certain conditions, and can greatly improve safety performance.
Owner:陕西瑞智新能源科技有限公司
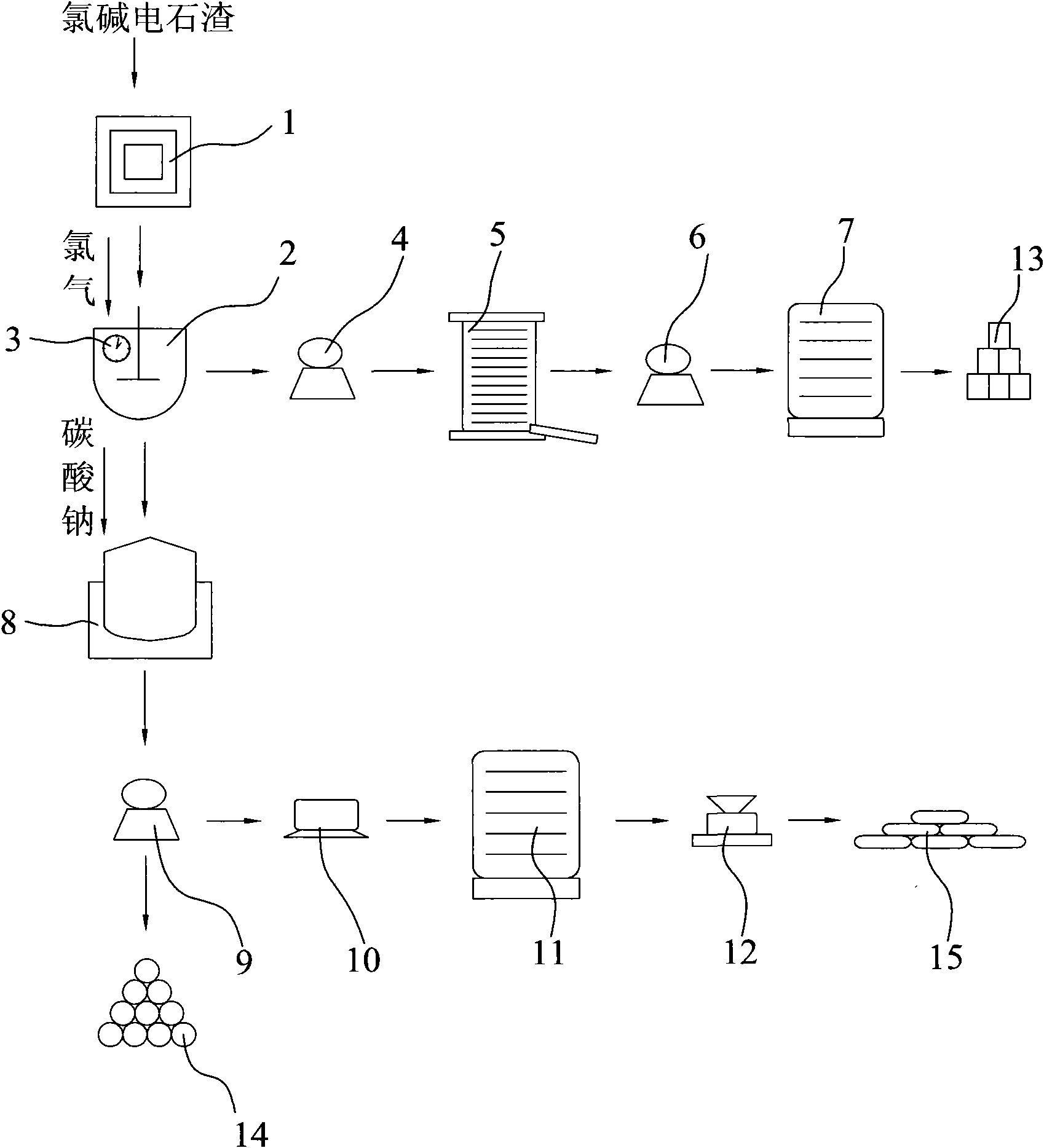
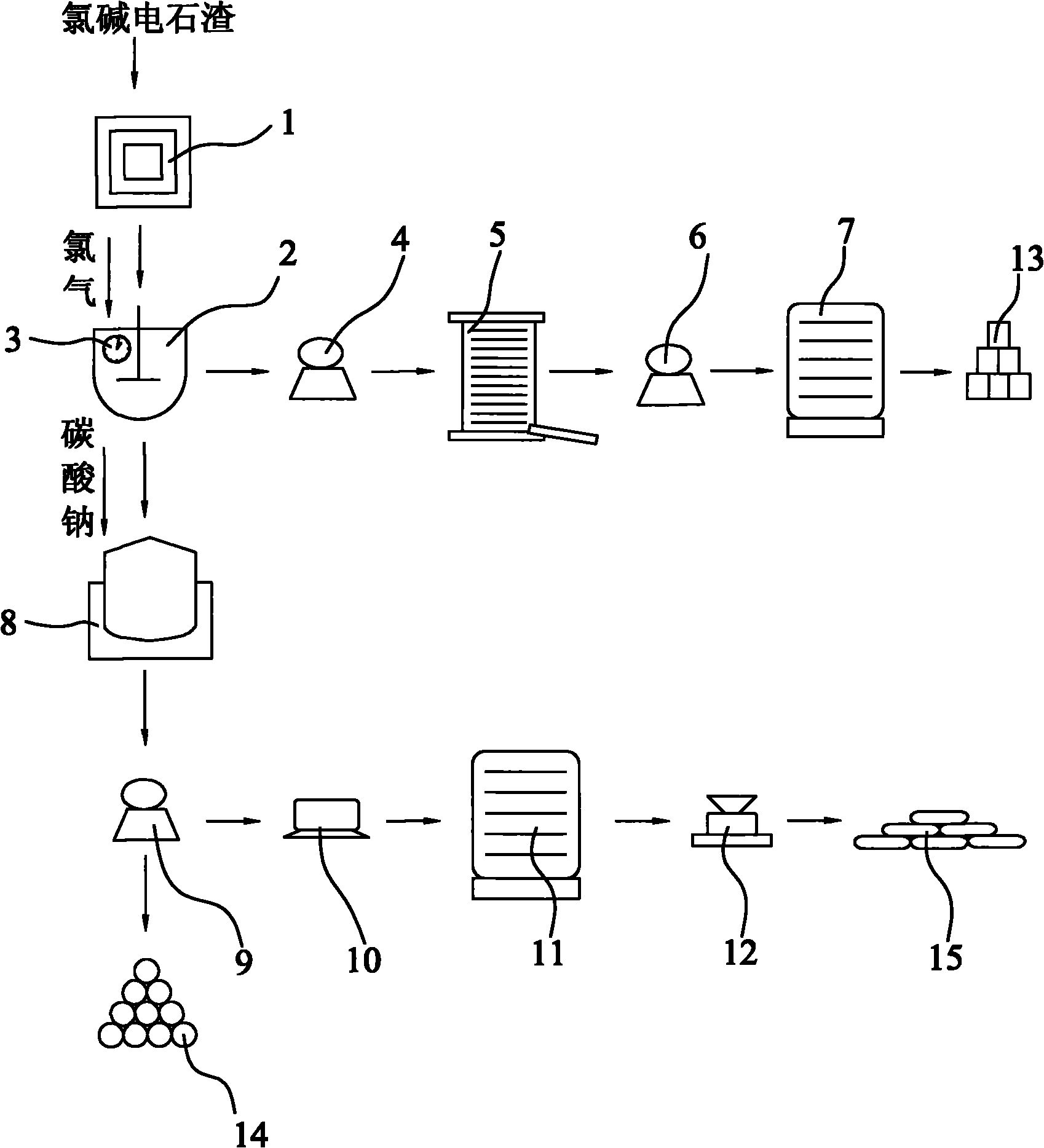
Method for preparation of calcium chlorate and co-production of potassium chlorate and calcium chloride using chlor-alkali calcium carbide slag
InactiveCN102086027AEasy to operateLess investmentChloratesCalcium/strontium/barium fluoridesSlagPotassium
The invention discloses a method for preparation of calcium chlorate and co-production of potassium chlorate and calcium chloride using chlor-alkali calcium carbide slag. In the method, calcium hydrate in the chlor-alkali calcium carbide slag is reacted with chlorine to generate reaction products containing calcium chlorate and calcium chloride; the reaction products are filtered to remove impurities; the obtained filtrate is processed by cooling and settlement, calcium chlorate solid is precipitated, and the mixture is filtered again to obtain calcium chlorate solid and calcium chloride solution; the reaction products containing calcium chlorate and calcium chloride can undergo a metathesis reaction with potassium chloride to generate a mixed solution of potassium chlorate and calcium chloride; the mixed solution is filtered to remove impurities, and distilled at reduced pressure to precipitate potassium chlorate crystal; then the resulting mixed solution is filtered to obtain potassium chlorate crystal and calcium chloride solution; calcium chloride solution is distilled at reduced pressure, and condensed to obtain calcium chloride crystal product.
Owner:汪晋强
Graphene-based flexible conductive film material and preparation method therefor
ActiveCN105869708AReduce sheet resistanceMeet application needsNon-conductive material with dispersed conductive materialCable/conductor manufacturePolyvinyl alcoholPolyethylene glycol
The invention discloses a graphene-based flexible conductive film material and a preparation method therefor. The conductive film material comprises a flexible substrate and a conductive material for covering the flexible substrate, wherein the conductive material is prepared from the following components in parts by weight: graphene oxide, nanometer tantalum powder, polyaniline, polyving akohol, modified alum, formaldehyde modified xylogen, sulfamic acid, trimethylamine-borane, nanometer active calcium carbonate, methylcellulose, polyethylene glycol, ammonium polyvanadate, lithium chlorate, dimethyl formamide, 0.5mol / L nitric acid and water. The square resistance of the conductive film prepared by the invention is relatively low, which is less than 10<omega> / m; the conductivity can reach greater than 525.4S / cm; after the conductive film is subjected to 200 times of bending, the retention rate of the conductivity of the conductive film can be still greater than 90.2%; and in addition, the graphene-based flexible conductive film material presents high conductivity and bending resistance, so that the conductive film material can satisfy the application requirements in the electronic field.
Owner:深圳市宝康电子材料有限公司
Oxygen Storage and Generation Using an Oxygen Generating Liquid
InactiveUS20120308475A1High oxygen contentEffective oxygen generatingEnergy inputOxygen respiratorsContinuous flowApplication areas
Provided are methods and devices for storing and generating oxygen from a low temperature oxygen generating liquid. The oxygen storage method may use lithium chlorate plus water to store oxygen wherein all solids that may be present enter solution for delivery as a liquid to a reaction vessel. The oxygen production method may be a batch process with steps to heat the liquid, boil out the water, thermally decompose the lithium chlorate and then rinse out the remaining product. The apparatus for oxygen generation may use multiple reaction vessels operating sequentially to produce a continuous flow of oxygen with a rinse step in a separate area from the heat application area to remove end product solid. The device for oxygen storage includes a storage vessel and is configured to heat the oxygen generating liquid using waste heat present in the rinse liquid.
Owner:API ENG
Ionic liquid doped water-soluble polythiophene composite film as well as preparation method and application thereof
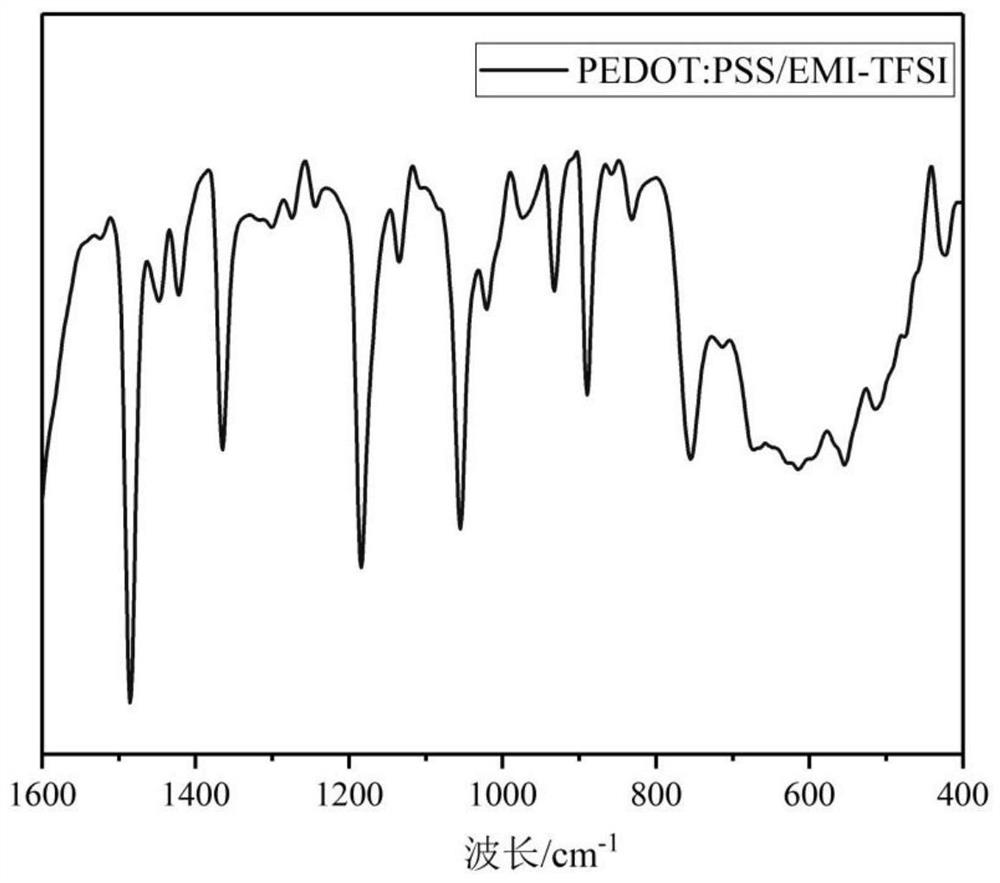
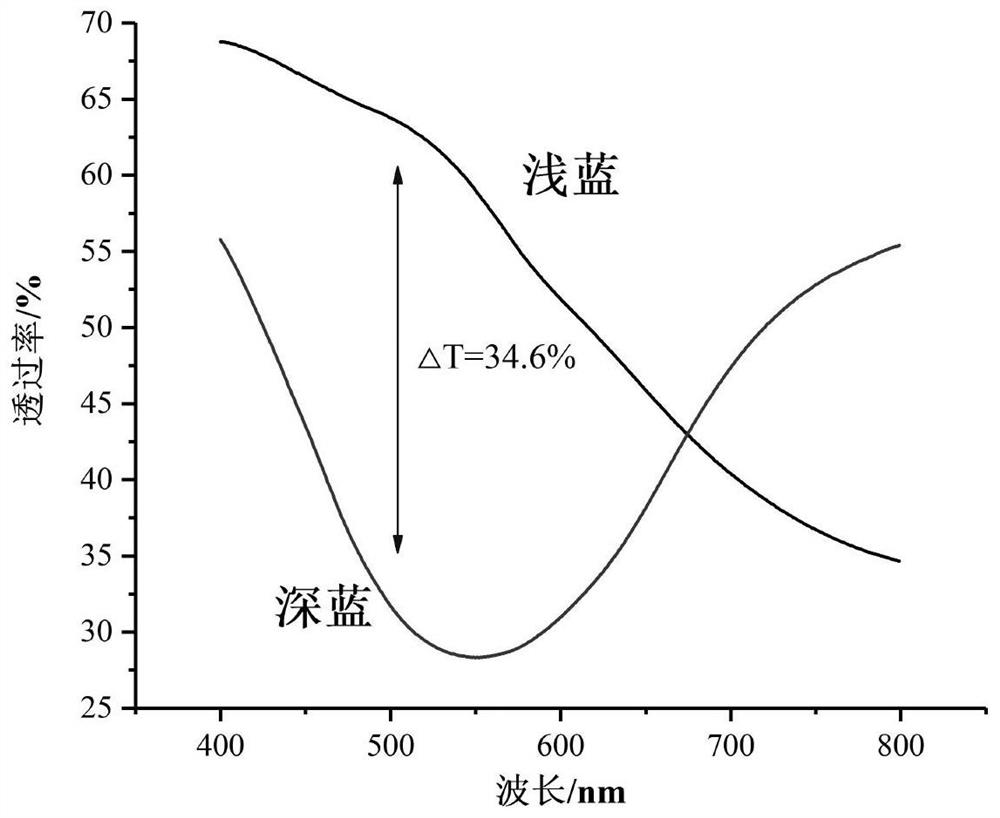
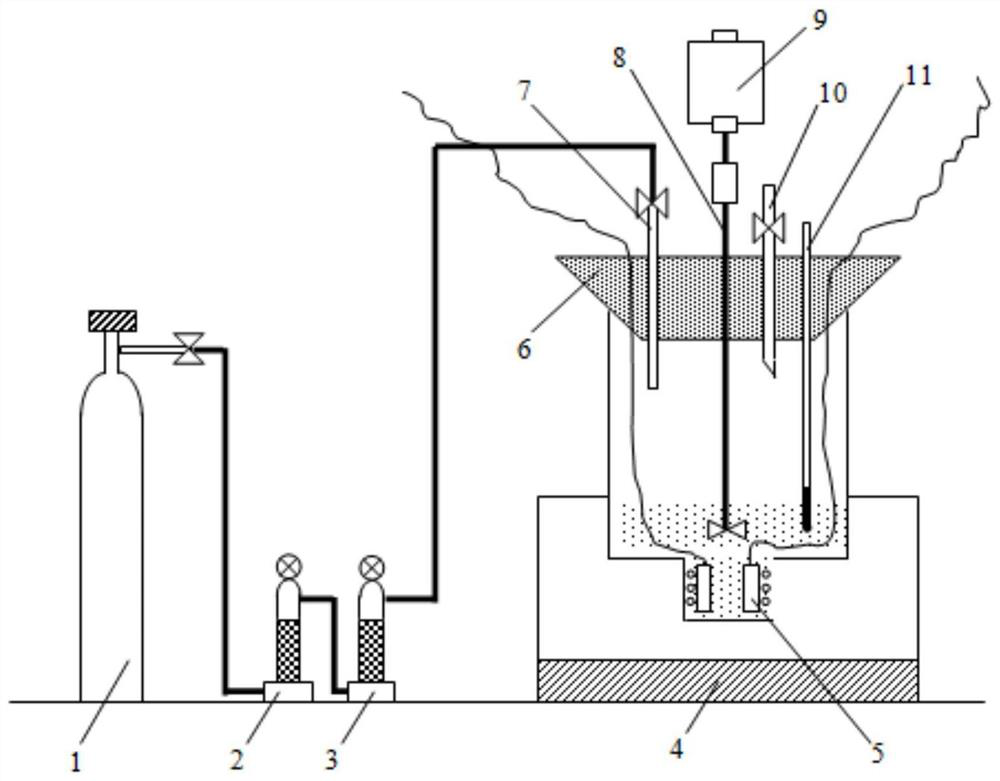
ActiveCN113737241AEvenly dispersedIncreased free-moving ionsElectrolytic organic material coatingComposite filmPolystyrene
The invention provides an ionic liquid doped water-soluble polythiophene composite film as well as a preparation method and application thereof, and belongs to the technical field of electrochromism. The preparation method comprises the following steps of doping sodium polystyrenesulfonate (PSS) into an aqueous solution containing 3, 4-ethylenedioxythiophene (EDOT), lithium perchlorate (LiClO4) and sodium dodecyl benzene sulfonate (SDBS) to obtain a deposition solution containing a large amount of uniformly dispersed anion water to replace a traditional organic solvent-based deposition solution; meanwhile, adding the ionic liquid to promote uniform dispersion of EDOT in the aqueous solution, and increasing free moving ions in the solution; and performing electrochemical deposition by using an electrochemical workstation to obtain the ionic liquid doped water-soluble polythiophene composite film. According to the preparation method, an in-situ electrochemical polymerization method is adopted, water-soluble deposition preparation of PEDOT is achieved through secondary doping, and the obtained film has high electrochromic performance and also has high cycling stability and electrochemical activity.
Owner:HUNAN UNIV OF TECH
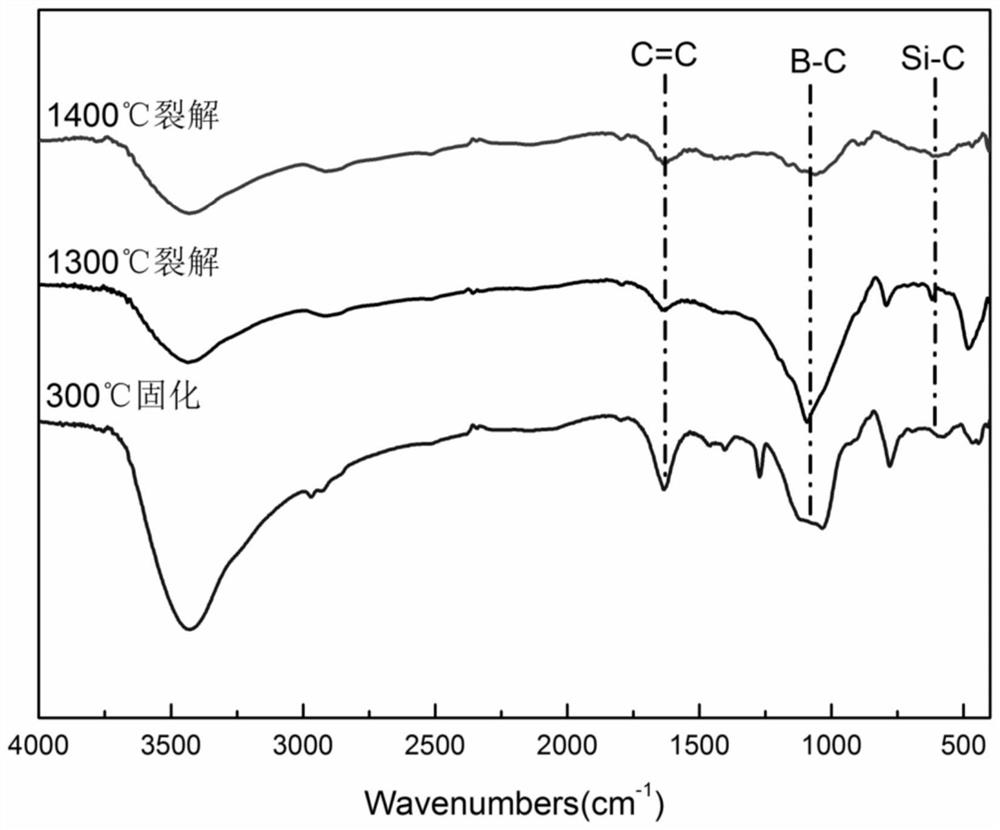
Method for electrochemically preparing boron-containing polysilane
ActiveCN109680297BLow priceHigh ceramic conversion rateElectrolysis componentsElectrolytic organic productionElectrochemical responseMetallic materials
The invention discloses a method for electrochemically preparing boron-containing polysilane, which belongs to the field of inorganic non-metallic materials. Under the condition of room temperature and inert gas protection, trihalosilane, allyl chloride and sodium borohydride are used as raw materials, anhydrous tetrahydrofuran is used as solvent, lithium perchlorate is used as electrolyte, and metal magnesium blocks are used as electrodes. The electrochemical reaction is carried out under the field to obtain boron-containing polysilane. The reaction conditions of the method are mild, safe and controllable, the raw material price is low, and the prepared precursor has a high ceramic conversion rate, which can be used to prepare silicon carbide-boron carbide or silicon-boron-carbon-nitrogen high-temperature ceramics, and is used in the preparation of high-performance high-temperature ceramics The field of materials has broad application prospects.
Owner:SHANGHAI UNIV
Method for preparing lithium hypochlorite through transformation of sodium hypochlorite
The invention discloses a method for preparing lithium hypochlorite through transformation of sodium hypochlorite. The method for preparing lithium hypochlorite through transformation of sodium hypochlorite comprises the following steps: step A, slowly adding hydrochloric acid into a flask filled with tert-butyl alcohol and sodium hypochlorite, then standing for liquid separation, taking a lower-layer water phase as a sodium hypochlorite recovery liquid, and stirring and washing an upper-layer yellow oily matter with pure water once to obtain tert-butyl hypochlorite and a stirring and washingliquid; and B, adding tert-butyl hypochlorite obtained in the step A into pure water to react with a lithium source, stopping the reaction when an upper organic phase becomes colorless, and separatingliquid to obtain a lower water phase which is a lithium hypochlorite solution and an upper organic phase which is tert-butyl alcohol and can be directly recycled. According to the method for preparing lithium hypochlorite through transformation of sodium hypochlorite, cheap sodium hypochlorite is selected to replace conventional highly toxic gas chlorine to prepare lithium hypochlorite, and the economic benefits and safety of the method are improved. Lithium hypochlorite dehydration adopts freeze drying, decomposition of lithium hypochlorite is avoided, and available chlorine of the product is improved.
Owner:GANFENG LITHIUM CO LTD
Preparing method of ozone heterogeneous oxidized solid catalyst
InactiveCN107088411AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionCerium
The invention relates to a preparing method of an ozone heterogeneous oxidized solid catalyst, and belongs to the technical field of environment-friendly and chemical catalysts. The preparing method comprises the steps of after using gamma-aluminium oxide, barite, brucite, serpentine, fly ash and coal gangue as carriers to expand pore sizes through lithium chlorate and beryllium acetylacetonate, adding a surfactant dimethyl chloride dioctadecyl to conduct activating treatment under the action of ultrasonic waves, then making the carriers conduct a hydrothermal reaction with a composite mineralizer borax and potassium sulfate, a catalytic activity active auxiliary tetrakis(2,2,6,6-tetramethyl-3,5-heptanedionato)cerium(IV), tris[4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedionato-O,O']europium, tris(2,2,6,6-tetramethyl-3,5-heptanedionato)gadolinium and tris[N,N-bis(trimethylsilyl)amide]erbium, catalytic activity center precursor lysine manganese, ammonium zirconium carbonate, gold potassium chloride and dichlorodiamminoplatinum in a hydrothermal reaction kettle under the action of an emulsion oleamide propyl trimethyl methyl sulfate ammonium, and after conducting drying to remove moisture, conducting calcination in a muffle furnace to obtain the ozone heterogeneous oxidized solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Recycling phase change material
InactiveCN107177347APhase change energy storage effect is goodGood effectHeat-exchange elementsSodium acetatePotassium fluoride
The invention discloses a phase change energy storage material capable of recycling, which comprises calcium dichloride, potassium fluoride, manganese nitrate, magnesium iodide, sodium sulfate, sodium acetate, lithium chlorate, sodium carbonate, Water, the proportion of each component of the recyclable phase change energy storage material is: in parts by weight, 5-10 parts of calcium dichloride, 8-12 parts of potassium fluoride, 13-17 parts of manganese nitrate, Magnesium iodide 11-15 parts, sodium sulfate 7-12 parts, sodium acetate 3-7 parts, lithium chlorate 10-15 parts, sodium carbonate 4-8 parts, water 30-35 parts. Through the above method, the present invention can achieve a very good phase change energy storage effect through the compound use of several inorganic substances, the material can be recycled, and it still has a very good effect after multiple cycles, saving the cost of use. Energy is saved.
Owner:PIONEER ENERGY JIANGSU
Chemical carbon dioxide gas generator
A chemical carbon dioxide gas generator comprising:a charge housing;a carbon dioxide gas penetrable charge, contained in the said housing, the charge comprisinga) 40-60 wt. % of a substance which upon decomposition generates carbon dioxide, which substance is selected from the group of magnesium carbonate, other carbonates, magnesium oxalate and other oxalates,b) 20-50 wt. % of an oxidiser selected from the group of sodium chlorate, potassium chlorate, lithium chlorate, other metal chlorates, sodium perchlorate, potassium perchlorate, lithium perchlorate, and other metal perchlorates,c) 1-20 wt. % of carbon or another fuel,d) 1-10 wt. % binder,said components a), b), c) and d) together forming 90-100 wt. % of the total weight of the charge;an ignition device for igniting the charge;a carbon dioxide gas treatment unit for reducing the content of one or more side-products—which may have been formed by the charge—in the generated carbon dioxide, and / or for cooling carbon dioxide gas generated by the charge; andan outlet for carbon dioxide gas generated by the charge.
Owner:NEDERLANDSE ORG VOOR TOEGEPAST-NATUURWETENSCHAPPELIJK ONDERZOEK (TNO)
Low-concentration lithium salt electrolyte and lithium secondary battery containing same
ActiveCN109935908BComplete structureProtect structural stabilityLi-accumulatorsSecondary cells servicing/maintenanceDifluorophosphateElectrolytic agent
The invention provides an electrolytic solution containing low-concentration lithium salt, and a lithium secondary battery containing the electrolytic solution. The electrolyte comprises lithium salt (I), lithium salt (II) and a solvent, and the lithium salt (I) is selected from the group consisting of lithium bisoxalate borate, lithium tetrafluorooxalate phosphate, lithium difluorophosphate, 2-trifluoroform One or more of base-4,5-dicyanoimidazolium lithium, lithium difluorooxalate borate, lithium chlorotrifluoroborate and lithium trioxalate phosphate, the lithium salt (II) is selected from lithium hexafluorophosphate, hexafluoro Lithium arsenate, lithium tetrafluoroborate, lithium perchlorate, LiCF 3 SO 3 and LiN(C x f 2x+1 SO 2 )(C y f 2y+1 SO 2 ), wherein x and y are each independently an integer of 0-5, wherein the total concentration of the lithium salt (I) and lithium salt (II) is 0.3-0.6 mol / L.
Owner:华彩(合肥)新能源科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com