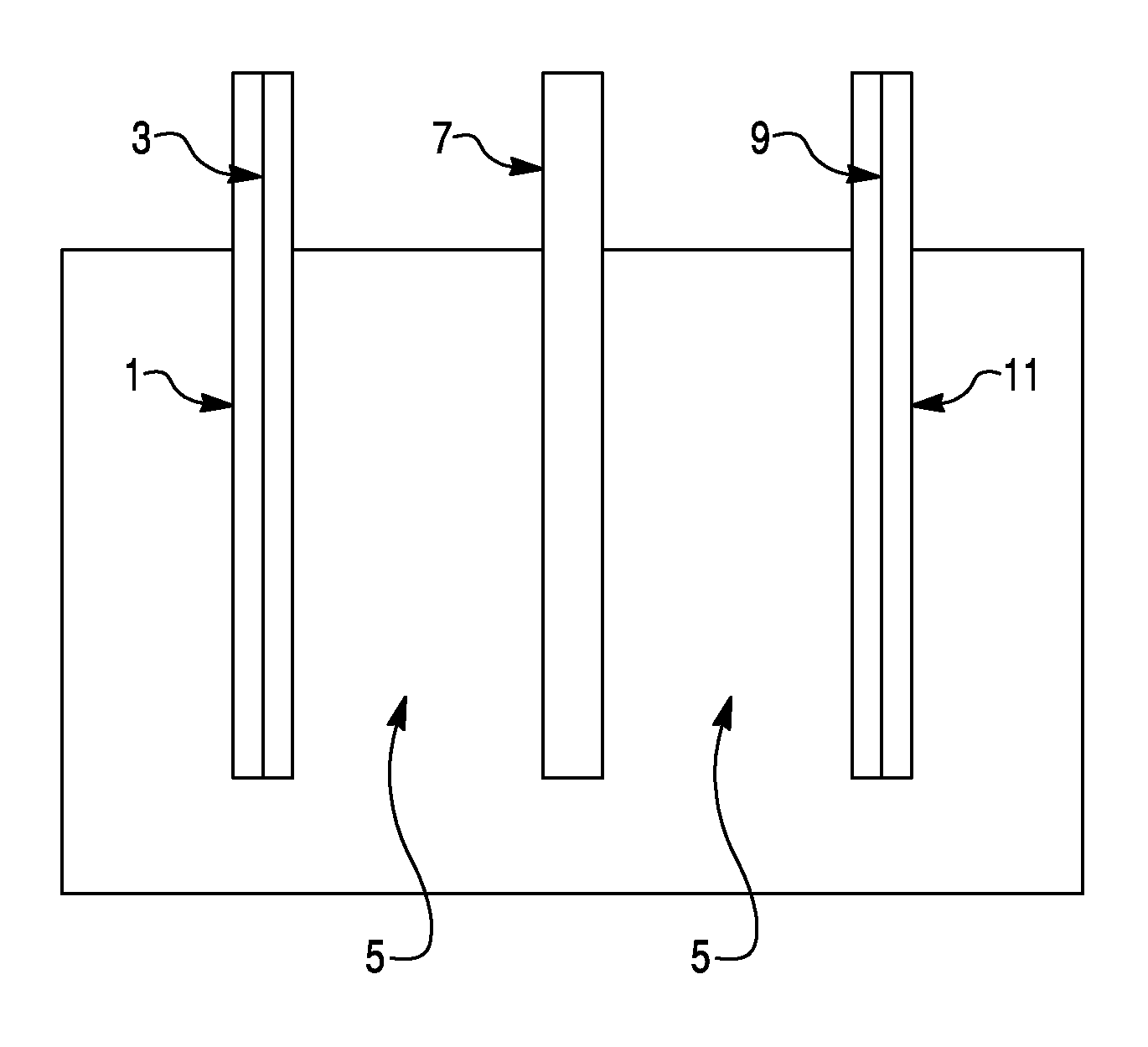
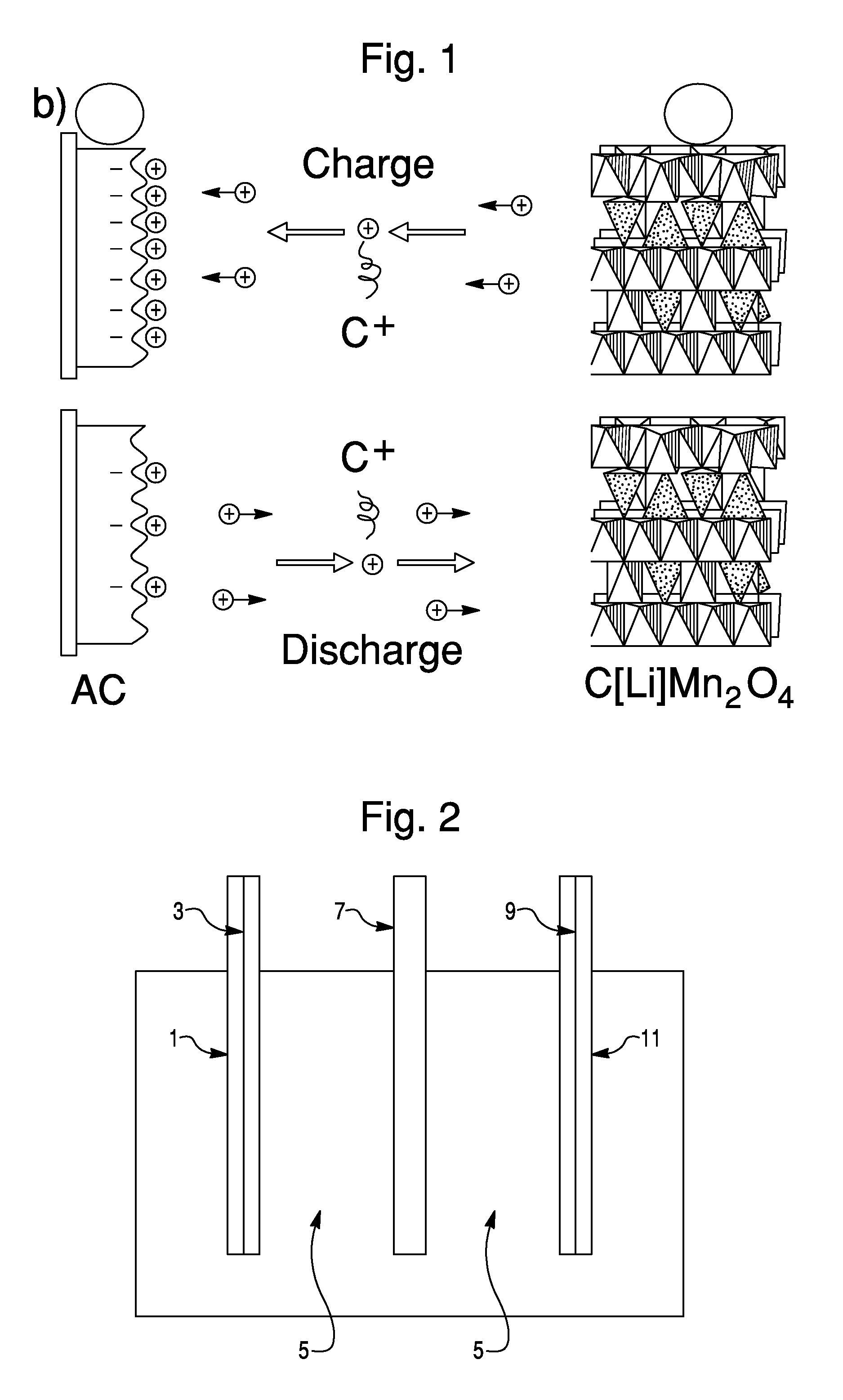
Sodium ion based aqueous electrolyte electrochemical secondary energy storage device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0090]A test cell was constructed with a λ-MO2-type active cathode material versus an activated carbon anode material in 1 M Na2SO4 in DI H2O electrolyte.
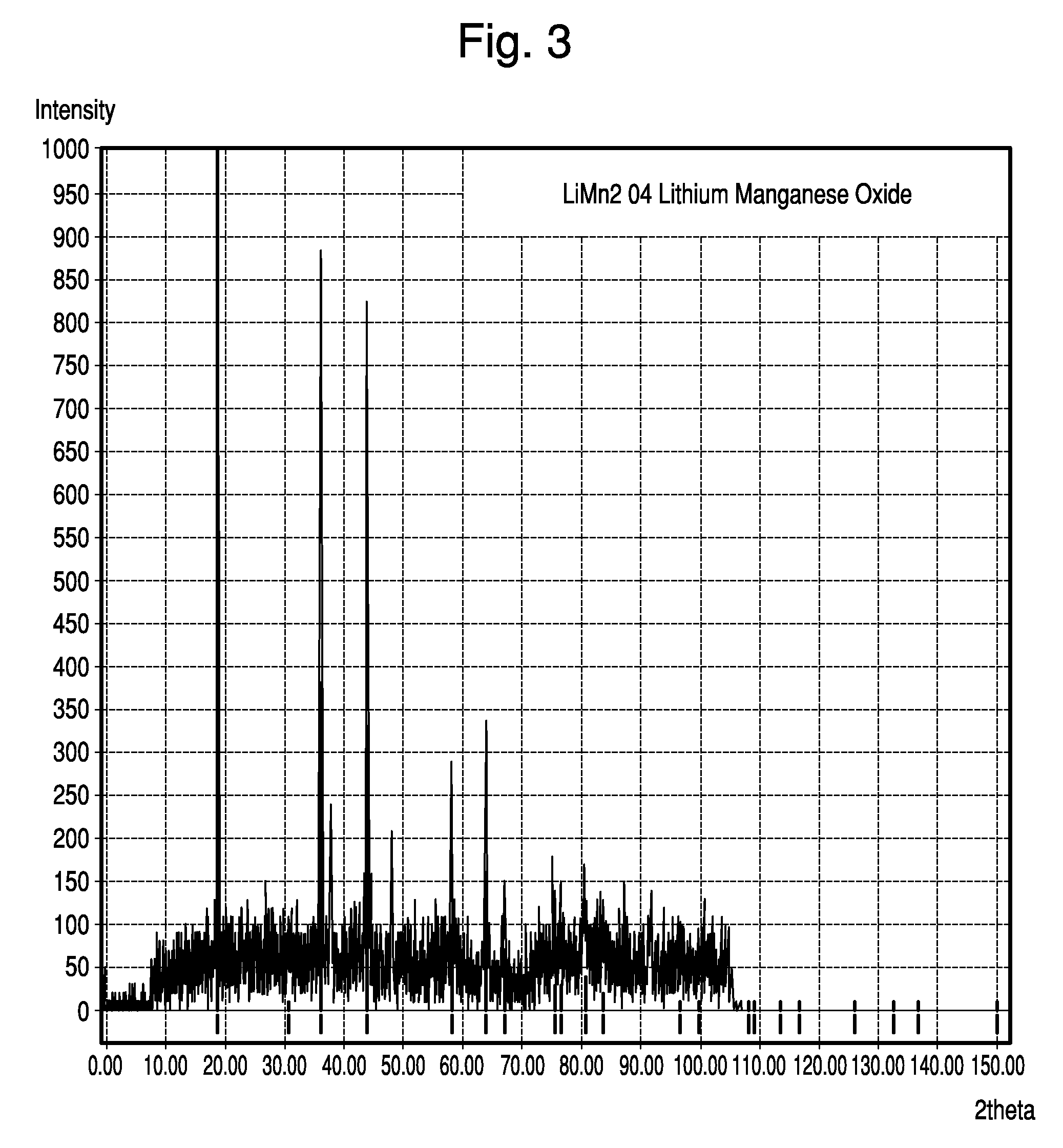
[0091]The active cathode material was made from Al-doped, Li-containing, cubic spinel MnO2. Specifically, the Li-containing cubic spinel was synthesized by thoroughly mixing Li2CO3, Mn2O3, and Al(OH)3 to proper mole ratios and firing at 750° C. for 24 hours. This material resulted in a spinel structure with the formula Li1.05Mn1.89Al0.06O4, as verified by X-ray diffraction analysis. X-ray spectra is shown in FIG. 3. As the X-ray data confirm, this material fits the well known cubic spinel LiMn2O4 structure, as archived by JCPDS card # 00-035-0782.
[0092]A composite cathode was formed by mixing about 80 wt % Li0.05Mn1.89Al0.06O4 initial active material, 10 wt % carbon black conductive diluent, and about 10% PTFE polymeric binder. This mixture was then pressed into a pellet, which was placed into a large electrochemical cell and biase...
example 2
[0107]A test cell similar to that described in Example 1 above was constructed with a NaMnO2 (birnassite structure) active cathode material, activated carbon anode material, and 1 M Na2SO4 in DI H2O electrolyte.
[0108]FIG. 19 shows the charge / discharge behavior (i.e., cell potential versus time through charge / discharge cycles) of the NaMnO2 (birnassite phase) active cathode material test cell. The system demonstrated a potential range of about 0.0 V to about 1.7 V.
example 3
[0109]A half cell similar to that described in Example 1 above was constructed with a Na2Mn3O7 (JCPDS structure: 078-0193) working electrode, a SCE reference electrode, and a Pt counter electrode. The half-cell was cycled between about −0.5 and 0.6 V vs. SCE. The data indicate that Na2Mn3O7 does display Na cation intercalation / deintercalation events and is stable between the potential range studied. The data shown in FIG. 20A show cyclic voltammargrams which demonstrate reversible capacity for Na2Mn3O7 in 1 M Na2SO4 in DI H2O electrolyte solution. FIG. 20B shows a potential versus time profile from a portion of the same test.
[0110]Results of these studies indicate that Na2Mn3O7 is a suitable active cathode material for use in embodiments of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com