Electrochemical energy cell system
a technology of electrochemical energy cell and electrochemical energy system, which is applied in the direction of cell components, final product manufacturing, sustainable manufacturing/processing, etc., can solve the problems of reducing capacity, increasing the probability of failure, and disadvantages of prior electrochemical energy system for standby applications, so as to maintain system availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Electrolyte Energy Cell System
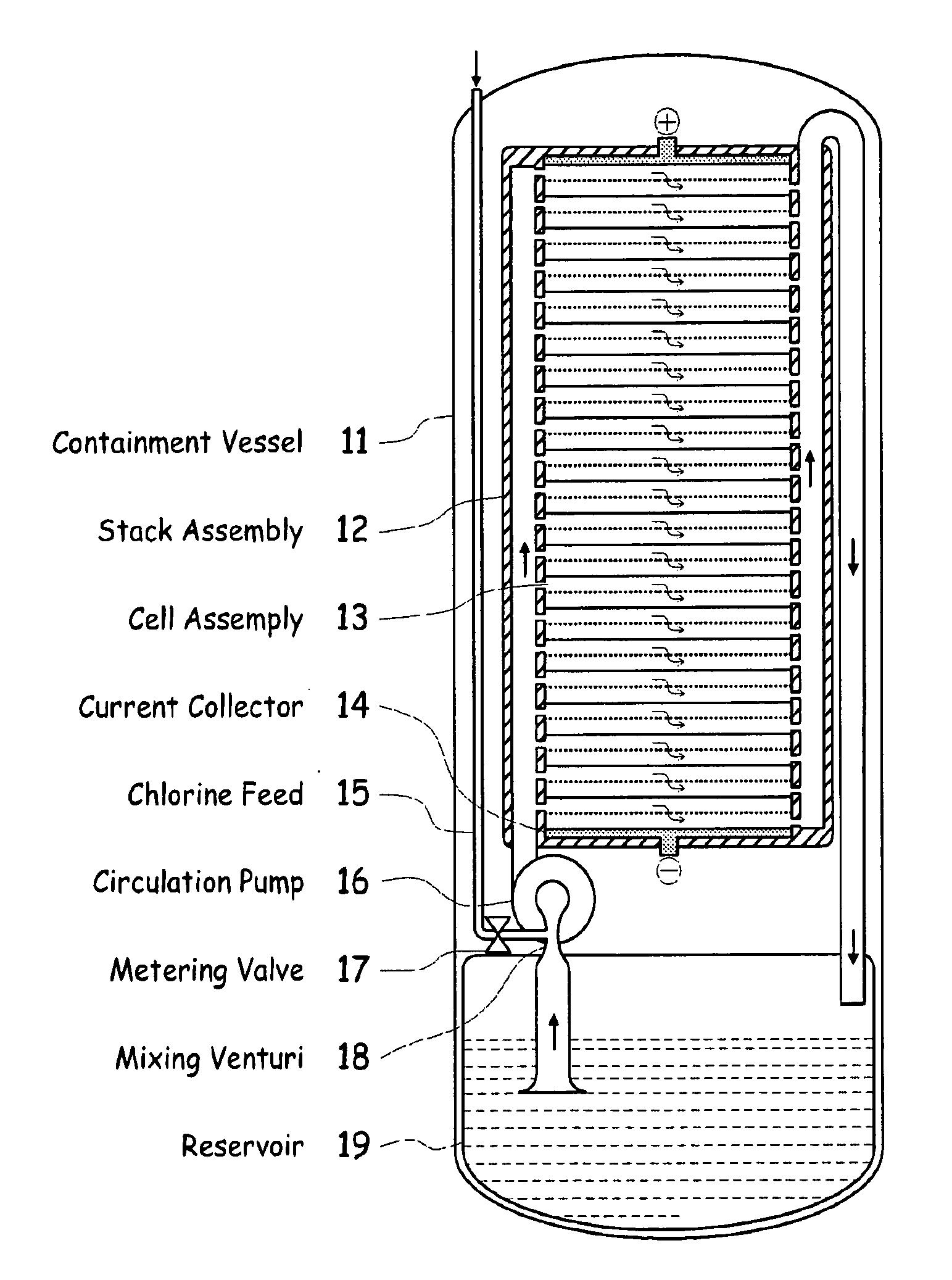
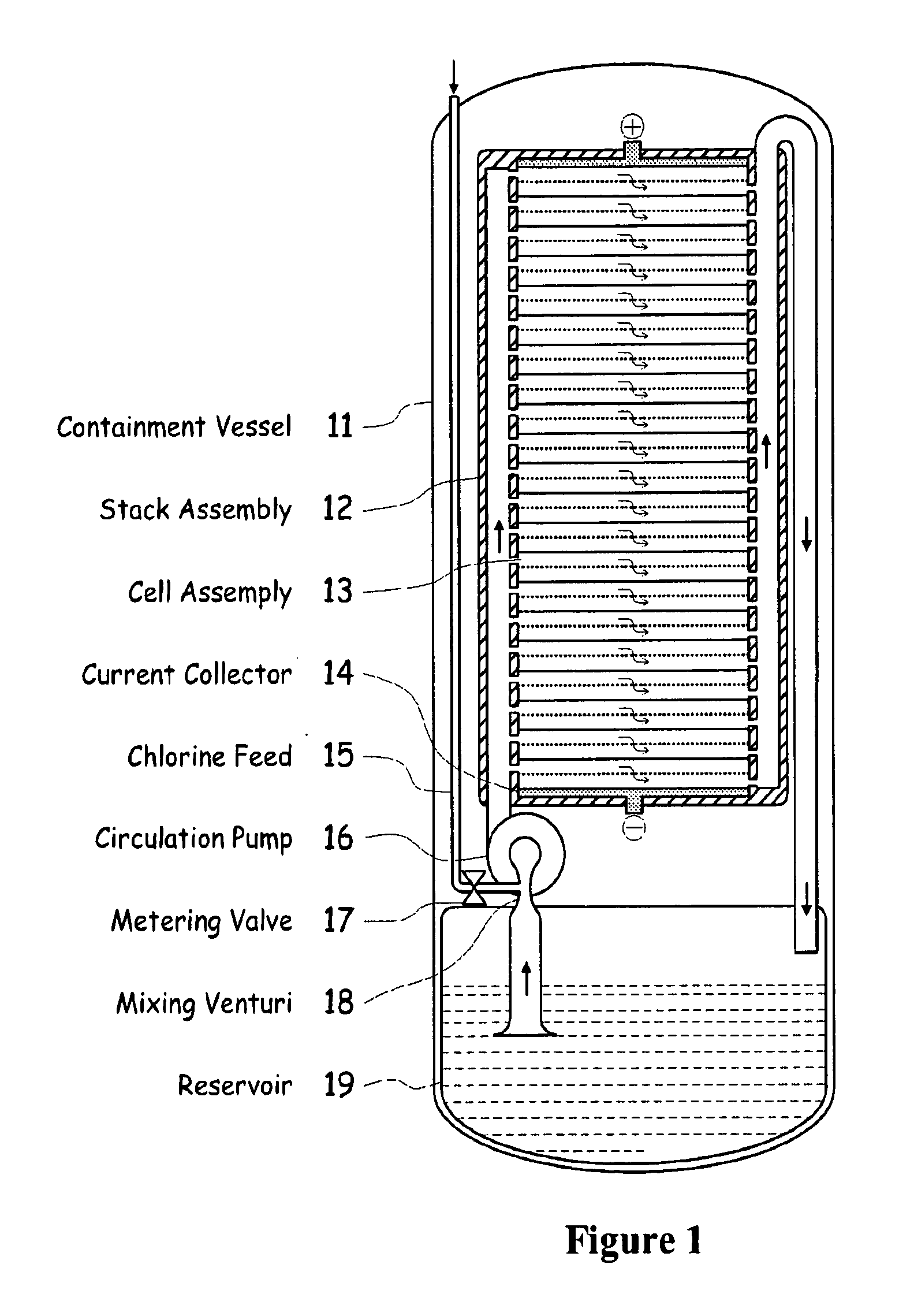
[0039]FIG. 1 illustrates a metal halogen electrochemical energy cell system according to the invention.
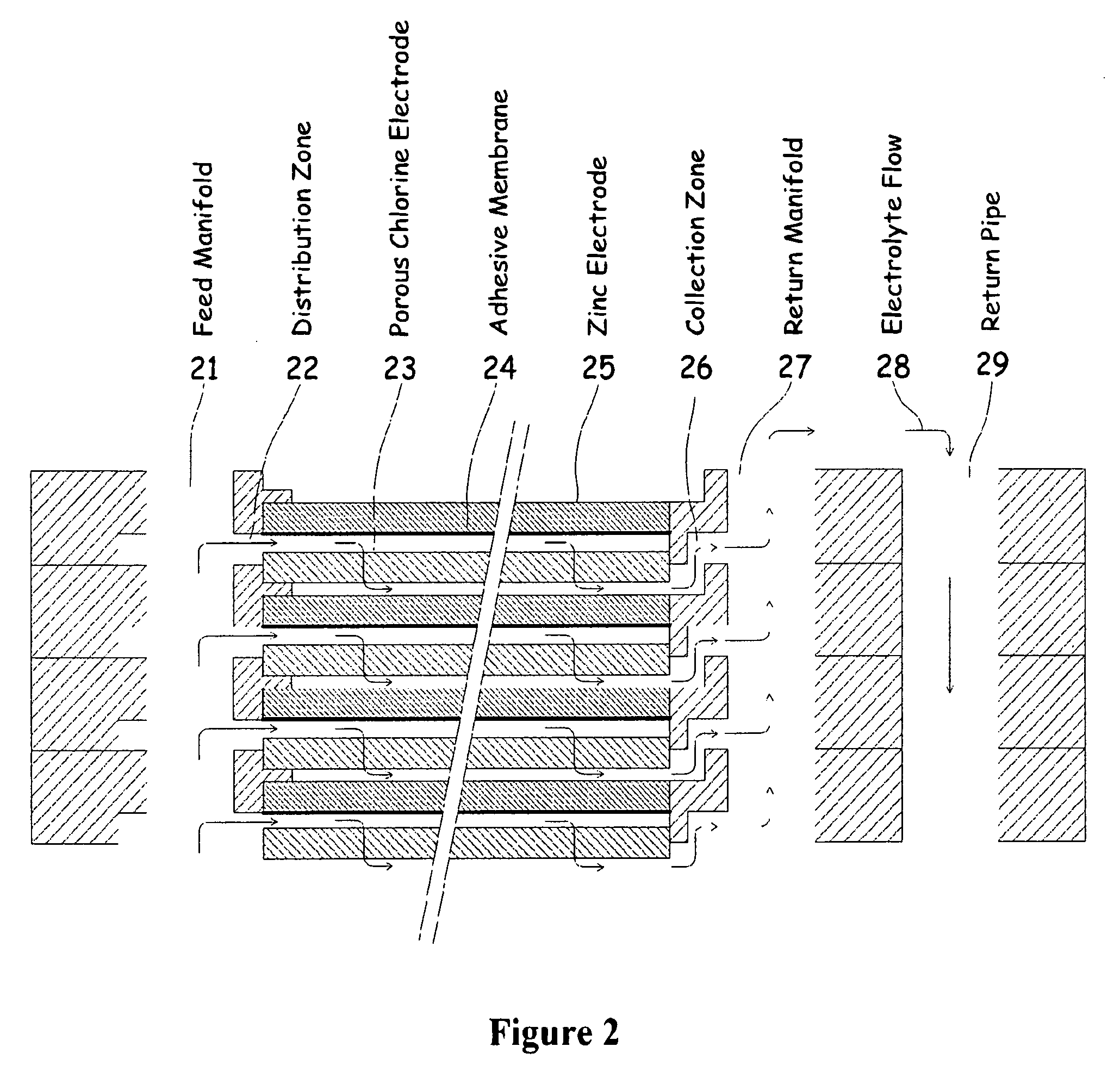
[0040]One embodiment of the invention that attempts to address some or all of these weaknesses and disadvantages is a metal halogen electrochemical energy cell system. This embodiment includes at least at least one positive and at least one negative electrode, a reaction zone between the positive electrode and the negative electrode, at least one electrolyte that includes a metal and a halogen, and a circulation pump that conveys the electrolyte through the reaction zone. The electrolyte and a halogen reactant can be mixed before, at, or after the pump, for example using a mixing venture. Preferably, the positive electrode is made of porous carbonaceous material, the negative electrode is made of zinc, the metal include zinc, the halogen includes chlorine, the electrolyte includes an aqueous zinc-chloride electrolyte, and the halogen reactant includes ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical potential | aaaaa | aaaaa |
| electrical potential | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com