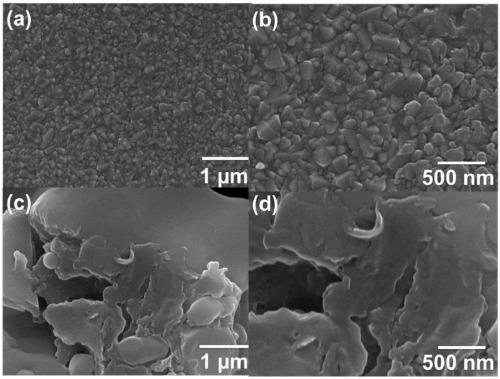
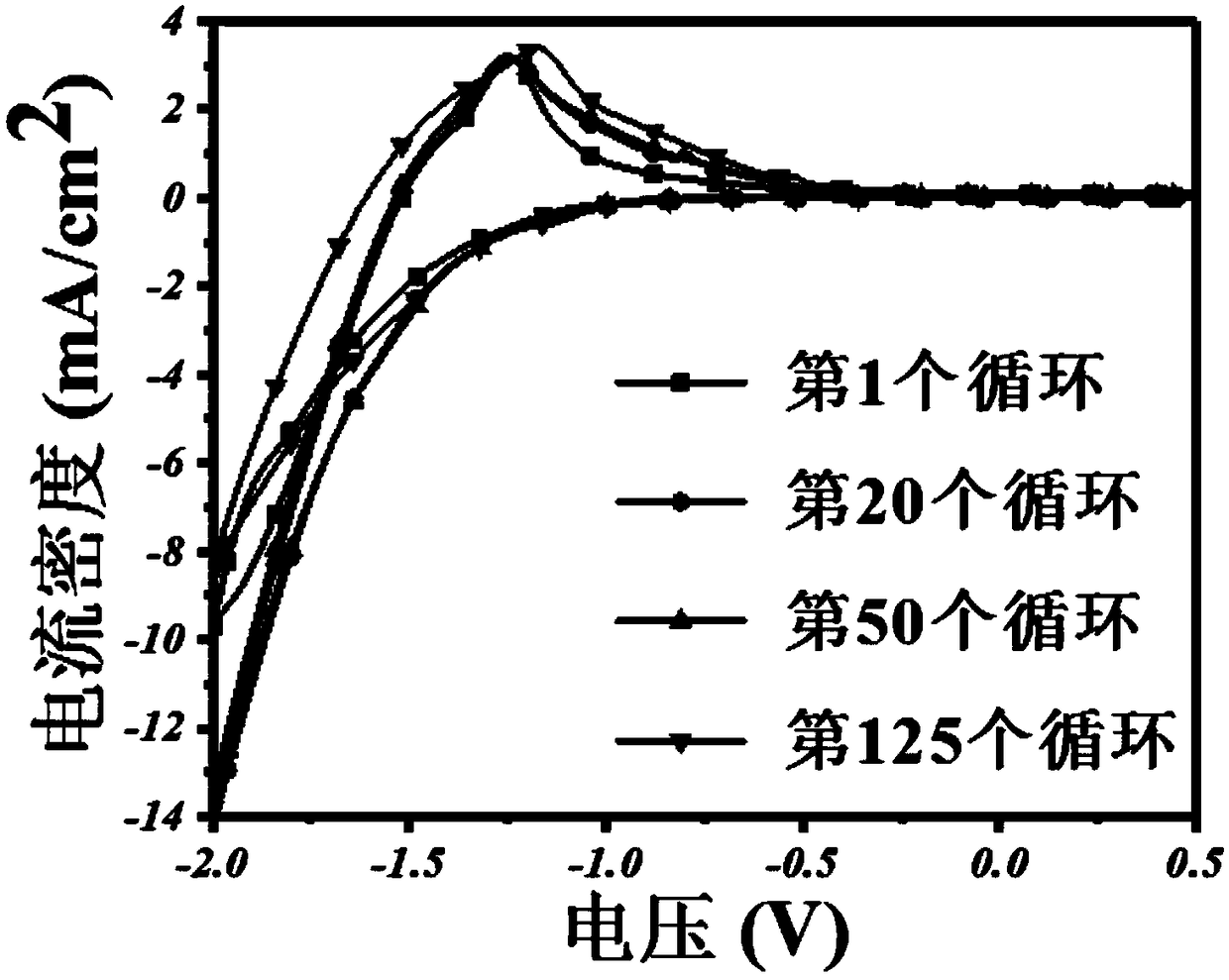
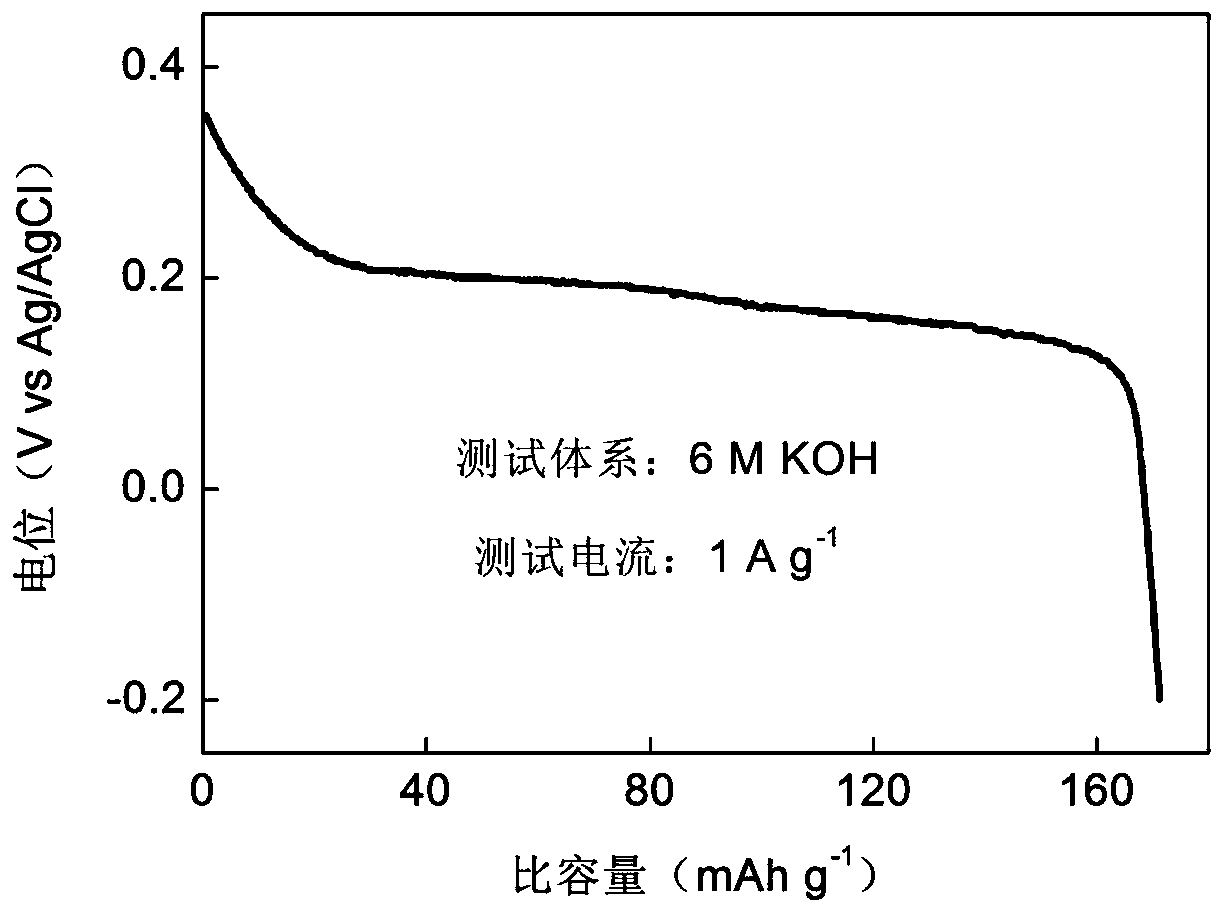
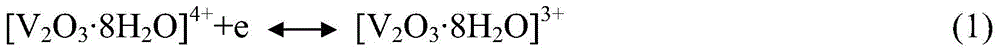Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Chloride electrolytes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
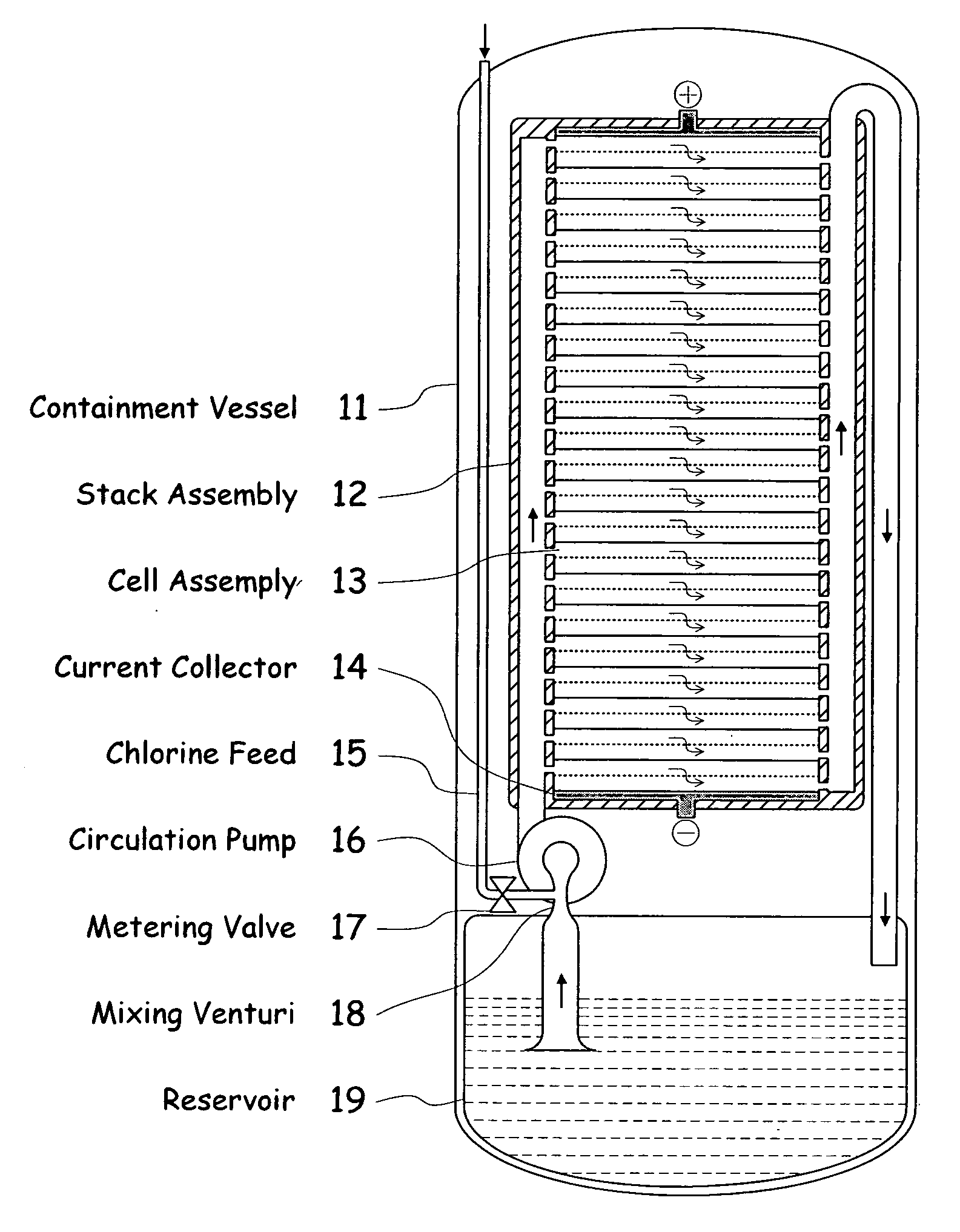
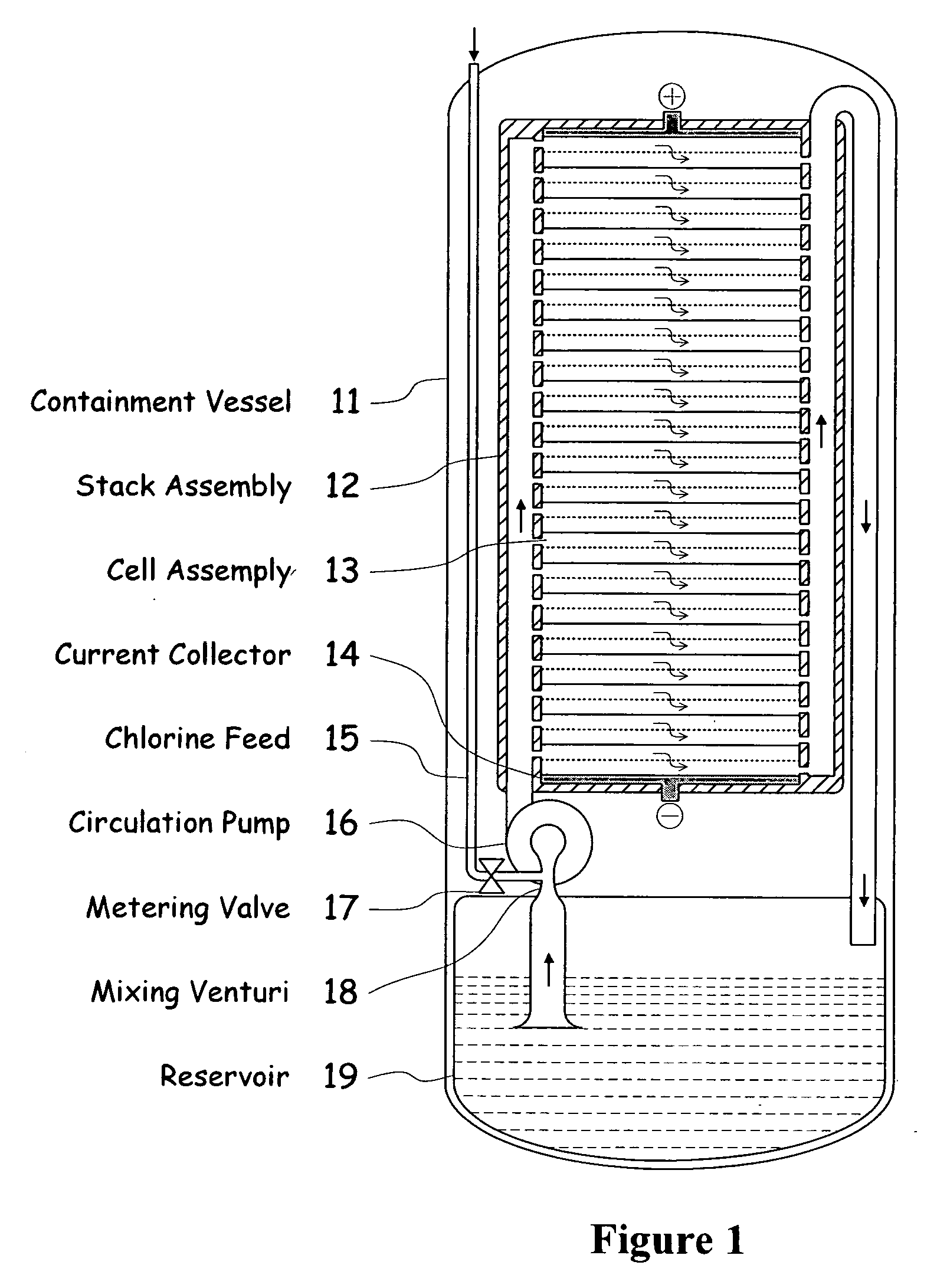
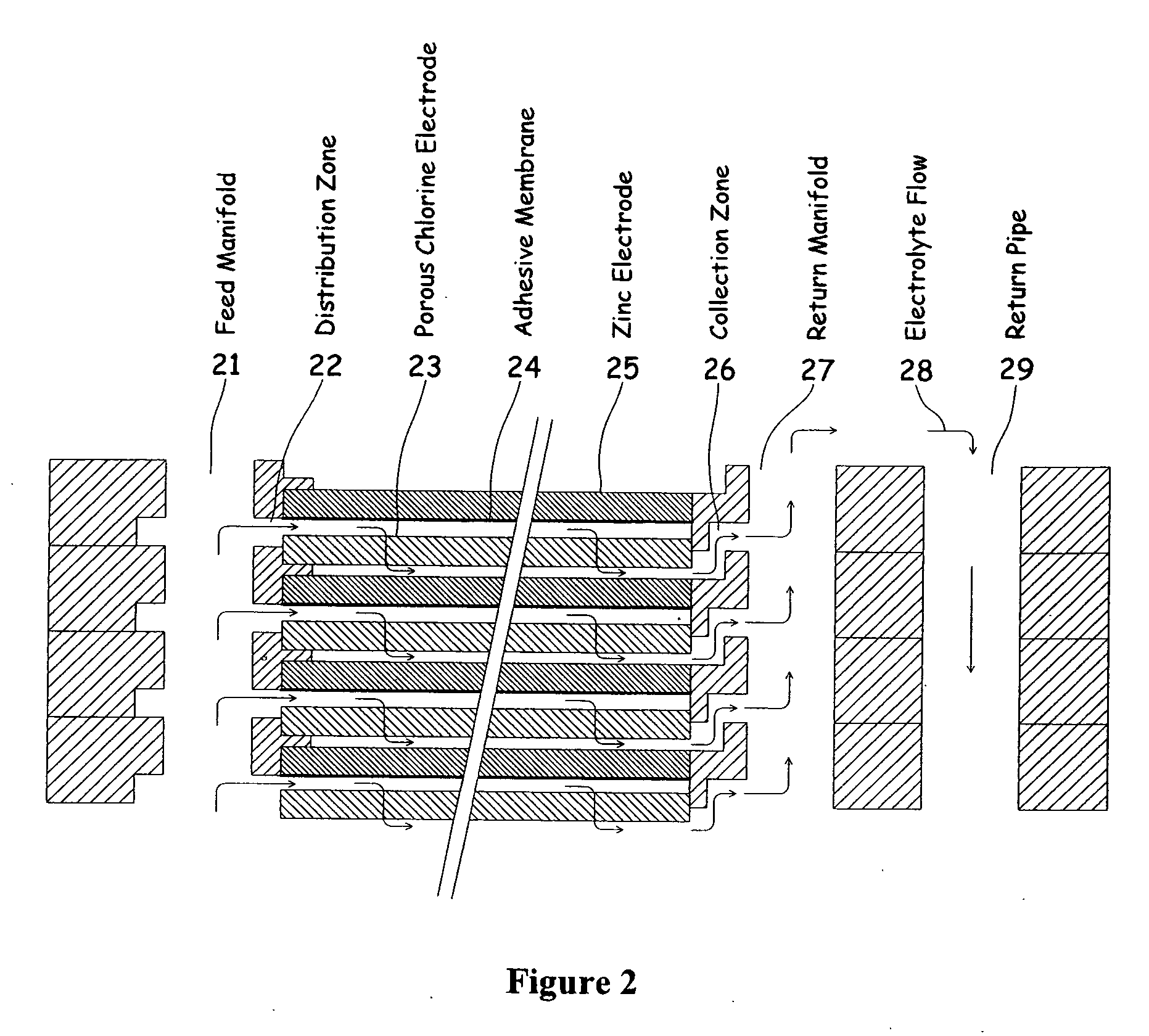
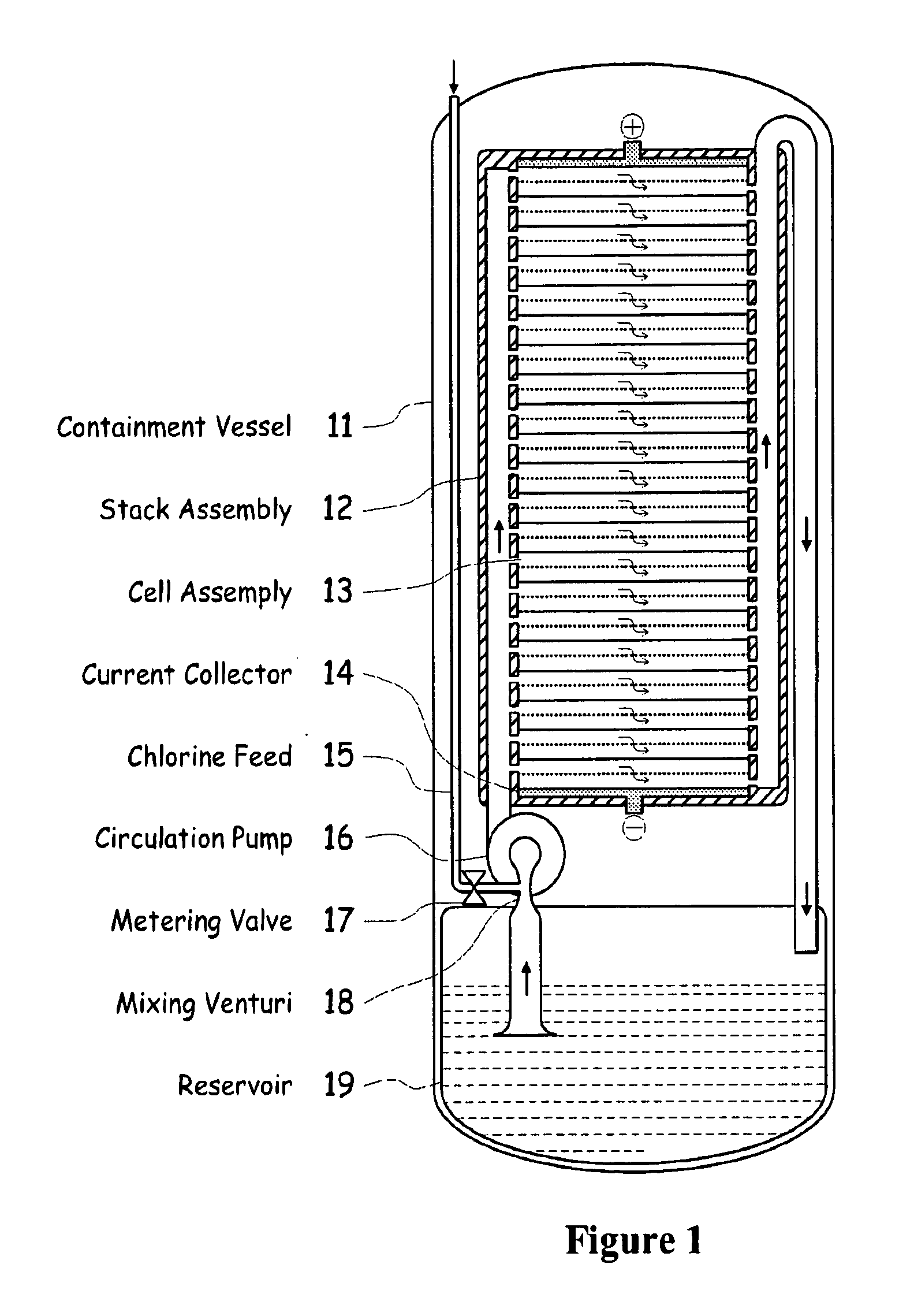
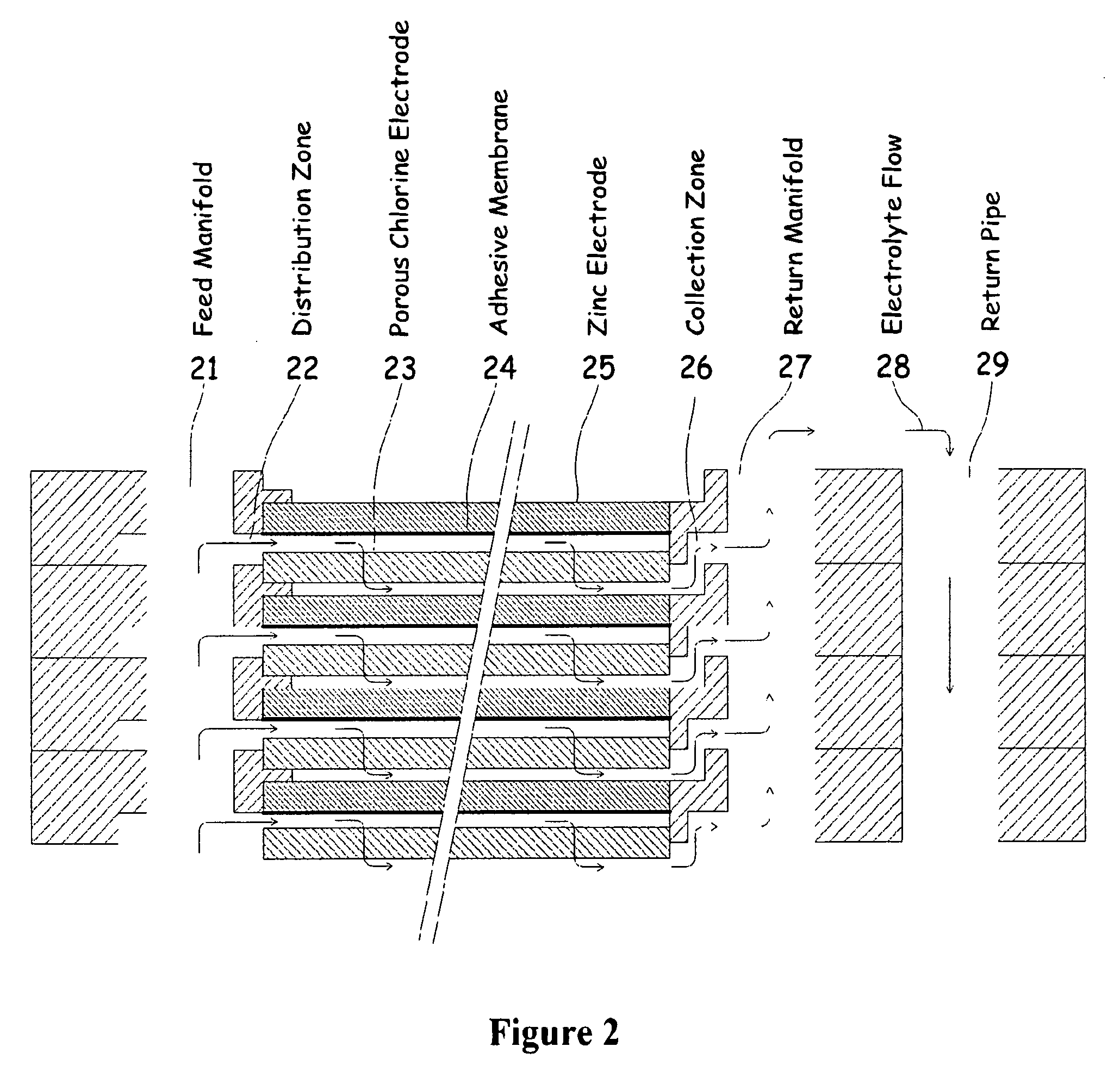
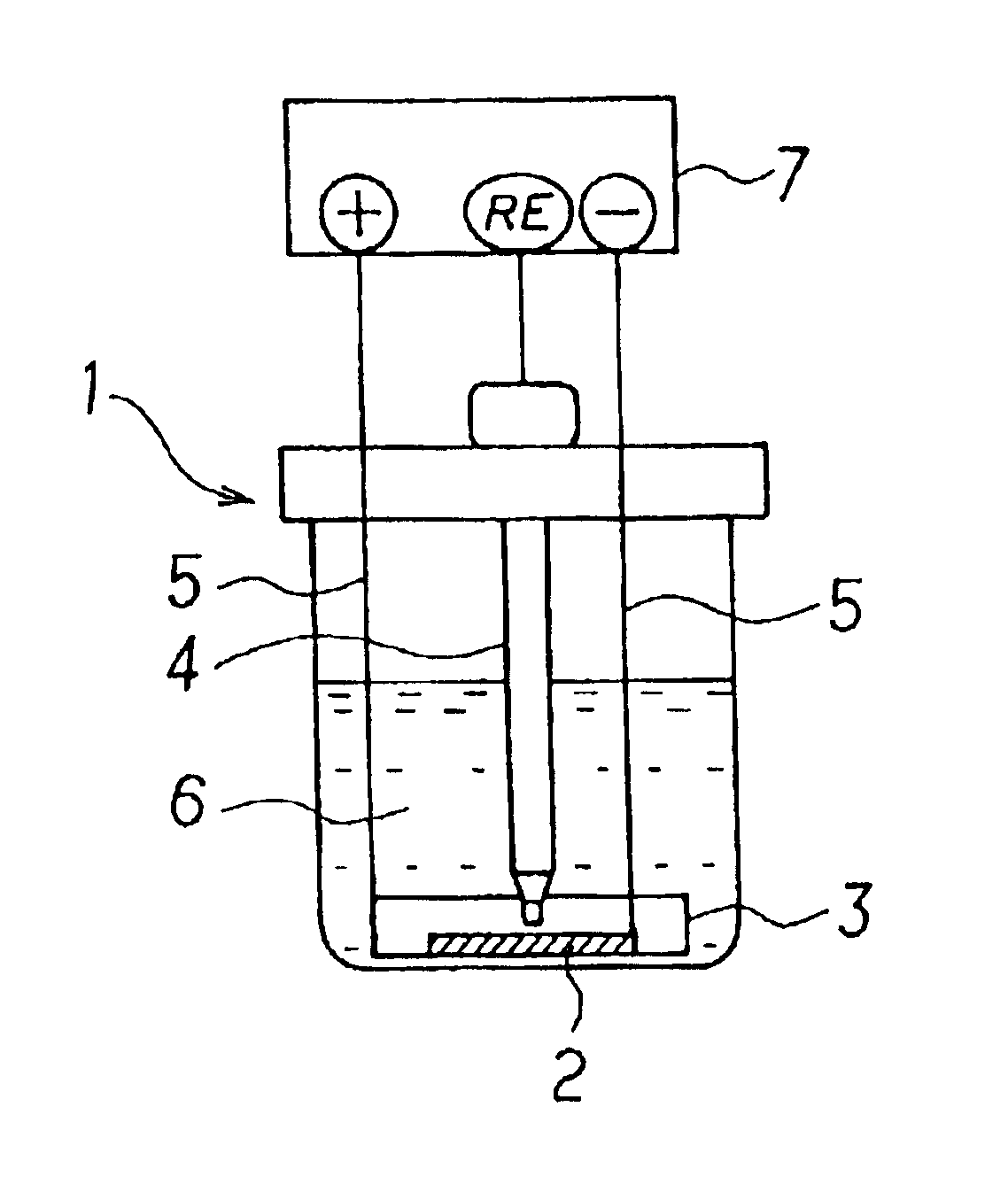
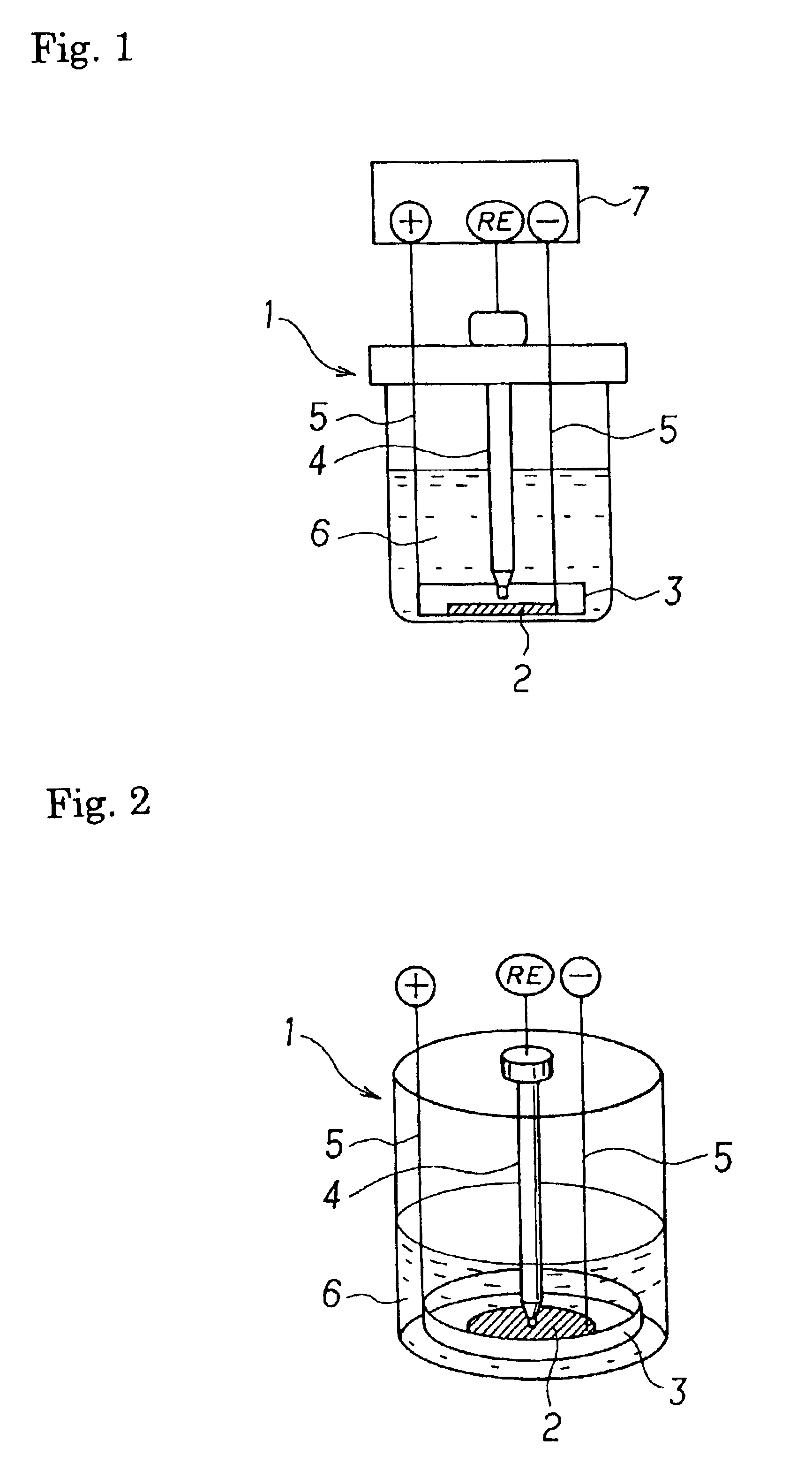
Electrochemical energy cell system
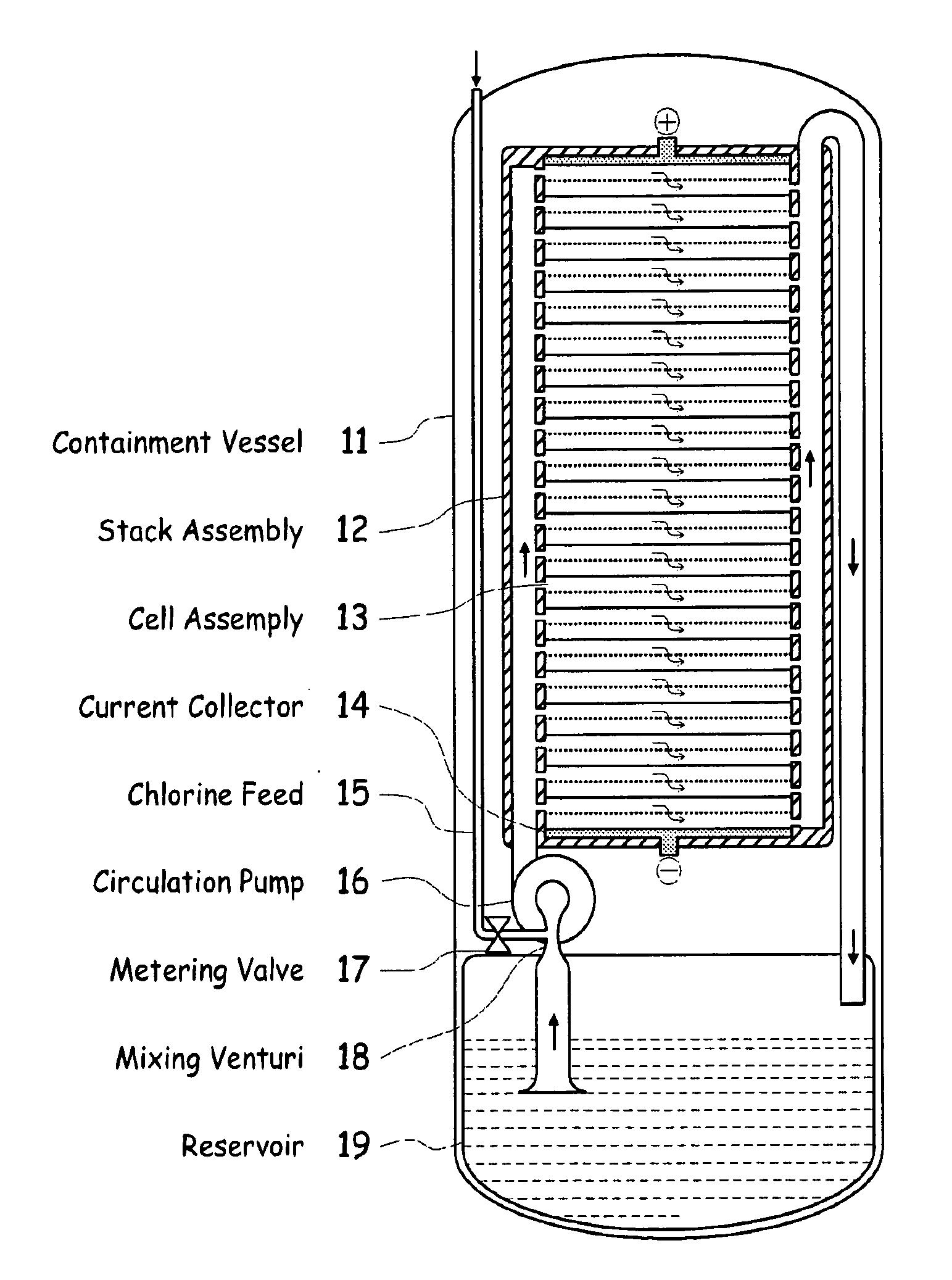
InactiveUS20090239131A1Maintain system availabilityAvoid electrochemical reactionsFuel and primary cellsElectrolyte moving arrangementsChloride electrolytesCell system
A metal halogen electrochemical energy cell system that generates an electrical potential. One embodiment of the system includes at least one cell including at least one positive electrode and at least one negative electrode, at least one electrolyte, a mixing venturi that mixes the electrolyte with a halogen reactant, and a circulation pump that conveys the electrolyte mixed with the halogen reactant through the positive electrode and across the metal electrode. Preferably, the negative electrodes are made of zinc, the metal is zinc, the positive electrodes are made of porous carbonaceous material, the halogen is chlorine, the electrolyte is an aqueous zinc-chloride electrolyte, and the halogen reactant is a chlorine reactant. Also, variations of the system and a method of operation for the systems.
Owner:PRIMUS POWER CORP
Liquid-phase plasma rock blasting method
InactiveCN105444631ATo achieve the purpose of blasting rockAchieve the desired effectBuilding repairsBlastingShock waveIon clusters
The invention discloses a liquid-phase plasma rock blasting method suitable for blasting rocks and concrete structures. According to the method, strong discharging is implemented in strong sodium chloride or potassium chloride electrolyte solutions for stimulating generation of high-density liquid-phase plasmas; in the high-density liquid-phase plasmas, due to the mutual effect of the electrical property of ion clusters, intense vibration occurs in the sections where ions exist in an instant, high temperature and high pressure are generated in the plasma sections, clustering shock waves of the ions are formed and extend outwards, and therefore the energy of ion waves is spread to the surrounding rocks, natural defects such as joints and fractures in the rocks or the concrete structures are enlarged, new cracks and weakness planes are formed until the rocks or the concrete structures are damaged, and the purpose of blasting the rocks or the concrete structures through the plasmas is realized. Compared with traditional explosive blasting methods, the liquid-phase plasma rock blasting method has the remarkable advantages that dust is avoided, fume is avoided, poisonous and harmful gas is avoided, throwing is avoided, and the noise is low.
Owner:CHINA UNIV OF MINING & TECH
Electrolytic method in diaphragm-type cell
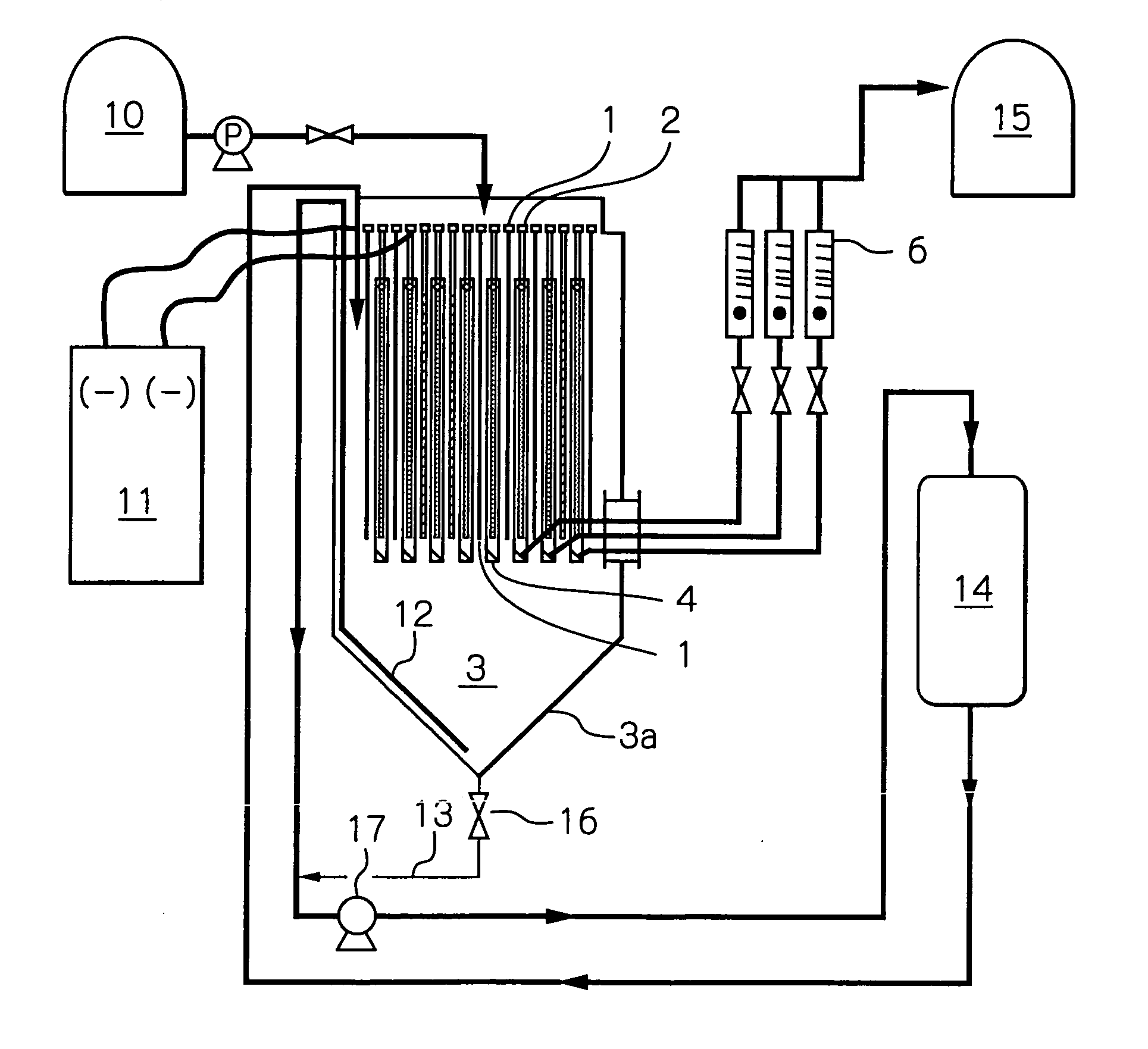
InactiveUS20050067299A1Improve leaching efficiencyReduce reaction efficiencyPhotography auxillary processesElectrolysis componentsPregnant leach solutionChloride electrolytes
In a metal-winning method, copper ore or copper-ore concentrates is effectively hydraulically leached in a chloride leach liquor and the resultant leached liquor is diaphragm-electrolyzed. A chloride electrolyte containing Br− ions and the leached metals is subjected to a diaphragm-electrolysis in an electrolytic cell comprising an anode compartment (4) and a cathode compartment (3). A portion of the electrolyte in the anode compartment (4) is withdrawn from below an anode (2) of the anode compartment (4) and is returned to the leaching step so as to increase the oxidation potential of the chloride leach liquor.
Owner:JX NIPPON MINING& METALS CORP +1
Electrochemical energy cell system
ActiveUS20100009243A1Reduce capacityAvailable capacityFuel and primary cellsElectrolyte moving arrangementsChloride electrolytesCell system
A metal halogen electrochemical energy cell system that generates an electrical potential. One embodiment of the system includes at least one cell including at least one positive electrode and at least one negative electrode, at least one electrolyte, a mixing venturi that mixes the electrolyte with a halogen reactant, and a circulation pump that conveys the electrolyte mixed with the halogen reactant through the positive electrode and across the metal electrode. Preferably, the positive electrode comprises porous carbonaceous material, the negative electrode comprises zinc, the metal comprises zinc, the halogen comprises chlorine, the electrolyte comprises an aqueous zinc-chloride electrolyte, and the halogen reactant comprises a chlorine reactant. Also, variations of the system and a method of operation for the systems.
Owner:PRIMUS POWER SOLUTIONS INC
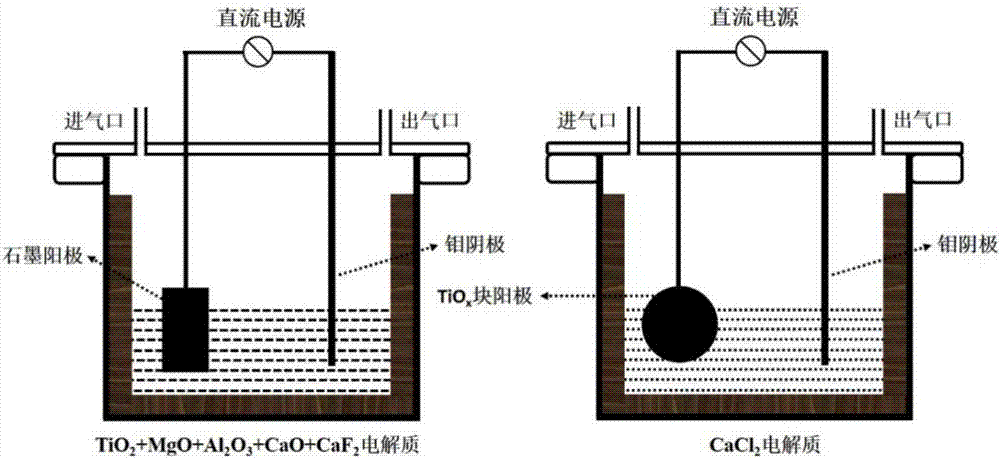
Two-step method for preparing high-purity titanium
The invention provides a two-step method for preparing high-purity titanium, and relates to the field of electrochemical metallurgy. The two-step method includes the two steps of preparation of low-valence TiOx (x<1) and extraction of high-purity titanium. Firstly, a TiOx (x<1) raw material is prepared with graphite or an inert electrode as the anode and a metal material as a cathode in an electrolyte system with oxides (TiO2+MgO+Al2O3+CaO) and a small quantity of fluorides as additives; and then in a chloride electrolyte system, high-purity titanium is extracted through fused salt with TiOx as the anode and a metal material as a cathode, and in the process, free-state [O] generated in an electrolyte can further oxide residual low-valence TiOx into high-valence TiO2 which returns to the first step to serve as an oxide titanium ion source raw material. The novel two-step method for extracting high-purity titanium through fused salt has the characteristics that the technique is simple, energy consumption is low and the titanium recovery rate is high, and can achieve industrial extraction of high-purity titanium.
Owner:UNIV OF SCI & TECH BEIJING
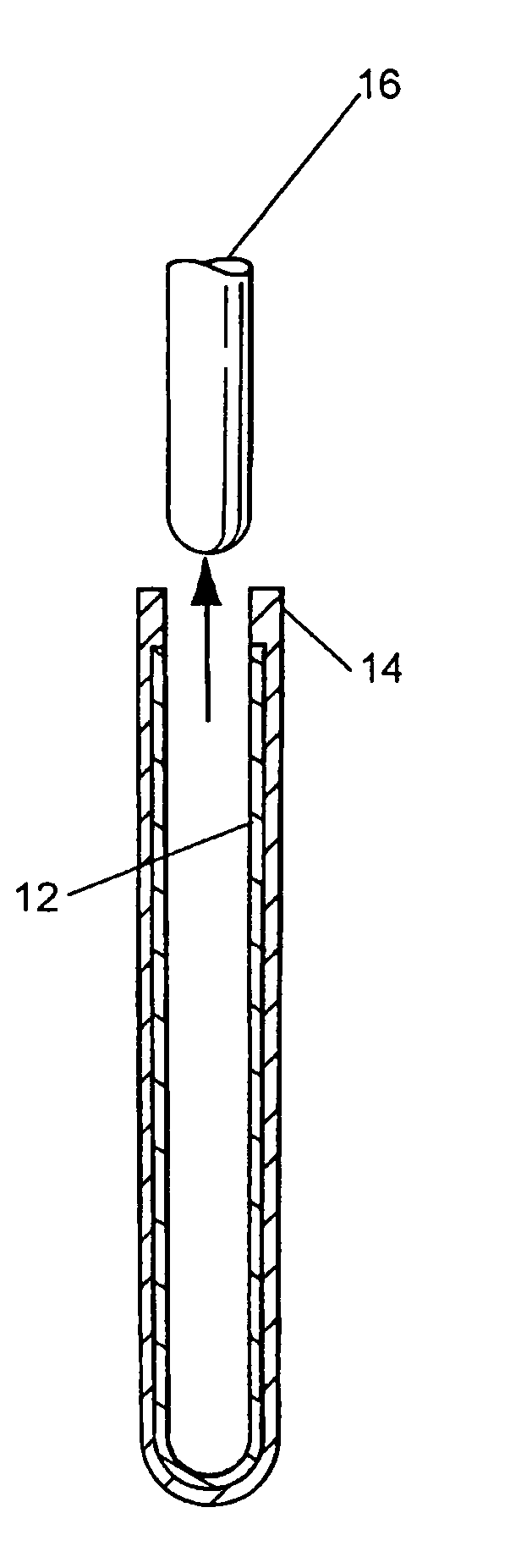
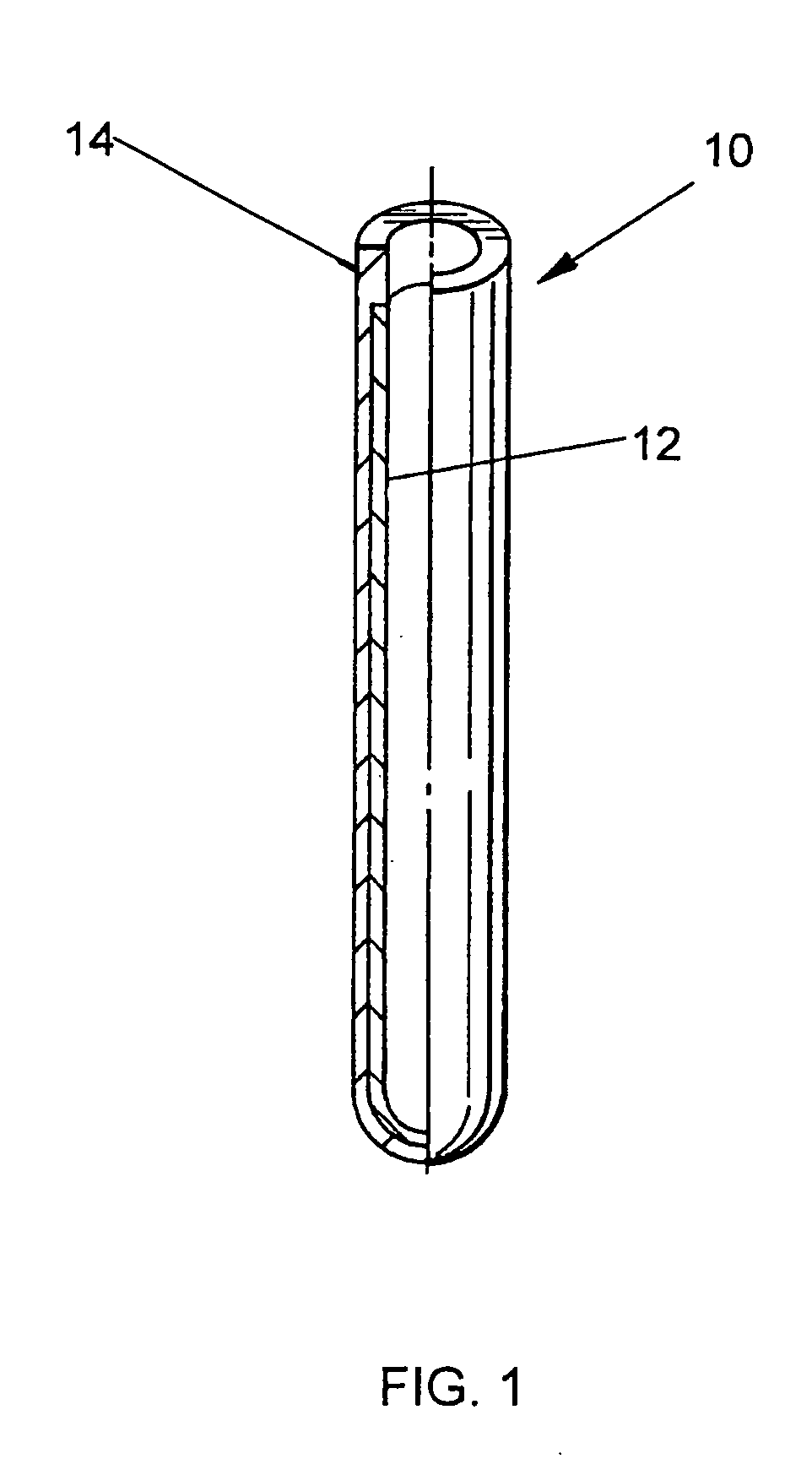
Clad tube for nuclear fuel
A rhenium lined niobium alloy tube for use as a clad tube for nuclear fuel in a nuclear reactor. The tube is produced by an electro deposit process. A graphite mandrel is placed in the electro deposit chamber as the cathode material. Refined rhenium stock is used as the anode material. The chamber is filled with the chloride electrolyte. The chamber is closed and the electrolyte bath is heated. Current and voltage applied across the anode and cathode cause the rhenium to be deposited on the mandrel. Refined niobium alloy is then used as the anode material and applied over the rhenium on the mandrel to a desired thickness. The part is removed from the chamber and ground to the desired outside diameter. The graphite mandrel is removed from the tube.
Owner:BWXT NUCLEAR OPERATIONS GRP
Decomposition method for molybdenite by wet process
InactiveCN101260461AImprove leaching rateReduce energy consumptionElectrolysis componentsElectrolysisUltrasound - action
The invention discloses a molybdenite whole-wet method decomposition method. The molybdenite, the chloride electrolyte and the water are added into a membraneless electrobath with an ultrasonic generator and are stirred to prepare the ore pulp with the ore pulp mass percentage ranging from 5 to 80 percent and the chloride electrolyte mass percentage ranging from 5 to 50 percent, the mixture is electrolyzed and oxidized under the action of ultrasonic and at a temperature ranging from 0 to 100 DEG C, in the electroanalysis process, the current density of the anode is controlled to be 50 to 1200 A / m<2>, the ultrasonic is emitted continuously or intermittently, the electrolyzed and oxidized molybdenite is converted into the molybdena or molybdate compound. By the ultrasonic field and the electric field coupling action, the method reinforces the wet method decomposition process of the molybdenite, overcomes the retardation of the oxide passive film generated in the oxidization leaching process of the conventional wet method, and improves the current efficiency and the Mo leaching rate of the prior membraneless electrooxidation leaching technique.
Owner:CENT SOUTH UNIV
Synthetic method for stannous chloride
ActiveCN105862068AOvercome the problem of slow responseReliable workmanshipElectrolysis componentsElectrolytic agentChloride ion binding
The invention relates to a method for synthesizing stannous chloride through a membrane electrolysis method and belongs to the technical field of inorganic chemical engineering. A catholyte and an anolyte of certain concentrations are prepared through diluted hydrochloric acid. A cathode plate and an anode plate which are made of refined tin are put into a cathode frame and an anode frame of an electrolytic cell correspondingly. Under the action of direct currents, anode tin loses electrons, stannous ions are formed and enter the anolyte to be combined with chlorine ions, and a stannous chloride electrolyte is formed. The stannous chloride electrolyte is put into a reactor containing a certain amount of tin for concentration. The stannous chloride is obtained after a concentrated solution is subjected to the purification, crystallization and solid-liquid separation processes. According to the novel method provided by the invention, the stannous chloride is generated through electrolysis, concentration, purification, crystallization and other processes, and chlorine gas, hydrogen chloride and other harmful gases are not generated in the electrolysis process. Meanwhile, the method for synthesizing the stannous chloride besides a concentrated hydrochloric acid method and a chlorine gas method is provided. The method for synthesizing the stannous chloride has the advantages of being high in operability, low in cost, environmentally friendly, high in safety and the like and can be used for industrialization popularization.
Owner:云南锡业锡化工材料有限责任公司
Electrochemical detection method for quinhydrone
InactiveCN101581694AImprove electrocatalytic performanceHigh peak current responseMaterial electrochemical variablesElectronic transmissionPhosphate
The invention provides an electrochemical detection method for quinhydrone, which comprises the following steps: jointly inserting a bare glassy carbon electrode, a multi-wall carbon nano tube and a room-temperature ionic liquid glue-modified glassy carbon electrode into an electrochemical detection cell filled with phosphate buffer solution of quinhydrone to be detected together with a reference electrode and a platinum counter electrode respectively, and carrying out electrochemical cyclic voltammetry to obtain cyclic voltammetry peak current response (Ip) of the quinhydrone which is 43 times of bare glassy carbon electrode peak current response; and carrying out electrochemical impedance scanning in an electrochemical detection cell of potassium chloride electrolyte solution containing red potassium prussiate / yellow prussiate probe molecules to obtain charge transfer impedance value of the multi-wall carbon nano tube and the room-temperature ionic liquid glue. The Rct of the charge transfer impedance value of the multi-wall carbon nano tube and the room-temperature ionic liquid glue is close to 0 omega, while the Rct of the charge transfer impedance value of the bare glassy carbon electrode is about 1,000 omega. Therefore, the multi-wall carbon nano tube and the room-temperature ionic liquid glue have good electric conductivity and greatly increase the electronic transmission speed. The method for detecting the quinhydrone on the surfaces of the multi-wall carbon nano tube and the room-temperature ionic liquid glue-modified glassy carbon electrode is convenient and quick, and realizes electrochemical detection for the quinhydrone in cosmetics.
Owner:NORTHWEST NORMAL UNIVERSITY
Low-chloride electrolyte
InactiveCN105143106ALithium hexafluorophosphateLi-accumulatorsPhysical chemistryLithium hexafluorophosphate
The present invention relates to a method for producing low-chloride lithium hexafluorophosphate starting from lithium fluoride and phosphorus pentafluoride and the use thereof in an electrolyte.
Owner:LANXESS DEUTDCHLAND GMBH
Visual self-driven ultraviolet photoelectric detector and preparation method thereof
InactiveCN106505125AHas commercial application prospectsShort response timeFinal product manufactureSemiconductor devicesExternal biasPhotovoltaic detectors
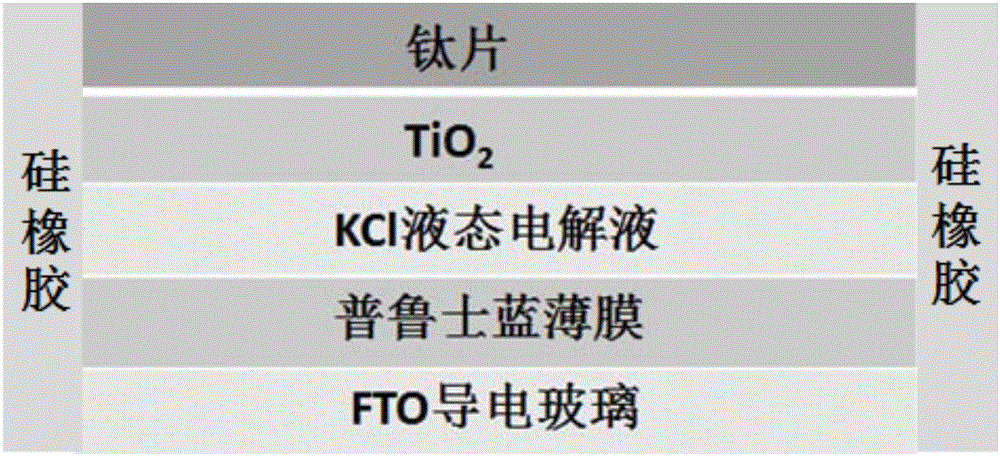
The invention discloses a visual self-driven ultraviolet photoelectric detector and a preparation method thereof. The visual self-driven ultraviolet photoelectric detector is composed of a titanium dioxide nanotube array with the titanium sheet acting as the substrate, potassium chloride electrolyte and Prussian blue with FTO conductive glass acting as the substrate. In the system, the titanium dioxide nanotube array acts as a photo-anode and the Prussian blue film acts as a counter electrode. The Prussian blue has excellent optical modulation characteristic so that the Prussian blue electrode can be used as an electrochromic display screen to realize visualization for ultraviolet light detection. Compared with the conventional ultraviolet light detector, the visual self-driven ultraviolet photoelectric detector has the advantages that (1) naked-eye visualization can be realized; (2) the detector is self-driven without external bias voltage; (3) the response time is short; (4) the sensitivity is high; and (5) the preparation cost is low, the technology is simple and the environment is friendly.
Owner:JINAN UNIVERSITY
Porphyrin detection method based on self-assembly monomolecular film
InactiveCN101936943AImprove the coordination effectHigh sensitivityMaterial analysis by electric/magnetic meansChloride electrolytesPhosphate
The invention relates to a porphyrin detection method based on a self-assembly monomolecular film. The method comprises the following steps of: firstly, dipping a phosphate modified electrode into a dichloromethane methylene solution of porphyrin with different concentrations for reaction and observing the change of porphyrin solution colors instantly; and eluting the electrode after each reaction, and then carrying out electrochemistry impedance scanning in a potassium ferricyanide probe molecule-containing potassium chloride electrolyte solution by using a three-electrode system, wherein the charge transfer impedance value (Rct) of the monomolecular self-assembly film and a logarithm (lg C) of the tetraphenyl porphyrin concentration are in a favorable linear relation. In the invention, the porphyrin is detected by combining a colorimetry and an AC impedance method, the porphyrin detection method has convenience and speediness, high sensitivity, simple operation and low cost.
Owner:NORTHWEST NORMAL UNIVERSITY
Rechargeable chloride battery
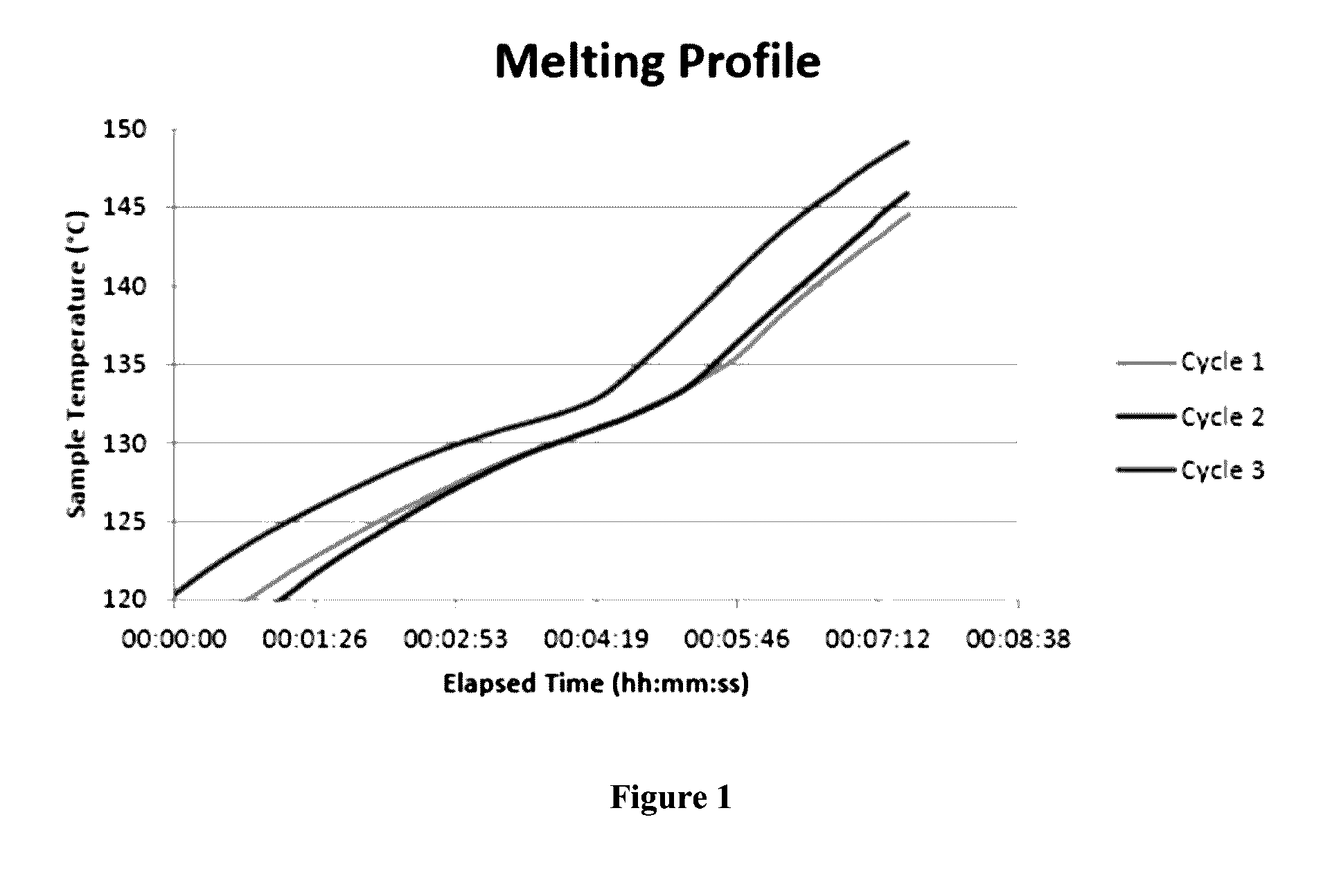
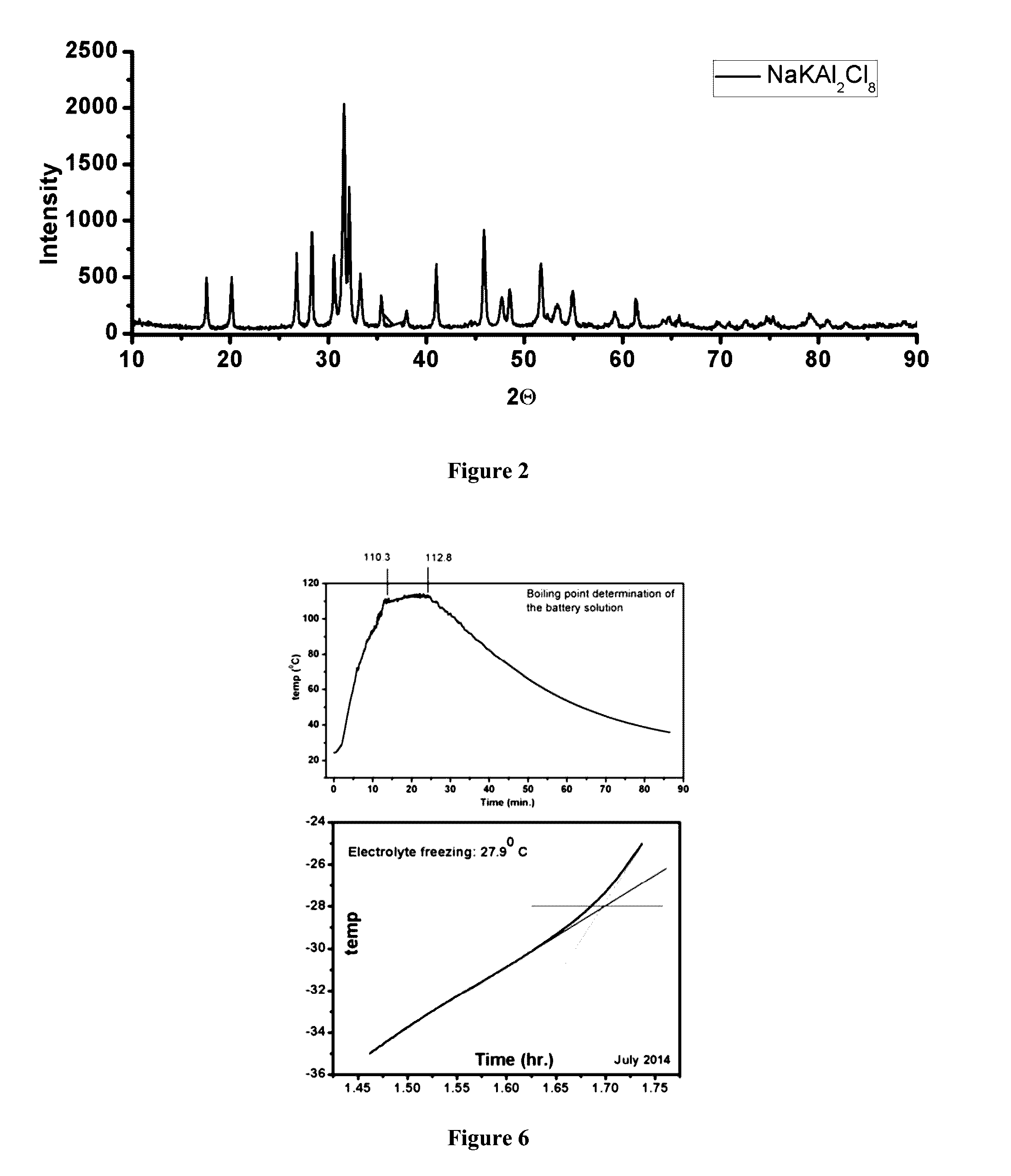
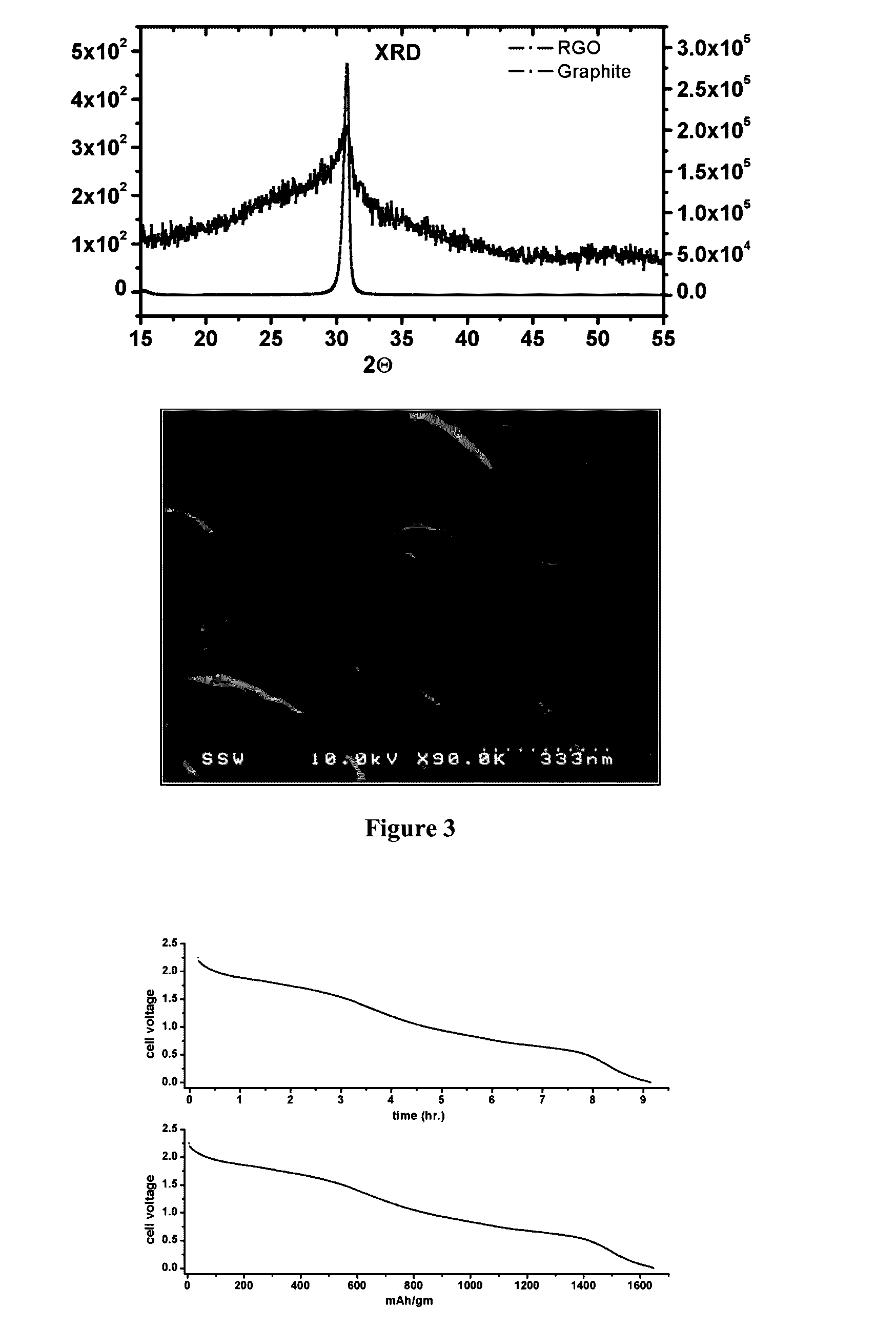
The present invention relates to storage batteries, also known as rechargeable batteries, using a chloride electrolyte, especially in which the electrolyte is a ternary chloroaluminate. In particular embodiments, the electrolyte is molten chloroaluminate and especially molten at a temperature of about 140° C. In another embodiment, the electrolyte is in a liquid state, especially at a temperature in the range of −27° C. to 110° C. The preferred electrodes are aluminum and graphite. The batteries have a variety of uses, particularly including storage of electricity for future use.
Owner:PROCESS RES ORTECH
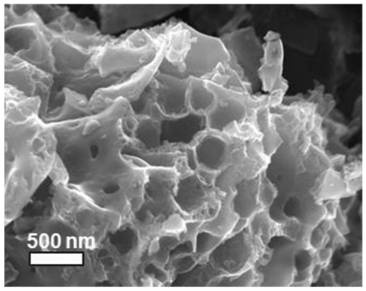
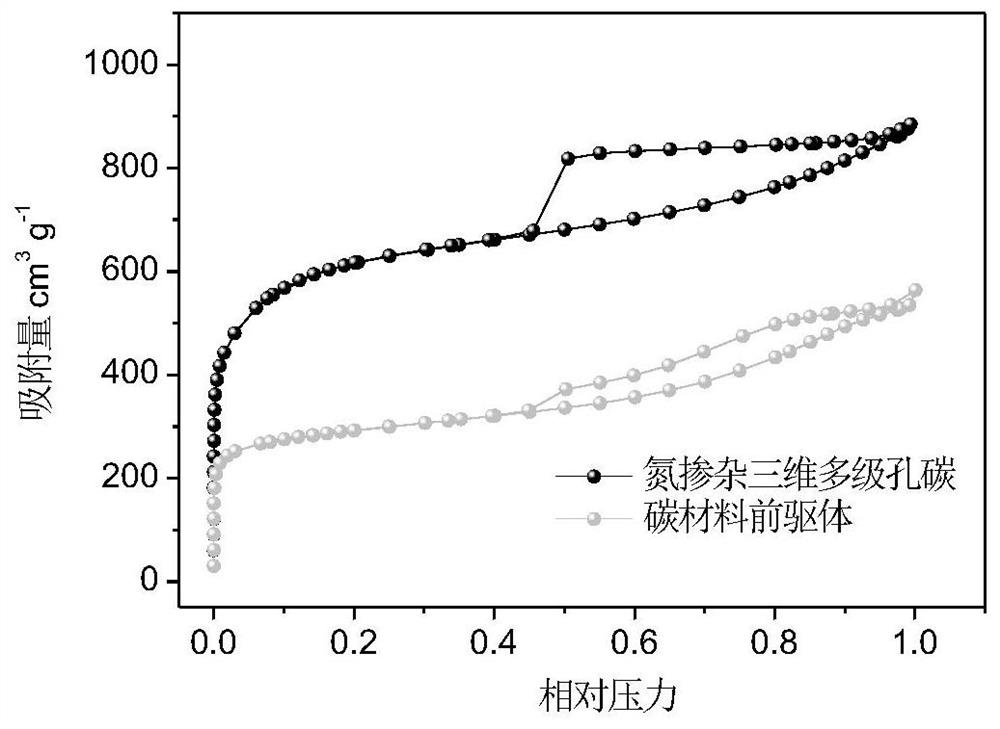
Method for preparing nitrogen-doped multi-dimensional hierarchical porous carbon material adaptive to sulfur positive electrode carrier of aluminum-sulfur battery
InactiveCN113013391APromote migrationEvenly distributedSecondary cellsPositive electrodesAluminium chlorideElectrolytic agent
The invention relates to a method for preparing a nitrogen-doped multi-dimensional hierarchical porous carbon material with high specific surface area and high pore volume. The nitrogen-doped multi-dimensional hierarchical porous carbon material is used as a sulfur positive electrode carrier adaptive to an aluminum-sulfur battery. The preparation method comprises the following specific steps: 1, preparing a polymer precursor with high nitrogen content through amine-aldehyde condensation polymerization; 2, carbonizing the precursor under the actions of high-temperature volatilization of zinc salt and the like to prepare a nitrogen-doped multi-dimensional hierarchical porous carbon material; 3, optimizing the pore channel structure of the nitrogen-doped three-dimensional hierarchical pore carbon material through potassium hydroxide etching; 4, loading elemental sulfur into the nitrogen-doped multi-dimensional hierarchical porous carbon through a sublimation method to form a composite positive electrode; 5, preparing a positive pole piece of the aluminum-sulfur battery; 6, performing battery assembly and performance evaluation. The discharge capacity of a battery assembled by the aluminum-sulfur battery positive electrode material prepared by the method and acetamide / aluminum chloride electrolyte is kept at 1000 mAh g <-1> or above after 50 cycles at 0.2 A g <-1>, and the discharge capacity is kept at 400 mAh g <-1> or above and the coulombic efficiency is kept at 96% or above after 700 cycles at 1A g <-1 >. The preparation method has the advantages of low raw material cost, environment friendliness, good structure optimization effect, easiness in modification, good cycle performance of the assembled aluminum-sulfur battery and the like. The method is applied to the field of aluminum-sulfur batteries.
Owner:BEIJING UNIV OF TECH
Alloyed galvanized steel plate having excellent slidability
InactiveUS20030175549A1Easy to slideHot-dipping/immersion processesDomestic articlesElectricityElectrolysis
An alloyed hot-dip galvanized steel sheet is obtained by forming a hot-dip galvanized layer on the surface of the steel sheet and then alloying the steel sheet. The steel sheet exhibits a potential of -850 mV or less when it is immersed in a zinc sulfate-sodium chloride electrolyte. Alternatively, when it is electrolyzed according to constant potential electrolysis process in a zinc sulfate-sodium chloride electrolyte at a potential in a range from -940 mV to -920 mV, the quantity of electricity consumed is less than or equal to 0.5 C / cm2. The steel sheet exhibits excellent processability and particularly excellent sliding property.
Owner:JFE STEEL CORP
Method for preparing wear-resistant super-hydrophobic coating by electro-deposition method
The invention provides a method for preparing a wear-resistant super-hydrophobic coating by an electro-deposition method. The method for preparing the wear-resistant super-hydrophobic coating by the electro-deposition method comprises the following steps of polishing a stainless steel substrate firstly, then preparing a zinc sulfate electrolyte, a potassium chloride electrolyte and a dopamine hydrochloride electrolyte with certain concentrations, sufficiently and uniformly stirring, and then taking the treated stainless steel substrate as a cathode, enabling a copper plate of the same size toserve as an anode, setting the voltage to be 10-30 V, conducting the deposition time for 10-60 minutes, and obtaining the final super-hydrophobic zinc / polydopamine / n-dodecanethiol coating through electro-deposition.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Alloyed galvanized steel plate having excellent slidability
InactiveUS6835466B2Easy to slideHot-dipping/immersion processesDomestic articlesElectricityElectrolysis
An alloyed hot-dip galvanized steel sheet is obtained by forming a hot-dip galvanized layer on the surface of the steel sheet and then alloying the steel sheet. The steel sheet exhibits a potential of -850 mV or less when it is immersed in a zinc sulfate-sodium chloride electrolyte. Alternatively, when it is electrolyzed according to constant potential electrolysis process in a zinc sulfate-sodium chloride electrolyte at a potential in a range from -940 mV to -920 mV, the quantity of electricity consumed is less than or equal to 0.5 C / cm<2>. The steel sheet exhibits excellent processability and particularly excellent sliding property.
Owner:JFE STEEL CORP
Preparation method of chloride composite electrolyte used for molten salt electrolysis
ActiveCN104611727AResolve continuitySolve the problem of raw materials for refiningComposite electrolyteAlkaline earth metal
The invention relates to a preparation method of a chloride composite electrolyte used for molten salt electrolysis. The preparation method comprises following steps: a refractory metal chloride is subjected to heating sublimation so as to obtain a gas material; the gas material is reacted with a granular solid alkali metal and / or a alkaline earth chloride so as to obtain a A2MCl6 type compound and / or a BMC16 type compound, wherein A is used for representing the alkali metal, B is used for representing the alkaline earth metal, and M is used for representing the refractory metal; the A2MCl6 type compound and / or the BMC16 type compound is reacted with the solid alkali metal and / or the alkaline earth chloride particles further so as to obtain a composite electrolyte with a low eutectic point, wherein reaction temperature is controlled, and the obtained composite electrolyte is in liquid states; and the composite electrolyte is separated from the solid alkali metal or the alkaline earth chloride particles, and is dropwise collected under the effect of gravity. Operation of the preparation method is simple and safe; chemical composition of the obtained product is stable; automatic separation of the product from the raw materials can be realized; continuous operation can be realized; and problems of volatilization of chloride electrolytes used for chloride system molten salt electrolysis or refractory metal refining, and raw material continuous adding are solved.
Owner:有研资源环境技术研究院(北京)有限公司
Clad tube for nuclear fuel
A rhenium lined niobium alloy tube for use as a clad tube for nuclear fuel in a nuclear reactor. The tube is produced by an electro deposit process. A graphite mandrel is placed in the electro deposit chamber as the cathode material. Refined rhenium stock is used as the anode material. The chamber is filled with the chloride electrolyte. The chamber is closed and the electrolyte bath is heated. Current and voltage applied across the anode and cathode cause the rhenium to be deposited on the mandrel. Refined niobium alloy is then used as the anode material and applied over the rhenium on the mandrel to a desired thickness. The part is removed from the chamber and ground to the desired outside diameter. The graphite mandrel is removed from the tube.
Owner:BWXT NUCLEAR OPERATIONS GRP
A preparation method of chloride composite electrolyte for molten salt electrolysis
ActiveCN104611727BLow steam pressureSolve the continuous join problemElectrolysisComposite electrolyte
The invention relates to a preparation method of a chloride composite electrolyte used for molten salt electrolysis. The preparation method comprises following steps: a refractory metal chloride is subjected to heating sublimation so as to obtain a gas material; the gas material is reacted with a granular solid alkali metal and / or a alkaline earth chloride so as to obtain a A2MCl6 type compound and / or a BMC16 type compound, wherein A is used for representing the alkali metal, B is used for representing the alkaline earth metal, and M is used for representing the refractory metal; the A2MCl6 type compound and / or the BMC16 type compound is reacted with the solid alkali metal and / or the alkaline earth chloride particles further so as to obtain a composite electrolyte with a low eutectic point, wherein reaction temperature is controlled, and the obtained composite electrolyte is in liquid states; and the composite electrolyte is separated from the solid alkali metal or the alkaline earth chloride particles, and is dropwise collected under the effect of gravity. Operation of the preparation method is simple and safe; chemical composition of the obtained product is stable; automatic separation of the product from the raw materials can be realized; continuous operation can be realized; and problems of volatilization of chloride electrolytes used for chloride system molten salt electrolysis or refractory metal refining, and raw material continuous adding are solved.
Owner:有研资源环境技术研究院(北京)有限公司
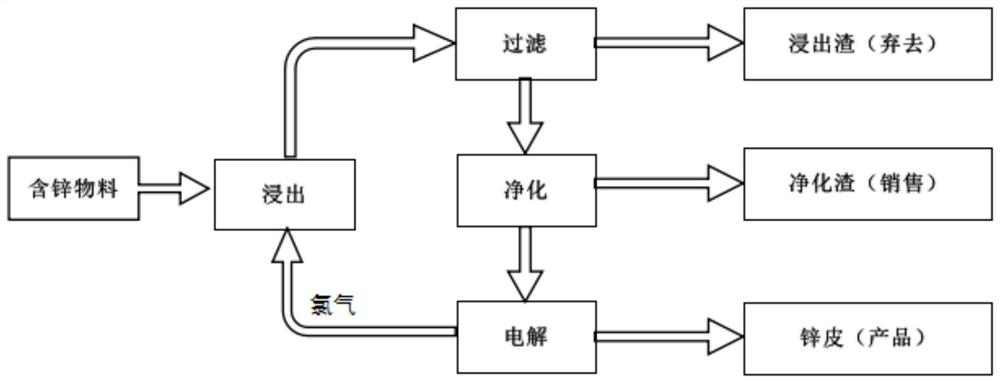
Zinc hydrometallurgy process adopting chloride system
PendingCN113584323AWide variety of sourcesLong electrolytic cyclePhotography auxillary processesProcess efficiency improvementElectrolytic agentChloride electrolytes
The invention discloses a zinc hydrometallurgy process adopting a chloride system, and belongs to the technical field of hydrometallurgy. The zinc hydrometallurgy process adopting the chloride system comprises the following steps of leaching zinc oxide and zinc sulfide raw materials by taking chlorine as a leaching agent, and obtaining an aqueous solution taking zinc chloride as a main component after leaching; removing impurities such as lead, copper and indium in the aqueous solution to obtain a zinc chloride electrolyte; pumping the electrolyte into an electrolytic bath for electrolysis; conveying gas separated out from an anode to a leaching procedure in a centralized mode through a pipeline, and leaching raw materials; and separating metal zinc out from a cathode, and stripping off the metal zinc from the surface of the cathode to obtain a product zinc plate. According to the zinc hydrometallurgy process adopting the chloride system provided by the invention, the chloride system is completely used in the whole circulation process, the chlorine serves as the leaching agent to leach zinc-containing materials, electrolysis can be conducted under the neutral-weak acid condition, the electrolysis period is long, the process route is simple, the trouble of faults such as crystallization separation is avoided, the product yield is high, the material source is wide, and good economic benefits and market prospects are achieved.
Owner:BAIYIN YUANDIAN TECHNOLOGY CO LTD
Novel lead-free zinc chloride battery pack
InactiveCN109687039AImprove discharge performanceHigh specific energy of the batteryFinal product manufactureZinc-halogen accumulatorsChloride electrolytesChloride
Provided is a novel lead-free zinc chloride battery pack. The novel lead-free zinc chloride battery pack includes a battery shell, a soft packaging discharging assembly and a sealing protection coverassembly; the soft packaging discharging assembly includes a plurality of soft packaging batteries which are connected in series, each soft packaging battery includes a soft packaging battery shell, aroll core assembly and a battery connection lug, electrolyte liquid containing cavities are formed inside the soft packaging battery shells, the roll core assemblies are arranged inside the electrolyte liquid containing cavities and are connected with the battery connection lugs, and the electrolyte liquid containing cavities are filled with zinc chloride electrolyte liquid. According to the novel lead-free zinc chloride battery pack, by arranging the battery shell, the soft packaging discharging assembly and the sealing protection cover assembly and filling the electrolyte liquid containingcavities with the zinc chloride electrolyte liquid, the zinc chloride electrolyte liquid replaces leady electrolyte liquid in traditional technologies to serve as an electrolyte, so that the novel lead-free zinc chloride battery pack has good discharging performance and has the advantages of being high in battery specific energy, simple in recovery process and long in service life.
Owner:惠州市佳呈得科技有限公司
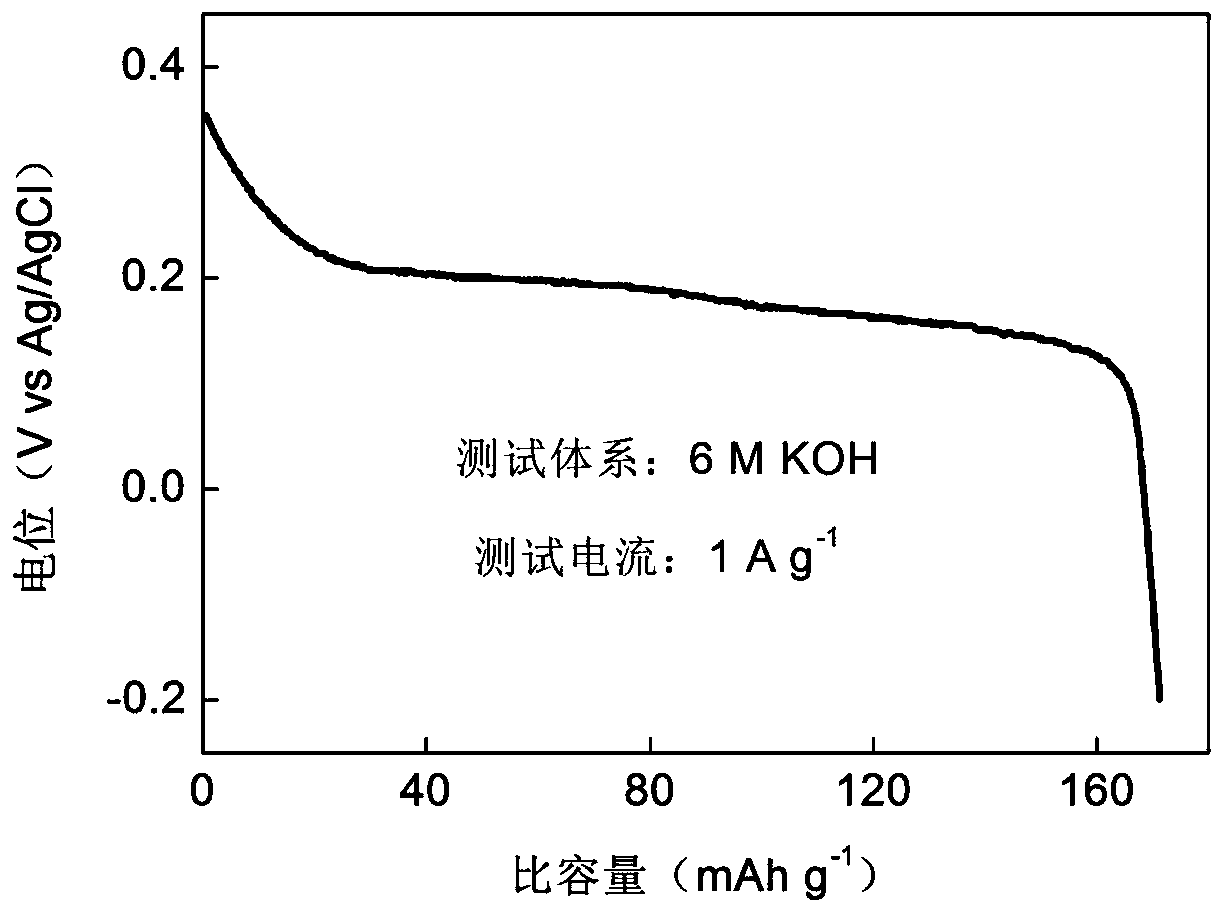
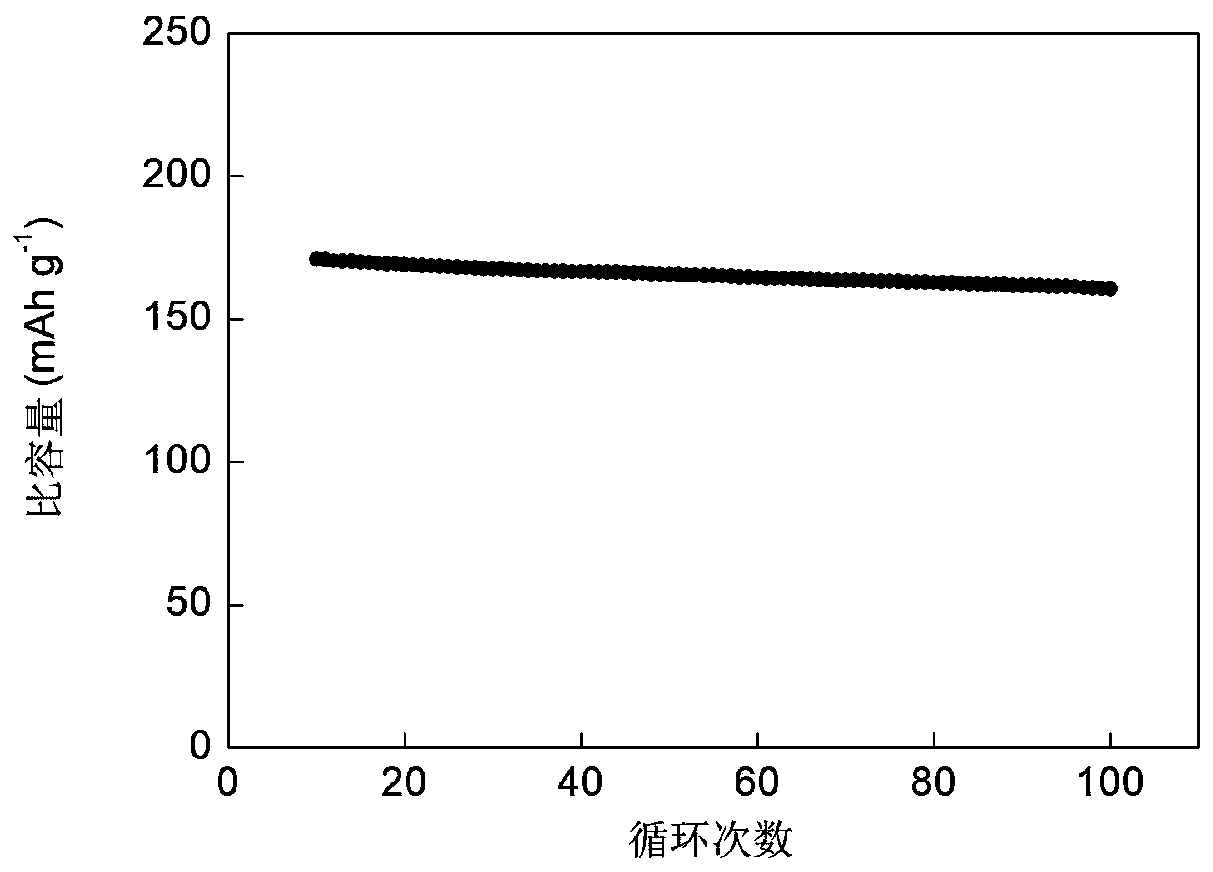
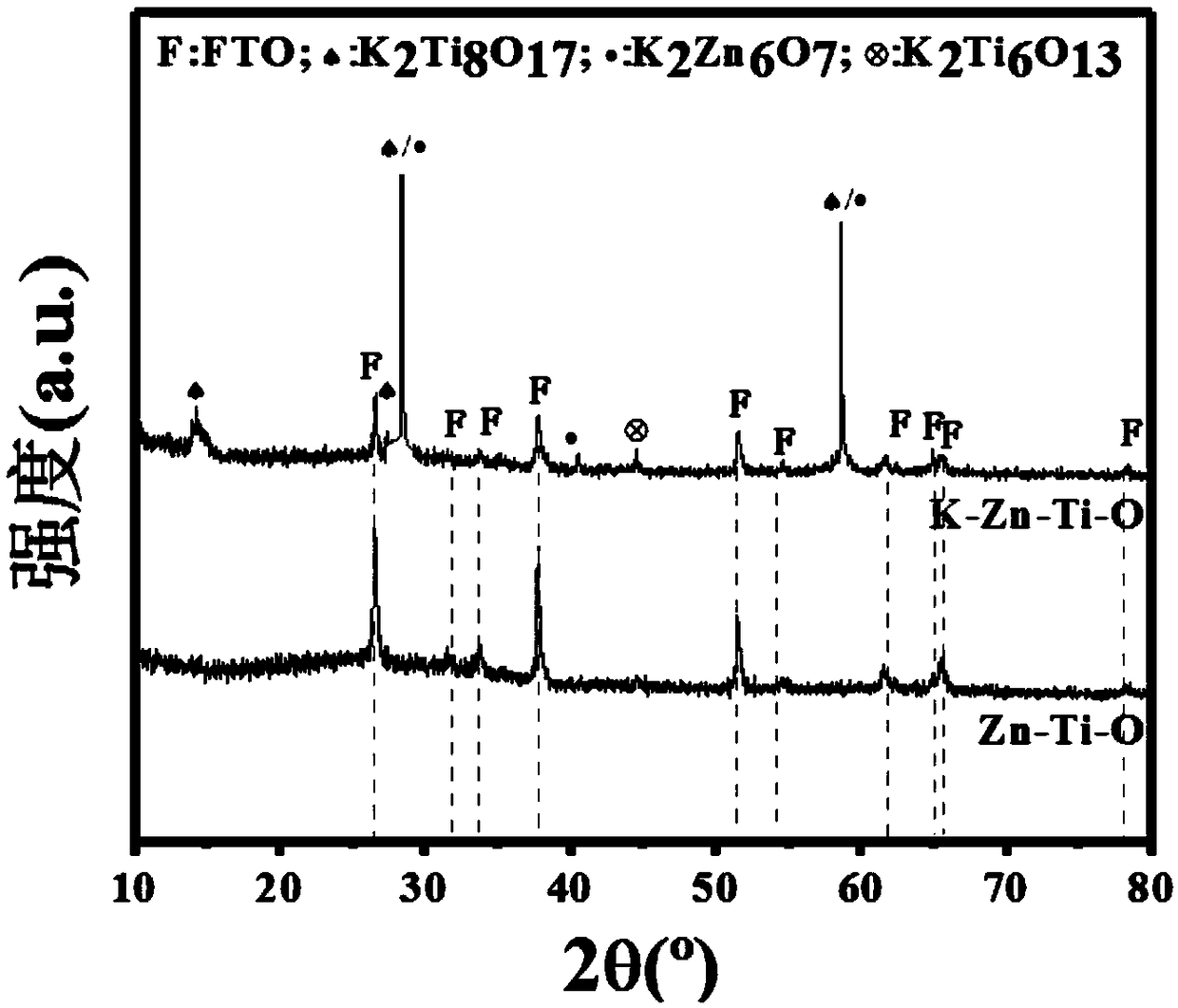
Zinc-titanium-oxygen composite film material with electrochromic effect, its application and preparation method
ActiveCN108931872BWith electrochromic effectImprove electrochromic repeatabilityTenebresent compositionsNon-linear opticsElectrolytic agentComposite film
Owner:SHANGHAI UNIV
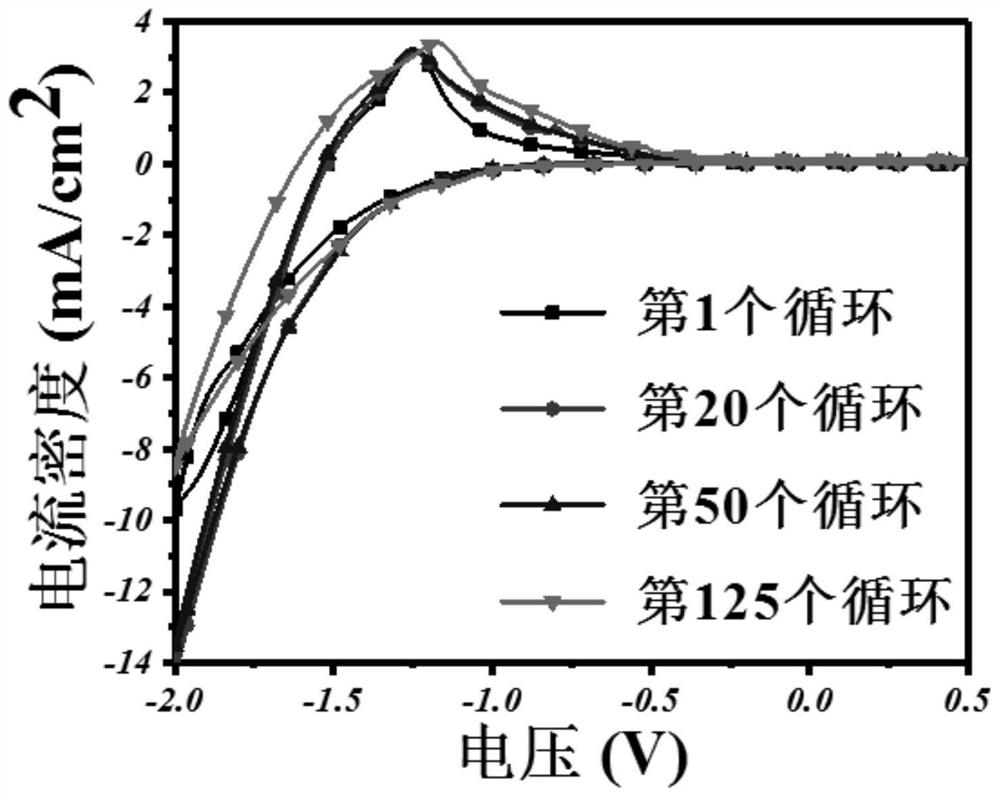
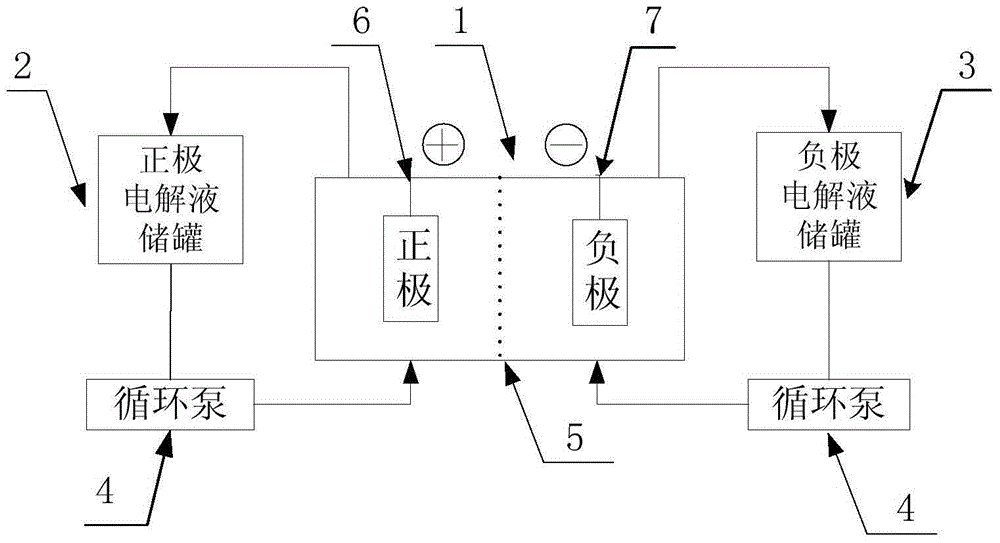
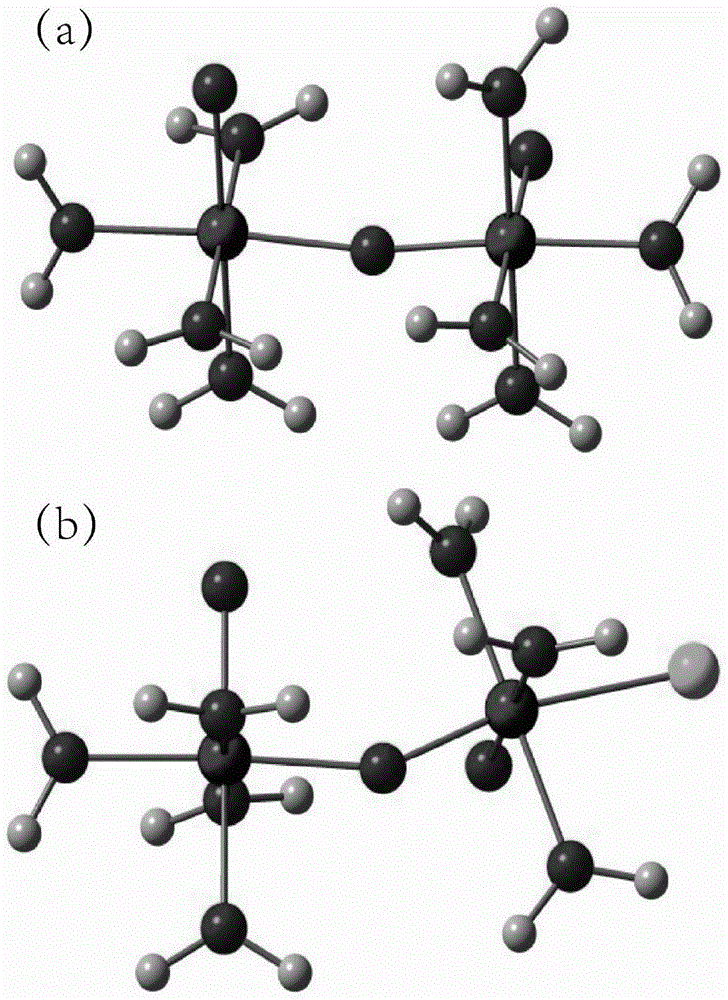
A vanadium/chloride electrolyte and a redox flow battery using the electrolyte
ActiveCN104979577BIncrease concentrationImprove stabilityRegenerative fuel cellsSupporting electrolyteWorking temperature
The present invention belongs to the technical field of flow energy storage batteries, and relates to a vanadium / chloride electrolyte and a redox flow battery using the vanadium / chloride electrolyte. According to the present invention, an electric conduction inert material is adopted as the electrode, the electrolyte adopts hydrochloric acid as the supporting electrolyte, and Cl<-> ions are introduced, such that the existing form of V is the stable dual-core vanadium ions [V2O3.4H2O]<4+> or dual-core vanadium-chlorine composite ion [V2O3Cl.3H2O]<3+> and is more stable than the V existing form [VO2(H2O)3]<+> in the electrolyte adopting sulfuric acid as the supporting electrolyte, and the stability of the vanadium cations is increased while the concentration of the vanadium cations in the electrolyte is improved so as to increase the energy density of the battery system; when the battery system electrolyte temperature is greater than 40 DEG C, the two existing forms of V are stable and no V2O5 precipitate can be generated; and the battery system work temperature range is wide, and the battery system can stably work within a temperature range of 0-65 DEG C so as to eliminate the electrolyte temperature control device, reduce the battery system cost, and improve the system efficiency.
Owner:HUANENG CLEAN ENERGY RES INST +1
Preparation method of flower-shaped nanometer nickel oxide
ActiveCN109755029AUnique shapeImprove electrochemical performanceHybrid capacitor electrodesHybrid/EDL manufactureFlower likeNickel substrate
The invention belongs to the technical field of electrochemical materials, and in particular relates to a preparation method of flower-shaped nanometer nickel oxide. The preparation method of the flower-shaped nanometer nickel oxide comprises the following steps: electrolyzing a nickel substrate in a chloride electrolyte to obtain a precursor; and performing heat treatment on the precursor to obtain the flower-shaped nanometer nickel oxide. According to the invention, electrochemical oxidation and heat treatment are combined, so that the nickel oxide with a flower-like stacked structure is obtained; and the single piece of nickel oxide has an ultra-thin structure, which is beneficial to improving the electrochemical performance of the material. The results of embodiments show that the nickel oxide prepared by the preparation method provided by the invention has a specific capacity of 170 mAh / g when the nickel oxide is used as a super-capacitor positive electrode material, and has excellent cycle stability.
Owner:YANSHAN UNIV
Zinc-titanyl composite thin film material with electrochromic effect, and application and manufacturing method thereof
ActiveCN108931872AWith electrochromic effectImprove electrochromic repeatabilityTenebresent compositionsNon-linear opticsComposite filmPotassium
The invention discloses a zinc-titanyl composite thin film material with an electrochromic effect, and application and a manufacturing method thereof. The zinc-titanyl composite thin film material haselectrochromism phenomenon in a potassium chloride electrolyte. A sol-gel method is used, molar ratio of titanium dioxide to zinc oxide is controlled to be 1:1 to prepare a sol precursor, and a F127three-stage telcomer is added in the precursor, and through sol coating and thin film heat treatment steps, a transparent zinc-titanyl composite thin film is prepared on an FTO conductive glass substrate. The thin film has electrochromism phenomenon, and a large ionic electrolyte can be used. A colored state of the zinc-titanyl composite thin film in the potassium chloride electrolyte is dusty blue, a color fading state is transparent, coloring / fading contrast reaches 37%, and cycle life is good. The zinc-titanyl composite thin film material has advantages of simple process, low cost, and stable electrochromic performance of the film material, and the like, and can be applied to the fields of aerospace, automobile industry, information technology, building materials and the like.
Owner:SHANGHAI UNIV
A kind of preparation method of flake-shaped nano-nickel oxide
ActiveCN109755029BUnique shapeImprove electrochemical performanceHybrid capacitor electrodesHybrid/EDL manufactureElectrolytic agentChloride electrolytes
The invention belongs to the technical field of electrochemical materials, and in particular relates to a preparation method of flower-shaped nanometer nickel oxide. The preparation method of the flower-shaped nanometer nickel oxide comprises the following steps: electrolyzing a nickel substrate in a chloride electrolyte to obtain a precursor; and performing heat treatment on the precursor to obtain the flower-shaped nanometer nickel oxide. According to the invention, electrochemical oxidation and heat treatment are combined, so that the nickel oxide with a flower-like stacked structure is obtained; and the single piece of nickel oxide has an ultra-thin structure, which is beneficial to improving the electrochemical performance of the material. The results of embodiments show that the nickel oxide prepared by the preparation method provided by the invention has a specific capacity of 170 mAh / g when the nickel oxide is used as a super-capacitor positive electrode material, and has excellent cycle stability.
Owner:YANSHAN UNIV
Method for preparing nano copper protoxide material by metal copper anodic oxidation method
In the chloride electrolyte said invention adds crystal growth inhibitor of Cu2O or / and crystal centre of Cu2O or carrier containing crystal centre or / and another nano material and controls the concentration of chloride salt in the electrolytic liquor, concentration of alkali, electric energy consumption and electrolytic temperature so as to can obtain the Cu2O powder body whose grain size is less than 100 nm or nano Cu2O with carrier or nano composite materials, such as nano Cu2O2, carbon nano tube and Cu2O-nano titanium dioxide.
Owner:HUAZHONG NORMAL UNIV
A kind of synthetic method of stannous chloride
ActiveCN105862068BOvercome the problem of slow responseReliable workmanshipElectrolysis componentsSynthesis methodsTin(II) chloride
The invention relates to a method for synthesizing stannous chloride through a membrane electrolysis method and belongs to the technical field of inorganic chemical engineering. A catholyte and an anolyte of certain concentrations are prepared through diluted hydrochloric acid. A cathode plate and an anode plate which are made of refined tin are put into a cathode frame and an anode frame of an electrolytic cell correspondingly. Under the action of direct currents, anode tin loses electrons, stannous ions are formed and enter the anolyte to be combined with chlorine ions, and a stannous chloride electrolyte is formed. The stannous chloride electrolyte is put into a reactor containing a certain amount of tin for concentration. The stannous chloride is obtained after a concentrated solution is subjected to the purification, crystallization and solid-liquid separation processes. According to the novel method provided by the invention, the stannous chloride is generated through electrolysis, concentration, purification, crystallization and other processes, and chlorine gas, hydrogen chloride and other harmful gases are not generated in the electrolysis process. Meanwhile, the method for synthesizing the stannous chloride besides a concentrated hydrochloric acid method and a chlorine gas method is provided. The method for synthesizing the stannous chloride has the advantages of being high in operability, low in cost, environmentally friendly, high in safety and the like and can be used for industrialization popularization.
Owner:云南锡业锡化工材料有限责任公司
Electrochemical oxidation method of sulfite in desulfurized fly ash
PendingCN112501634AReduce generationSimple stepsElectrolysis componentsChloride electrolytesPhysical chemistry
The invention provides an electrochemical oxidation method of sulfite in desulfurized fly ash, and belongs to the technical field of electrochemistry, aiming at solving the problems in the existing desulfurized fly ash treatment technology. The method comprises the following steps: (1) putting desulfurized fly ash and water into an electrolytic cell in proportion, uniformly mixing, adding an ammonia-ammonia chloride electrolyte solution, and adjusting the pH value of a turbid liquid by using an alkali; (2) putting electrodes at the two ends of the electrolytic cell, and carrying out electrolysis at stirring conditions; (3) filtering a turbid solution obtained after electrolysis, drying a filtrate. According to the method for treating sulfite in desulfurized fly ash by adopting the electrochemical method, the advantages are that: the operation is simple, no extra waste liquid or waste is generated, and the oxidation efficiency is high.
Owner:ANSTEEL GRP MINING CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com