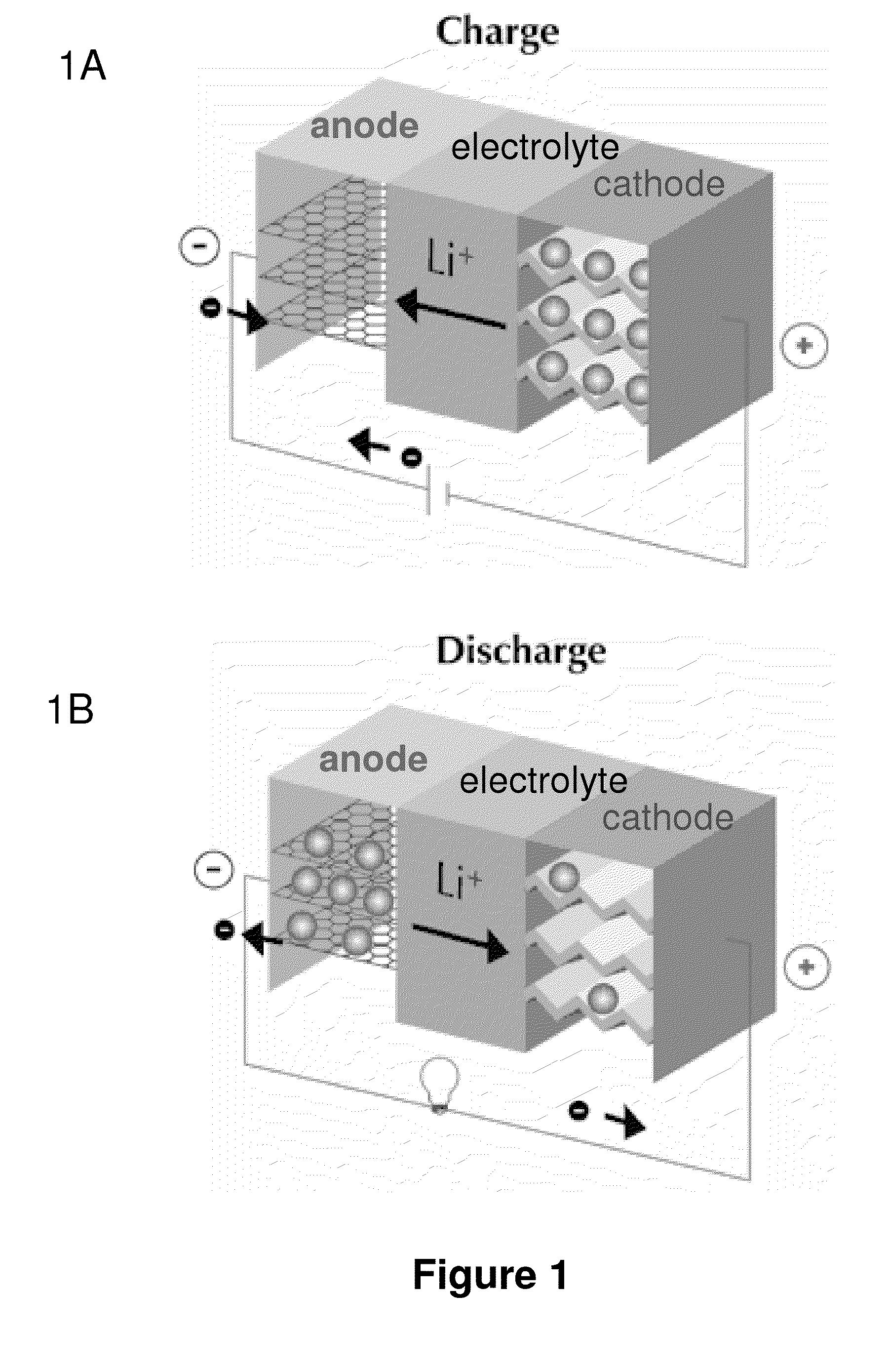
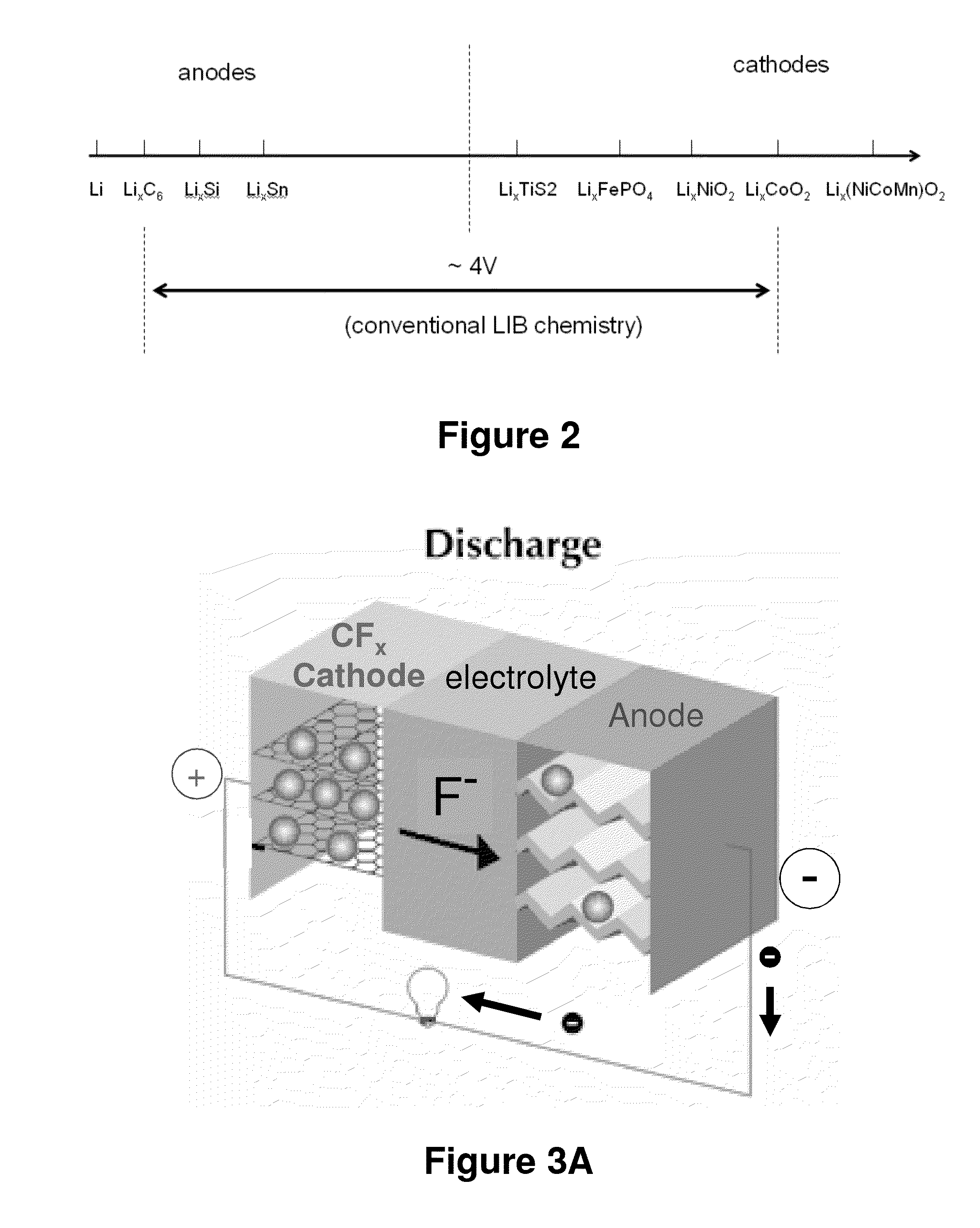
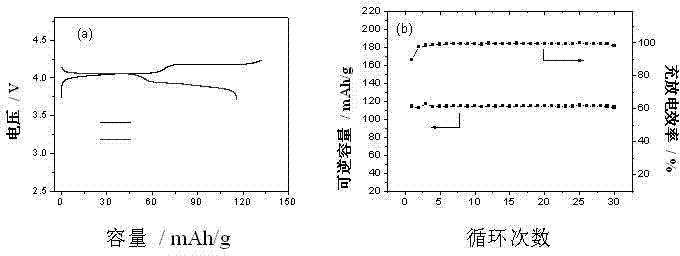
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
477results about "Aqueous electrolytes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
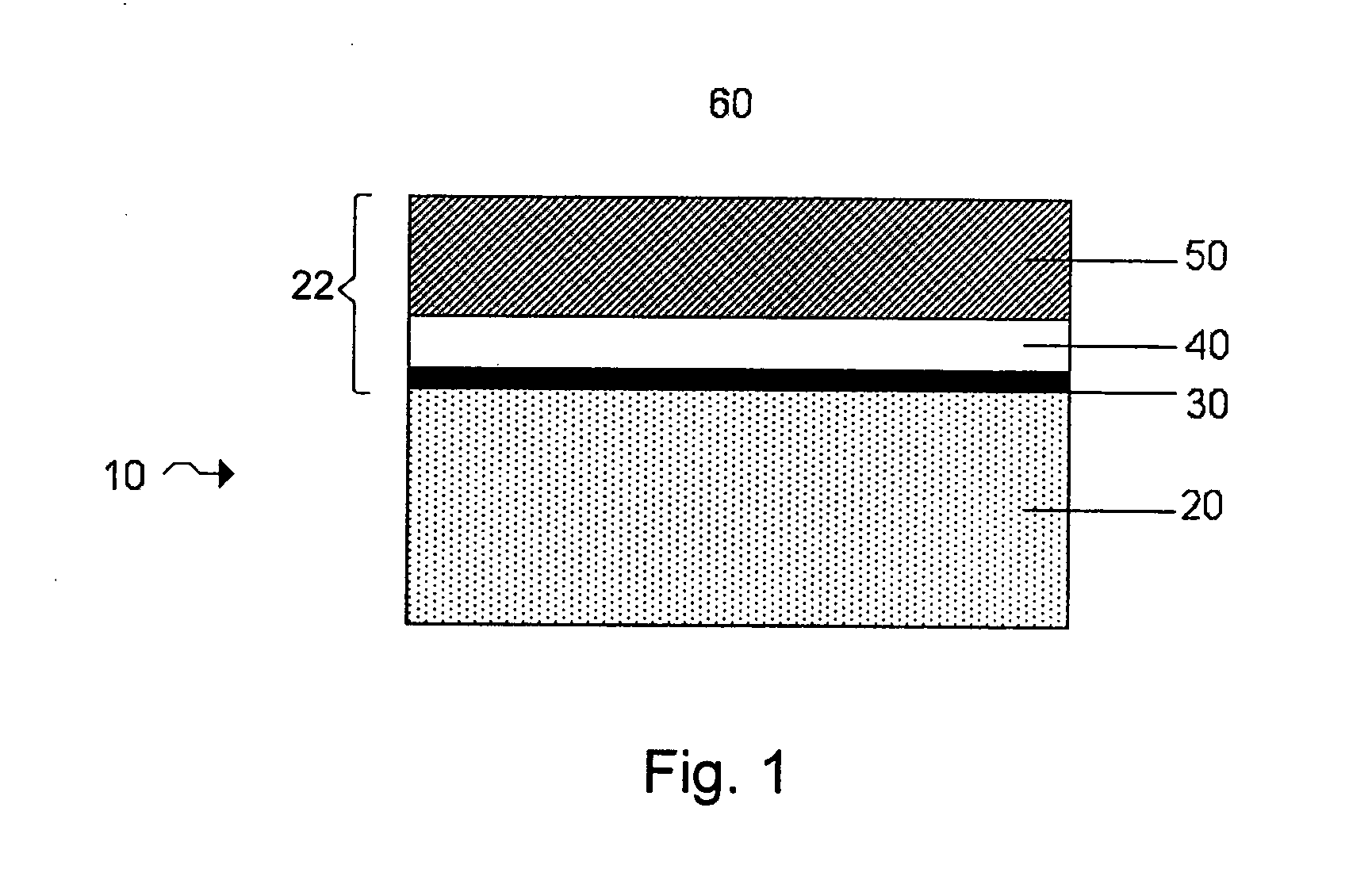
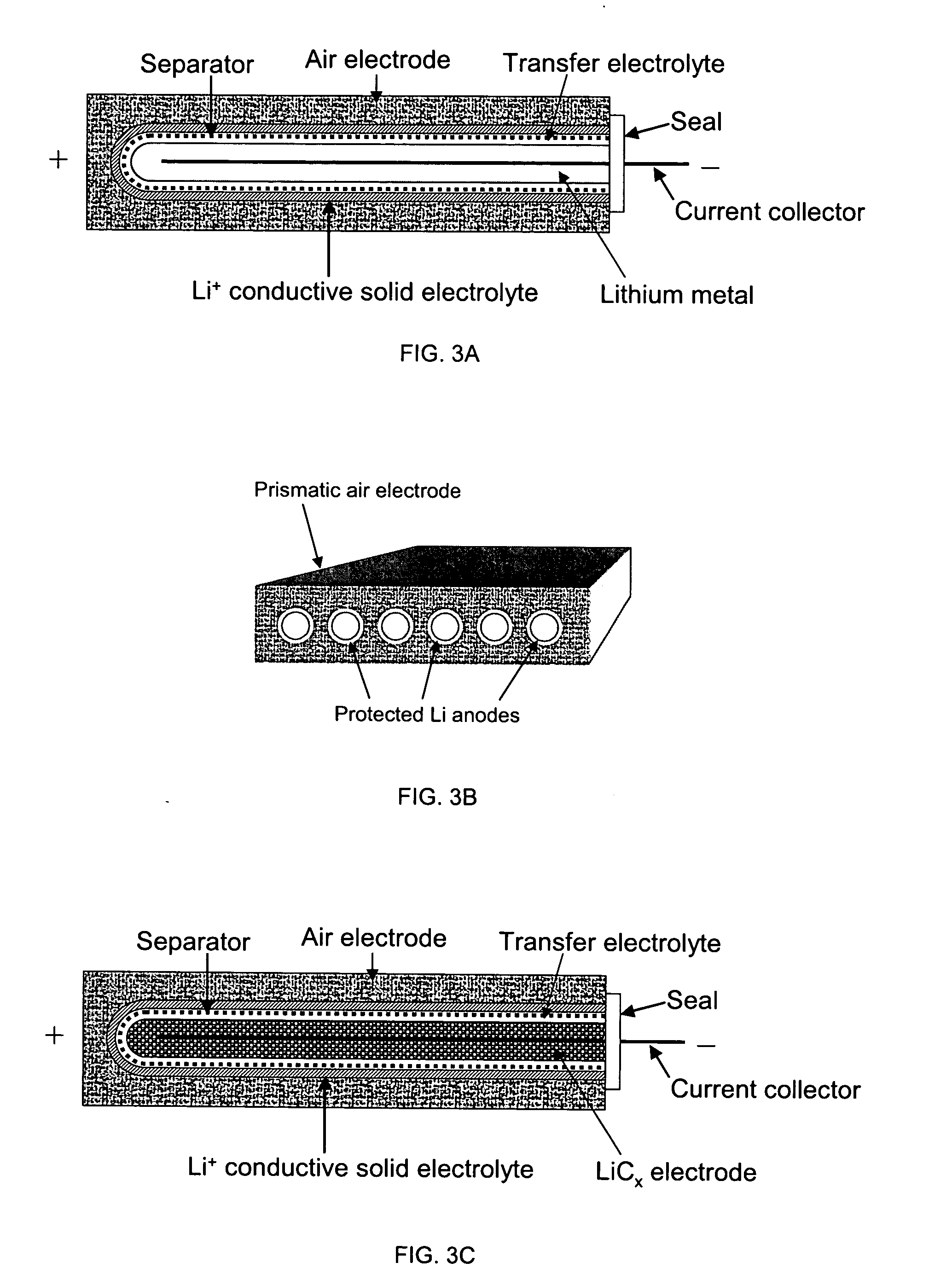
Protected active metal electrode and battery cell structures with non-aqueous interlayer architecture
ActiveUS7282295B2Avoid harmful reactionsFinal product manufactureElectrode carriers/collectorsMetal electrodesBattery cell
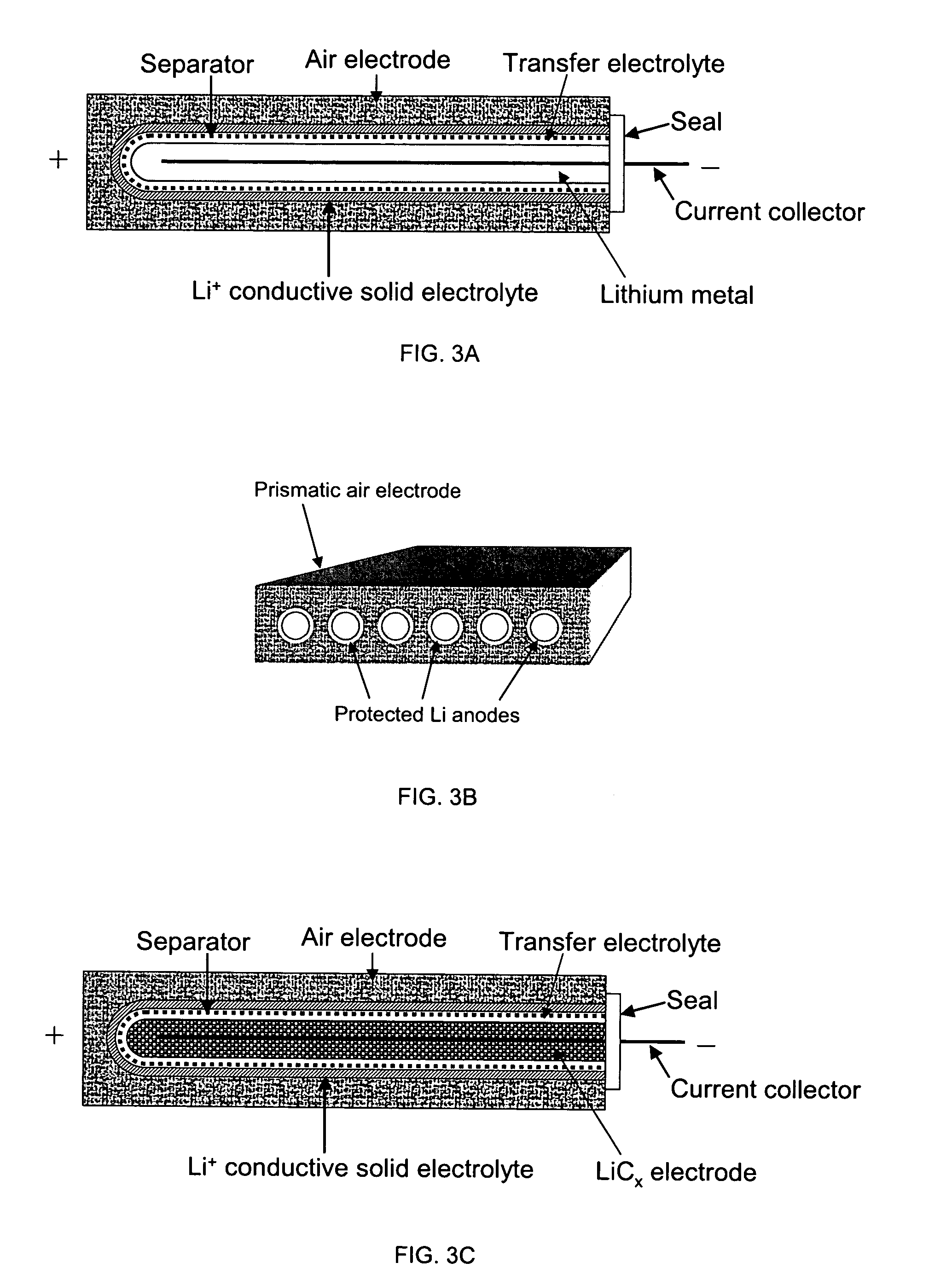
Active metal and active metal intercalation electrode structures and battery cells having ionically conductive protective architecture including an active metal (e.g., lithium) conductive impervious layer separated from the electrode (anode) by a porous separator impregnated with a non-aqueous electrolyte (anolyte). This protective architecture prevents the active metal from deleterious reaction with the environment on the other (cathode) side of the impervious layer, which may include aqueous or non-aqueous liquid electrolytes (catholytes) and / or a variety electrochemically active materials, including liquid, solid and gaseous oxidizers. Safety additives and designs that facilitate manufacture are also provided.
Owner:POLYPLUS BATTERY CO INC
Rechargeable lithium/water, lithium/air batteries
InactiveUS20070221265A1Improve protectionEasy to controlFinal product manufacturePV power plantsHigh energyOptoelectronics
Electrochemical cells, and more specifically, rechargeable batteries comprising lithium anodes for use in water and / or air environments, as well as non-aqueous and non-air environments, are presented. In one embodiment, an electrochemical cell includes an anode comprising lithium and a multi-layered structure positioned between the anode and an electrolyte of the cell. A multi-layered structure can include at least a first single-ion conductive material layer (e.g., a lithiated metal layer), and at least a first polymeric layer positioned between the anode and the single-ion conductive material. The invention also can provide an electrode stabilization layer positioned within the electrode, i.e., between one portion and another portion of an electrode, to control depletion and re-plating of electrode material upon charge and discharge of a battery. Advantageously, electrochemical cells comprising combinations of structures described herein are not only compatible with environments that are typically unsuitable for lithium, but the cells may be also capable of displaying long cycle life, high lithium cycling efficiency, and high energy density.
Owner:SION POWER CORP
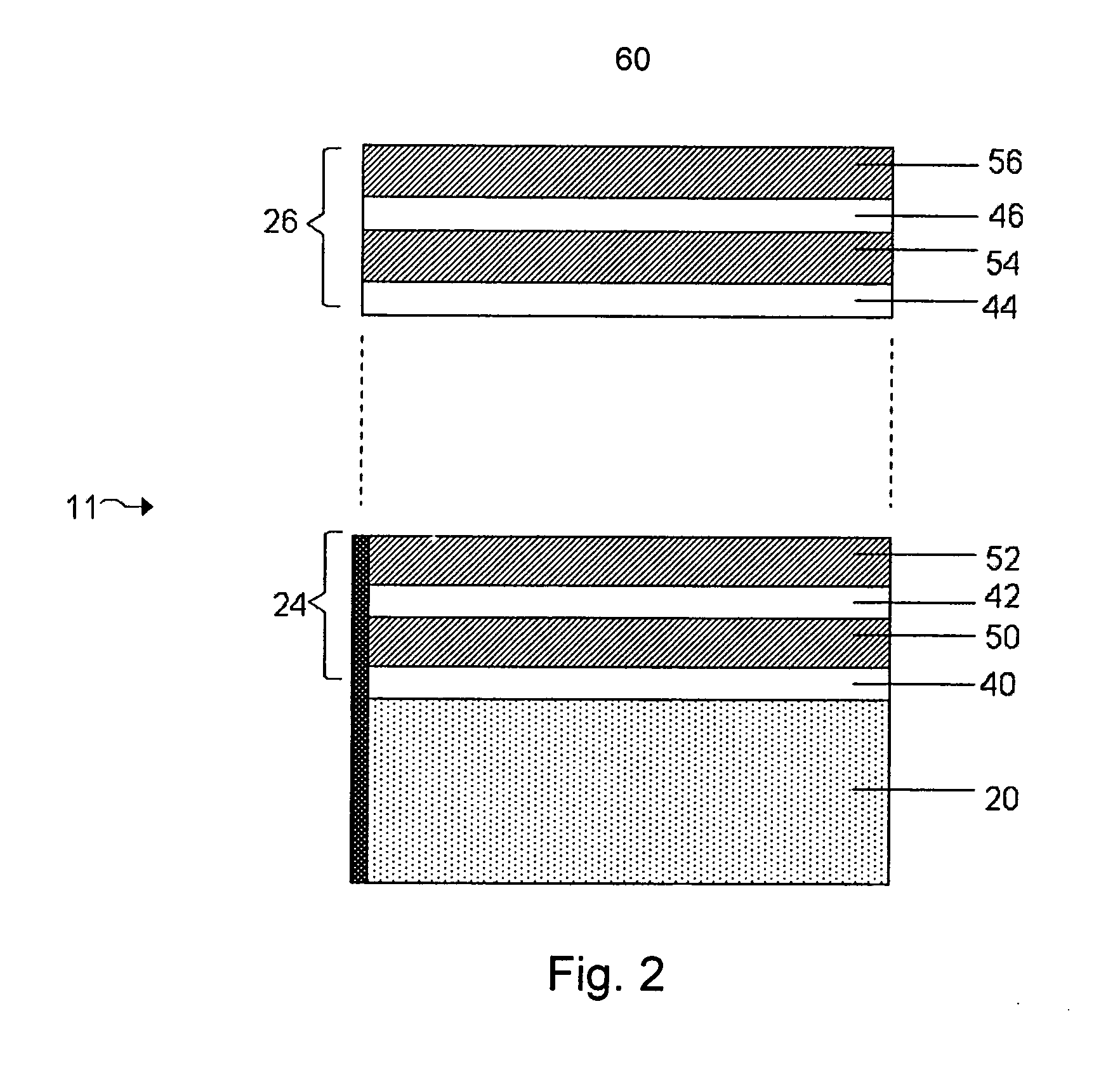
Protected active metal electrode and battery cell structures with non-aqueous interlayer architecture
ActiveUS20050175894A1Avoid harmful reactionsHybrid capacitor separatorsHybrid capacitor electrolytesMetal electrodesBattery cell
Active metal and active metal intercalation electrode structures and battery cells having ionically conductive protective architecture including an active metal (e.g., lithium) conductive impervious layer separated from the electrode (anode) by a porous separator impregnated with a non-aqueous electrolyte (anolyte). This protective architecture prevents the active metal from deleterious reaction with the environment on the other (cathode) side of the impervious layer, which may include aqueous or non-aqueous liquid electrolytes (catholytes) and / or a variety electrochemically active materials, including liquid, solid and gaseous oxidizers. Safety additives and designs that facilitate manufacture are also provided.
Owner:POLYPLUS BATTERY CO INC
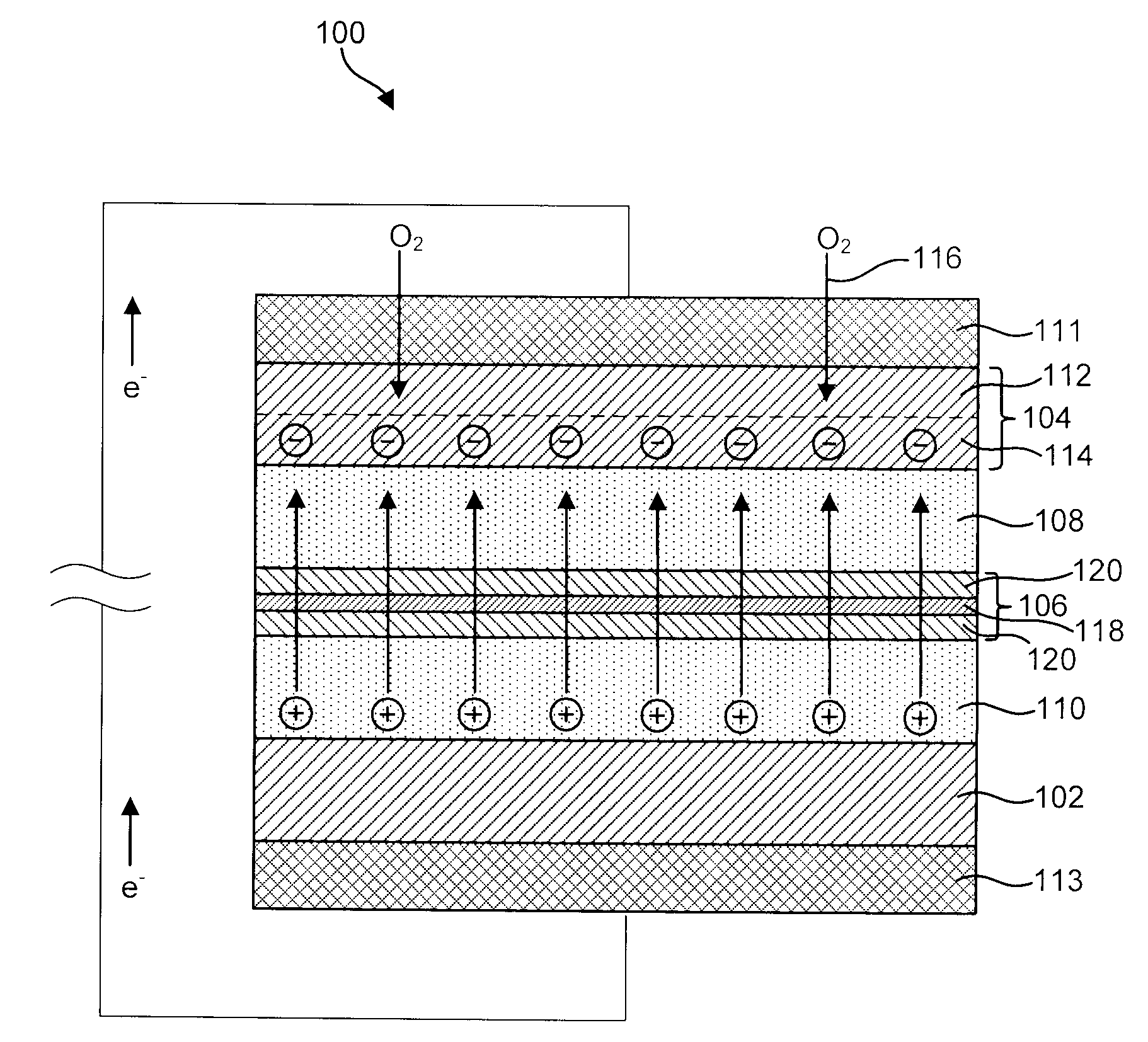
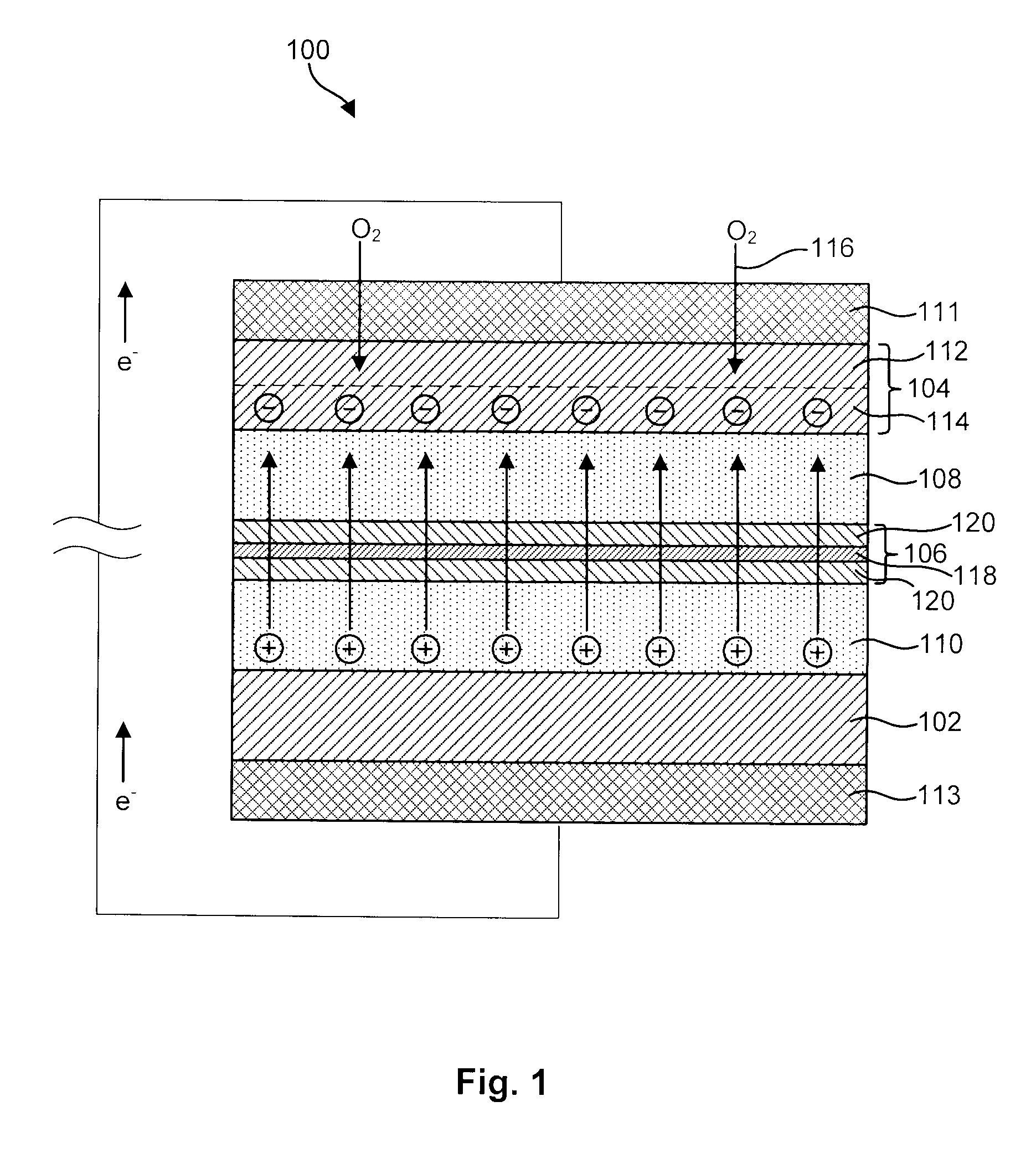
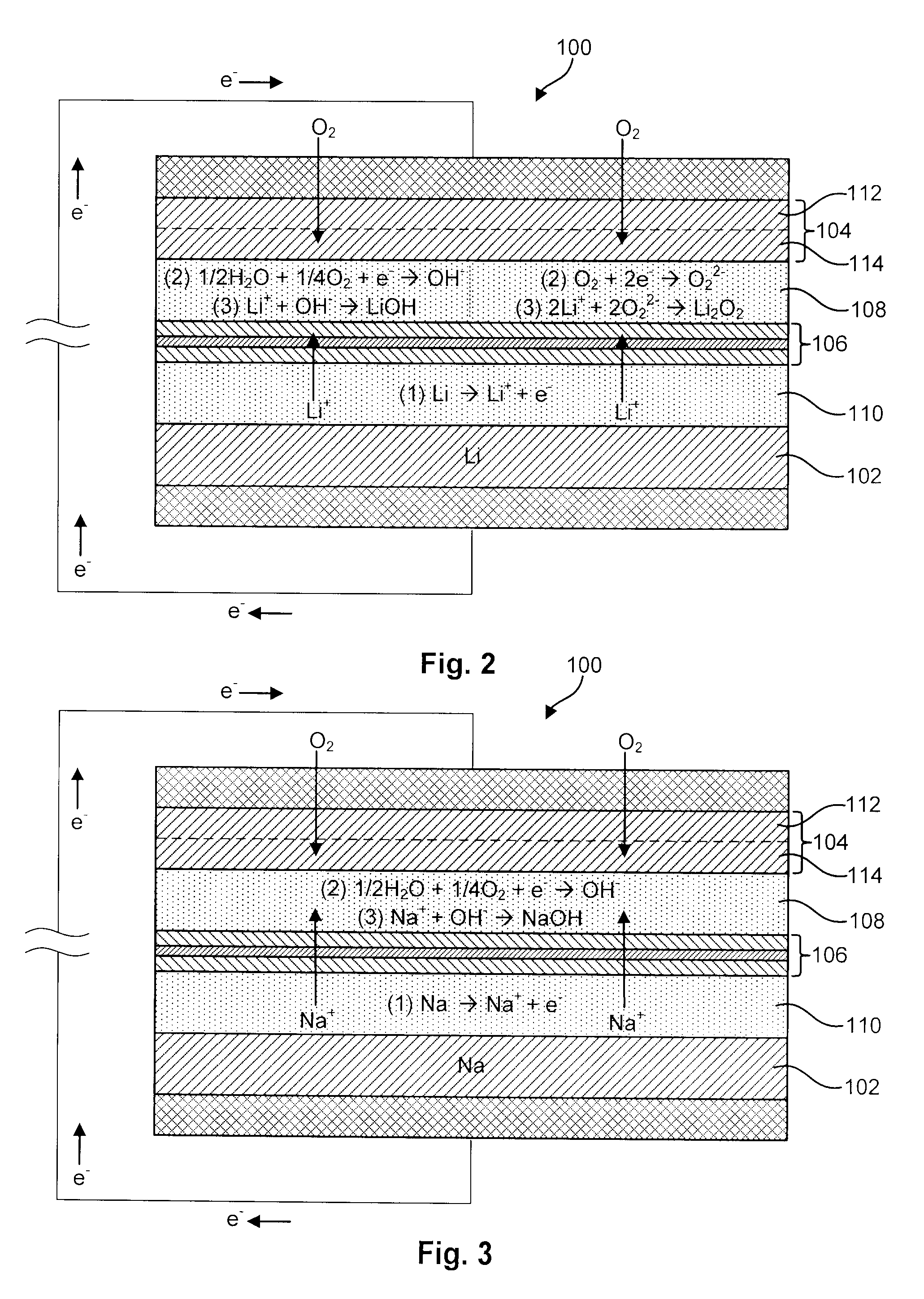
Advanced Metal-Air Battery Having a Ceramic Membrane Electrolyte Background of the Invention
ActiveUS20080268327A1Reduce oxygenReduce layeringFuel and primary cellsSolid electrolytesOxygenCeramic membrane
A metal-air battery is disclosed in one embodiment of the invention as including a cathode to reduce oxygen molecules and an alkali-metal-containing anode to oxidize the alkali metal (e.g., Li, Na, and K) contained therein to produce alkali-metal ions. An aqueous catholyte is placed in ionic communication with the cathode to store reaction products generated by reacting the alkali-metal ions with the oxygen containing anions. These reaction products are stored as solutes dissolved in the aqueous catholyte. An ion-selective membrane is interposed between the alkali-metal containing anode and the aqueous catholyte. The ion-selective membrane is designed to be conductive to the alkali-metal ions while being impermeable to the aqueous catholyte.
Owner:FIELD UPGRADING USA INC
Sodium ion based aqueous electrolyte electrochemical secondary energy storage device
InactiveUS20090253025A1Preserve electroneutralityHybrid capacitor electrolytesCapacitor and primary/secondary cellsSODIUM CATIONAqueous electrolyte
A secondary hybrid aqueous energy storage device includes an anode electrode, a cathode electrode which is capable of reversibly intercalating sodium cations, a separator, and a sodium cation containing aqueous electrolyte, wherein an initial active cathode electrode material comprises an alkali metal containing active cathode electrode material which deintercalates alkali metal ions during initial charging of the device.
Owner:CARNEGIE MELLON UNIV
Electrode protection in both aqueous and non-aqueous electrochemical cells, including rechargeable lithium batteries
ActiveUS20070224502A1Preventing electronic communicationAvoid communicationFinal product manufactureElectrode carriers/collectorsHigh energyConductive materials
Electrode protection in electrochemical cells, and more specifically, electrode protection in both aqueous and non-aqueous electrochemical cells, including rechargeable lithium batteries, are presented. In one embodiment, an electrochemical cell includes an anode comprising lithium and a multi-layered structure positioned between the anode and an electrolyte of the cell. A multi-layered structure can include at least a first single-ion conductive material layer (e.g., a lithiated metal layer), and at least a first polymeric layer positioned between the anode and the single-ion conductive material. The invention also can provide an electrode stabilization layer positioned within the electrode, i.e., between one portion and another portion of an electrode, to control depletion and re-plating of electrode material upon charge and discharge of a battery. Advantageously, electrochemical cells comprising combinations of structures described herein are not only compatible with environments that are typically unsuitable for lithium, but the cells may be also capable of displaying long cycle life, high lithium cycling efficiency, and high energy density.
Owner:SION POWER CORP
Electrically rechargeable, metal-air battery systems and methods
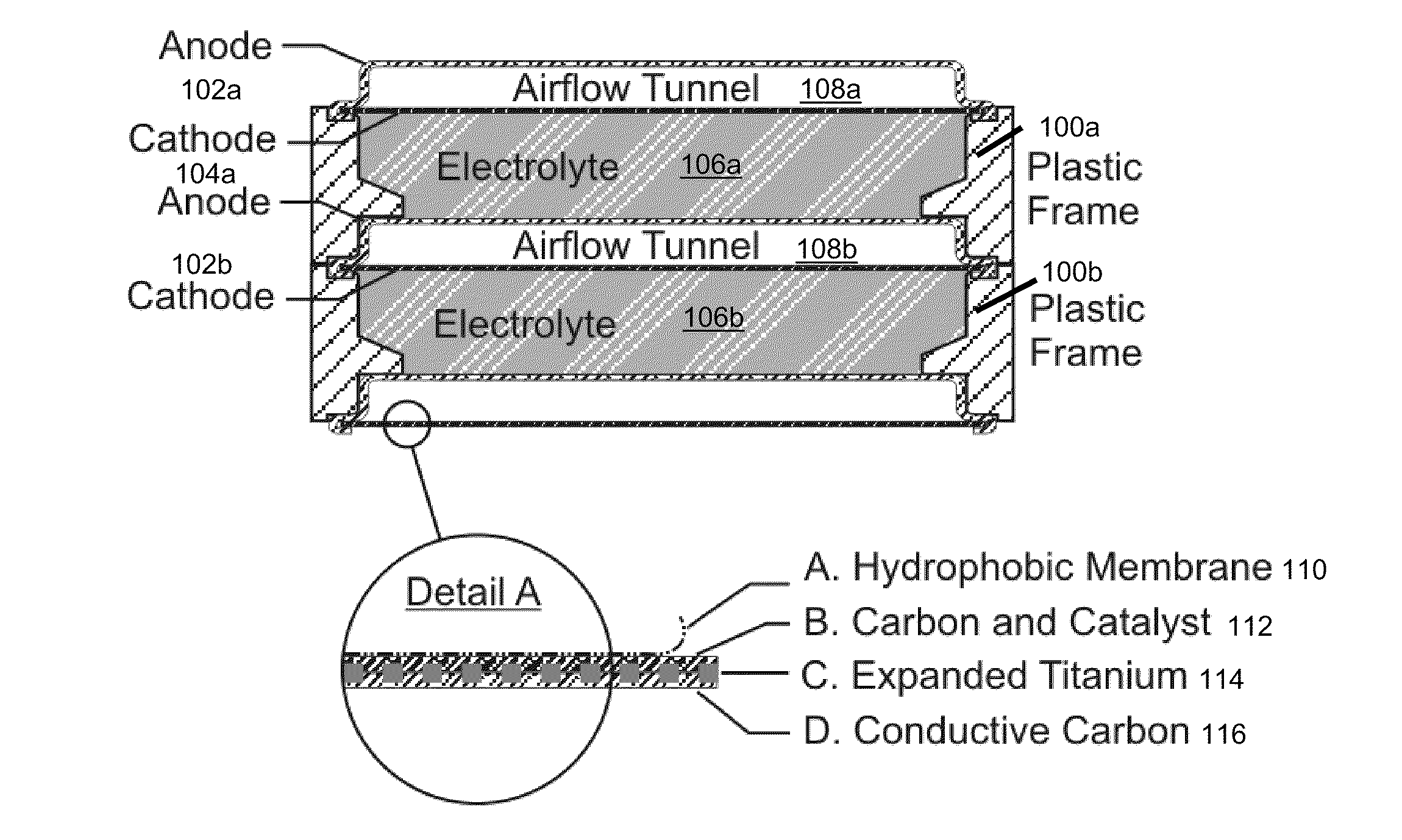
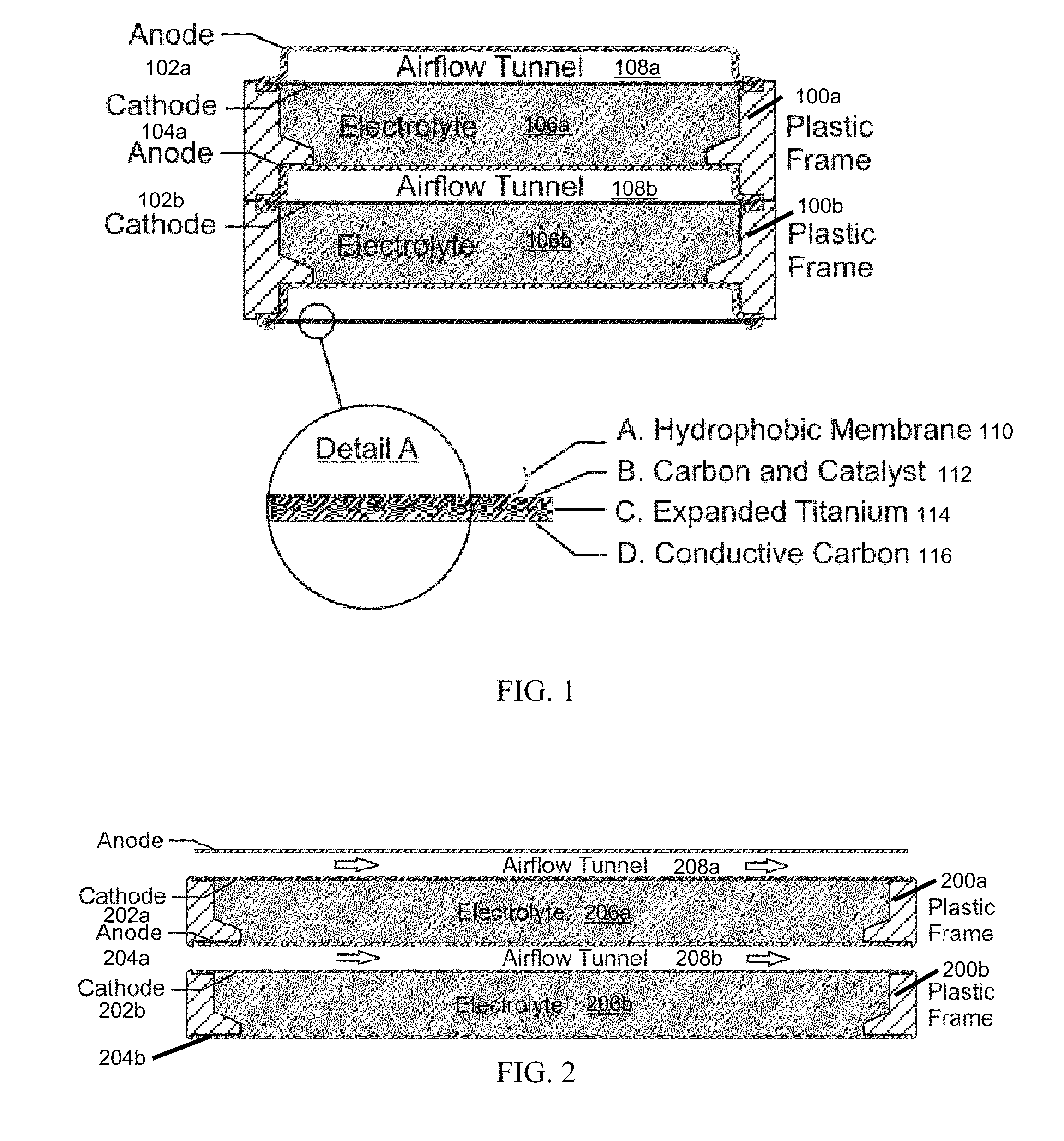
InactiveUS20120021303A1Solution to short lifeAffordable and practicalElectrolyte moving arrangementsFuel and secondary cellsElectricityElectrical battery
The invention provides for a fully electrically rechargeable metal-air battery systems and methods of achieving such systems. A rechargeable metal air battery cell may comprise a metal electrode an air electrode, and an aqueous electrolyte separating the metal electrode and the air electrode. In some embodiments, the metal electrode may directly contact the electrolyte and no separator or porous membrane need be provided between the air electrode and the electrolyte. Rechargeable metal air battery cells may be electrically connected to one another through a centrode connection between a metal electrode of a first battery cell and an air electrode of a second battery cell. Air tunnels may be provided between individual metal air battery cells. In some embodiments, an electrolyte flow management system may be provided.
Owner:EOS ENERGY STORAGE
Protection of anodes for electrochemical cells
InactiveUS20110014524A1Light weightElectrode carriers/collectorsNegative electrodesLithium metalReactive gas
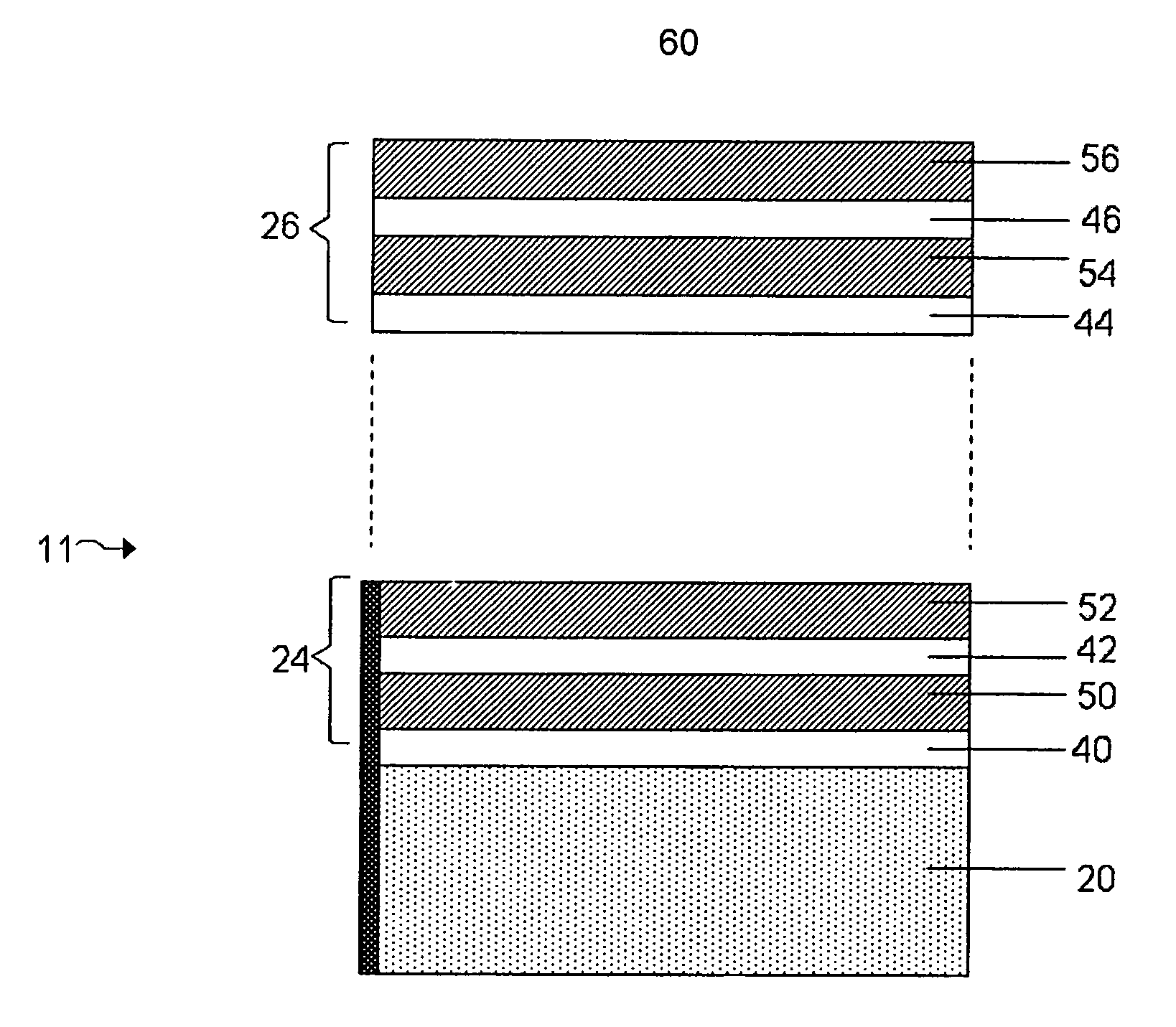
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
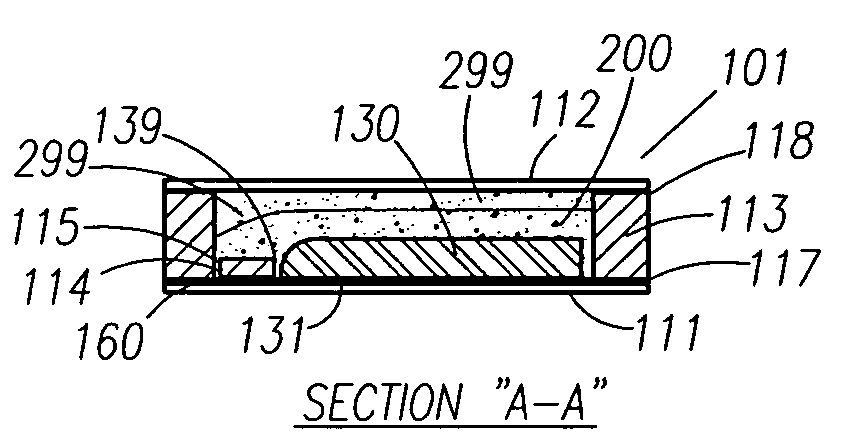
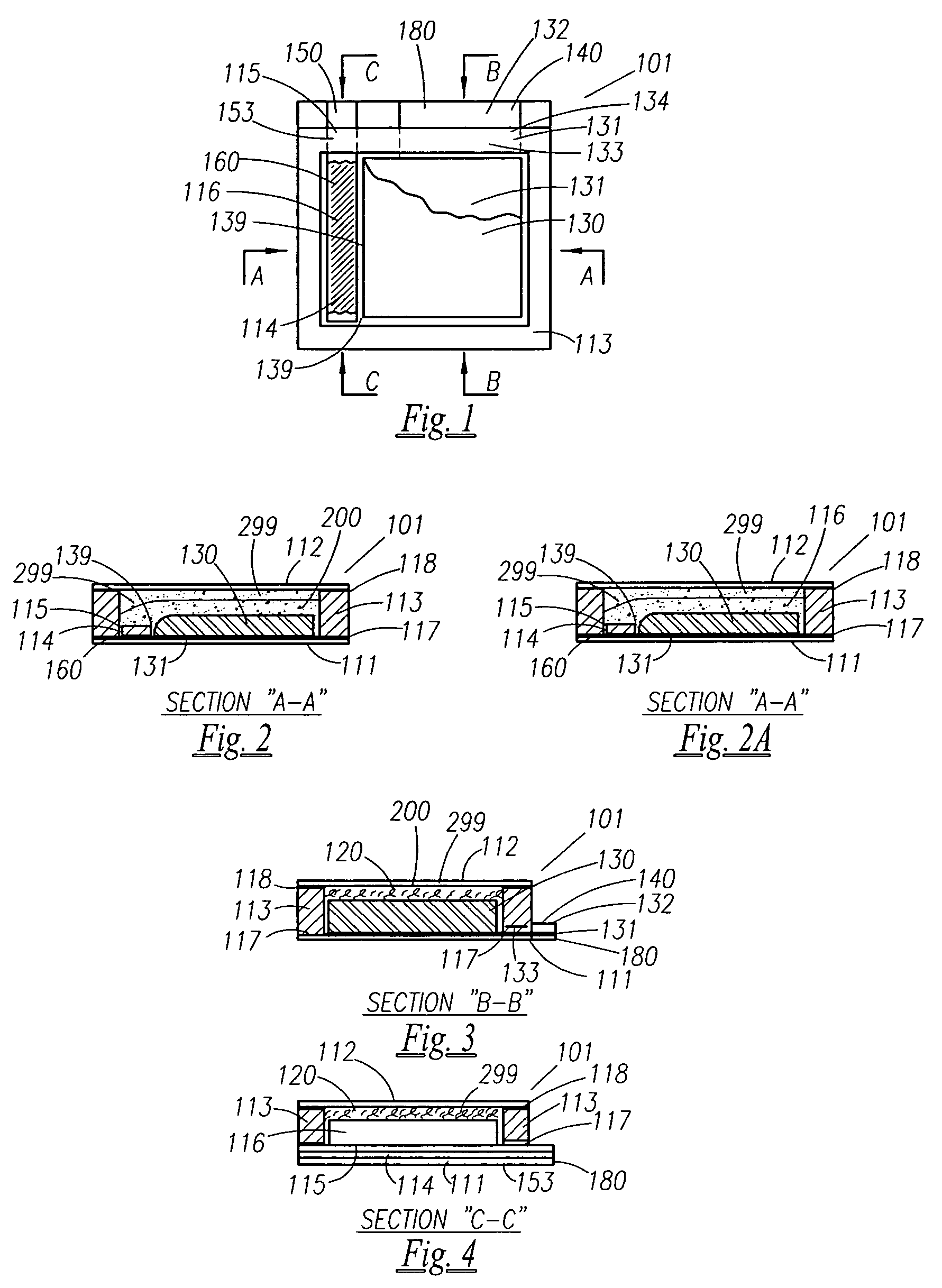
Thin printable electrochemical cell utilizing a "picture frame" and methods of making the same
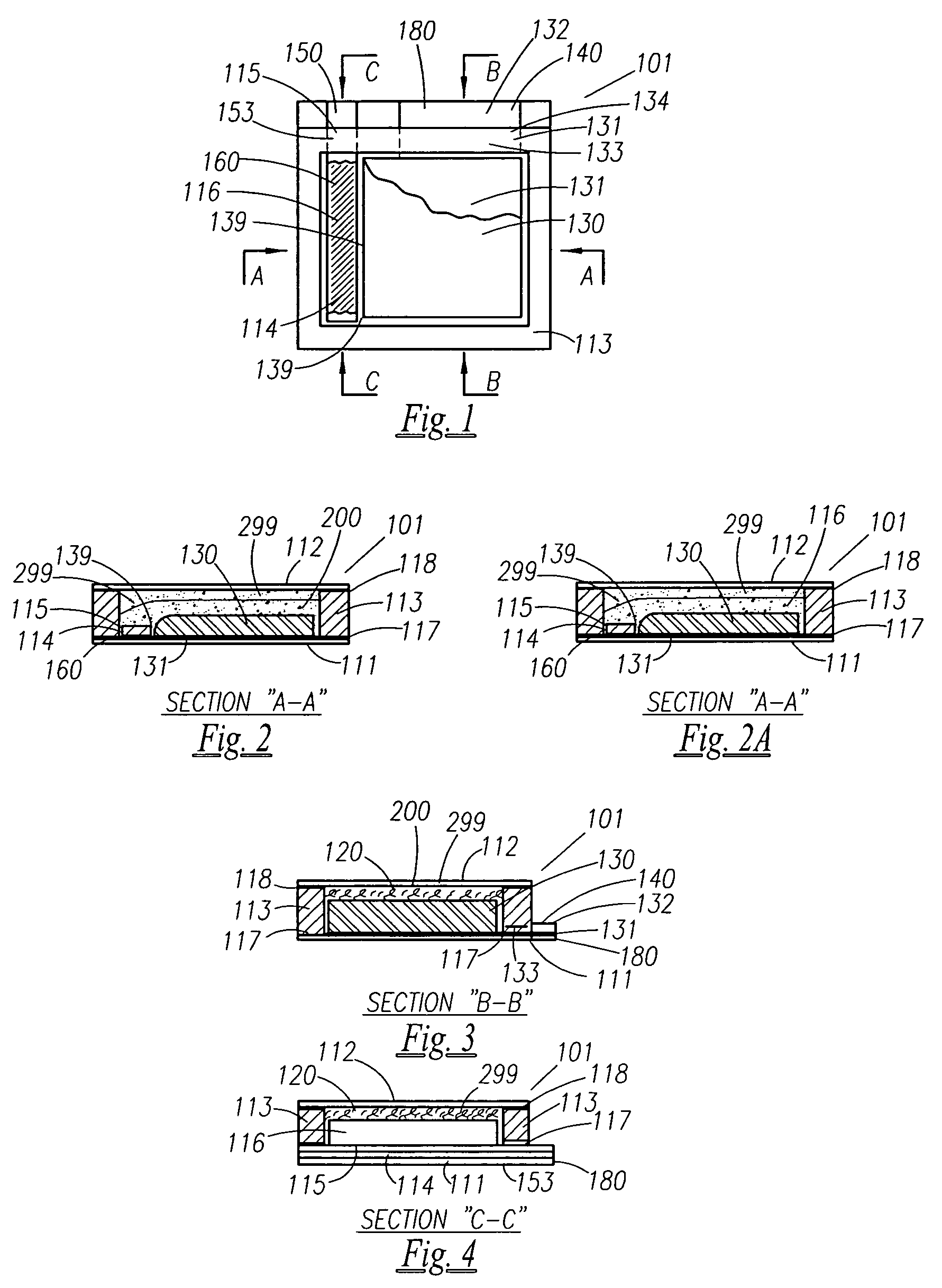
A thin printed flexible electrochemical cell, and its method of manufacture, using a “picture frame” structure sealed, for example, with a high moisture and oxygen barrier polymer film and featuring, for example, a printed cathode deposited on an optional, highly conductive carbon printed cathode collector with a printed or a foil strip anode placed adjacent to the cathode. A viscous or gelled electrolyte is dispensed and / or printed in the cell, and a top laminate can then be sealed onto the picture frame. Such a construction could allow the entire cell to be made on a printing press, for example, as well as gives the opportunity to integrate the battery directly with an electronic application, for example.
Owner:BLUE SPARK INNOVATIONS LLC
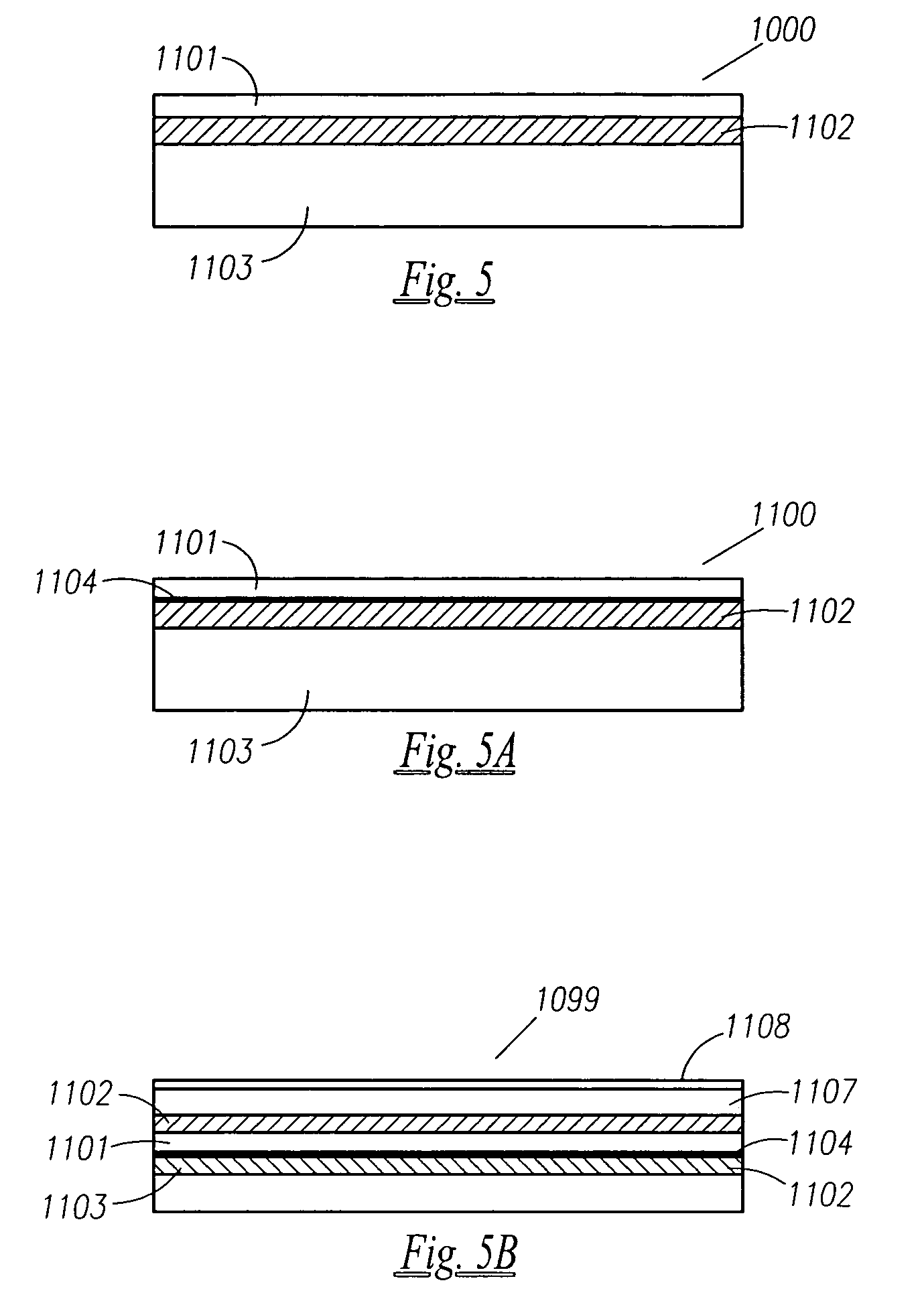
Functionally improved battery and method of making same
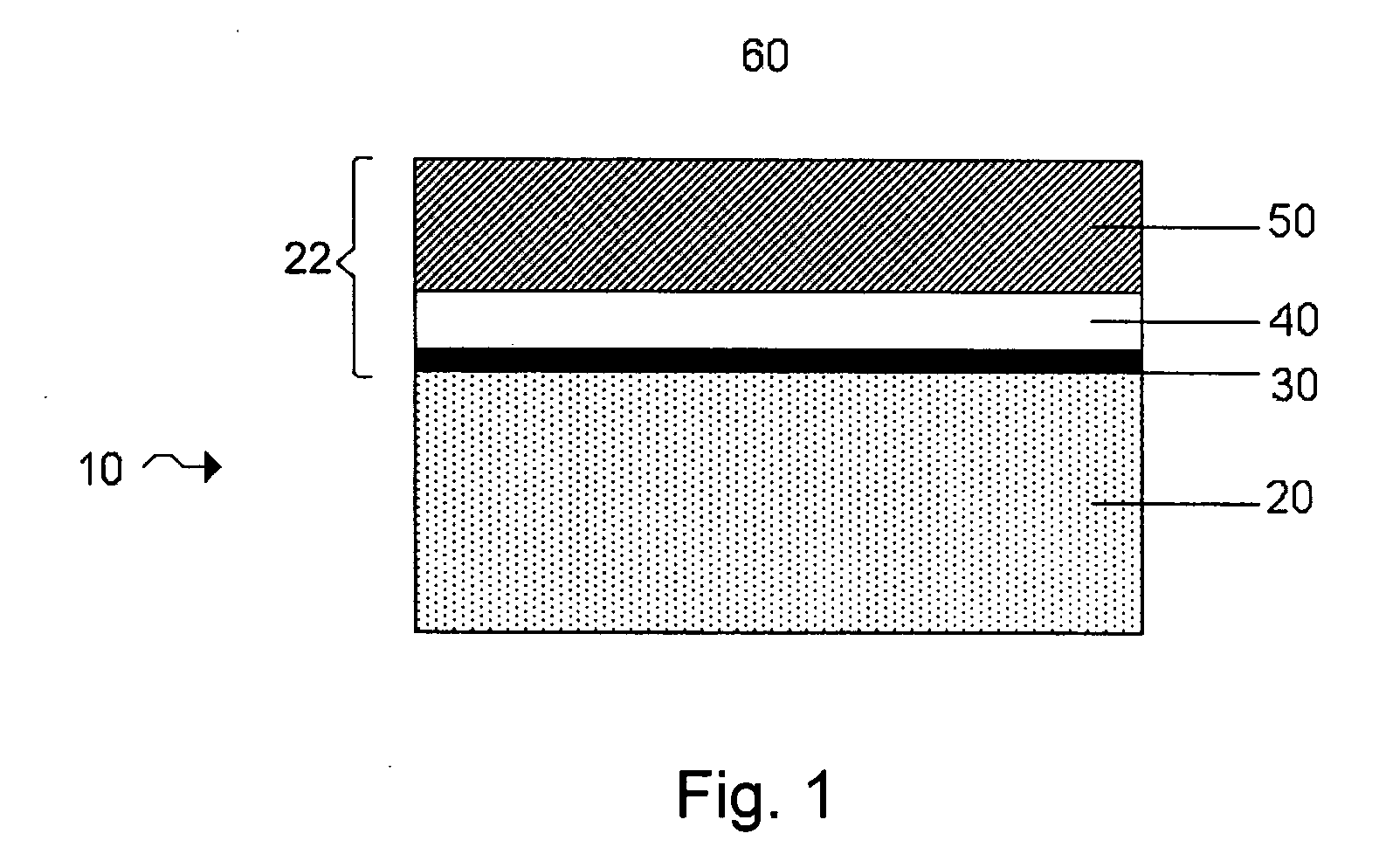
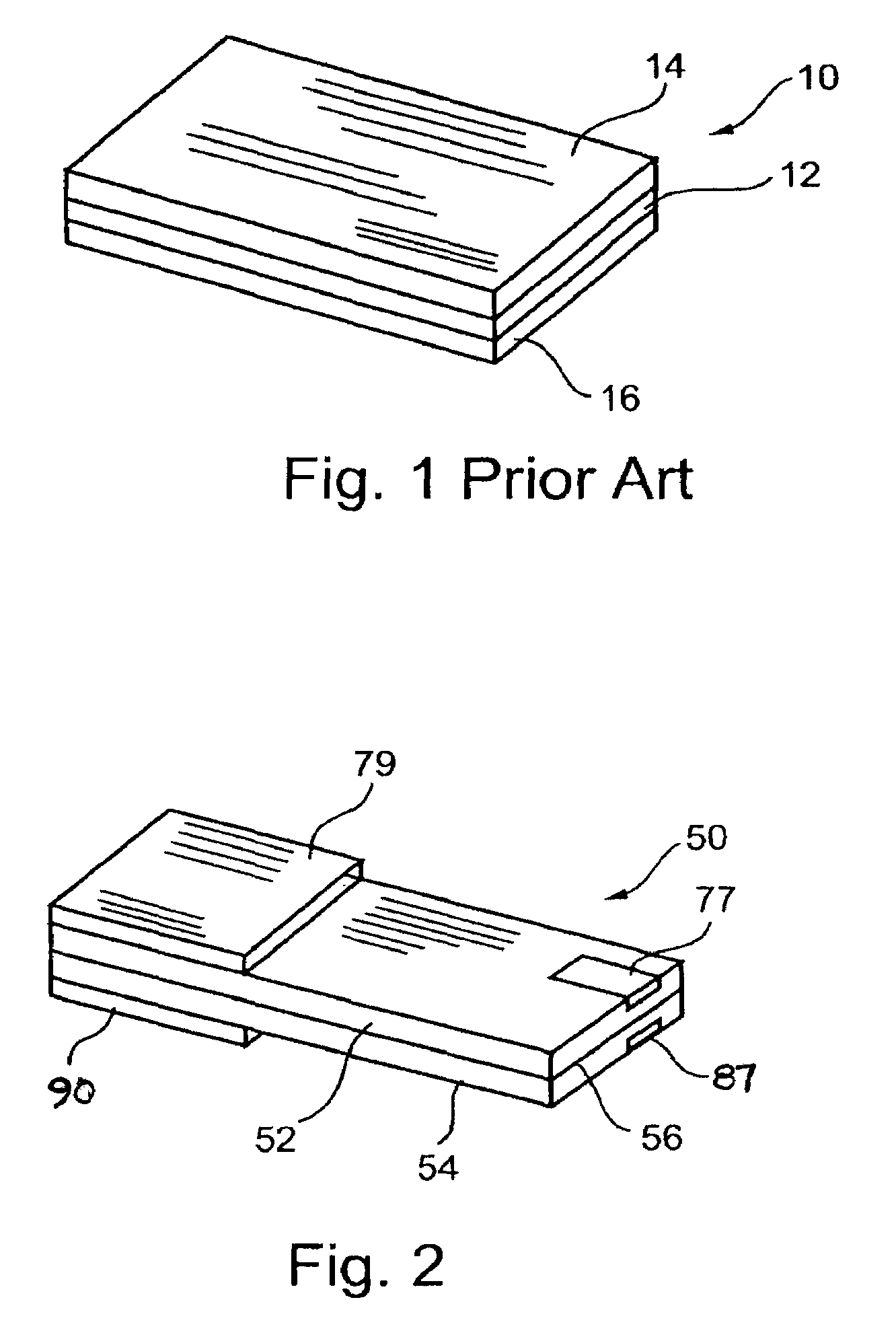
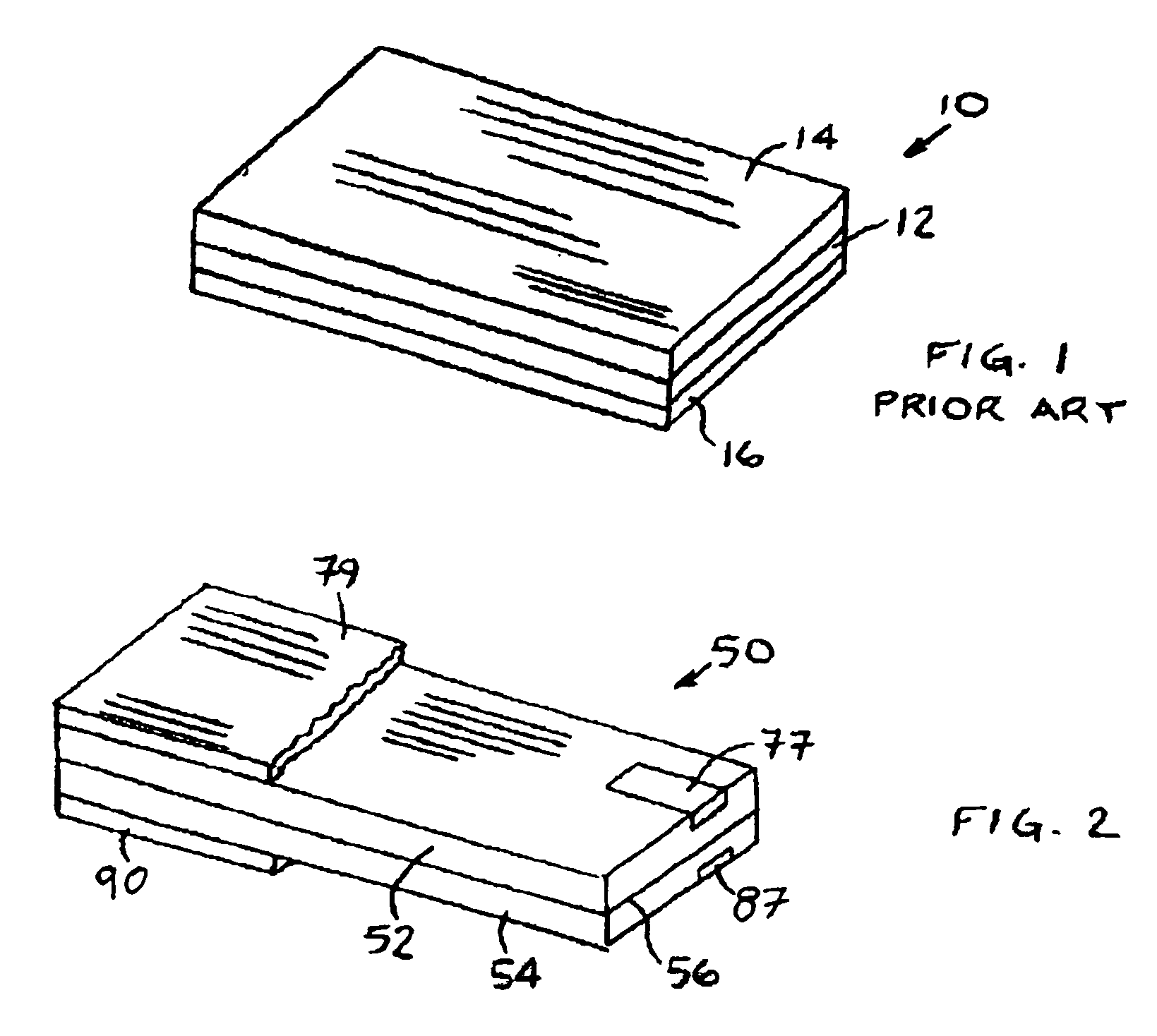
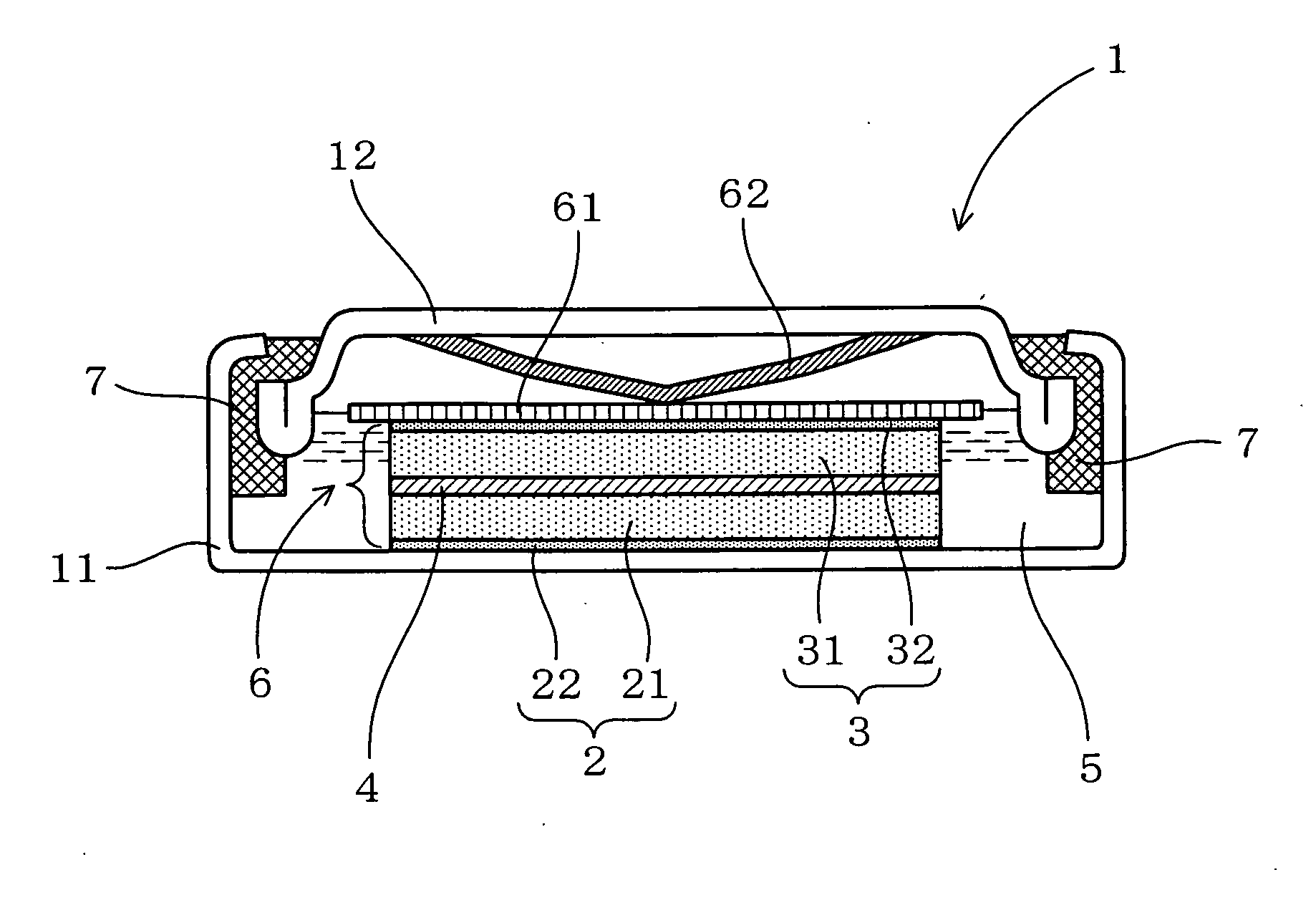
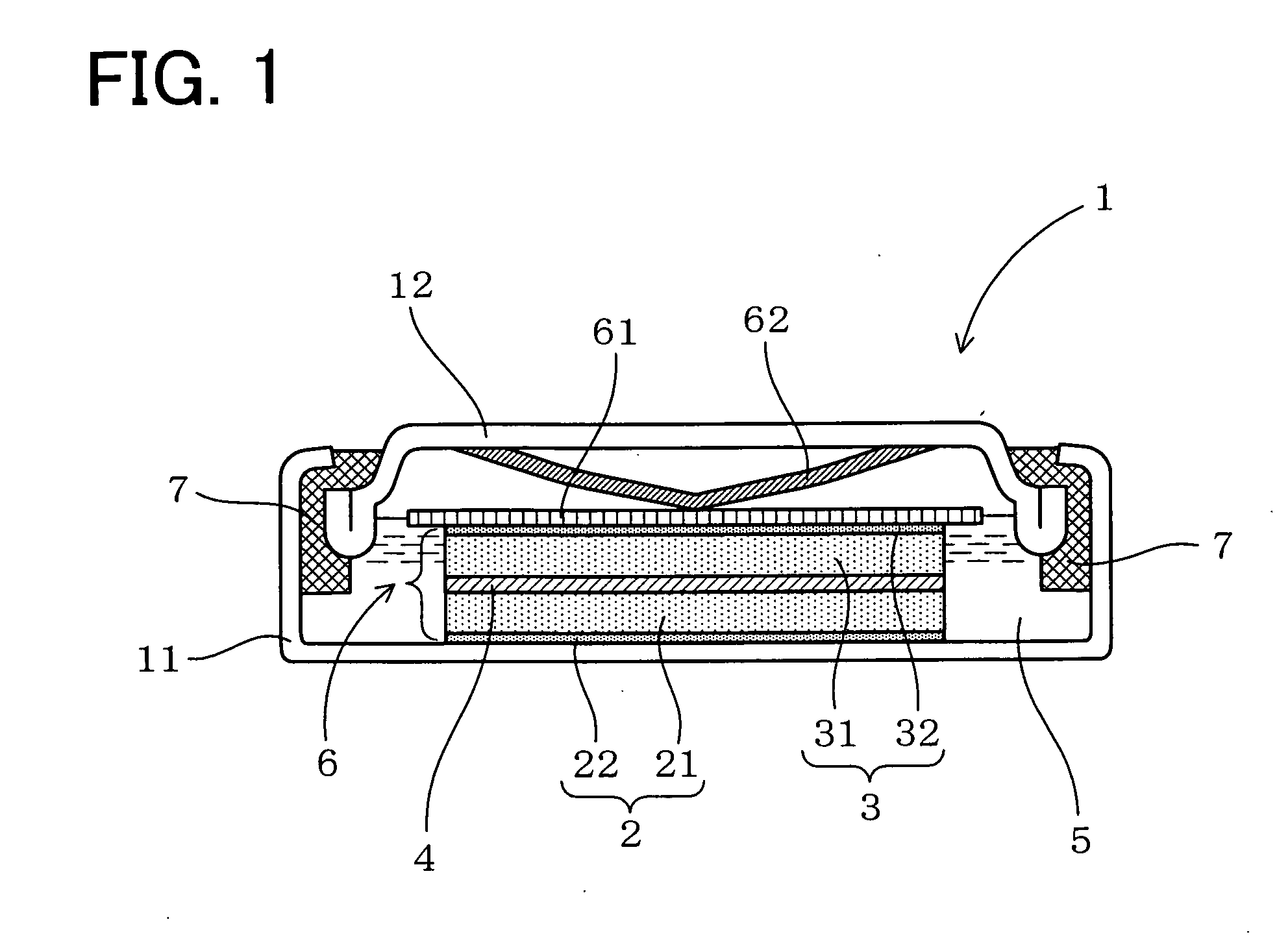
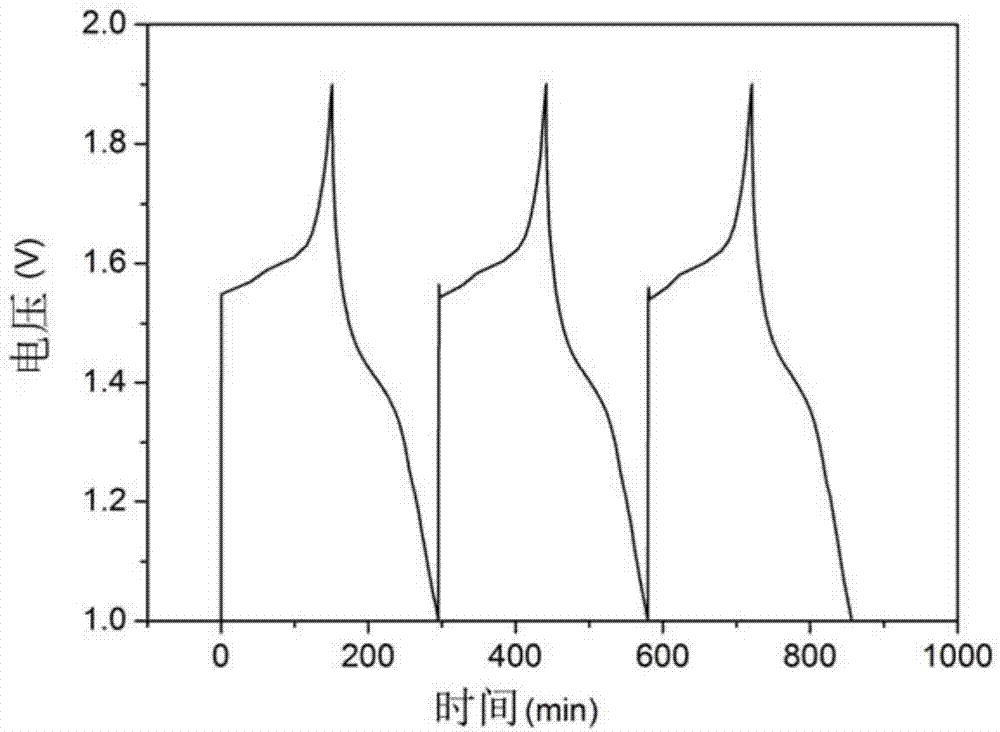
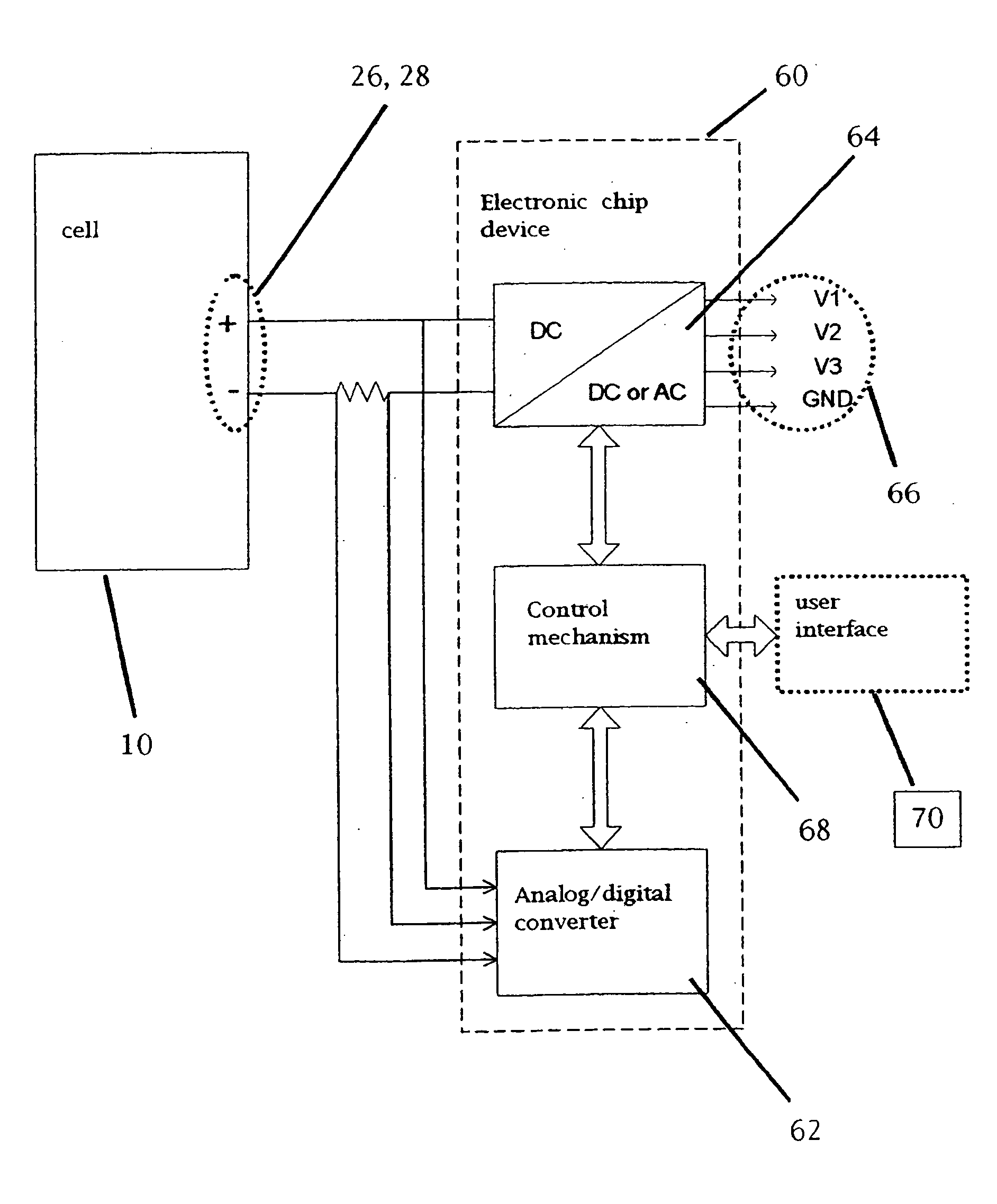
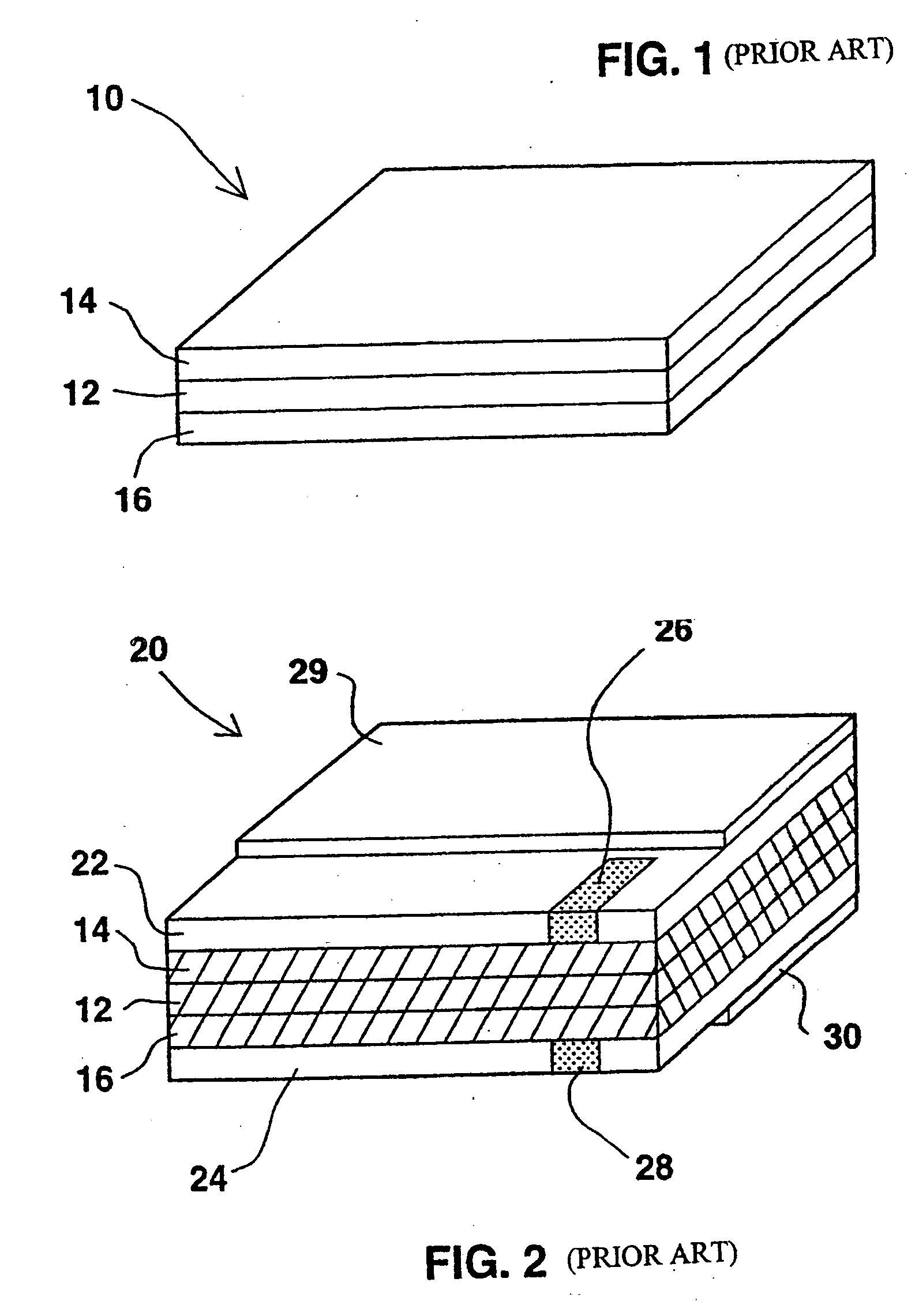
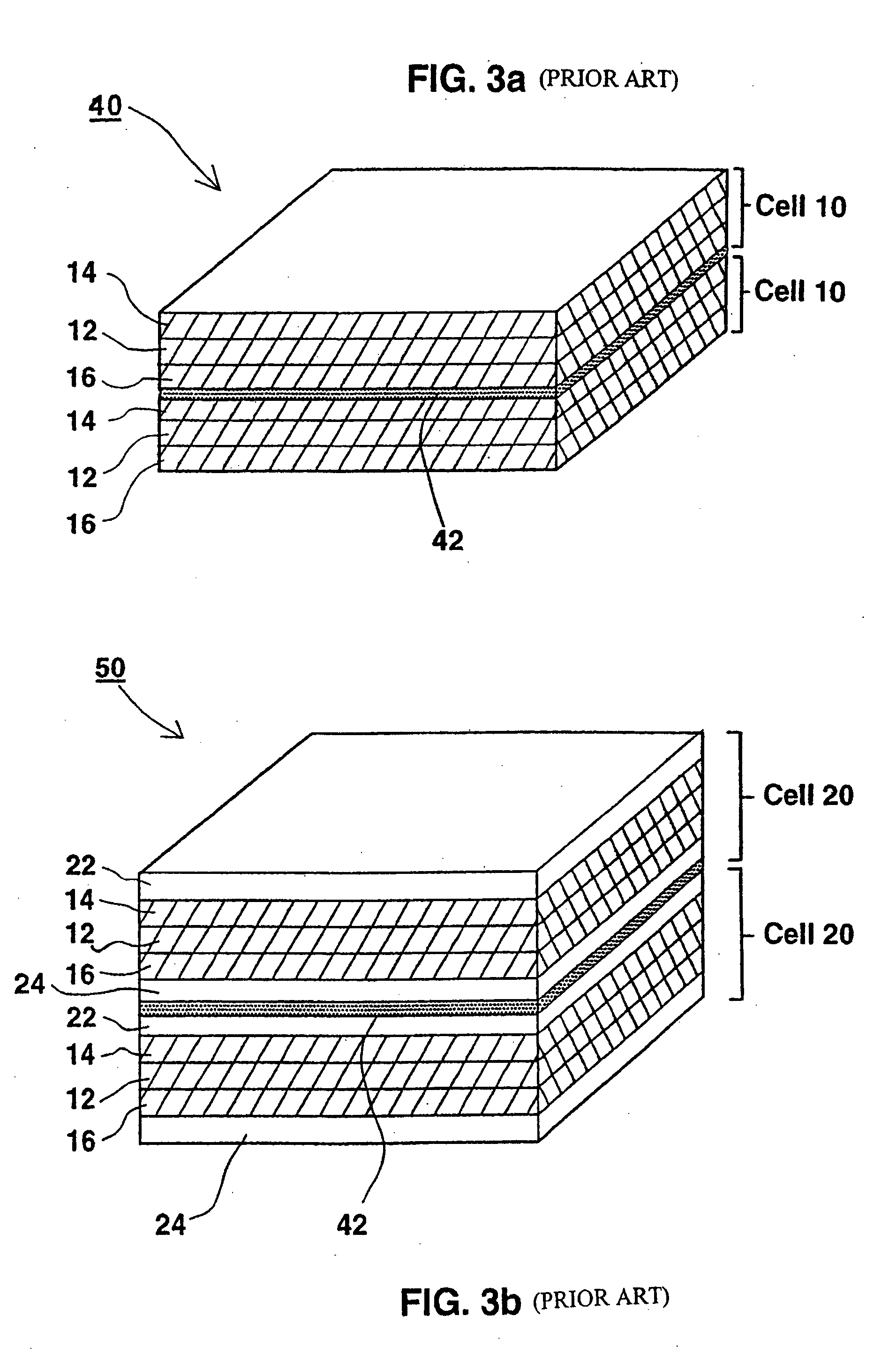
InactiveUS6855441B1Extended service lifePower outputBatteries circuit arrangementsSemiconductor/solid-state device detailsElectrical batteryLiquid state
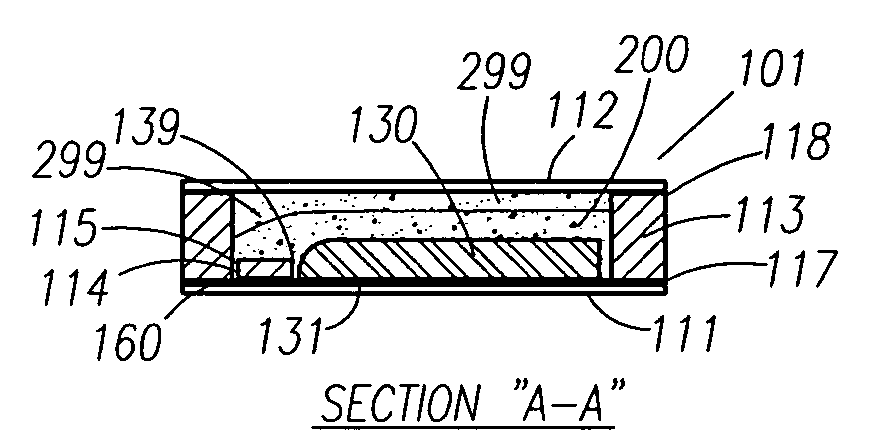
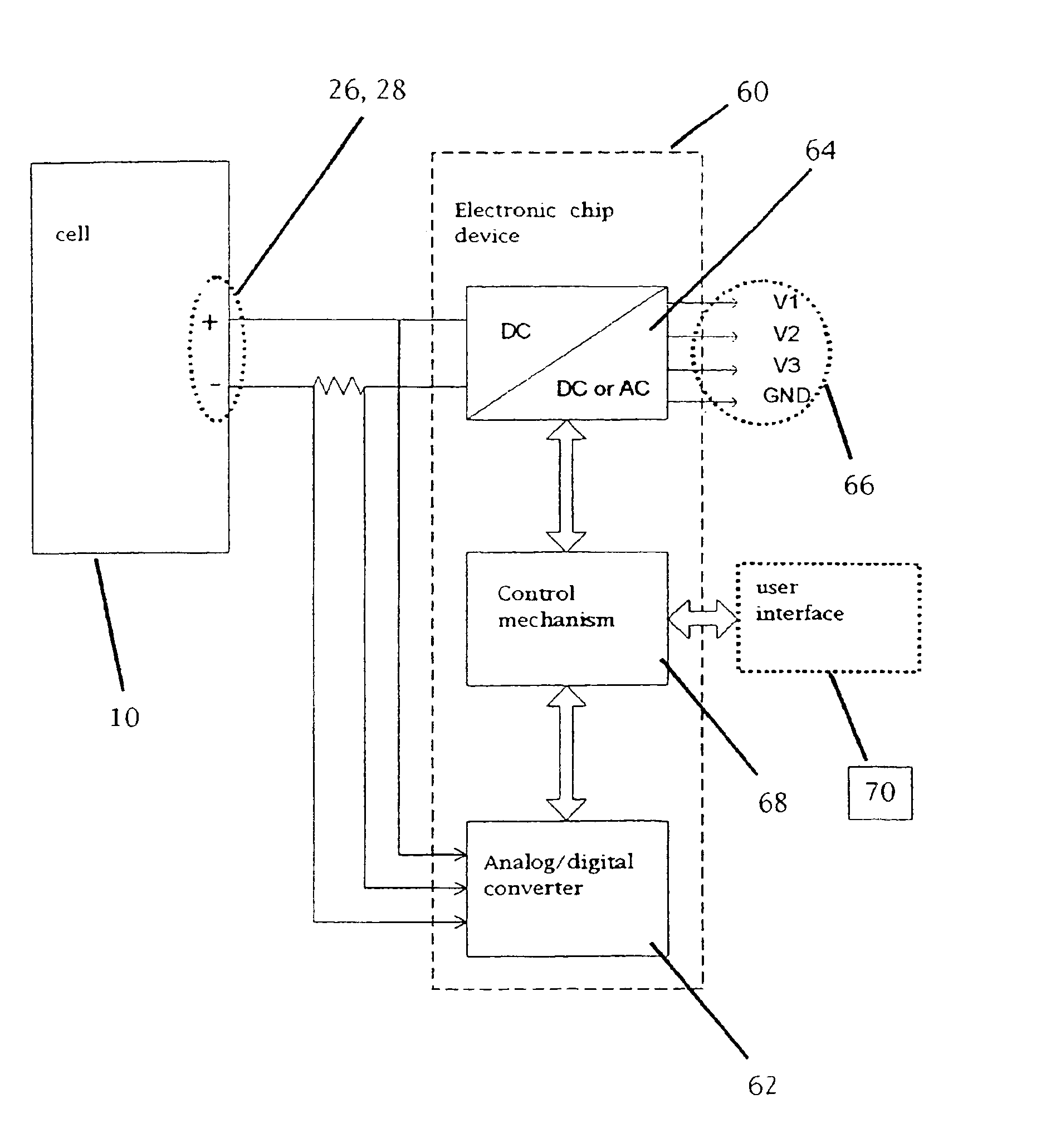
A functionally improved battery is disclosed. The battery includes a flexible thin layer open liquid state electrochemical cell (10) and an electronic chip device (60) integrally formed on or within the electrochemical cell (10). The cell (10) includes a first layer of insoluble negative pole (14), a second layer of insoluble positive pole (16) and a third layer of aqueous electrolyte (12). The third layer (12) is disposed between the first (14) and second (16) layers. The third layer (12) includes a diliquescent material for keeping the cell wet, an electroactive soluble material for ionic conductivity and a watersoluble polymer for viscosity. The viscosity adheres the first (14) and second (16) layer to the third layer (12). The chip (60) serves to improve the functionality of the battery.
Owner:POWER PAPER
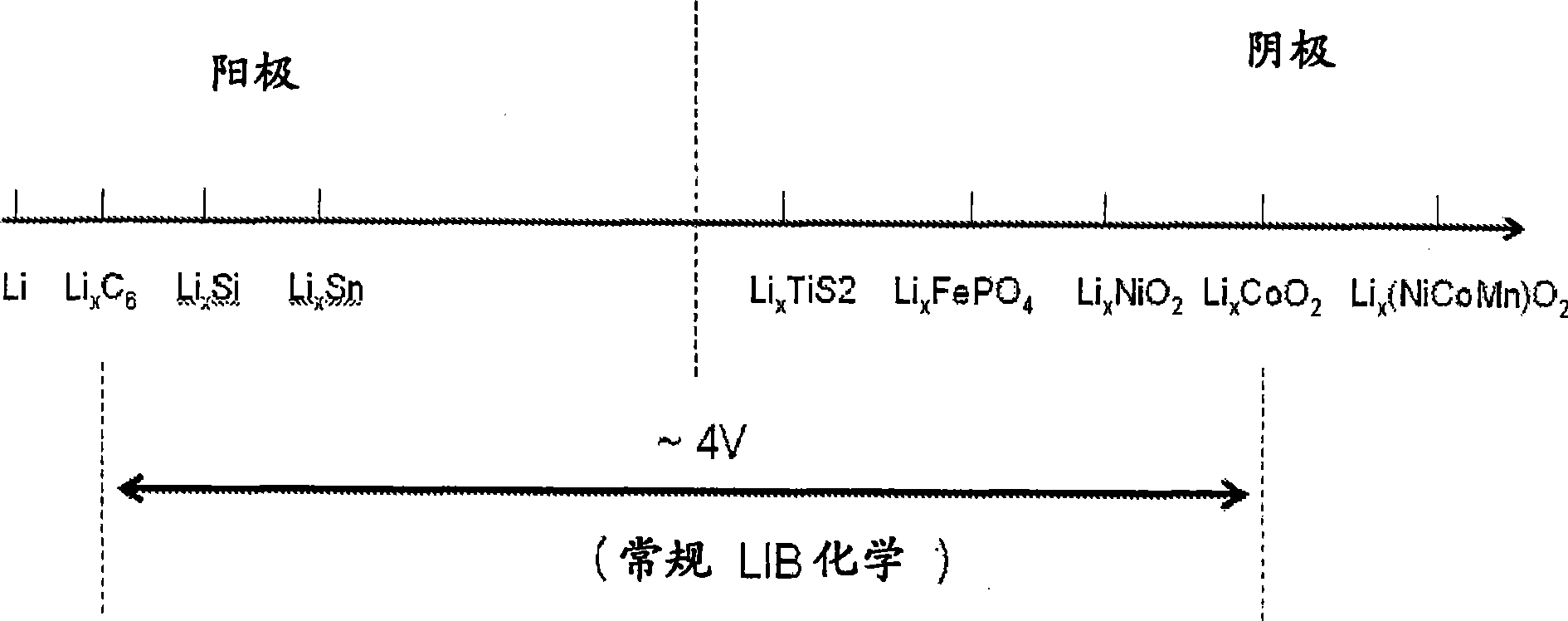
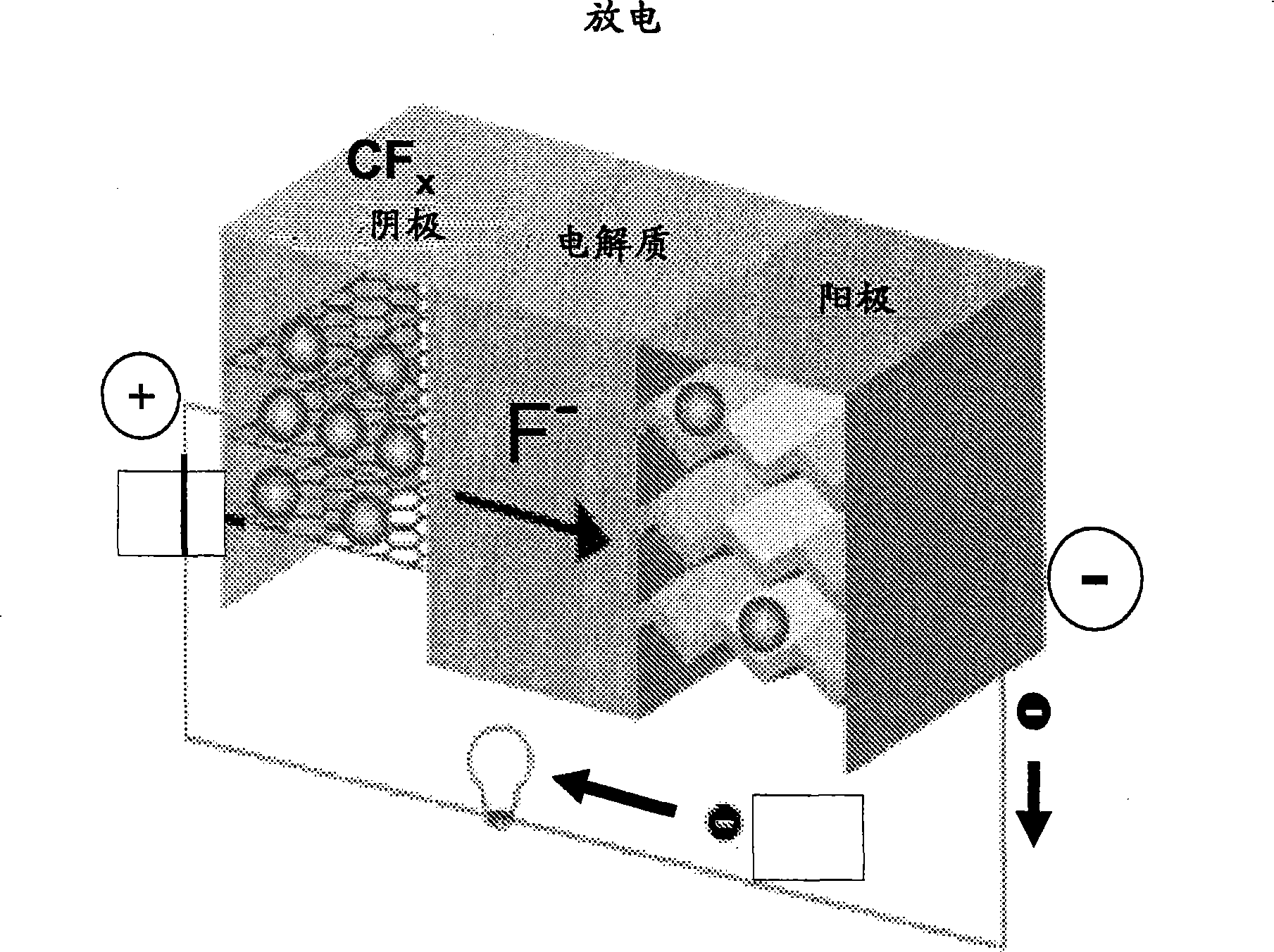
Fluoride ion electrochemical cell
ActiveUS20090029237A1Improve performanceHigh energyAlkaline accumulatorsLead-acid accumulatorsMetallic lithiumState of art
The present invention provides electrochemical cells capable of good electronic performance, particularly high specific energies, useful discharge rate capabilities and good cycle life. Electrochemical cells of the present invention are versatile and include primary and secondary cells useful for a range of important applications including use in portable electronic devices. Electrochemical cells of the present invention also exhibit enhanced safety and stability relative to conventional state of the art primary lithium batteries and lithium ion secondary batteries. For example, electrochemical cells of the present invention include secondary electrochemical cells using anion charge carriers capable of accommodation by positive and negative electrodes comprising anion host materials, which entirely eliminate the need for metallic lithium or dissolved lithium ion in these systems.
Owner:CALIFORNIA INST OF TECH +1
Alkali Metal-Sulfur Batteries Having High Volumetric and Gravimetric Energy Densities
ActiveUS20170207484A1Large capacityImprove the immunityFinal product manufactureElectrode carriers/collectorsCarbon compositesSlurry
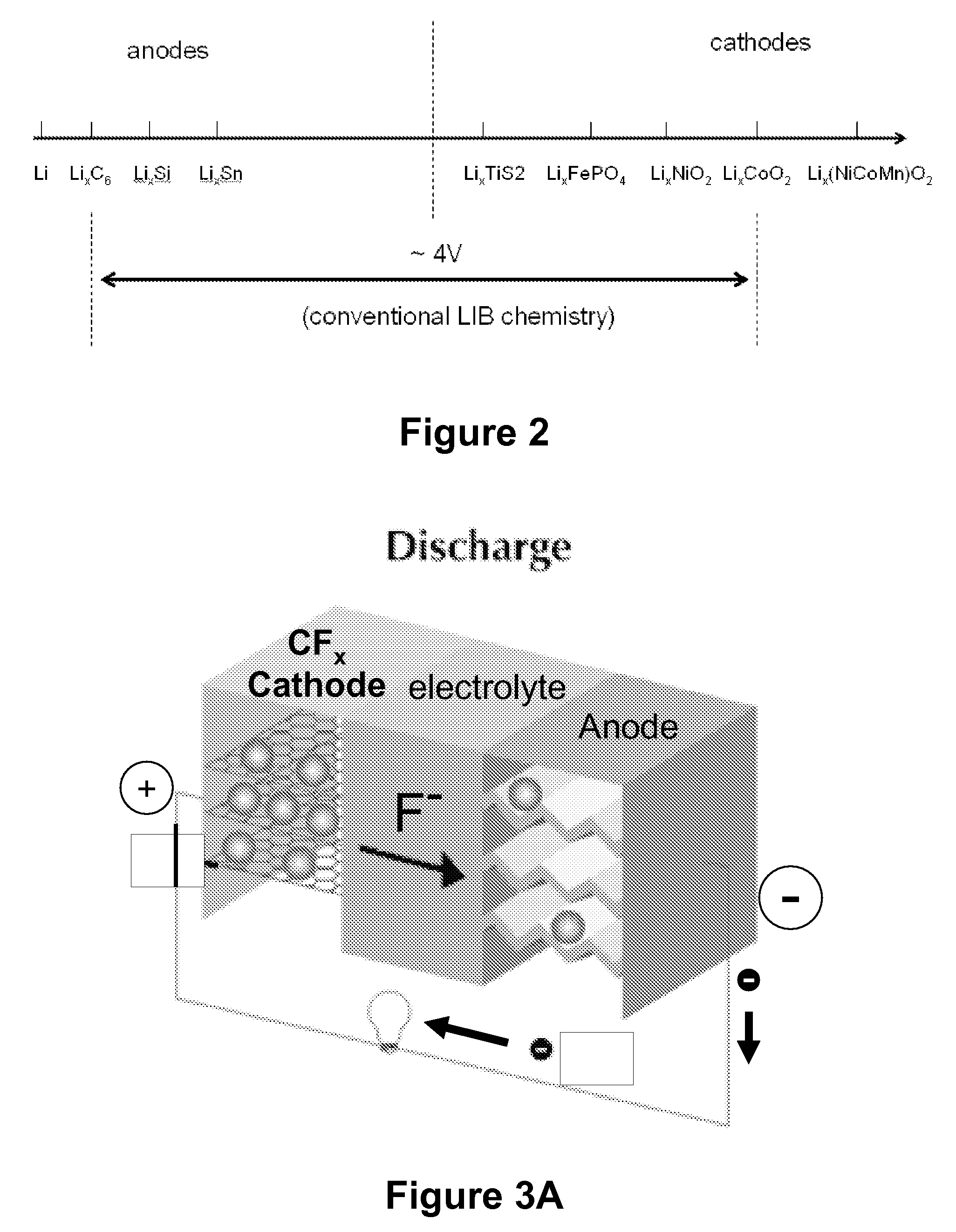
Provided is an alkali metal-sulfur battery, comprising: (a) an anode; (b) a cathode having (i) a cathode active material slurry comprising a cathode active material dispersed in an electrolyte and (ii) a conductive porous structure acting as a 3D cathode current collector having at least 70% by volume of pores and wherein cathode active material slurry is disposed in pores of the conductive porous structure, wherein the cathode active material is selected from sulfur, lithium polysulfide, sodium polysulfide, sulfur-polymer composite, sulfur-carbon composite, sulfur-graphene composite, or a combination thereof; and (c) a separator disposed between the anode and the cathode; wherein the cathode thickness-to-cathode current collector thickness ratio is from 0.8 / 1 to 1 / 0.8, and / or the cathode active material constitutes an electrode active material loading greater than 15 mg / cm2, and the 3D porous cathode current collector has a thickness no less than 200 μm (preferably thicker than 500 μm).
Owner:GLOBAL GRAPHENE GRP INC
Thin printable electrochemical cell utilizing a “picture frame” and methods of making the same
Owner:BLUE SPARK INNOVATIONS LLC
Thin layer electrochemical cell with self-formed separator
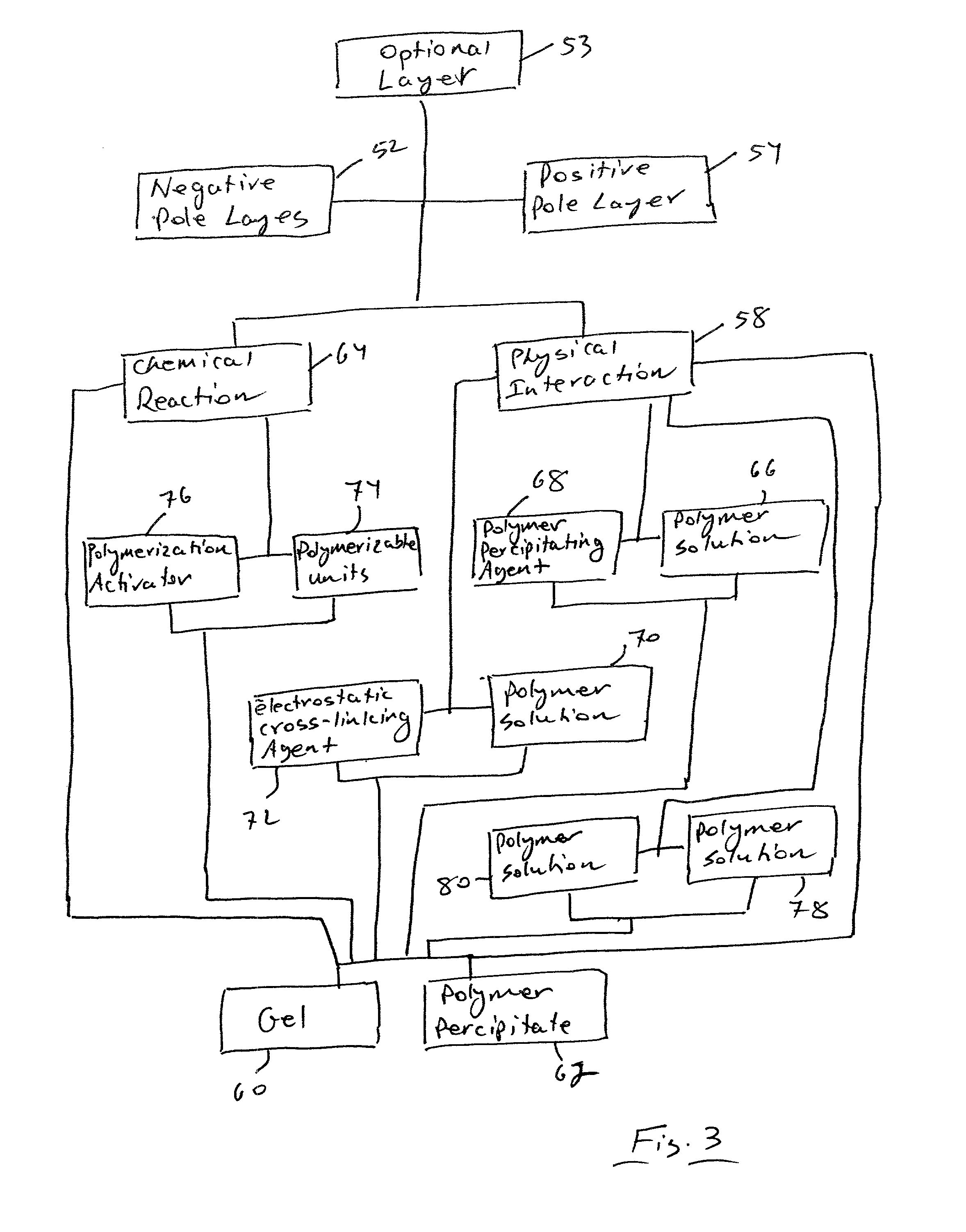
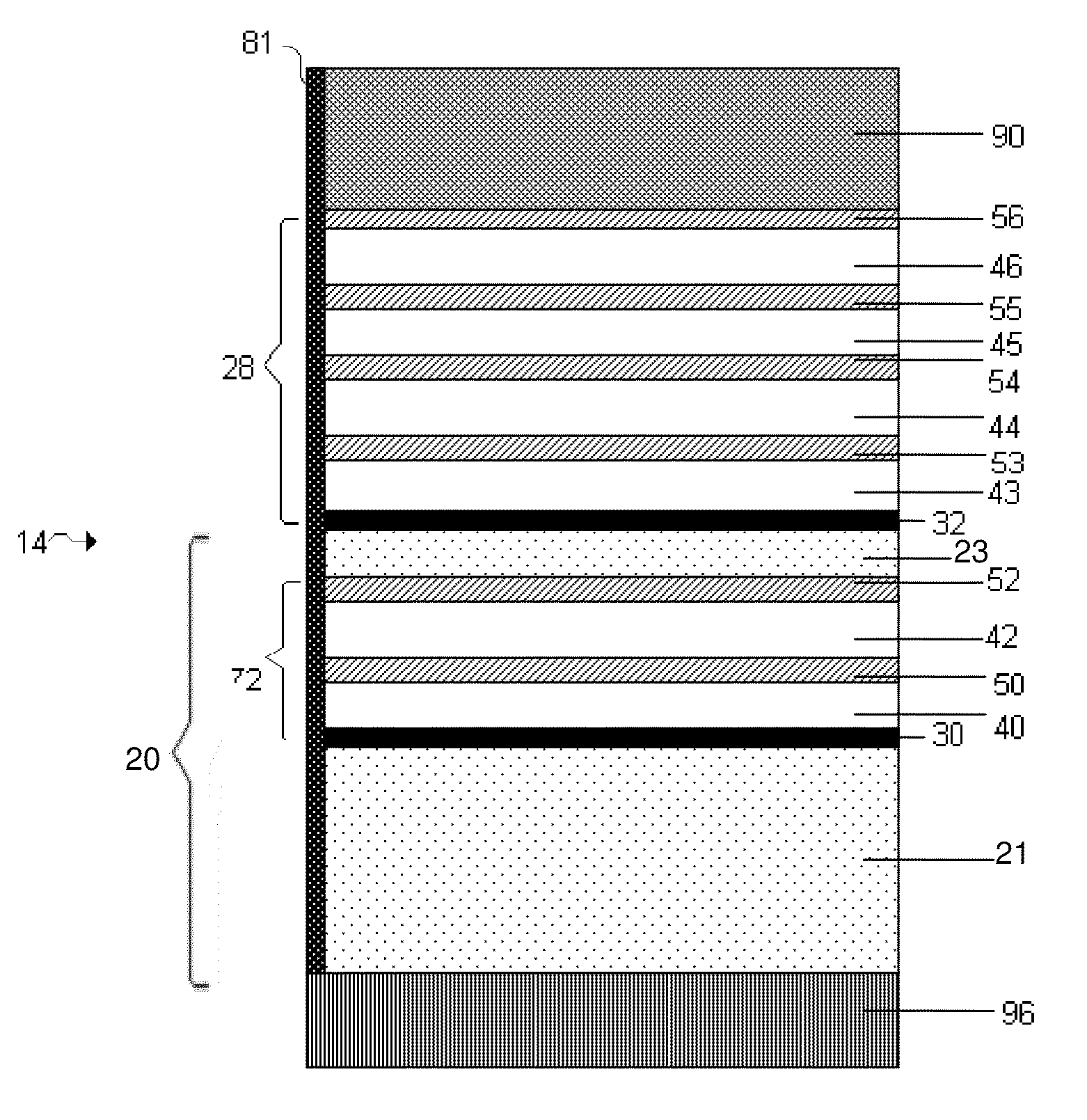
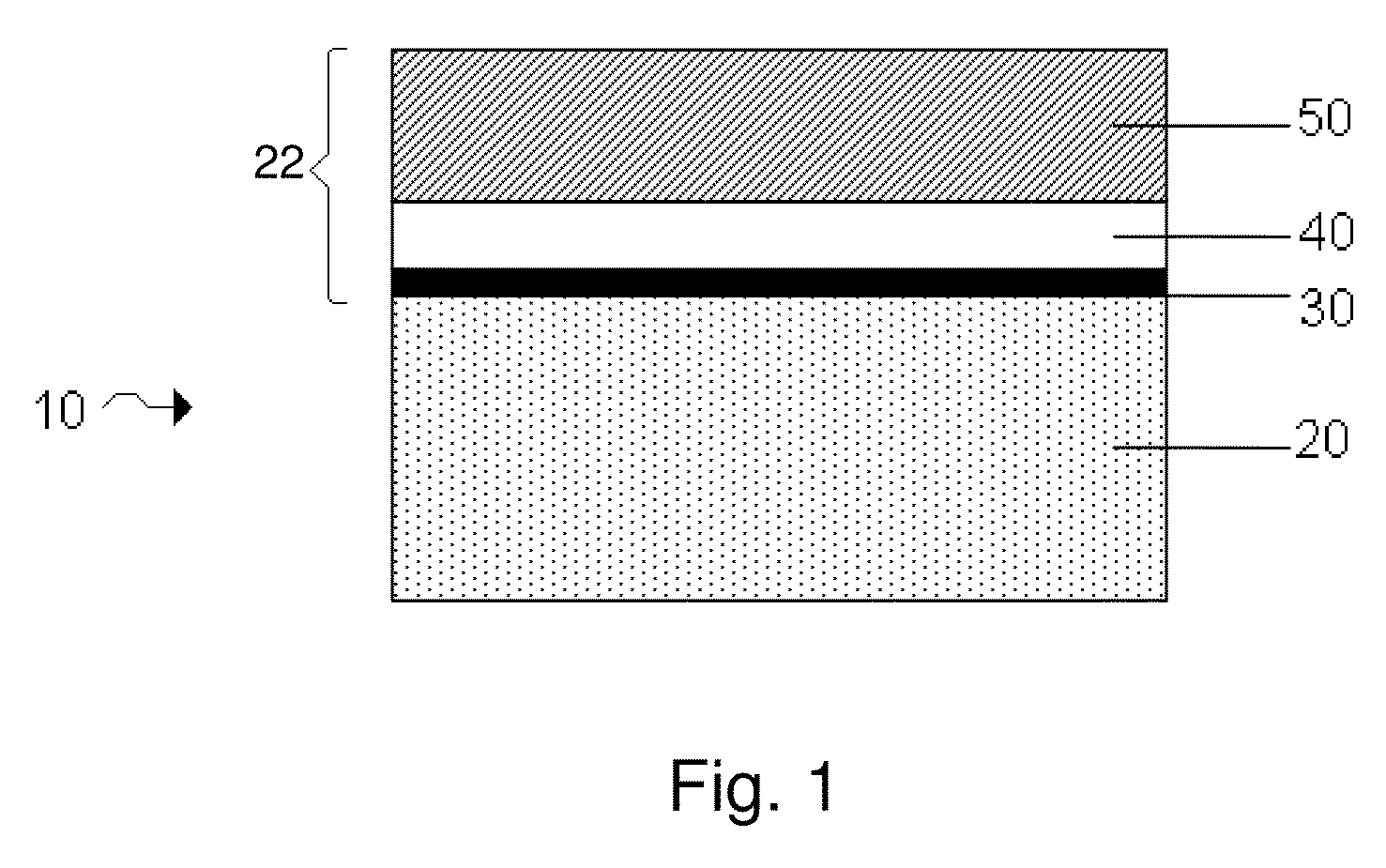
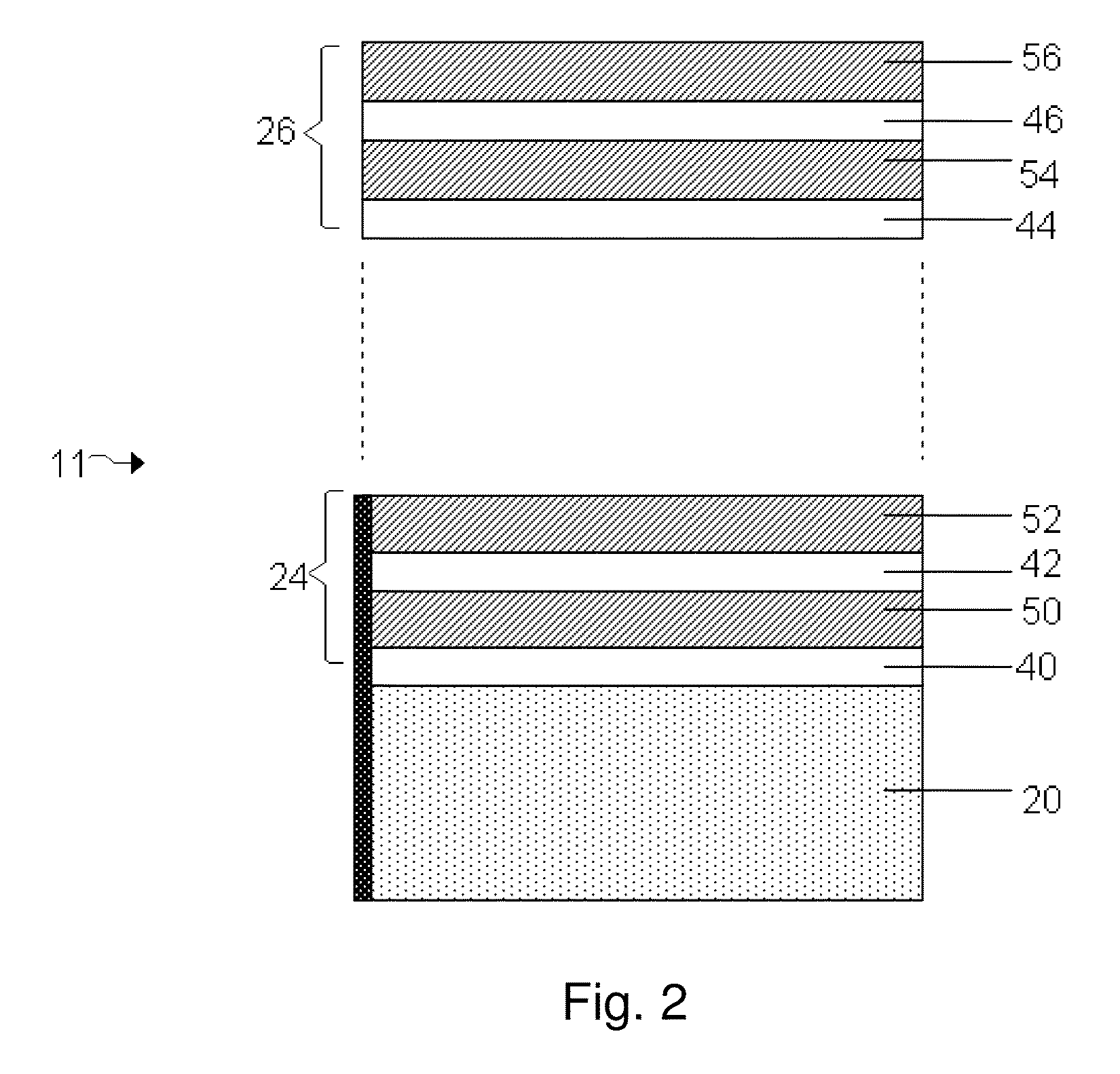
InactiveUS7335441B2Enhanced interactionReduce in quantityFinal product manufactureElectrode carriers/collectorsSelf formingThin layer
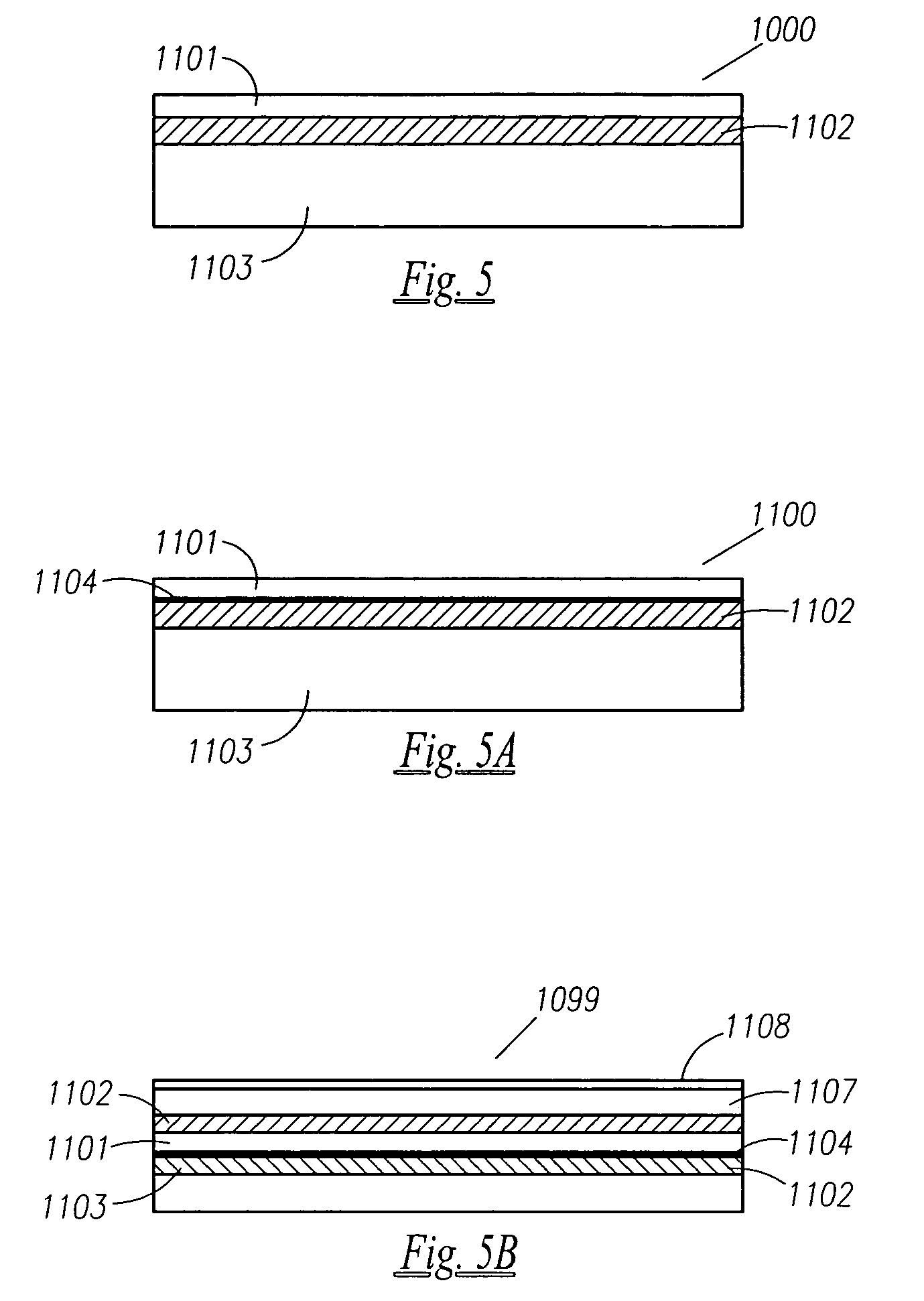
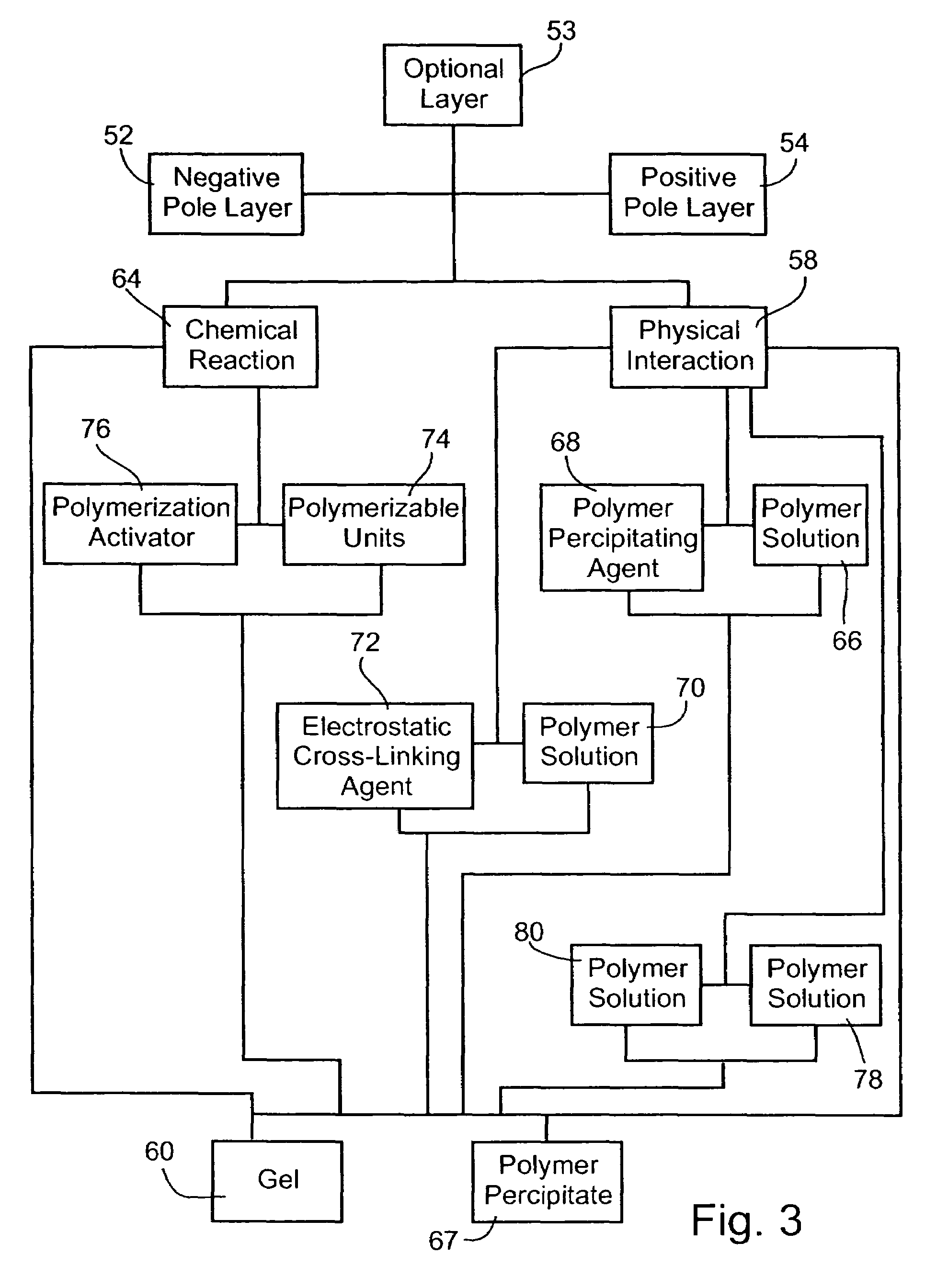
A method of forming an electrochemical cell is disclosed. The method comprises contacting a negative pole layer and a positive pole layer one with the other or with an optional layer interposed therebetween. The pole layers and the optional layer therebetween are selected so as to self-form an interfacial separator layer between the pole layers upon such contacting.
Owner:POWER PAPER
Thin layer electrochemical cell with self-formed separator
InactiveUS7022431B2Reduce manufacturing stepsIncrease contactFinal product manufactureElectrode carriers/collectorsSelf formingThin layer
A method of forming an electrochemical cell is disclosed. The method comprises contacting a negative pole layer and a positive pole layer one with the other or with an optional layer interposed therebetween. The pole layers and the optional layer therebetween are selected so as to self-form an interfacial separator layer between the pole layers upon such contacting.
Owner:POWER PAPER
Electrode protection in both aqueous and non-aqueous electrochemical cells, including rechargeable lithium batteries
ActiveUS20090291353A1Improve protectionEasy to controlFinal product manufacturePrimary cellsHigh energyConductive materials
Electrode protection in electrochemical cells, and more specifically, electrode protection in both aqueous and non-aqueous electrochemical cells, including rechargeable lithium batteries, are presented. In one embodiment, an electrochemical cell includes an anode comprising lithium and a multi-layered structure positioned between the anode and an electrolyte of the cell. A multi-layered structure can include at least a first single-ion conductive material layer (e.g., a lithiated metal layer), and at least a first polymeric layer positioned between the anode and the single-ion conductive material. The invention also can provide an electrode stabilization layer positioned within the electrode, i.e., between one portion and another portion of an electrode, to control depletion and re-plating of electrode material upon charge and discharge of a battery. Advantageously, electrochemical cells comprising combinations of structures described herein are not only compatible with environments that are typically unsuitable for lithium, but the cells may be also capable of displaying long cycle life, high lithium cycling efficiency, and high energy density.
Owner:SION POWER CORP
Water based lithium secondary battery
InactiveUS20100136427A1Large capacityInhibit currentAlkaline accumulatorsCell electrodesWater basedStrong acids
The present invention provides a water based lithium secondary battery that can inhibit deteriorations in capacity owing to charge-and-discharge operations and maintain a high capacity even after it is charged and discharged repeatedly. The water based lithium secondary battery includes a positive electrode, a negative electrode, a separator sandwiched between these, and an aqueous electrolyte solution obtained by dissolving an electrolyte made of a lithium salt in a water based solvent. As the water based solvent, a pH buffer solution is employed. The buffer solution is obtained by dissolving an acid and its conjugate base's salt, a base and its conjugate acid's salt, a salt made from a weak acid and a strong base, a salt made from a weak base and a strong acid, or a salt made from a weak acid and a weak base in water.
Owner:TOYOTA CENT RES & DEV LAB INC
Aqueous secondary battery
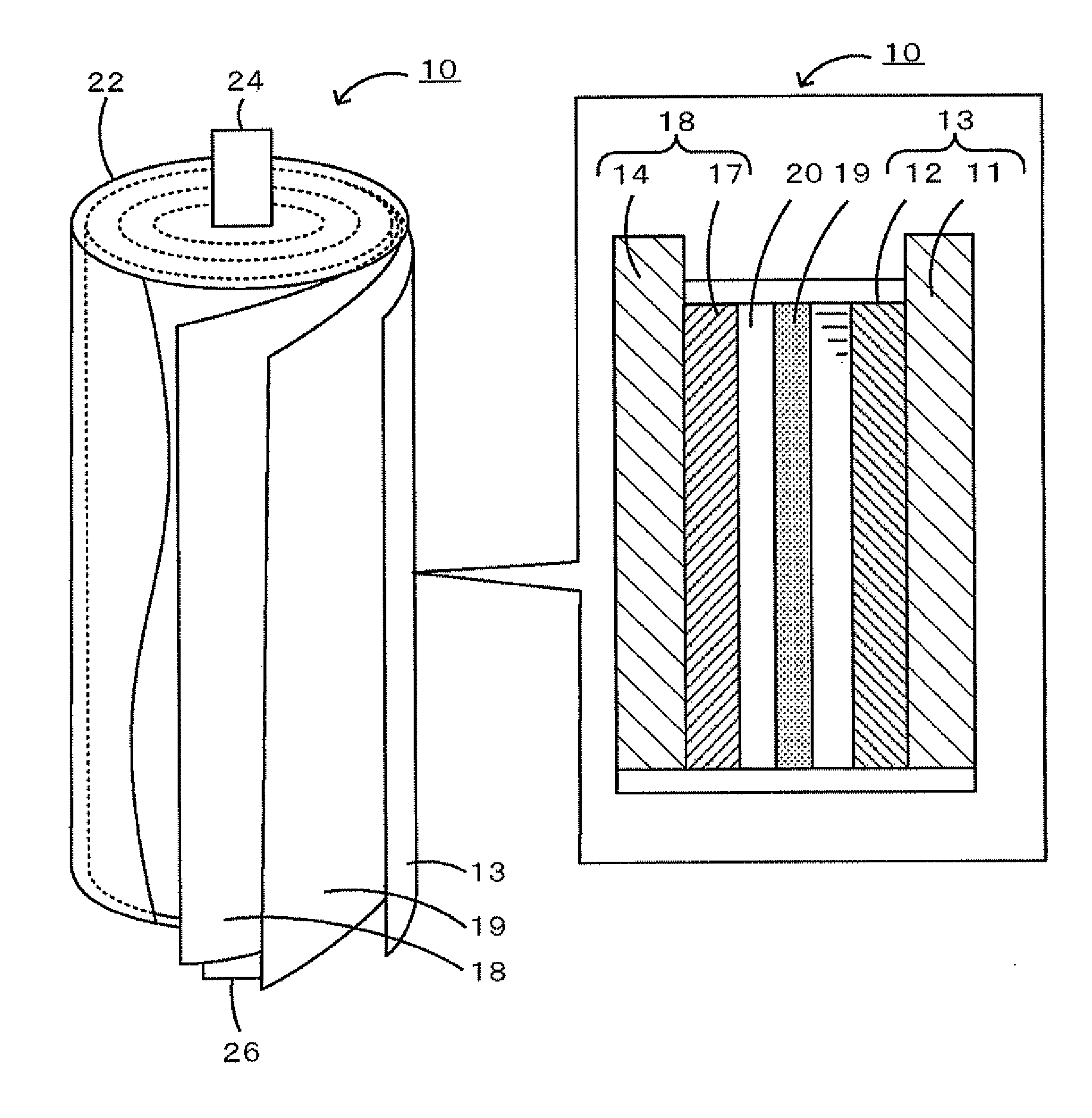
ActiveUS20110086266A1Increase energy densityLittle interactionAlkaline accumulatorsCell electrodesAqueous solutionElectrolyte
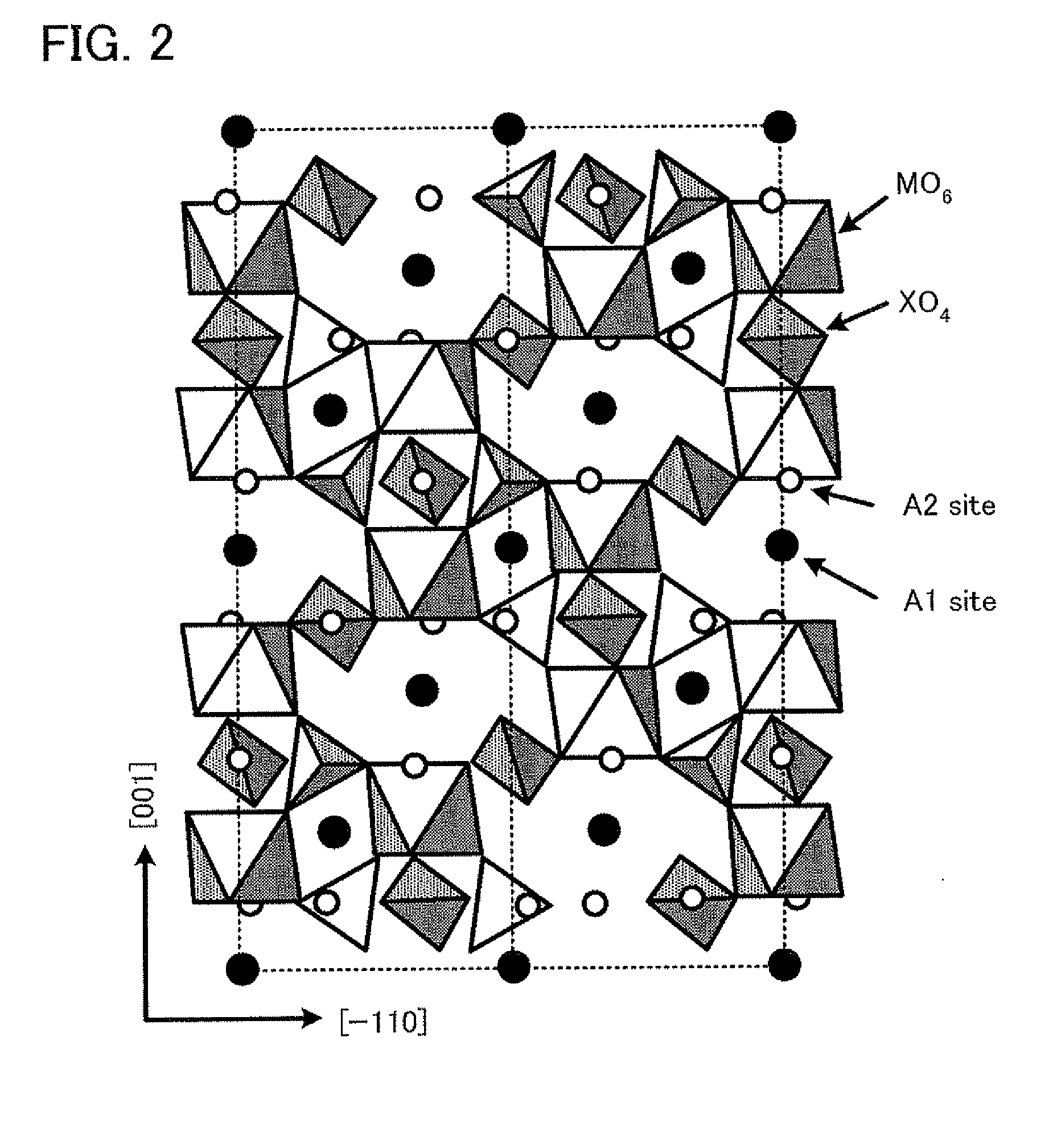
An aqueous secondary battery 10 according to the present invention includes a positive electrode containing a NASICON-type positive-electrode active material that can insert and extract sodium as a positive-electrode active material 12, a negative electrode containing a negative-electrode active material 17 that can insert and extract sodium, and an electrolyte solution 20 disposed between the positive electrode and the negative electrode, the electrolyte solution 20 being an aqueous solution in which sodium is dissolved. The NASICON-type positive-electrode active material is, for example, Na3V2(PO4)3, and the electrolyte solution 20 is an aqueous solution in which sodium is dissolved. The negative-electrode active material 17 is preferably a NASICON-type negative-electrode active material (for example, LiTi2(PO4)3 or NaTi2(PO4)3).
Owner:TOYOTA CENT RES & DEV LAB INC
Electrochemical energy cell system
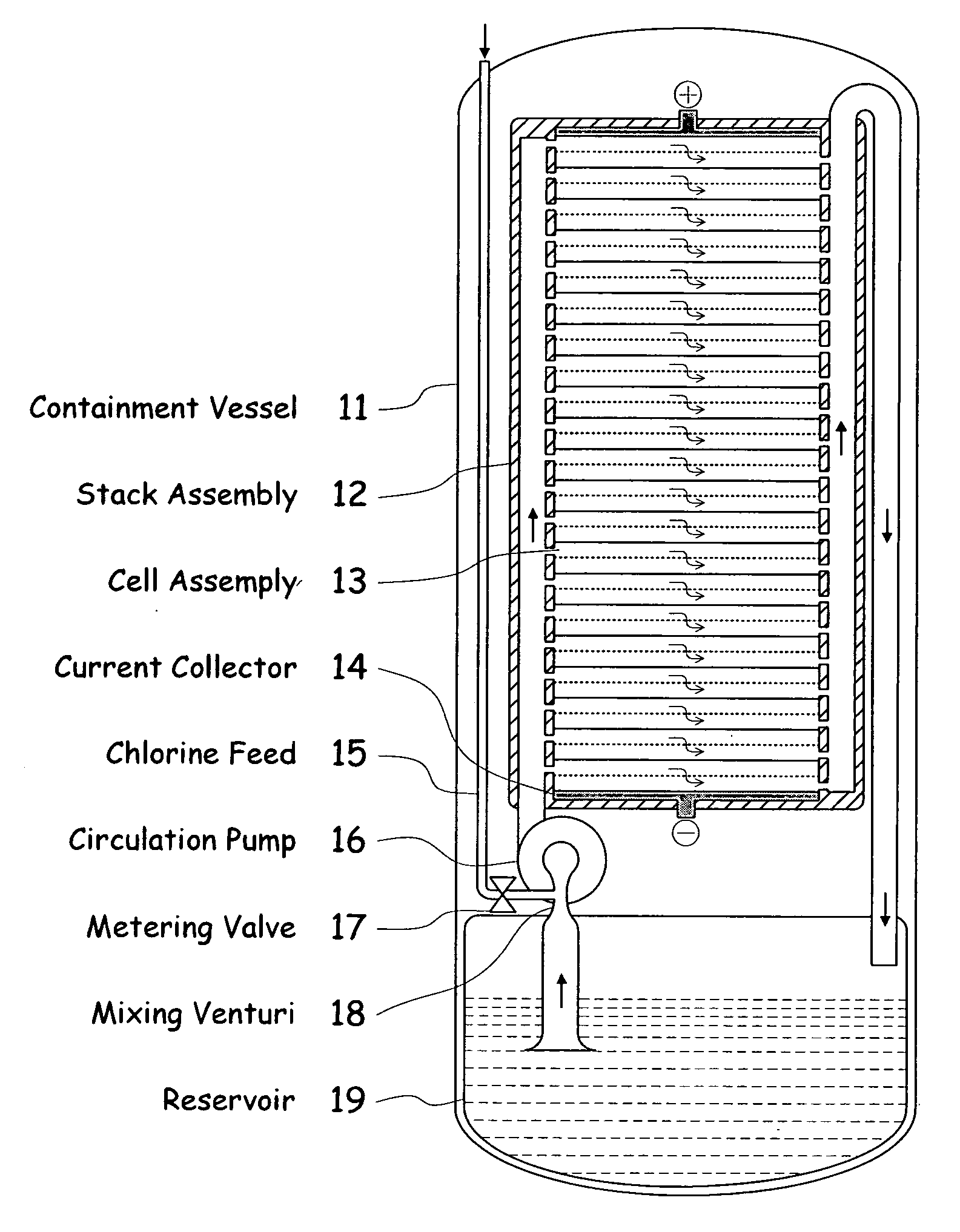
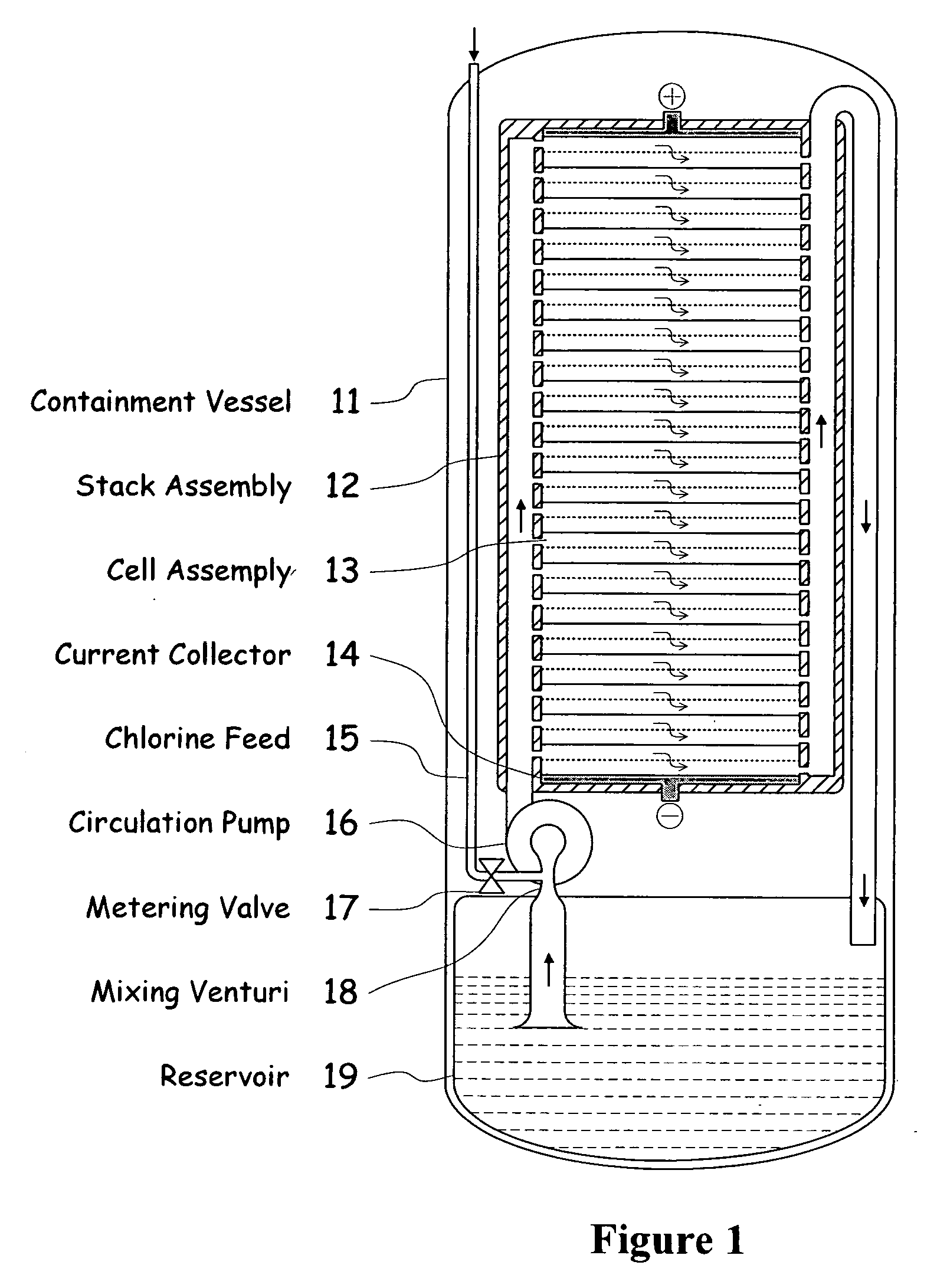
InactiveUS20090239131A1Maintain system availabilityAvoid electrochemical reactionsFuel and primary cellsElectrolyte moving arrangementsChloride electrolytesCell system
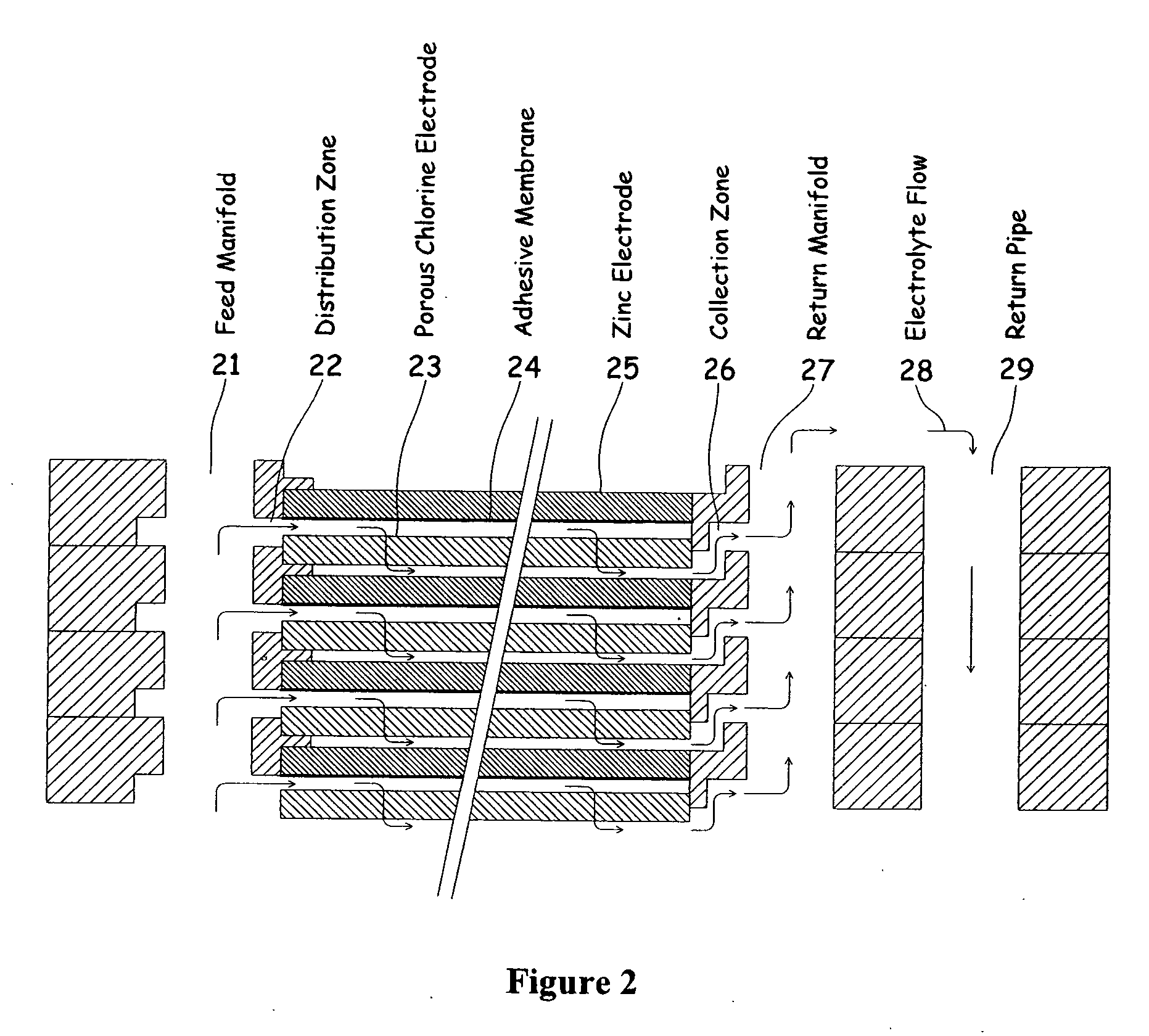
A metal halogen electrochemical energy cell system that generates an electrical potential. One embodiment of the system includes at least one cell including at least one positive electrode and at least one negative electrode, at least one electrolyte, a mixing venturi that mixes the electrolyte with a halogen reactant, and a circulation pump that conveys the electrolyte mixed with the halogen reactant through the positive electrode and across the metal electrode. Preferably, the negative electrodes are made of zinc, the metal is zinc, the positive electrodes are made of porous carbonaceous material, the halogen is chlorine, the electrolyte is an aqueous zinc-chloride electrolyte, and the halogen reactant is a chlorine reactant. Also, variations of the system and a method of operation for the systems.
Owner:PRIMUS POWER CORP
Rechargeable zinc-ion batteries having flexible shape memory
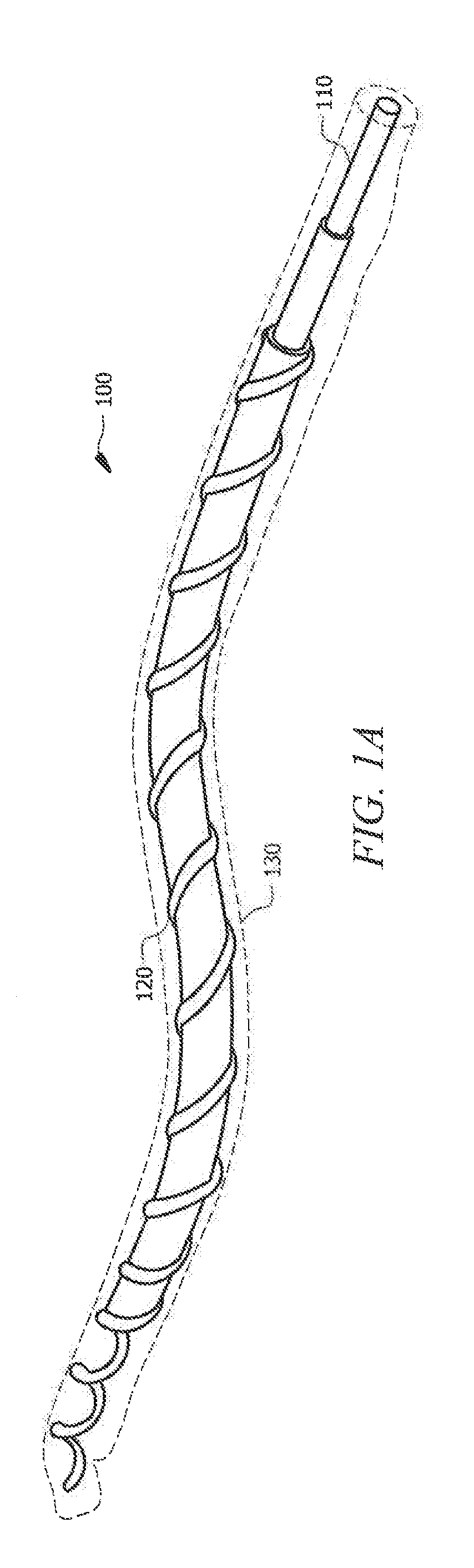
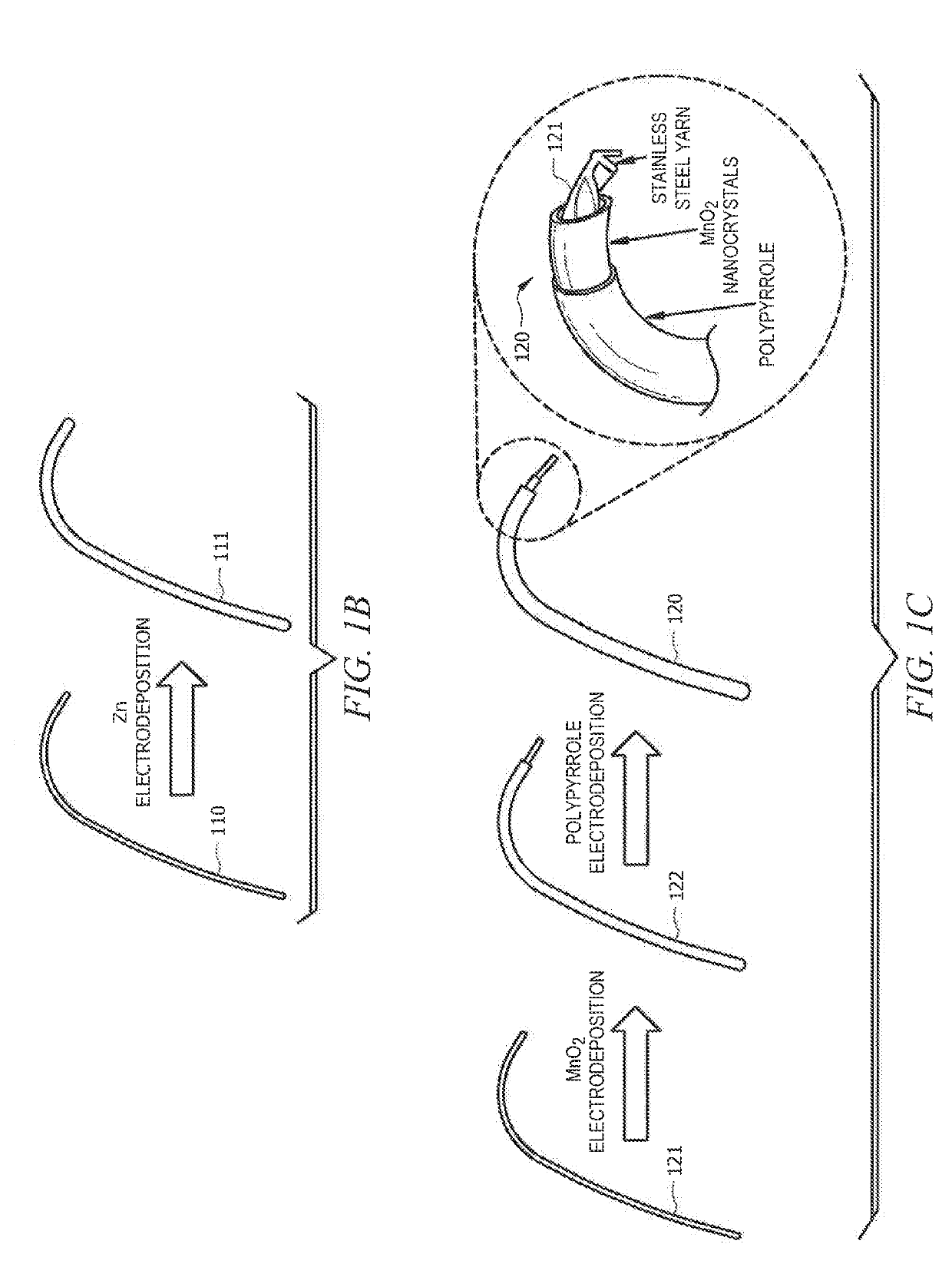
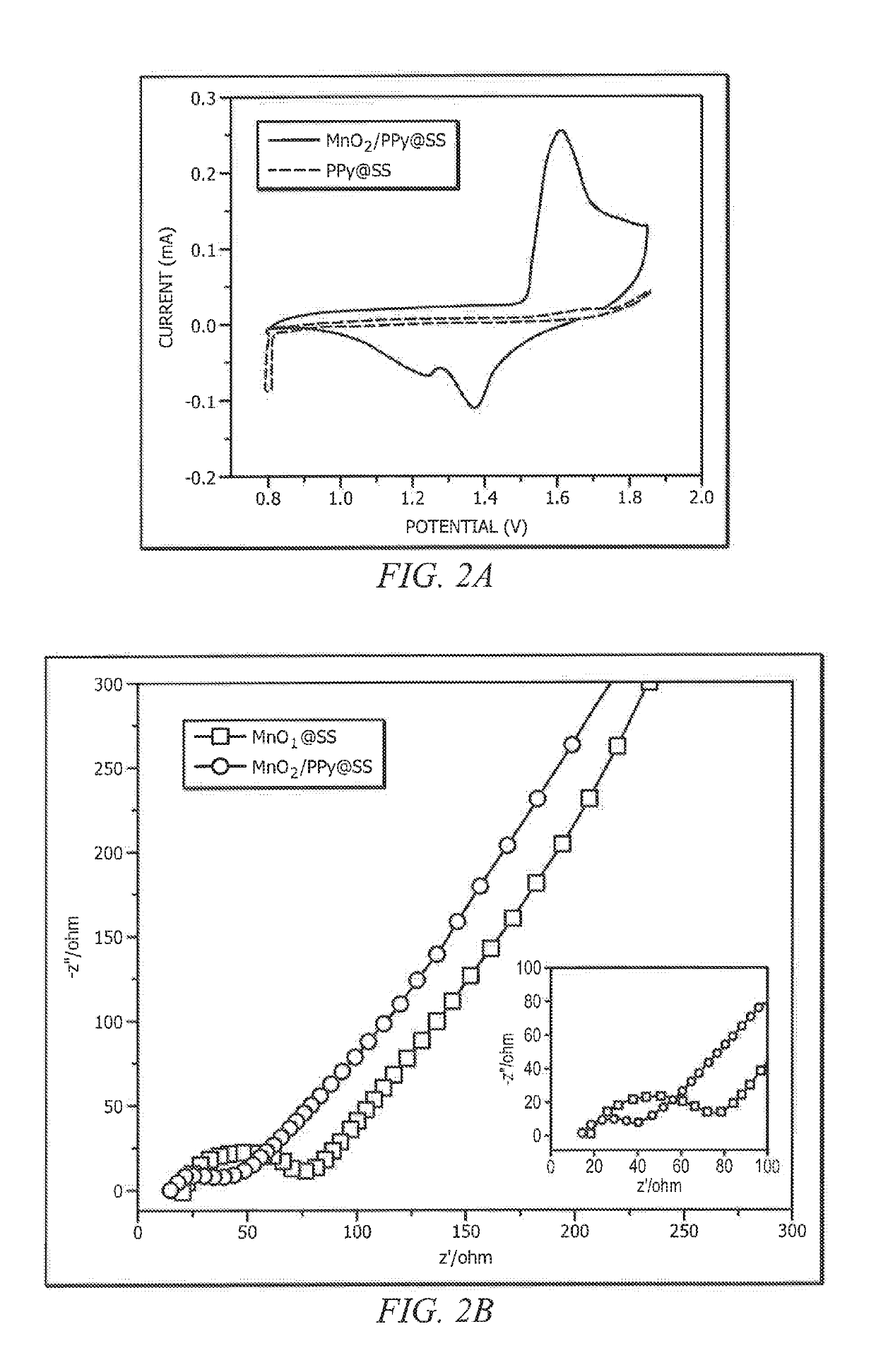
ActiveUS20190140270A1Increased mechanical flexibilityPromote recoveryElectrochemical processing of electrodesFinal product manufactureFiberYarn
Systems and methods which provide flexible zinc ion (Zn-ion) battery configurations with shape memory are described. For example, embodiments of flexible shape memory yarn batteries (SMYBs) may be fabricated using shape memory material wire, filament, and / or fiber and flexible conductive material yarn as flexible substrate materials. In accordance with some embodiments, Nickel-Titanium-based alloy wire may be coated with a zinc material to provide a flexible anode electrode for a SMYB. Additionally or alternatively, flexible stainless steel (SS) yarn may be coated with a manganese dioxide (MnO2) material to provide a flexible cathode electrode for a SMYB of embodiments. An aqueous electrolyte may be combined with the flexible cathode and anode electrodes to provide a SMYB in accordance with the concepts herein. The aqueous electrolyte may, for example, comprise a polymer gel electrolyte (e.g., gelatin-borax polymer gel electrolyte).
Owner:CITY UNIVERSITY OF HONG KONG
Sodium Based Aqueous Electrolyte Electrochemical Secondary Energy Storage Device
ActiveUS20110052945A1Hybrid capacitor electrolytesCapacitor and primary/secondary cellsSODIUM CATIONAqueous electrolyte
A secondary hybrid aqueous energy storage device includes an anode electrode, a cathode electrode which is capable of reversibly intercalating sodium cations, a separator, and a sodium cation containing aqueous electrolyte, wherein an initial active cathode electrode material comprises an alkali metal containing active cathode electrode material which deintercalates alkali metal ions during initial charging of the device.
Owner:AQUION ENERGY
Chargeable zinc ion battery and method for manufacturing same
ActiveCN104272523AImproved high current characteristicsImprove cycle lifeFinal product manufactureElectrode carriers/collectorsElectrical batteryManganese
The invention discloses a chargeable zinc ion battery and a method for manufacturing same. The chargeable zinc ion battery comprises an anode, a cathode and electrolyte, wherein the active material of the cathode contains Zn. The chargeable zinc ion battery is characterized in that the active material of the anode contains carbon based manganese dioxide composite material which means attaching manganese dioxide material onto the surface of carbon based material. The carbon based manganese dioxide composite material added anode material of the zinc ion battery improves the heavy current property of the battery, and prolongs the circulation life of the battery. Mn.2+ added in the electrolyte co-reacts with the carbon based manganese dioxide composite material to increase the battery capacity.
Owner:SHENZHEN CITY THROUGH SCI & TECH OF NEW ENERGY CO LTD
Cosolvent electrolytes for electrochemical devices
ActiveUS20140308544A1Control rateHigh voltageOrganic electrolyte cellsSecondary cells charging/dischargingHydrogenDissolution
A system and method for stabilizing electrodes against dissolution and / or hydrolysis including use of cosolvents in liquid electrolyte batteries for three purposes: the extension of the calendar and cycle life time of electrodes that are partially soluble in liquid electrolytes, the purpose of limiting the rate of electrolysis of water into hydrogen and oxygen as a side reaction during battery operation, and for the purpose of cost reduction.
Owner:NATRON ENERGY INC
Functionally improved battery and method of making same
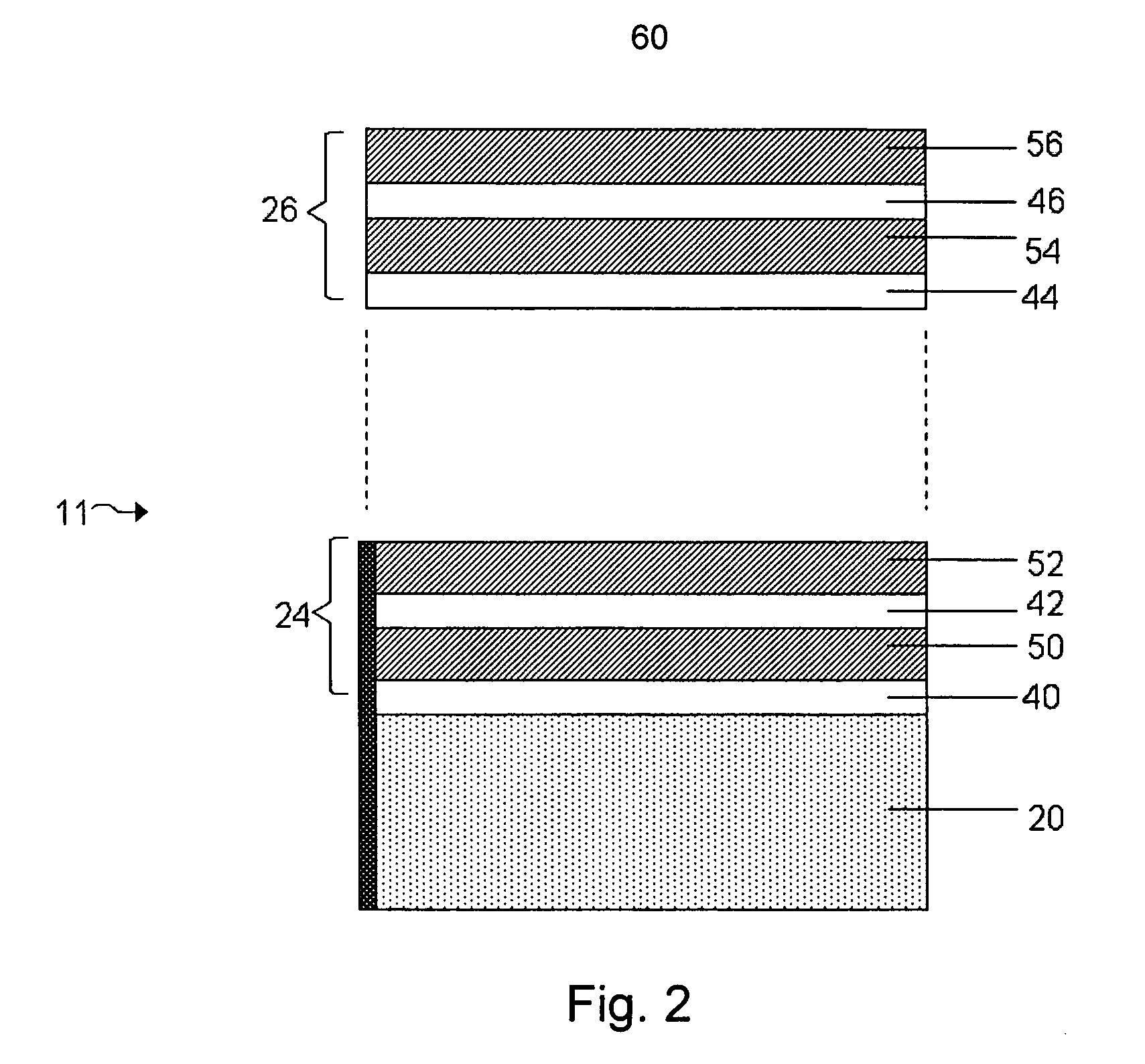
InactiveUS20050118464A1Extended service lifePower outputBatteries circuit arrangementsFinal product manufactureLiquid stateThin layer
A functionally improved battery is disclosed. The battery includes a flexible thin layer open liquid state electrochemical cell and an electronic chip device integrally formed on or within the electrochemical cell. The cell includes a first layer of insoluble negative pole, a second layer of insoluble positive pole and a third layer of aqueous electrolyte. The third layer is disposed between the first and second layers. The third layer includes and includes a deliquescent material for keeping the cell wet, an electroactive soluble material for ionic conductivity and a watersoluble polymer for viscosity. The viscosity adheres the first and second layer to the third layer. The chip serves to improves a functionality of the battery.
Owner:POWER PAPER
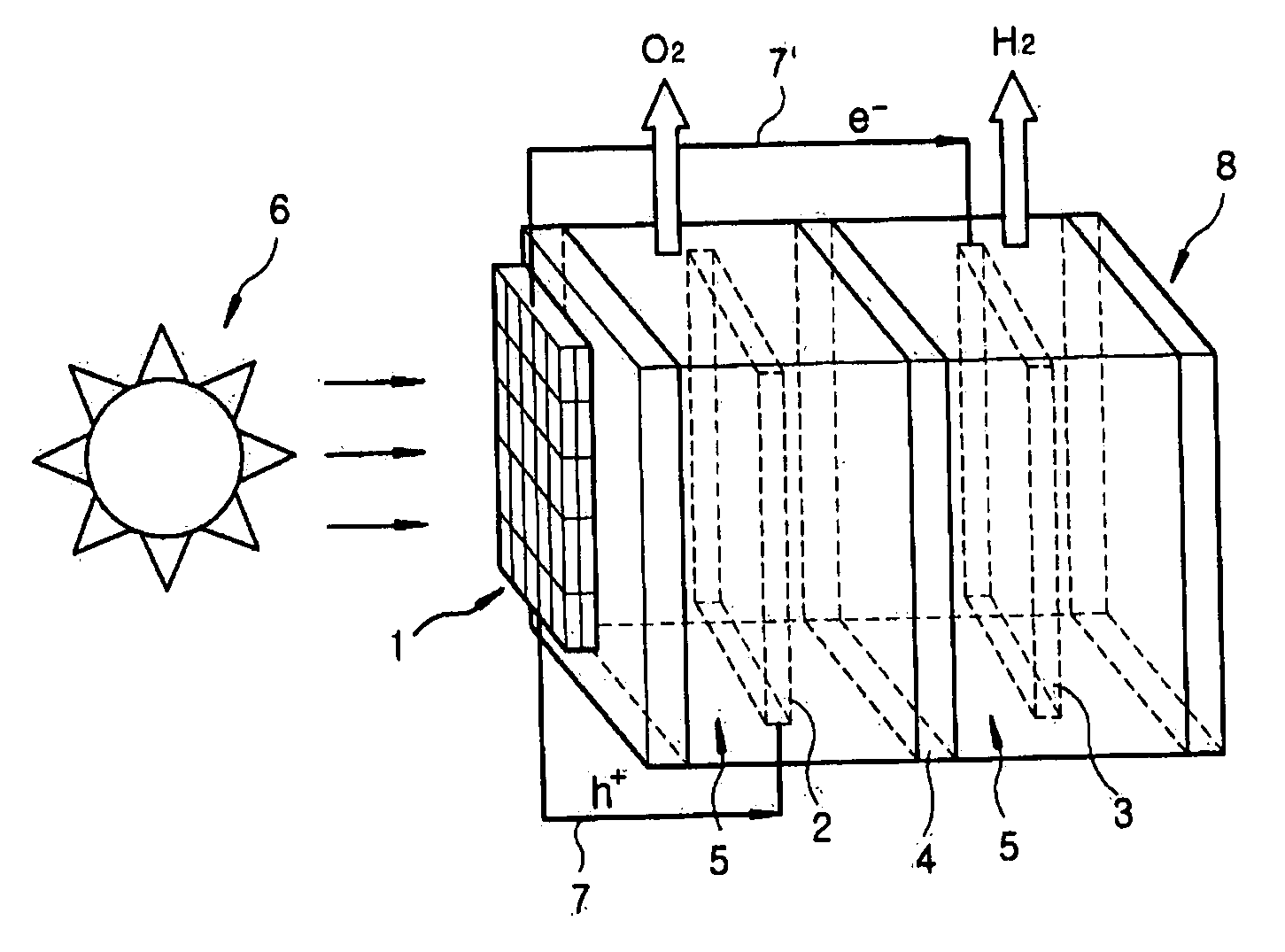
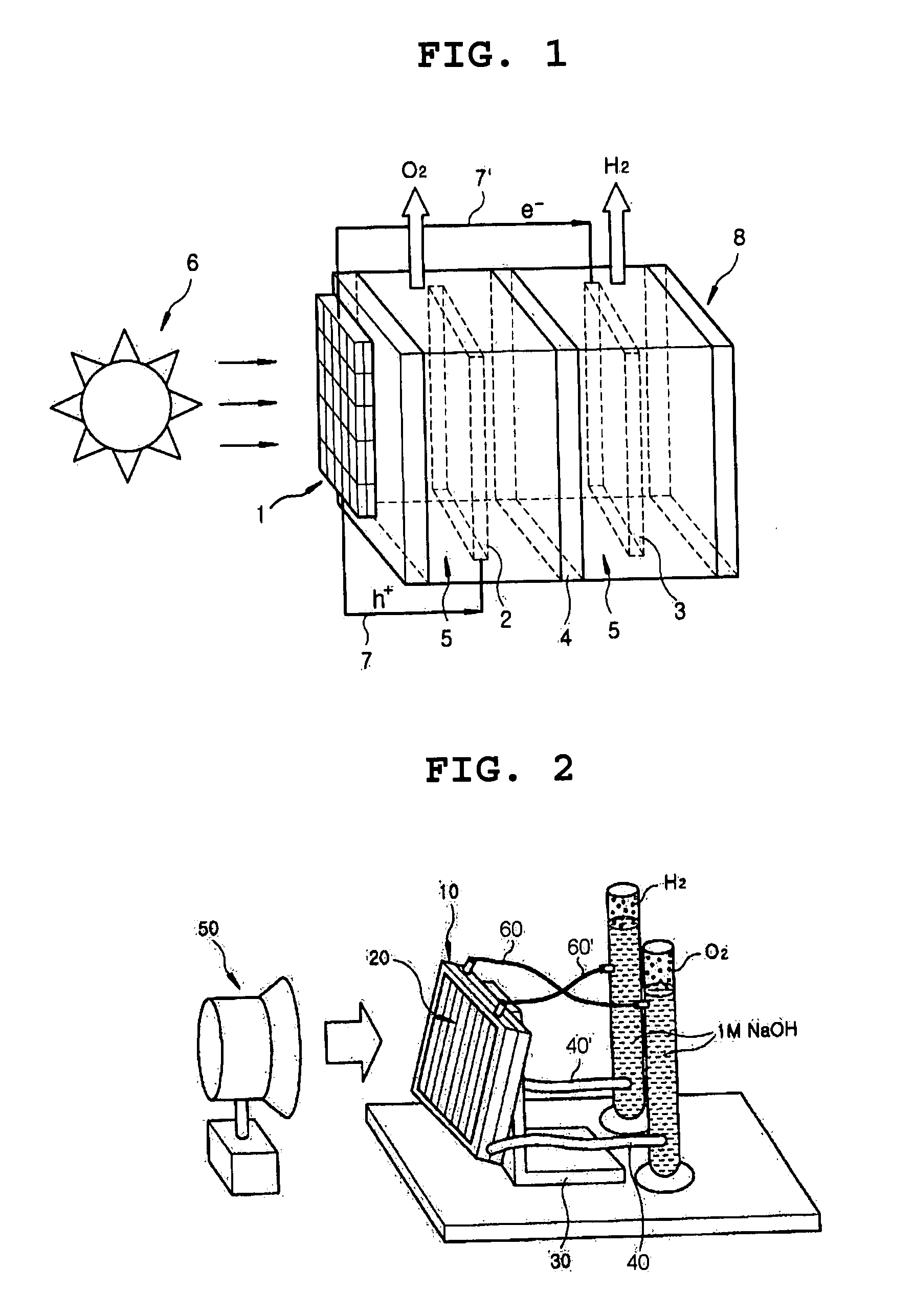
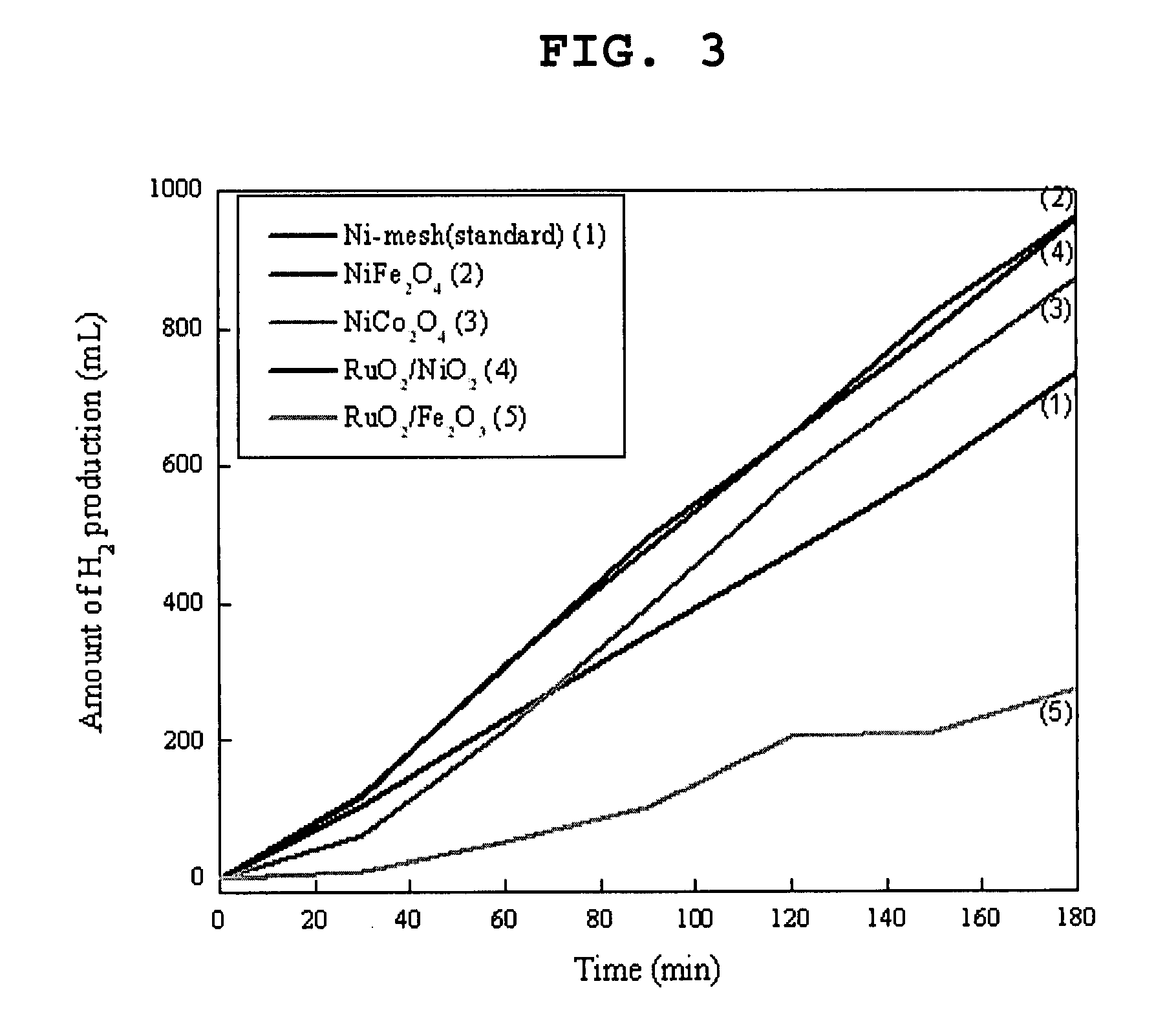
Photoelectrochemical system for hydrogen production from water
The present invention provides a photoelectrochemical (PEC) system for the production of hydrogen from water, which comprises (A) an electrolytic bath comprising an electrode for catalytic oxidation, an electrode for catalytic reduction, an ion separation film disposed between the two electrodes, and an aqueous electrolyte solution into which the two electrodes and the ion separation film are immersed, and (B) a photoelectrode positioned at the outside of the electrolytic bath and electrically connected to the two electrodes. The inventive PEC system is characterized by disposing a photoelectrode at the position which does not contact aqueous electrolyte solution, thus preventing the lowering of the photoelectrode activities, and maximizing the hydrogen production efficiency.
Owner:KOREA INST OF SCI & TECH
Fluoride ion electrochemical cell
The present invention provides electrochemical cells capable of good electronic performance, particularly high specific energies, useful discharge rate capabilities and good cycle life. Electrochemical cells of the present invention are versatile and include primary and secondary cells useful for a range of important applications including use in portable electronic devices. Electrochemical cells of the present invention also exhibit enhanced safety and stability relative to conventional state of the art primary lithium batteries and lithium ion secondary batteries. For example, electrochemical cells of the present invention include secondary electrochemical cells using anion charge carriers capable of accommodation by positive and negative electrodes comprising anion host materials, which entirely eliminate the need for metallic lithium or dissolved lithium ion in these systems.
Owner:CALIFORNIA INST OF TECH +1
Rechargeable aqueous zinc ion battery
ActiveCN107221716AImprove cycle stabilityLow equipment requirementsFinal product manufactureCell electrodesHigh rateElectrical battery
The invention belongs to the field of a novel chemical power supply and a new energy material, and particularly relates to a novel rechargeable aqueous zinc ion battery taking a vanadate salt as an electrode active material. The rechargeable aqueous zinc ion battery comprises a positive electrode, a negative electrode, a separator and an electrolyte, wherein the separator is arranged between the positive electrode and the negative electrode, the electrolyte contains negative ions and positive ions and has ion conductivity, an active material of the positive electrode takes the vanadate salt to which zinc ions are intercalated / de-intercalated as main, the electrolyte takes a zinc soluble salt as a solute and water as a solvent, the concentration of the electrolyte is (0.1-5)mol / L, and the electrolyte is a liquid-state or gel-state material with ion conductivity. The material shows excellent high-rate performance, cycle stability and long lifetime when assembled to form the aqueous zinc ion battery, is a potential application material of the high-power and long-lifetime zinc ion battery and has a wide application prospect in an aspect of energy storage on a large scale.
Owner:WUHAN UNIV OF TECH
Multi-stage micro-nano structural material, preparation method thereof, battery containing multi-stage micro-nano structural material
ActiveCN103746112AExcellent energy storageHigh specific capacityMaterial nanotechnologyCell electrodesMicro nanoHigh energy
The invention discloses a material with a multi-stage micro-nano structural material on surface. The multi-stage micro-nano structural material comprises a porous conductive substrate, and primary nano-rods or primary micron-piece arrays, wherein the primary nano-rods or primary micron-piece arrays grow vertical to the substrate, a plurality of ferroferric oxide secondary nano-rods radially grow on each primary micron-rod, or a plurality of ferroferric oxide secondary nano-rods grow perpendicular to each primary micron-piece on the corresponding micron-piece. The material can be used as a negative electrode material for a battery, in particular an alkali battery; the battery using the negative electrode material has unique advantages, such as high energy storage characteristics, large specific capacity, high energy density, high power density and high cycling performance, which can be kept very well under high-and-low current density.
Owner:BEIJING UNIV OF CHEM TECH
Lithium ion fluoride electrochemical cell
InactiveUS20140030559A1Improve performanceImprove securityElectrode manufacturing processesElectrode carriers/collectorsCharge carrierHost material
Electrochemical cells of the present invention are versatile and include primary and secondary cells useful for a range of important applications including use in portable electronic devices. Electrochemical cells of the present invention also exhibit enhanced safety and stability relative to conventional state of the art primary lithium batteries and lithium ion secondary batteries. For example, electrochemical cells of the present invention include secondary electrochemical cells using a combination of anion and cation charge carriers capable of accommodation by positive and negative electrodes independently comprising host materials.
Owner:CALIFORNIA INST OF TECH
High energy density charge-discharge lithium battery
InactiveCN102738442ACell seperators/membranes/diaphragms/spacersCell electrodesLithium metalHigh energy
The invention, belonging to the technical field of electrochemistry, particularly discloses a high energy density charge-discharge lithium battery. The lithium battery comprises a separator, a cathode, an anode and an electrolyte, wherein the separator is solid, lithium ions can reversibly pass through the separator, the cathode is made of lithium metal or lithium alloy, the electrolyte of the cathode side comprises common organic electrolyte, polymer electrolyte, ionic liquid electrolyte or a mixture thereof, the anode is made of common cathode material of a lithium ion battery, and the electrolyte of the anode side comprises an aqueous solution containing lithium salt or a hydrogel electrolyte. Compared with the traditional lithium ion battery, the charge-discharge lithium battery disclosed by the invention has higher voltage, and the energy density of the charge-discharge lithium battery is more than 30% higher. The high energy density charge-discharge lithium battery can be used in the fields of electricity storage and release, etc.
Owner:FUDAN UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com