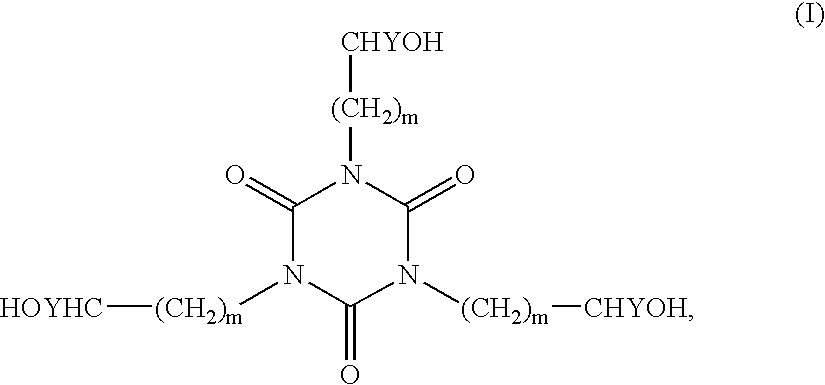
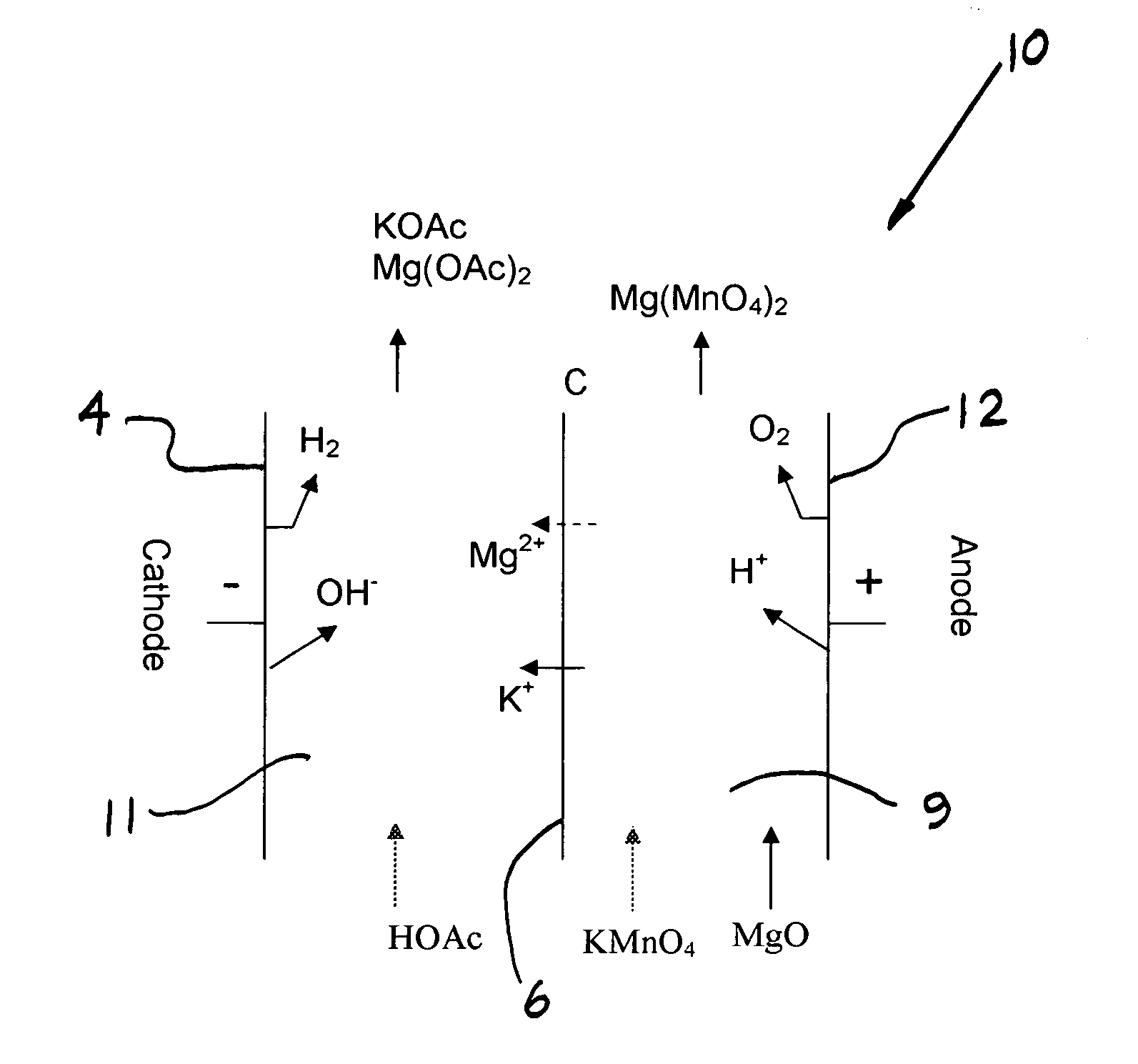
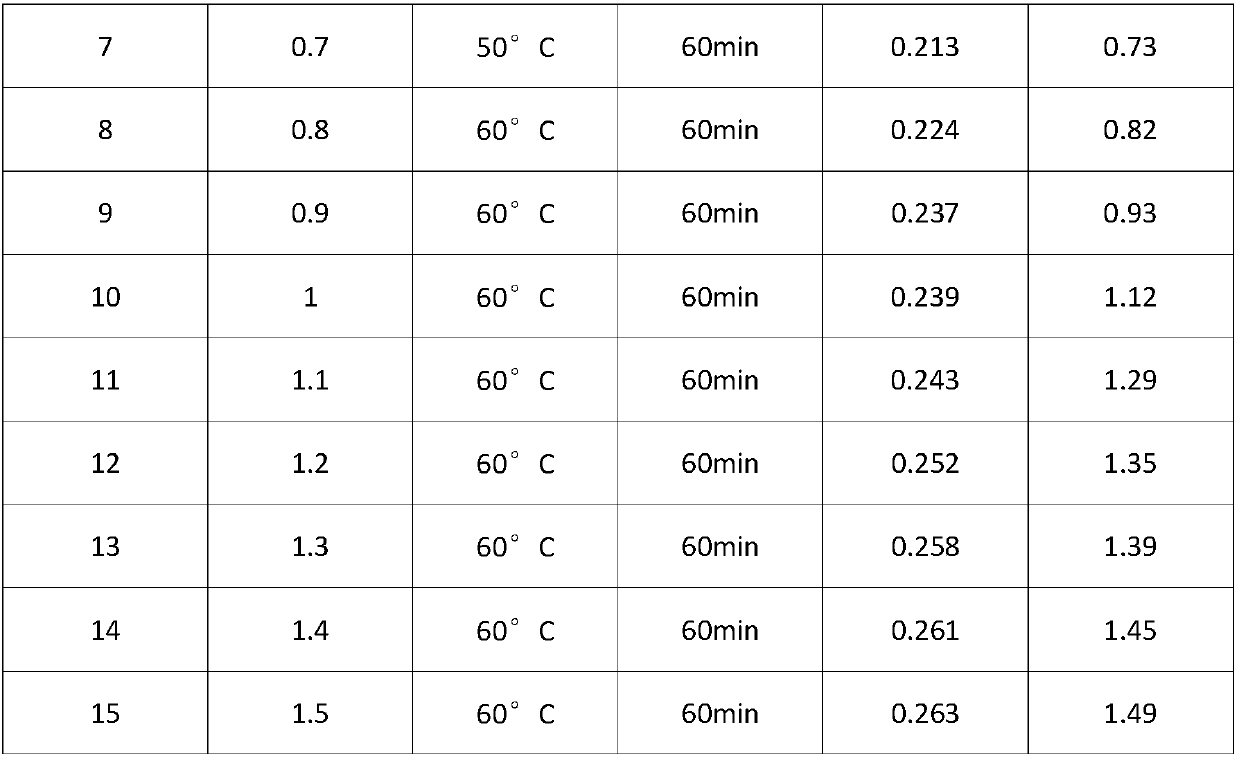
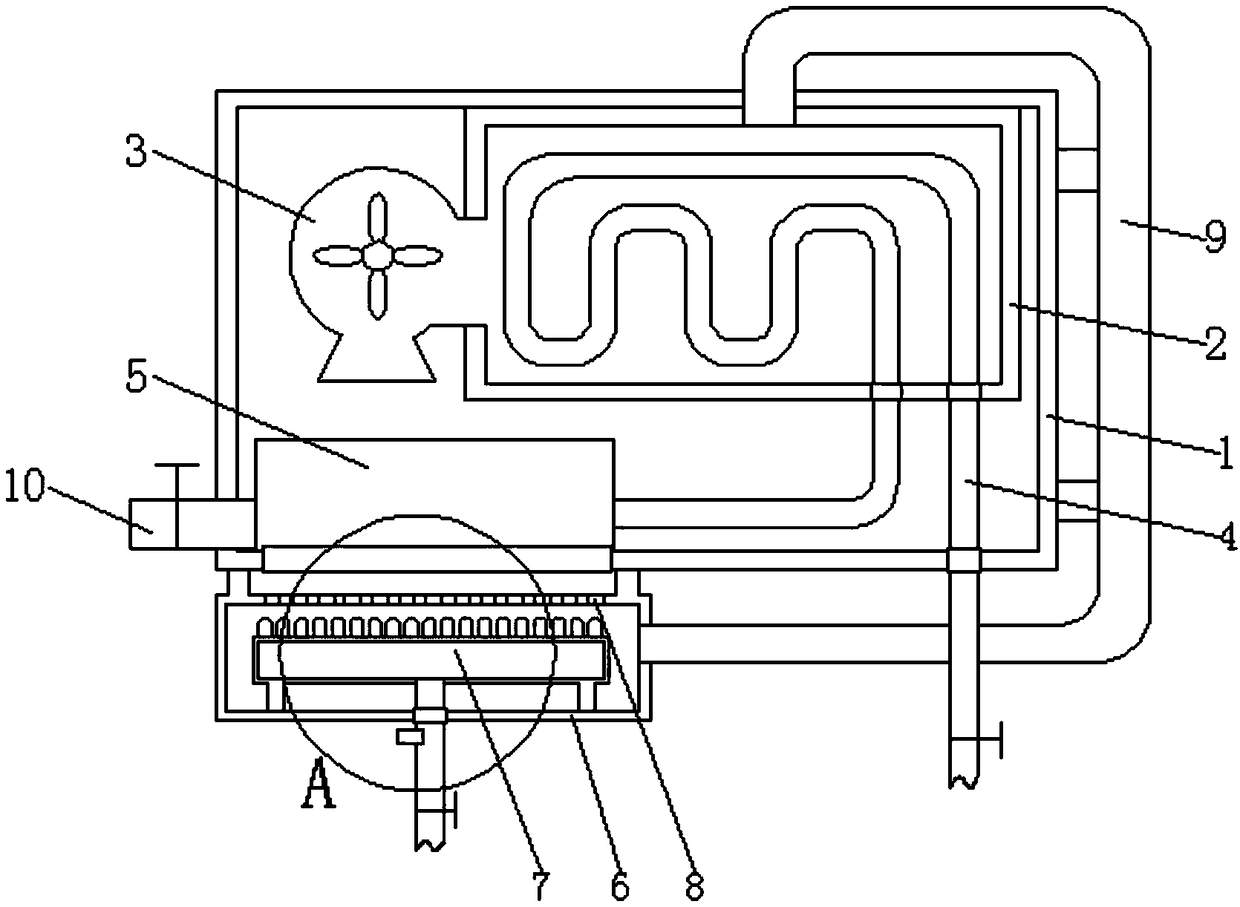
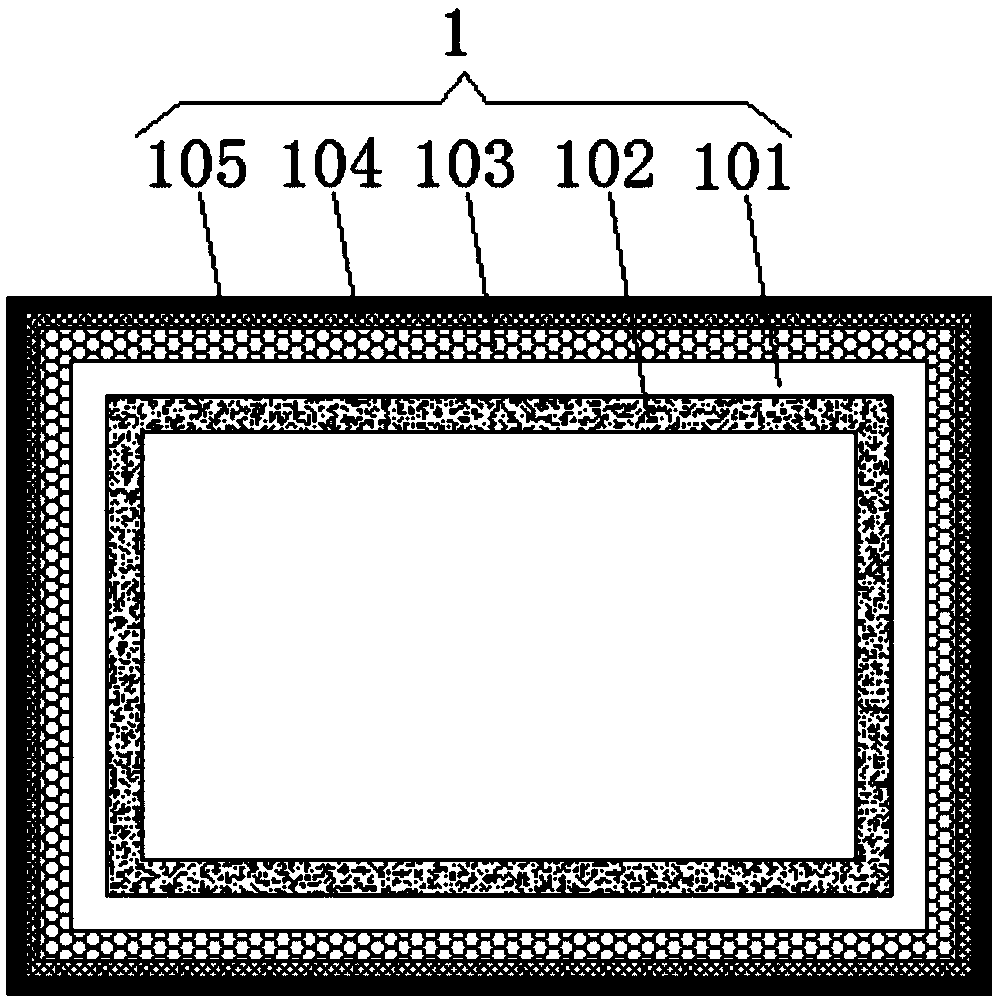
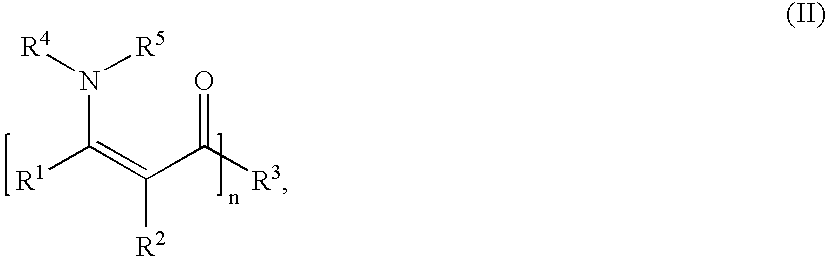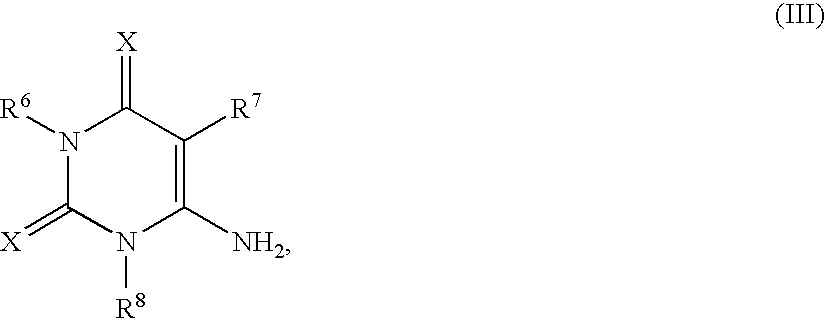Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
61results about "Chlorates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chemical composition and method
The invention relates to a novel composition useful as a feed at production of chlorine dioxide, said composition being an aqueous solution comprising from about 1 to about 6.5 moles / liter of alkali metal chlorate, from about 1 to about 7 moles / liter of hydrogen peroxide and at least one of a protective colloid, a radical scavenger or a phosphonic acid based complexing agent, wherein the pH of the aqueous solution is from about 1 to about 4. The invention also concerns a process for producing chlorine dioxide using the novel composition.
Owner:EKA CHEM AC LTD
Strontium peroxide catalyzed oxygen generating compositions
InactiveUS6866794B1Improved chemical oxygen generation compositionImprove performance uniformityChloratesOther chemical processesReaction rateOxygen
The oxygen generating compositions are formed from a metal powder as a fuel, strontium peroxide as a chlorine suppressant, a catalyst, a reaction rate modifier, and an oxygen source selected from the group consisting of alkali metal chlorates, alkali metal perchlorates, and mixtures thereof. The oxygen generating compositions can optionally also further comprise a transition metal oxide catalyst, and can optionally further include a binder as a pressing aid for forming an oxygen generating oxygen generating block or core. The oxygen generating compositions can be formed from zero to about 15% by weight of metal powder as a fuel, about 0.1%-20% by weight strontium peroxide, from zero to about 15% by weight of a transition metal oxide catalyst, from zero to about 5% of an optional binder, and the remainder of an oxygen source selected from the group consisting of alkali metal chlorates, alkali metal perchlorates, and mixtures thereof.
Owner:BE INTPROP
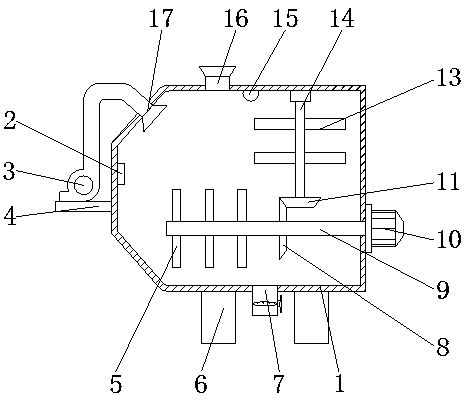
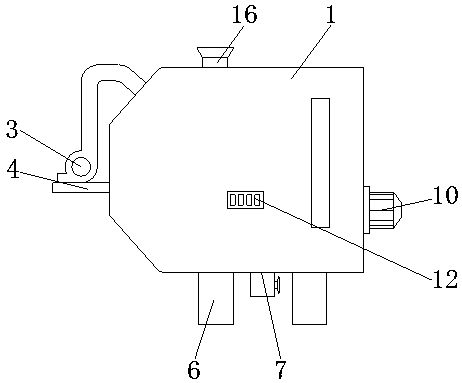
Potassium chlorate production stirring device
PendingCN107626248AIncrease the mixing areaReduce use costChloratesShaking/oscillating/vibrating mixersBiochemical engineeringMoving frame
The invention discloses a potassium chlorate production stirring device which comprises a tank body, wherein a feeding tube is connected with the left side of the top of the tank body; a discharge tube is communicated with the bottom of the right side of the tank body; a long hole is formed in the top of the tank body; casings are fixedly connected with both sides of the top of the tank body; support columns are fixedly connected with the tops of the casings; a transverse plate is fixedly connected with the tops of the support columns; a vertical plate is fixedly connected with the bottom of the transverse plate; a first motor is fixedly connected with the bottom of the front side of the vertical plate. Due to matched use of a second motor, a stirring rod and stirring blades, materials inside the inner cavity of the tank body can be stirred; due to matched use of the first motor, a round disc, a column, the moving frame, a moving frame, a circular plate, a fan-shaped gear and a transverse rod, the stirring rod is moved leftwards and rightwards, the stirring area of the stirring rod inside the inner cavity of the tank body is increased, then the device has the advantage of efficientstirring, the workload is reduced, the use cost of the user is reduced, and convenience of use is achieved.
Owner:LEIYANG JINYUE SCI & TECH DEV
Method for producing oxygen by microwave heated oxygen candle and oxygen candle and microwave device
The invention discloses a preparing method of oxygen through microwave heating oxygen candle as well as oxygen candle and microwave device, which comprises the following steps: (1) blending 80-86% chlorate, 4-10% sensitizing catalyst, 0-6% chloride-inhibiting agent and 0-8% adhesive together; pressurizing to prepare oxygen candle with density at 1.5-2g / cm3; (2) placing oxygen candle into microwave device to heat continuously; guiding oxygen from outlet on the microwave device.
Owner:TSINGHUA UNIV
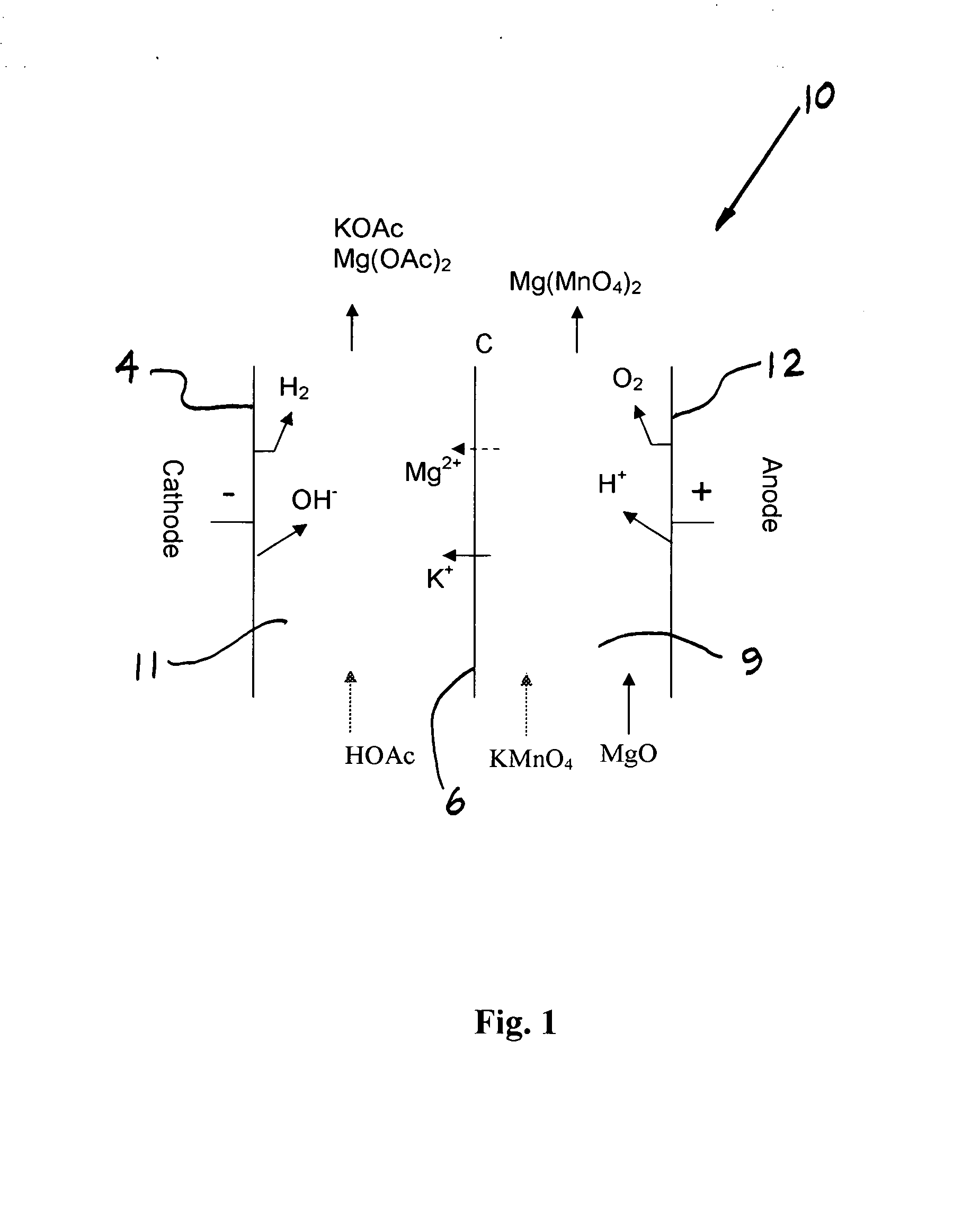
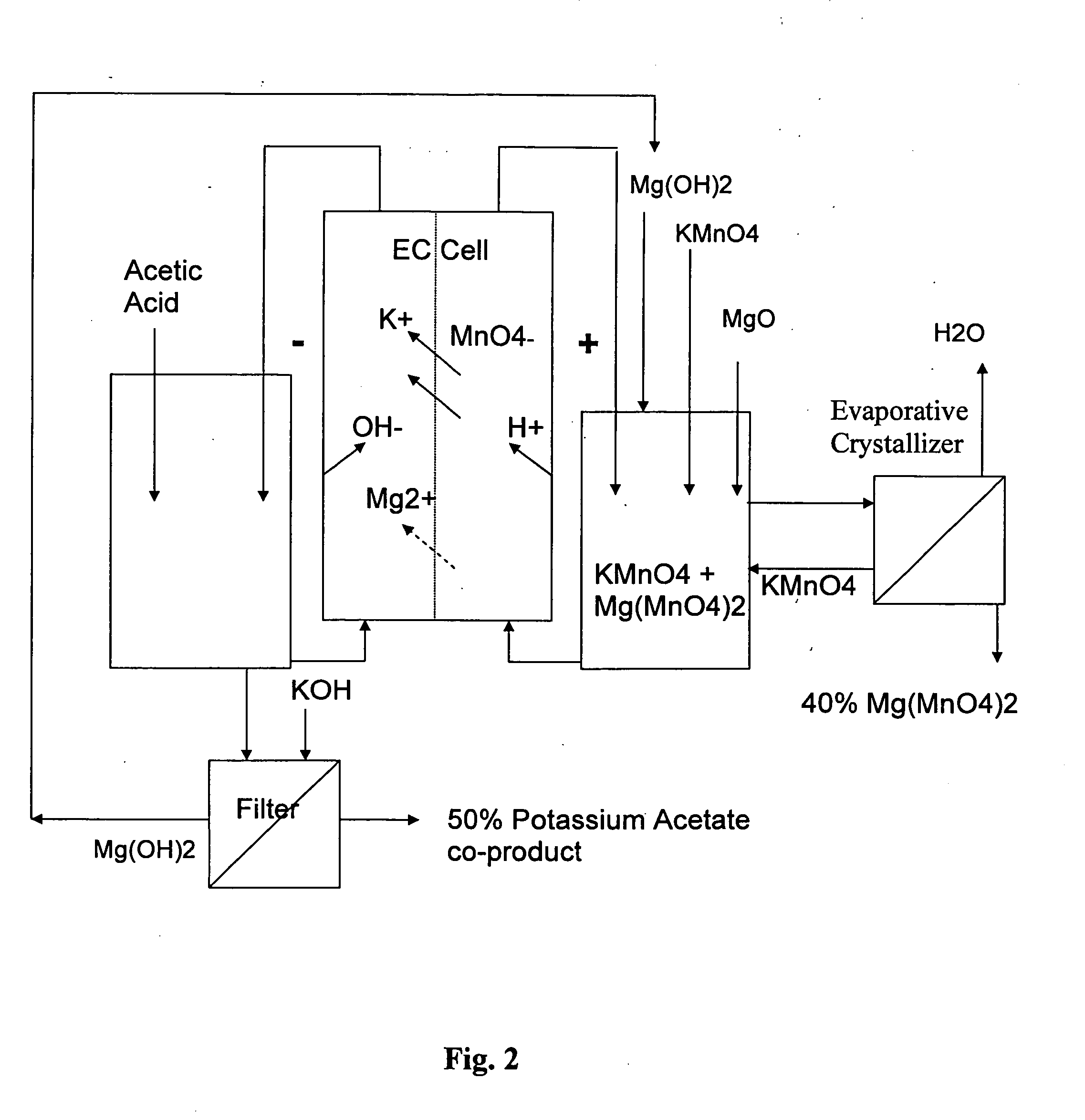
Electrochemical methods for making highly soluble oxidizing agents
InactiveUS20070012570A1Good water solubilityImprove solubilityElectrolysis componentsVolume/mass flow measurementSolubilityHydration reaction
Methods for preparing oxidizing agents having enhanced water solubility properties, such as magnesium permanganate, calcium permanganate and ammonium peroxydisulfate are prepared from oxidizing agents having more limited water solubility properties, such as potassium permanganate and potassium peroxydisulfate by electrochemical means employing oxidant stable, cationic permselective ion-exchange membranes that are also suitable for transporting a preponderance of cations with lower water of hydration, such as potassium over other more highly hydrated cations, such as sodium, magnesium and calcium used to replace the leaving potassium ion, and form more soluble oxidizer salt solutions. The methods may be practiced in multi-compartmentalized electrolytic cells, such as metathesis electrodialysis cells. The methods of the invention are also more attractive economically over previous technologies by simultaneously generating a value-added co-product without costly reagents, while avoiding the disposal of unwanted waste by-products, and the like.
Owner:CARUS CORP
Novel zinc-containing calcium-aluminium double salts
Neutral calcium-aluminum double salts of the formula (A):Ca2m(Zn2n)Al2(OH)6+2(2m+2n−1)An.oH2O (A),where the following apply for m and n:m=0.5 to 3 and 0.5m≧n>0; An=CO3, where this may be replaced completely or partially by at least one of the following groups selected from OH, ClO4 and H3CS(═O)2O (triflate) and o=0 to 3. The present invention further relates to their preparation, use in compositions and stabilizer systems and uses thereof.
Owner:NABALTEC AG
Method for producing sodium chlorate through low-temperature vacuum evaporation and crystallization
InactiveCN101941679AReduce material requirementsAvoid pollutionChloratesChemical industrySodium chlorate
The invention relates to a method for producing sodium chlorate through low-temperature vacuum evaporation and crystallization, belonging to the field of chlor-alkali chemical industry and solving the technical problem of providing the method for producing the sodium chlorate with lower production cost. The method for producing the sodium chlorate comprises a steps of crystallizing and separating sodium chlorate from sodium chlorate electrolyte. A method crystallizing and separating the sodium chlorate from the sodium chlorate electrolyte comprises the following steps of: a, mixing the sodium chlorate electrolyte with a right amount of mother solution to obtain a mixed solution with the density of 1.30-1.40 g / cm<3>, heating in vacuum, and boiling the mixed solution at 35-45 DEG C, wherein the mother solution is a solution remained after the sodium chlorate is crystallized, precipitated and separated from the sodium chlorate electrolyte, the concentration of NaCl contained in the mother solution is less than or equal to 130 g / L; b, stopping heating when the density of the mixed solution is 1.50-1.60 g / cm<3>; and c, discharging, cooling, filtering, washing and drying to obtain the sodium chlorate.
Owner:茂县鑫盐化工有限公司
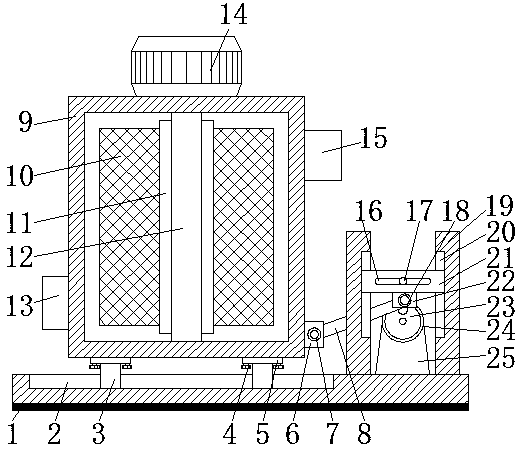
Potassium chlorate mixer capable of efficiently mixing materials
PendingCN107626249AFacilitates effective mixingWorks well when mixedChloratesShaking/oscillating/vibrating mixersEngineeringPotassium chlorate
The invention discloses a potassium chlorate mixer capable of efficiently mixing materials. The mixer comprises a laying plate, wherein a sliding chute is formed in the top of the laying plate; sliding rods are arranged on the two sides of the cavity of the sliding chute; a mixing box is arranged at the tops of the sliding rods; a material outlet is formed in the bottom of the left side of the mixing box; a material inlet is formed in the top of the right side of the mixing box; a stirring motor is arranged at the top of the mixing box; a rotating shaft is arranged at the outlet end of the stirring motor; the bottom of the rotating shaft penetrates through and extends to the cavity of the mixing box; stirring blades are arranged on the two sides of the rotating shaft. The mixer can efficiently mix potassium chlorate through matching among the sliding chute, the sliding rods, a first movable block, a first movable shaft, a shifting rod, the stirring blades, the rotating shaft, the stirring motor, a sliding rail, a bulge, a second movable shaft, a movable plate, a sliding groove, a shifting board, a second movable block, a rotating plate, a stirring motor and a rotating rod, and is better in effect when being used for mixing potassium chlorate.
Owner:LEIYANG JINYUE SCI & TECH DEV
Production technology of anti-caking sodium chlorate
InactiveCN106757134AGood anti-caking effectReduce dosageChloratesElectrolysis componentsSodium chlorateFiltration
The invention discloses a production technology of anti-caking sodium chlorate. The production technology comprises the following steps of: (1) performing crystal separation on sodium chlorate electrolyte to obtain sodium chlorate; (2) preparing a saturated sodium chlorate solution from the sodium chlorate obtained in the step (1) for standby application, taking and placing 500ml of the saturated sodium chlorate solution in a beaker, adding 2-5g of a surfactant, performing stirring to enable the surfactant to dissolve in the saturated sodium chlorate solution, then adding 500g of sodium chlorate crystals, performing uniform stirring, and performing standing for ten minutes to obtain a feed liquid; (3) performing vacuum filtration on the feed liquid obtained in the step (2), and placing the obtained sodium chlorate crystals in which the surfactant is adsorbed in a constant-temperature drying oven of 12-15 DEG C for drying; and (4) loading the dried sodium chlorate in a plastic sample bag, and performing sealing. The production technology disclosed by the invention is good in anti-caking effect, small in quantity of reagents, and low in cost.
Owner:HUNAN HENGGUANG CHEM
Oxygen-generating liquid composition
InactiveUS20110140038A1Increase contentEffective oxygen generating compoundChloratesOther chemical processesOxygenLiquid composition
An oxygen-generating liquid composition is described comprising compositions of water plus lithium chlorate as a saturated solution and as a mixture of saturated solution plus precipitated solids. The composition further comprises catalysts. The oxygen is produced via thermal decomposition. Uses of the composition include generation of oxygen for power production or for breathable air. A principal benefit is that the composition is an easily handled liquid stored in un-pressurized tank.
Owner:API ENG
A special anti-caking agent for potassium chlorate
The invention relates to an anticaking agent special for potassium chlorate. The anticaking agent consists of hydrophilic white carbon black and hydrophobic white carbon black, wherein the weight ratio of the hydrophilic white carbon black to the hydrophobic white carbon black is (1-2) to (1-2). Compared with the prior art, the anticaking agent special for the potassium chlorate has the advantages that: the two types of white carbon black, namely the hydrophilic white carbon black and the hydrophobic white carbon black, are adopted, the hydrophilic white carbon black can absorb water inside apotassium chlorate product and the hydrophobic white carbon black can prevent water outside the potassium chlorate product from entering the potassium chlorate product, so dual anticaking assurance for the potassium chlorate product is realized; and the test shows that an anticaking effect is extremely good and the potassium chlorate product cannot be caked for more than six months.
Owner:SUZHOU XINENG ENVIRONMENTAL SCI & TECH CO LTD
Method of preparing, storing, transporting and testing chlorine dioxide solutions
InactiveUS20120214248A1Easy to storeEasy to transportChloratesChlorous acidChlorine dioxidePhysical chemistry
A method for determining the concentration of chlorine dioxide in a chlorine dioxide solution having the following steps:(1) isolating two samples, Sample 1 and Sample 2, from the chlorine dioxide solution;(2) stripping the chlorine dioxide from Sample 1;(3) completely converting the chlorine dioxide in Sample 2 to other chlorine species;(4) transporting the chlorine dioxide samples either within the facility or outside the facility to a testing site;(5) separately determining the concentration of the chlorine containing species in each of Samples 1 and 2; and(6) calculating the concentration of chlorine dioxide in said chlorine dioxide solution based upon the information obtained in step (5).
Owner:SAMPSON ALLISON H +1
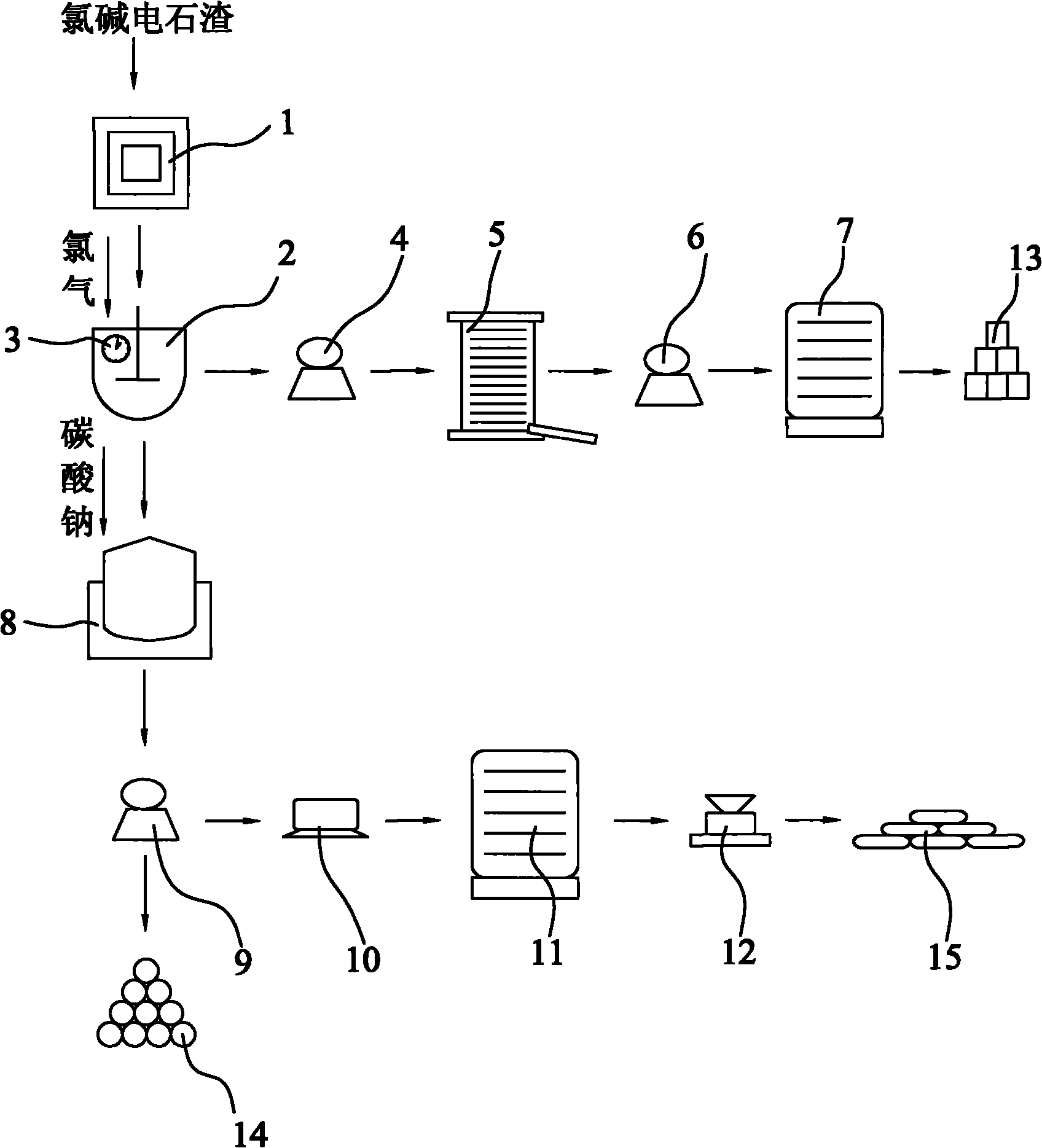
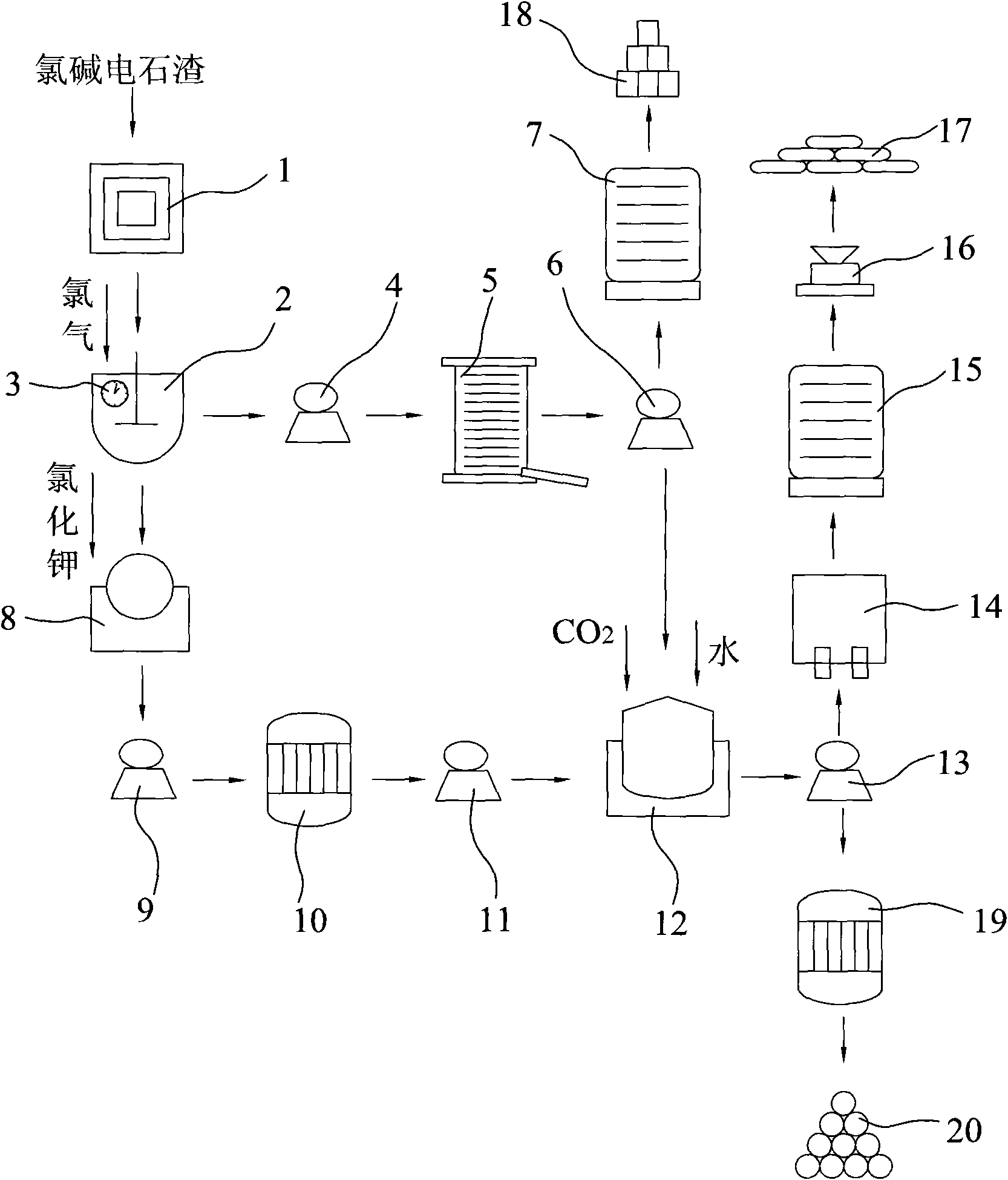
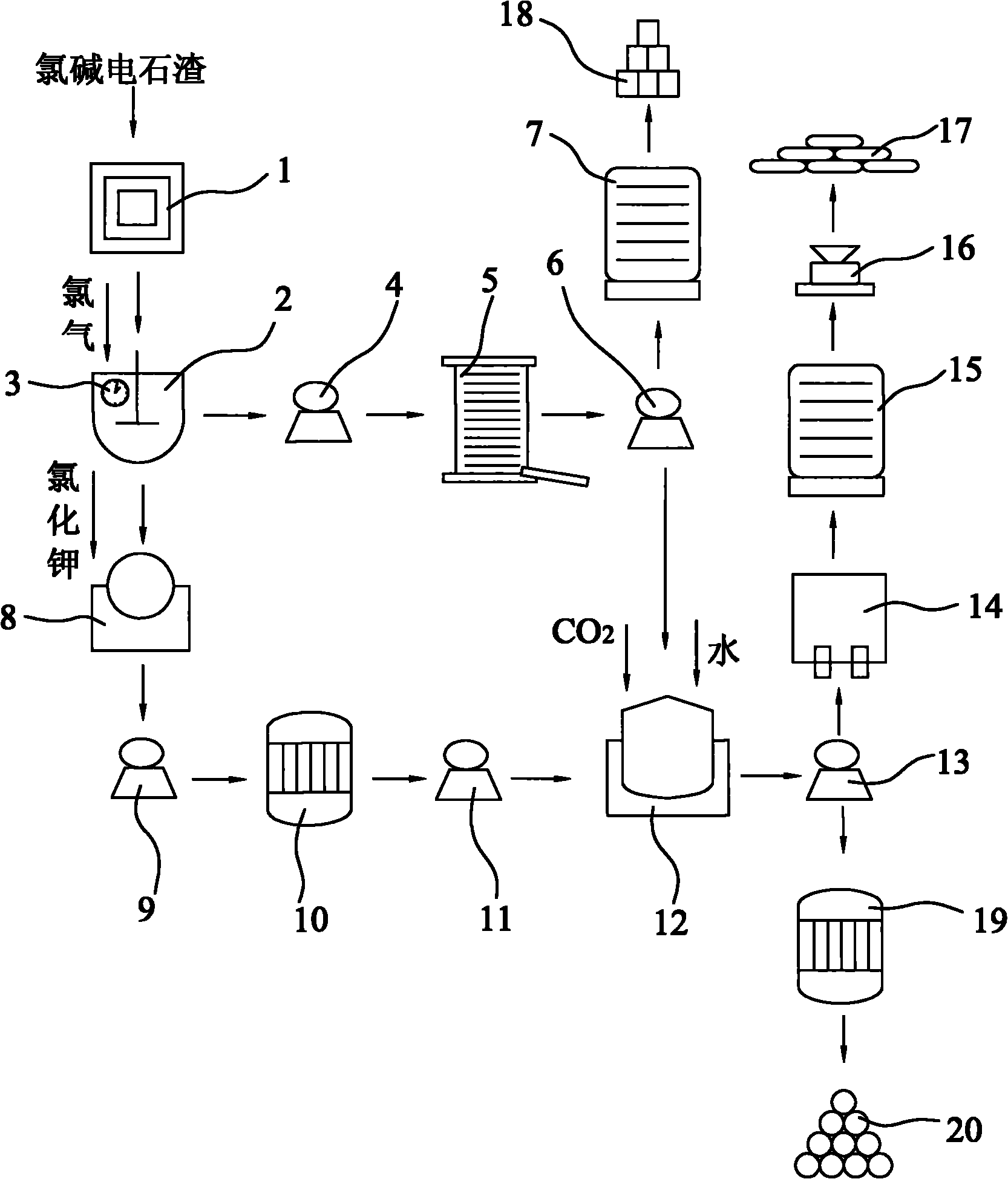
Method for co-production of light calcium carbonate and ammonium chloride in preparation of potassium chlorate by carbide slag
InactiveCN101823695AEasy to operateLess investmentChloratesCalcium/strontium/barium carbonatesSlagDecomposition
The invention discloses a method for co-production of light calcium carbonate and ammonium chloride in the preparation of potassium chlorate by carbide slag. In the method, calcium hydroxide in chlor-alkali carbide slag reacts with chlorine gas to produce reaction products with calcium chlorate and calcium chloride; the reaction products are filtered to remove impurities, solid calcium chlorate can be separated out through settlement under the reduced temperature, and then solid calcium chlorate and calcium chloride filtrate can be obtained through filtration again; the reaction products with the calcium chlorate and the calcium chloride can also react with potassium chloride for double decomposition to produce mixed solution of potassium chlorate and calcium chloride; the mixed solution is filtered to remove impurities, potassium chlorate crystals can be separated out through distillation under the reduced pressure, and potassium chlorate crystals and calcium chloride solution can be obtained through filtration again; and then the calcium chloride solution after being treated with a surfactant react with carbon dioxide and ammonia in a carbonation tower for carbonation, thereby obtaining the light calcium carbonate and ammonium chloride.
Owner:汪晋强
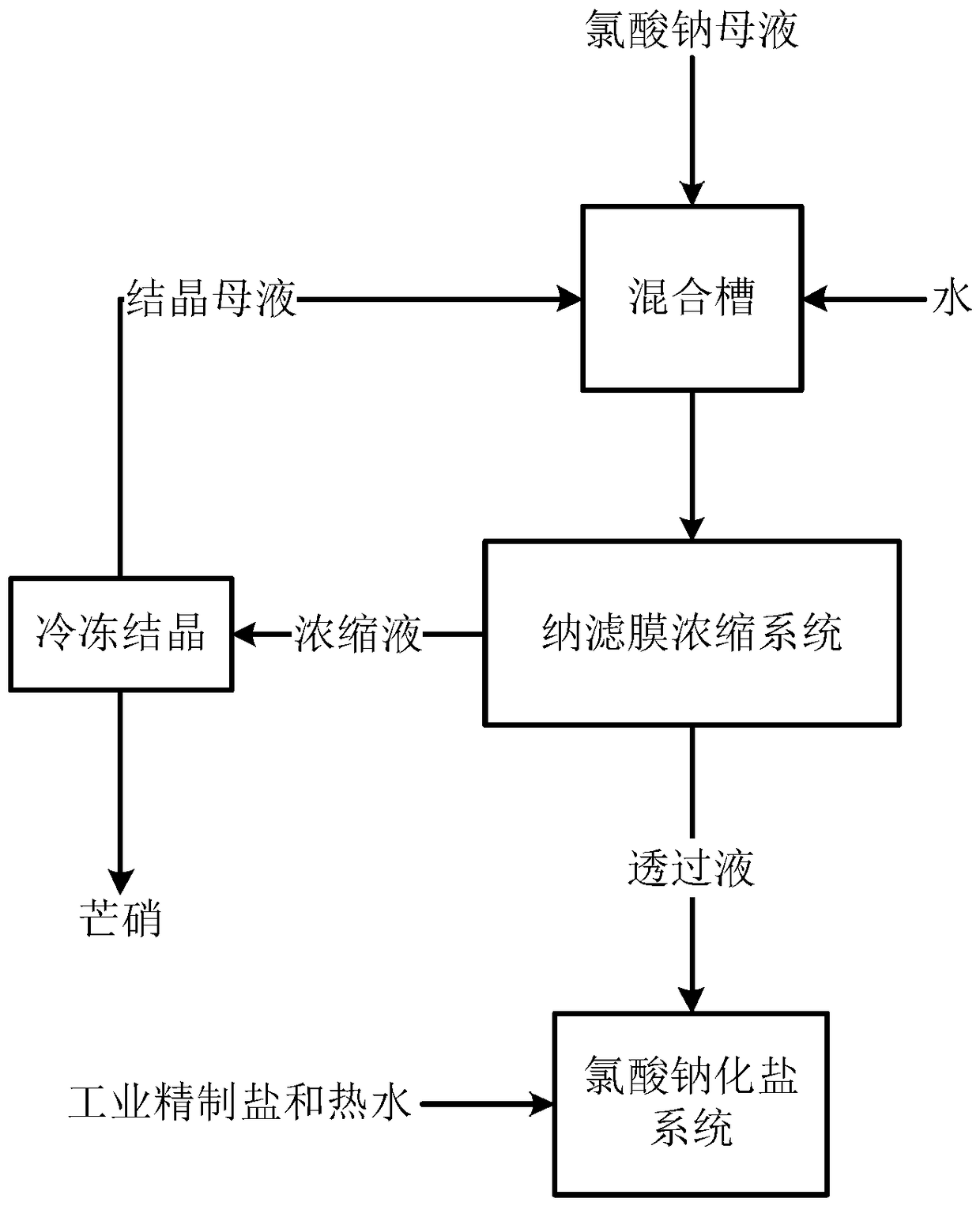
Process for freezing and denitrating sodium chlorate mother liquor by aid of membrane methods
InactiveCN108529562AMeet the requirements of feedImprove product added valueChloratesAlkali metal sulfite/sulfate purificationMembrane methodFiltration membrane
The invention discloses a process for freezing and denitrating sodium chlorate mother liquor by the aid of membrane methods. The process includes steps of (1), diluting the sodium chlorate mother liquor, to be more specific, adding the sodium chlorate mother liquor into water and diluting the sodium chlorate mother liquor to allow the concentration of sodium chlorate in the sodium chlorate motherliquor to be lower than 200 g / L so as to obtain sodium chlorate diluent; (2), carrying out pretreatment including rough filtration, pH (potential of hydrogen) value regulation, dechlorination and finefiltration on the sodium chlorate diluent to obtain sodium chlorate pretreated liquor; (3), carrying out concentration, to be more specific, feeding the sodium chlorate pretreated liquor into a nano-filtration membrane concentration system by the aid of booster pumps and acquiring permeated liquor and concentrated liquor; (4), carrying out freezing and denitrating, to be more specific, feeding the concentrated liquor into a freezing system, crystallizing the concentrated liquid, and carrying out solid and liquid separation by the aid of a centrifugal machine to obtain mirabilite and crystallization mother liquor; (5), carrying out recycling by means of mixing the crystallization mother liquor and the sodium chlorate mother liquor with each other. The process for freezing and denitrating the sodium chlorate mother liquor by the aid of the membrane methods has the advantages that the process is simple, is low in cost and has a high additional value, new chemical impurities can be prevented from being introduced into the sodium chlorate mother liquor, and impurities in the sodium chlorate mother liquor can be effectively removed by the aid of the process.
Owner:HUNAN HENGGUANG TECH
Method for producing calcium chlorate and co-producing fine calcium carbonate and sodium chloride by using carbide slag
InactiveCN102101652AEasy to operateLess investmentCalcium/strontium/barium carbonatesChloratesSlagSurface-active agents
The invention discloses a method for producing calcium chlorate and co-producing fine calcium carbonate and sodium chloride by using carbide slag, comprising the following steps of: generating a reaction product containing the calcium chlorate and calcium chloride by reacting calcium hydroxide contained in chlor-alkali carbide slag with chlorine; filtering and decontaminating the reaction product, reducing temperature, subsiding, and then separating out a calcium chlorate solid; then filtering to obtain the calcium chlorate solid and calcium chloride filter liquor; treating the calcium chloride filter liquor by using a surface active agent, and then reacting with sodium carbonate in a corrosion-resisting reactor to obtain a calcium carbonate sediment and sodium chloride mixed solution; and filtering and separating the calcium carbonate and the sodium chloride.
Owner:汪晋强
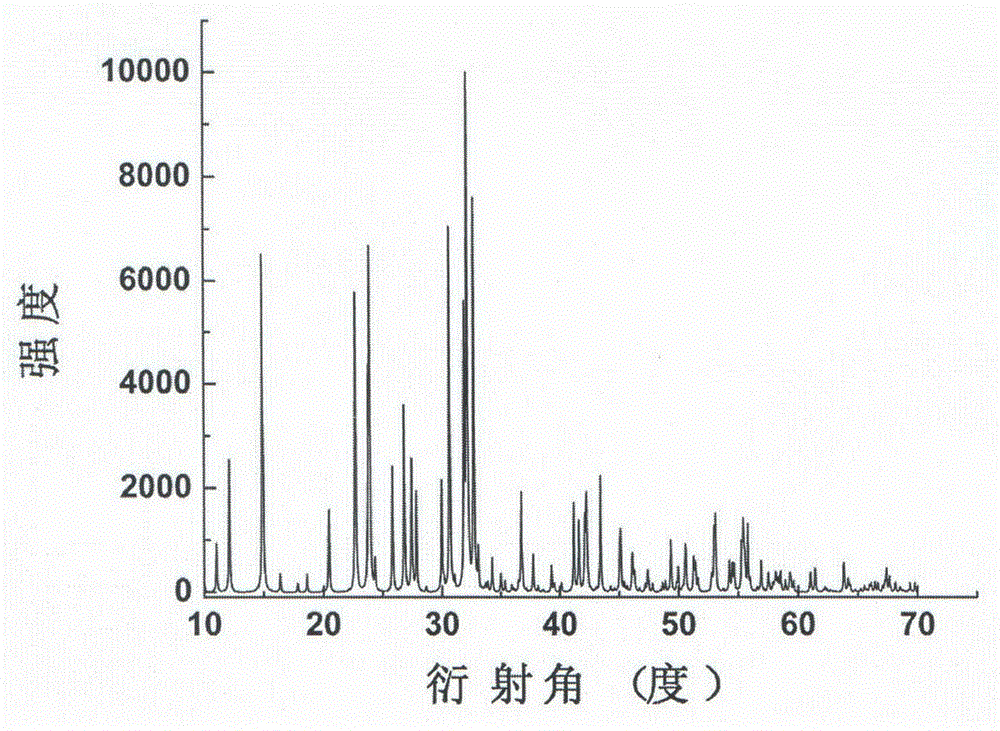
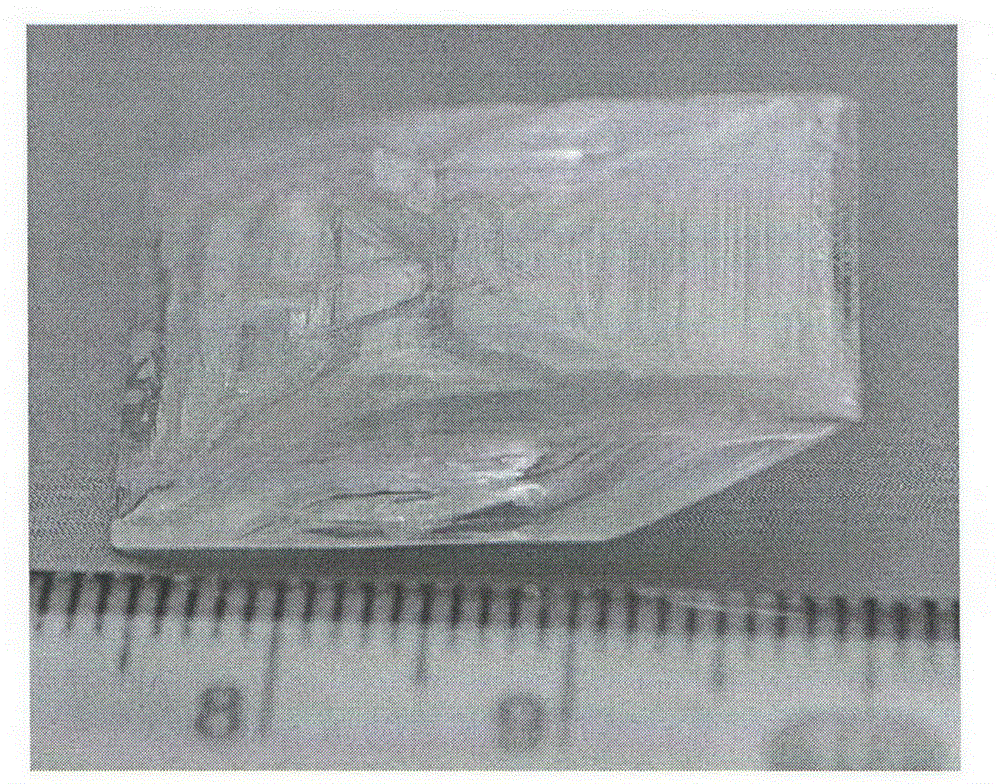
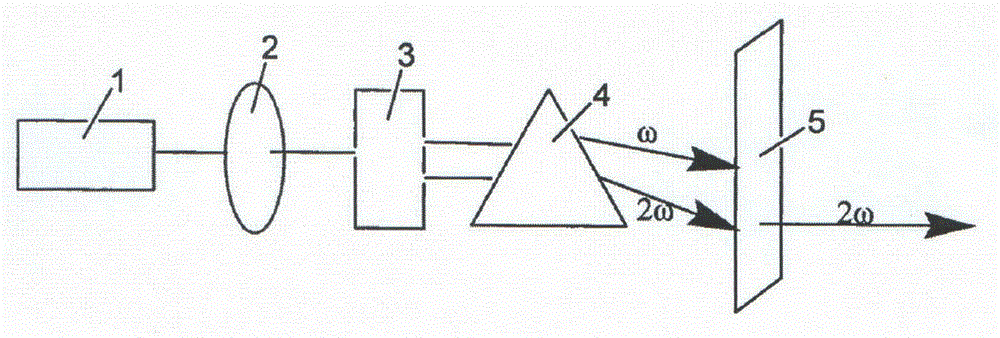
Compound lead chlorate, lead chlorate infrared nonlinear optical crystal, and preparation method and uses of lead chlorate infrared nonlinear optical crystal
ActiveCN105621361AStrong frequency doubling effectEasy to prepareChloratesPolycrystalline material growthNonlinear optical crystalCrystal system
The present invention relates to a compound lead chlorate, a lead chlorate infrared nonlinear optical crystal, and a preparation method and uses of the lead chlorate infrared nonlinear optical crystal. According to the present invention, the chemical formula of the compound is Pb2.14OCl2.28, the molecular weight is 545.802, and the compound is prepared by using a solid-state reaction method; the chemical formula of the compound crystal is Pb2.14OCl2.28, the molecular weight is 545.802, the compound crystal does not have a symmetry center and belongs to an orthorhombic crystal system, the space group is Fmm2, the cell parameters comprise that a is 5.829(3)angstrom, b is 16.056(6)angstrom and c is 35.528(15)angstrom, and the crystal grows by using a compound melt method or adding a co-solvent; the obtained crystal material has a strong phase-matched frequency doubling effect (SHG), the Kurtz powder frequency doubling testing results show that the powder frequency doubling effect of the crystal material is 4 times the frequency doubling effect of potassium dihydrogen phosphate (KDP), the phase matching can be achieved, and the penetration wave band is 0.34-7 [mu]m; the laser damage threshold of the crystal material is 10 times the laser damage threshold of the current commercial infrared nonlinear optical crystal material AgGaS2; and the obtained crystal material does not contain crystalline water, has characteristics of air stability and good thermal stability, and has important application values in the fields of laser frequency conversion, electro-optic modulation, photorefractive information processing, and the like.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Method for producing potassium chlorate and co-producing superfine calcium carbonate and ammonium chloride by utilizing carbide slag
InactiveCN102086026AEasy to operateLess investmentChloratesCalcium/strontium/barium carbonatesSlagDecomposition
The invention discloses a method for producing potassium chlorate and co-producing superfine calcium carbonate and ammonium chloride by utilizing carbide slag, which comprises the following steps of: reacting calcium hydroxide in chlor-alkali carbide slag with chlorine to generate a reaction product containing calcium chlorate and calcium chloride; filtering the reaction product to remove impurities, cooling and precipitating to separate out solid calcium chlorate, and filtering to obtain the solid calcium chlorate and calcium chloride filtrate; performing a double decomposition reaction of the reaction product containing the calcium chlorate and the calcium chloride and potassium chloride to generate mixed solution of the potassium chlorate and calcium chloride; filtering the mixed solution to remove impurities, distilling under reduced pressure, separating out potassium chlorate crystals, and filtering to obtain the potassium chlorate crystals and solution of calcium chloride; and treating the solution of calcium chloride by using a surfactant, and carburizing carbon dioxide and ammonia water in a carbonizer to obtain lightweight calcium carbonate and ammonium chloride.
Owner:汪晋强
Drying device for processing potassium chlorate
InactiveCN107670068AImprove drying efficiencyChloratesDrying gas arrangementsPhysical chemistryUltraviolet lights
The invention discloses a drying device for processing potassium chlorate. The drying device for processing potassium chlorate comprises a box, wherein the upper end of the left side of a cavity of the box is fixedly connected with an ultraviolet lamp; the left end of the top of the cavity of the box is fixedly connected with a lamp; the right end of the top of the cavity of the box is movably connected with a second rotating shaft through a bearing; and the outer surface of the second rotating shaft is successively fixedly connected with a second stirring blade and a second bevel gear from top to bottom. The middle of the top of a supporting seat is fixedly connected with a fan, under the actions of a pipe and a sprayer, potassium chlorate in the box can be dried by blowing, meanwhile, under the actions of a motor, a first rotating shaft, a first stirring blade, a first bevel gear, the second bevel gear, the second rotating shaft and the second stirring blade, the potassium chlorate in the box can be stirred, the efficiency of drying of the device to the potassium chlorate is improved, and the problems that an existing drying device for processing the potassium chlorate is unreasonable in structure, and thus efficiency of drying to the potassium chlorate is low are solved.
Owner:LEIYANG JINYUE SCI & TECH DEV
Aqueous solution and method of prolonging life of residual chlorine in aqueous solution
ActiveCN101562981AFull effectGive full play to the washing effectBiocideAnimal repellantsHalogenDisinfectant
An aqueous solution which retains a high residual chlorine concentration over long and has excellent disinfectant (bactericidal) ability. The aqueous solution contains at least one member selected from the group consisting of halogen acids and salts thereof and further contains active oxygen, wherein the halogen acids are at least one member selected from the group consisting of hypochlorous acid, chlorous acid, chloric acid, and perchloric acid, the total amount of the at least one member selected from the group consisting of the halogen acids and salts thereof and contained in the aqueous solution is 10-50,000ppm, and the total amount of the active oxygen contained in the aqueous solution is 0.1-1,000ppm.
Owner:イーデオ +1
Sodium chlorate crystallization evaporation water closed circulation process system and method
ActiveCN110975314ANo need to worry about pollutionGuarantee product qualityChloratesEvaporator accessoriesSodium chlorateProcess engineering
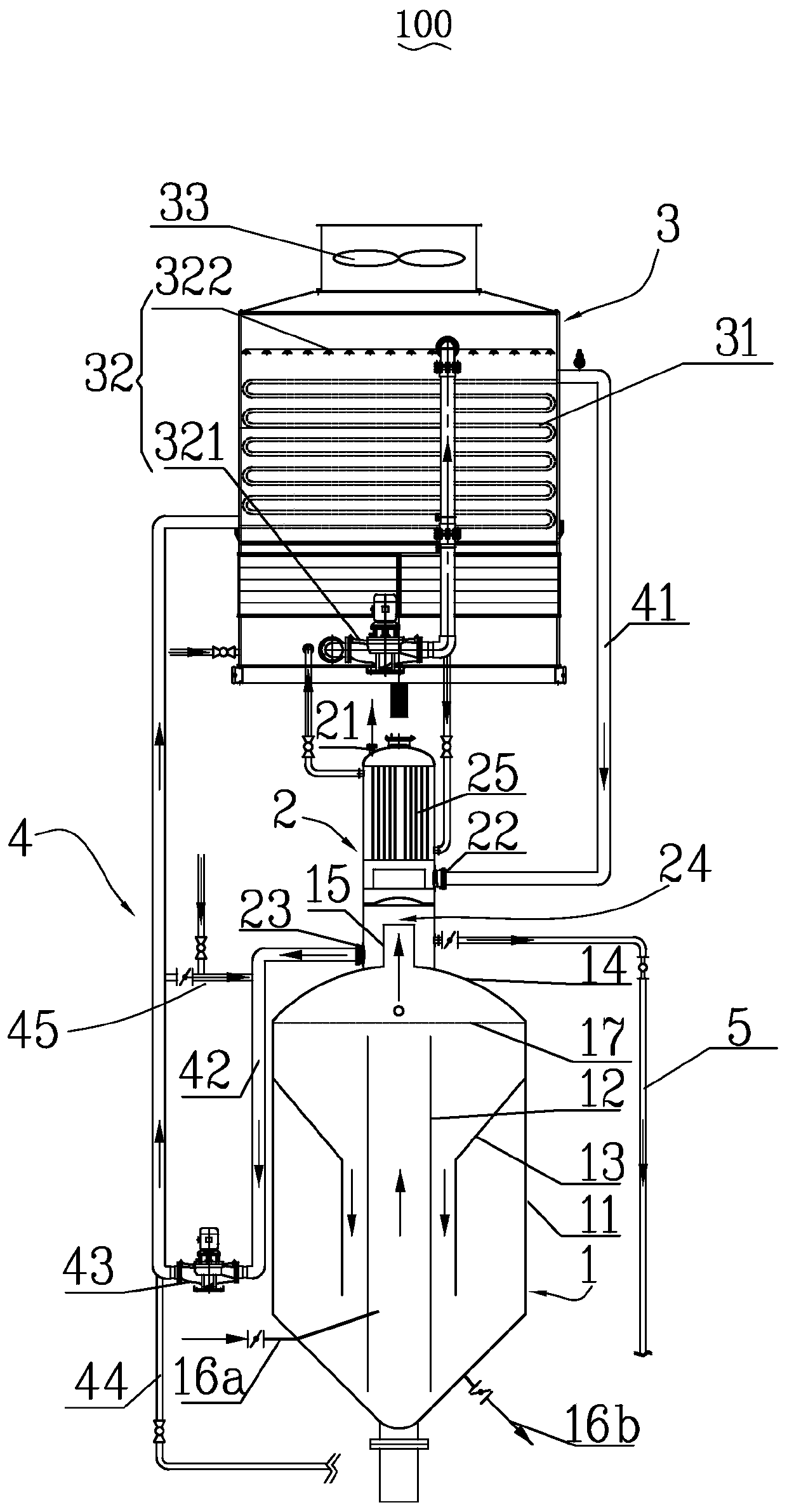
The invention discloses a sodium chlorate crystallization evaporation water closed circulation process system which comprises an evaporation crystallizer, an atmospheric condenser connected with the evaporation crystallizer, a closed water cooling tower with a heat exchange tube bundle and an overflow tube connected to the atmospheric condenser, a water outlet of the heat exchange tube bundle is connected with a water inlet of the atmospheric condenser through a first circulating water pipe, a water outlet of the atmospheric condenser is connected with a water inlet of the heat exchange tube bundle through a second circulating water pipe, and a process circulating water pump is arranged on the second circulating water pipe. And the overflow pipe is used for conveying process circulating water to a sodium chlorate salt system. According to the sodium chlorate crystallization evaporation water closed circulation process system provided by the invention, process circulating water independently circulates in a closed environment, so that entrainment in the electrolyte evaporation process is adapted, the environment is protected, and the product quality is ensured. The invention also provides a sodium chlorate crystallization evaporation water closed circulation process method.
Owner:HUNAN HENGGUANG TECH
Method for preparing sodium chlorate from industrial salt
InactiveCN108588746AImprove output per unit timeIncrease productionChloratesElectrolysis componentsEvaporationElectrolytic cell
The invention discloses a method for preparing sodium chlorate from industrial salt. The method comprises the following steps of: (1) mixing industrial salt with water to prepare industrial salt water, filtering and purifying the industrial salt water, putting industrial salt water into an electrolytic cell after processing to perform electrolysis, thereby obtaining a sodium chlorate solution; performing vacuum low-temperature continuous evaporation to obtain a mixture of sodium chlorate crystal and a sodium chlorate mother solution, and separating the sodium chlorate crystal from the sodium chlorate mother solution for the obtained mixture, adding anti-blocking substances in a separating process, feeding the separated-out sodium chlorate mother solution into a treatment slot for treatingsulfate radicals, adding a flocculating agent to stand after treatment, and performing dehydration separation after standing. A proportion of calcium chloride to barium chloride is controlled, so thathigh yield within unit time in a production and electrolysis process for sodium chlorate is realized, and power consumption of unit yield is reduced; and meanwhile, the prepared chlorate has a very good anti-blocking effect.
Owner:汶川县湘宁氯酸盐有限责任公司
Stability improvement of aluminum hydroxide in PVC compound
InactiveUS20060014875A1Easy compoundEasy to handleChloratesOther chemical processesPerchlorate saltAlkaline earth metal
An aluminum hydroxide composition is disclosed, having diminished tendency to cause discoloration on heating at 177° C. of a plastic composition whose major polymeric component is polyvinyl chloride, comprising aluminum hydroxide and an amount, effective in diminishing discoloration, of at least one inorganic perchlorate salt selected from the group consisting of alkali metal perchlorates and alkaline earth metal perchlorates.
Owner:GALATA CHEM LLC
Method for producing chlorate in different grain diameters by three-section continuous low-temperature vacuum evaporative crystallization
ActiveCN107840309AImprove use valueCompliant productChloratesSolution crystallizationSlurryVacuum evaporation
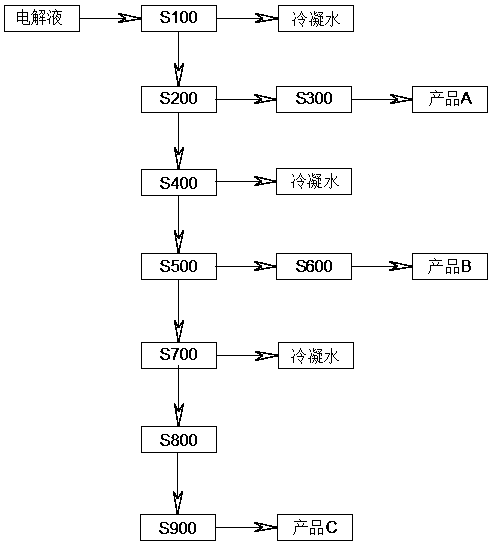
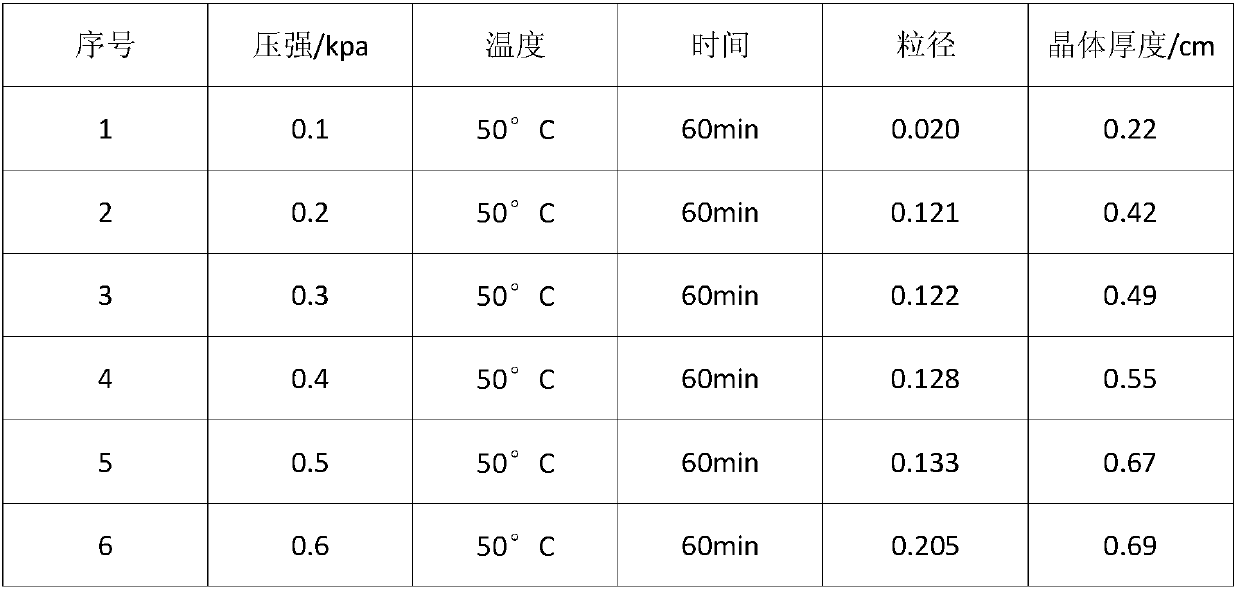
The invention discloses a method for producing chlorate in different grain diameters by three-section continuous low-temperature vacuum evaporative crystallization, comprising the following steps: performing low-temperature vacuum evaporative continuous crystallization on A, performing continuous crystallization on a chlorate electrolyte with concentration of 480 / L-620g / L at the pressure of 0.81KPa to 0.86 KPa and the temperature of 48-52 DEG C, so as to obtain an evaporated concentrated crystal-slurry mixture X; then performing centrifugal separation, and drying, then packaging chlorate solids by adopting a full-automatic quantitative packaging machine A to obtain a crude chlorate product A with particle size of greater than 0.2mm. the method has the advantages that the particle size of crystals of chlorate is indirectly controlled by controlling the vacuum degree and different temperatures in the evaporation and crystallization process by adopting a manner of continuous low-temperature vacuum evaporation, so as to prepare the expected chlorate product according to requirement, the use value is high, and products and percent of pass better meeting the requirement can be achieved in comparison with existing layered crystallization.
Owner:四川集康化工有限公司
Gas generant
PCT No. PCT / JP95 / 02732 Sec. 371 Date Aug. 28, 1996 Sec. 102(e) Date Aug. 28, 1996 PCT Filed Dec. 27, 1995 PCT Pub. No. WO96 / 20147 PCT Pub. Date Jul. 4, 1996A gas generant for an air, which is improved in the defects of gas generants using sodium azide in a practical use and has stable combustion capability. And a molecular compound comprising (a) a gas generant component, (b) an oxidant component and (c) a reaction accelerator component, preferably represented by the composition formula (I).M.mX.nY(I)[wherein, M is Al Mg, Ca, Cr, Cu, Zn, Mn, Fe, Co, Sr, Ni and another metal components; X is a nitrogen-containing compound having 0 or 1 carbon atom; Y is an anion such as NO3 and ClO; m is a number of 1 to 3; and n is a number 2 to 3].
Owner:DAICEL CHEM IND LTD
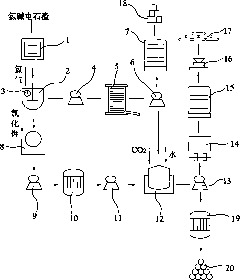
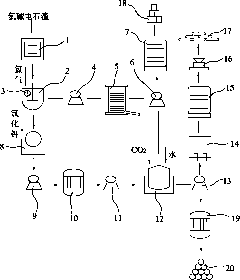
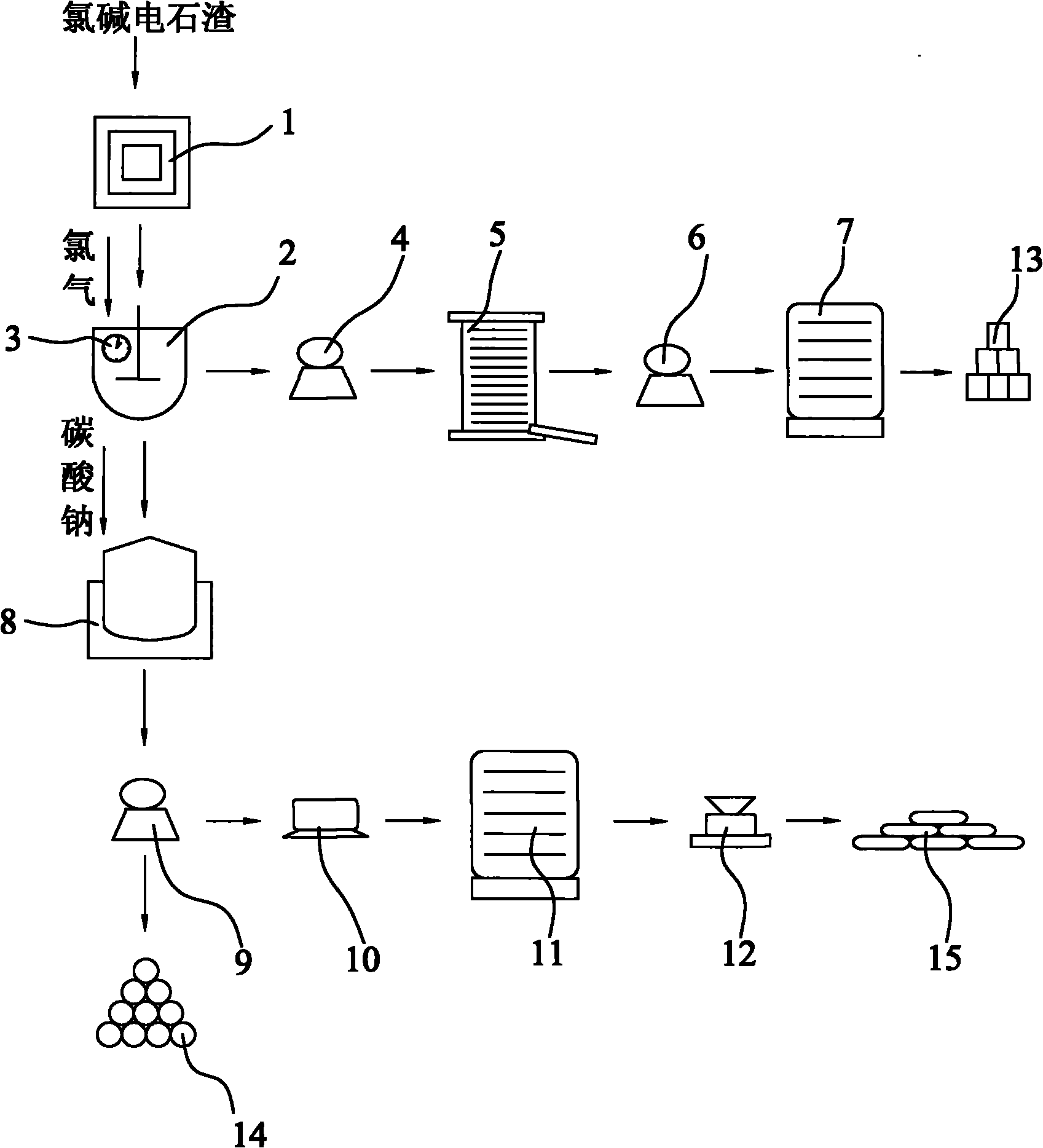
Method for producing potassium chlorate from calcium carbide waste residues
The invention relates to a method for producing potassium chlorate from calcium carbide waste residues. The method comprises the following steps: by taking calcium carbide waste residues as raw materials, adding water into the calcium carbide waste residues to prepare an emulsion, pumping the emulsion into a reaction kettle, continuously stirring, introducing chlorine and oxygen, stirring for reacting, performing press-filtering with a filter press after the reaction is over, removing filter residues, keeping filtrate, adding potassium chloride into the solution, continuously stirring, filtering, and drying the filtering residues to obtain potassium chlorate. By using the method, a great amount of calcium carbide waste residues can be consumed, so that the land occupation pressure caused by piling can be relieved, and the environment pollution can be decreased; and the waste recycling can be realized.
Owner:毛竹青
Potassium chlorate production equipment and method
The invention relates to potassium chlorate production equipment and a potassium chlorate production method. The equipment comprises an alkaline sodium chloride dissolving device and a potassium chloride dissolving device. The potassium chloride dissolving device is communicated with a double decomposition reaction facility through a first filter device. The alkaline sodium chloride dissolving device is communicated with an electrolytic tank. The electrolytic tank is communicated with a chlorine gas removing device and the double decomposition reaction facility. The double decomposition reaction facility is communicated with a crystallizer. The crystallizer is communicated with a centrifugal device. The centrifugal device is communicated with the alkaline sodium chloride dissolving device. The crystallizer is communicated with a solution concentrating device. The alkaline sodium chloride dissolving device and the potassium chloride dissolving device are both connected to a flow meter. Through a liquid-liquid system configuration mode, metering in potassium chloride production is precise; the quality is easy to control; there is no sandwiched material; the solid wastes can be easily removed, the generation of harmful solid waste is prevented; by arranging a chlorine gas removing device, the acid and alkali raw materials are saved; by arranging the solution concentrating device, excess water in the solution can be removed, and the added swirl concentrating system increases the crystallization efficiency.
Owner:汝城县三鑫电化有限公司
Method for co-production of ultra-fine calcium carbonate and ammonium chloride in preparation of potassium chlorate by carbide slag
InactiveCN101823696AEasy to operateLess investmentChloratesCalcium/strontium/barium carbonatesSal ammoniacCarbonation
The invention discloses a method for co-production of ultra-fine calcium carbonate and ammonium chloride in preparation of potassium chlorate by the carbide slag. In the method, calcium hydroxide in chlor-alkali carbide slag reacts with chlorine gas to produce reaction products with calcium chlorate and calcium chloride; the reaction products are filtered to remove impurities, solid calcium chlorate can be separated out through settlement under the reduced temperature, and then solid calcium chlorate and calcium chloride filtrate can be obtained through filtration again; the reaction products with the calcium chlorate and the calcium chloride can also react with potassium chloride for double decomposition to produce mixed solution of potassium chlorate and calcium chloride; the mixed solution is filtered to remove impurities, potassium chlorate crystals can be separated out through distillation under the reduced pressure, and potassium chlorate crystals and calcium chloride solution can be obtained through filtration again; and then the calcium chloride solution after being treated with a surfactant react with carbon dioxide and ammonia in a carbonation tower for carbonation, thereby obtaining the light calcium carbonate and ammonium chloride.
Owner:南通亚伦化工有限公司
Method for recycling and utilizing sodium chlorate production low-temperature waste heat
InactiveCN109353989AImprove recycling ratesTo achieve the purpose of warming upChloratesEnergy inputSodium chlorateCombustion
The invention discloses a method for recycling and utilizing sodium chlorate production low-temperature waste heat, and relates to the technical field of heat recycling and utilization. The method isimplemented by the aid of a heating system, a preheating system and a waste heat recycling system. The heating system is used for carrying out heating reaction on raw materials and comprises a combustion device. The combustion device is fixed to the bottom of a bottom frame, flame holes are formed in the top of the bottom frame, the bottom frame is fixedly connected with a side of the bottom of anouter frame, a feed pipe is fixedly connected with the bottom of the outer frame, and a container vessel is fixedly connected with a side of the bottom of the inner wall of the outer frame; the raw materials can be delivered into the container vessel via the feed pipe in heating procedures, then the combustion device can be started, accordingly, flame can penetrate the flame holes, and the container vessel can be heated. The method has the advantages that the waste heat can be recycled and reused by the aid of the method, and effects of high heat recycling and utilization rates can be realized; the heating system, the preheating system and the waste heat recycling system are convenient to use, the waste heat can be sufficiently utilized, energy loss can be reduced, and the work efficiencycan be improved.
Owner:SICHUAN MJSNOW SALT CHEM IND
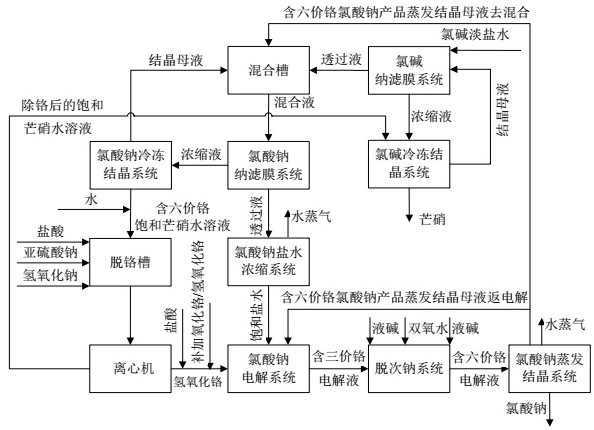
Chlor-alkali and sodium chlorate combined denitration process
ActiveCN113247921AReduce sodium sulfate contentReduce the mass percentage of Glauber's saltChloratesElectrolysis componentsElectrolytic agentSodium chlorate
The invention discloses a sodium chlorate and chlor-alkali combined denitration process. Chlor-alkali light salt brine is subjected to secondary denitration through a chlor-alkali nanofiltration membrane and a sodium chlorate nanofiltration membrane, so that the content of sodium sulfate in an electrolyte is smaller than or equal to 6 g / L, the content of sodium chloride is increased to 100-120 g / L, the electrolytic efficiency is increased to 91-92%, the content of mirabilite in a sodium chlorate product is smaller than or equal to 0.02%, the content of sodium chlorate in a mirabilite product is smaller than or equal to 5 mg / kg, and the service life of the nanofiltration membrane is prolonged to 3 years. Chromium oxide / chromium hydroxide is used to replace heavy-toxicity sodium dichromate, and closed-loop recycling of chromium is achieved. The process is suitable for enterprises for preparing caustic soda from brine in northern dry areas. Pipeline-based salt supply is directly conducted on a sodium chlorate electrolysis system through light salt brine, and heat of low-grade electrolyte is fully utilized.
Owner:HUNAN HENGGUANG TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com