Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2813results about "Oxygen preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
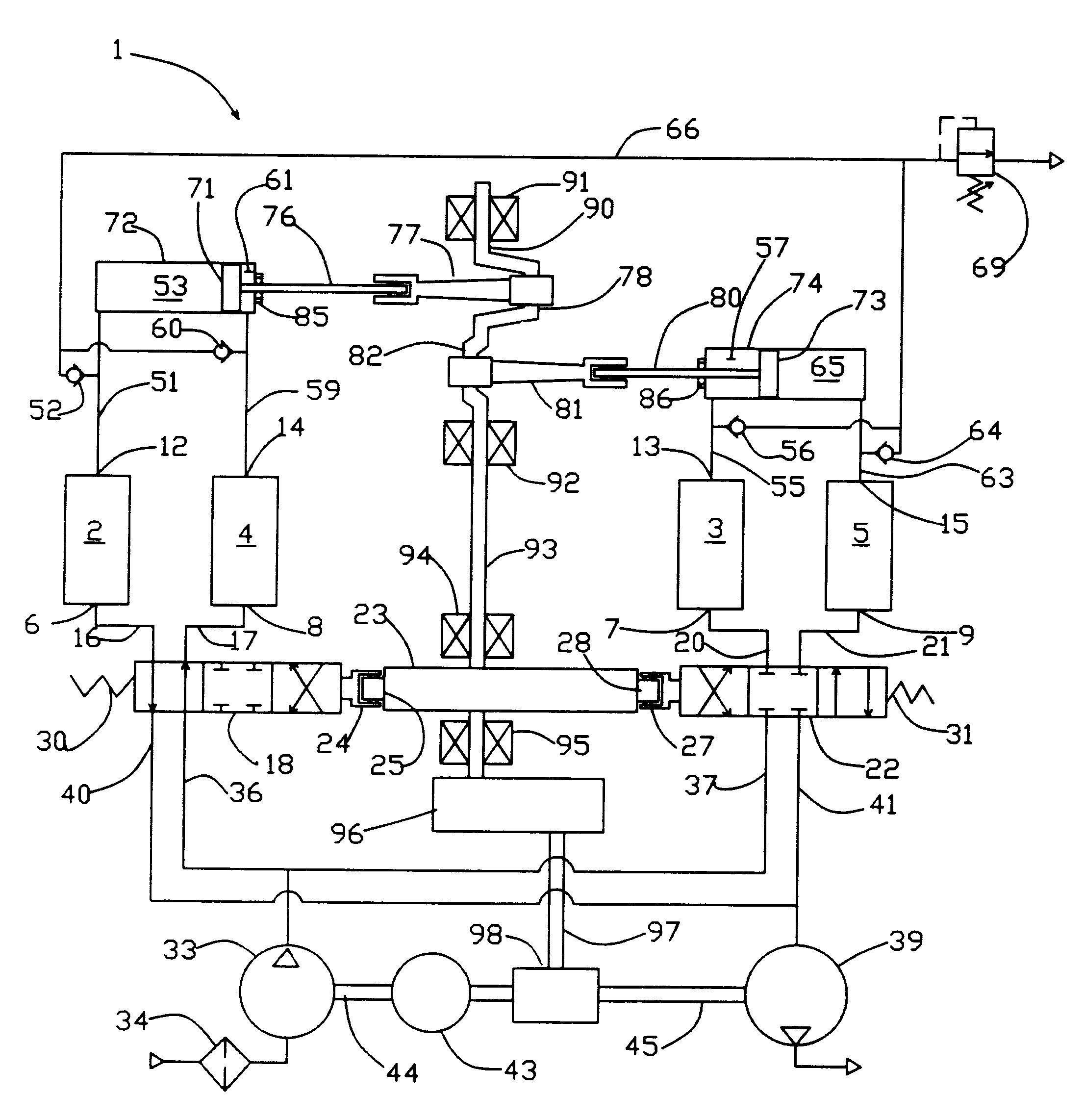
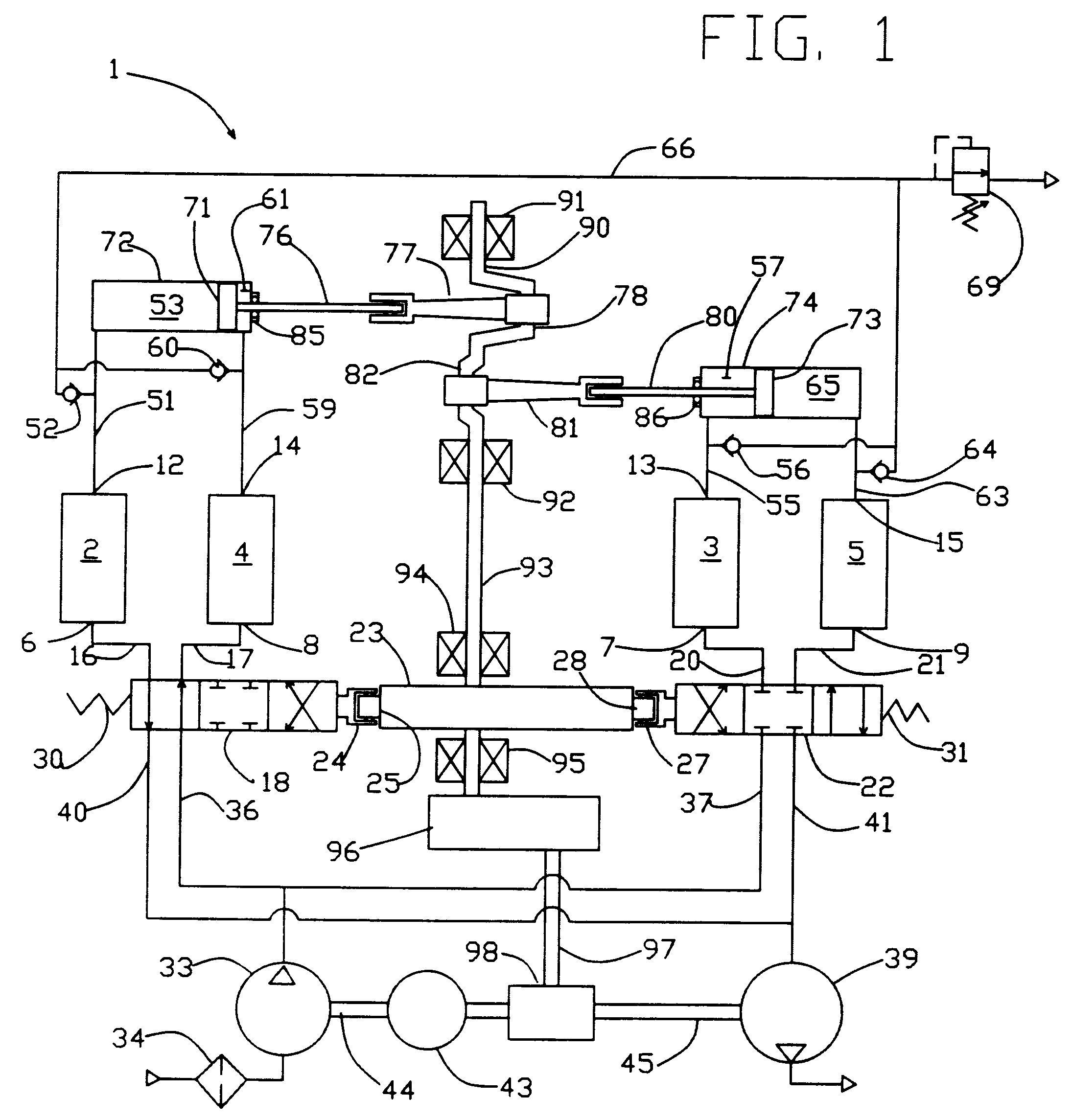
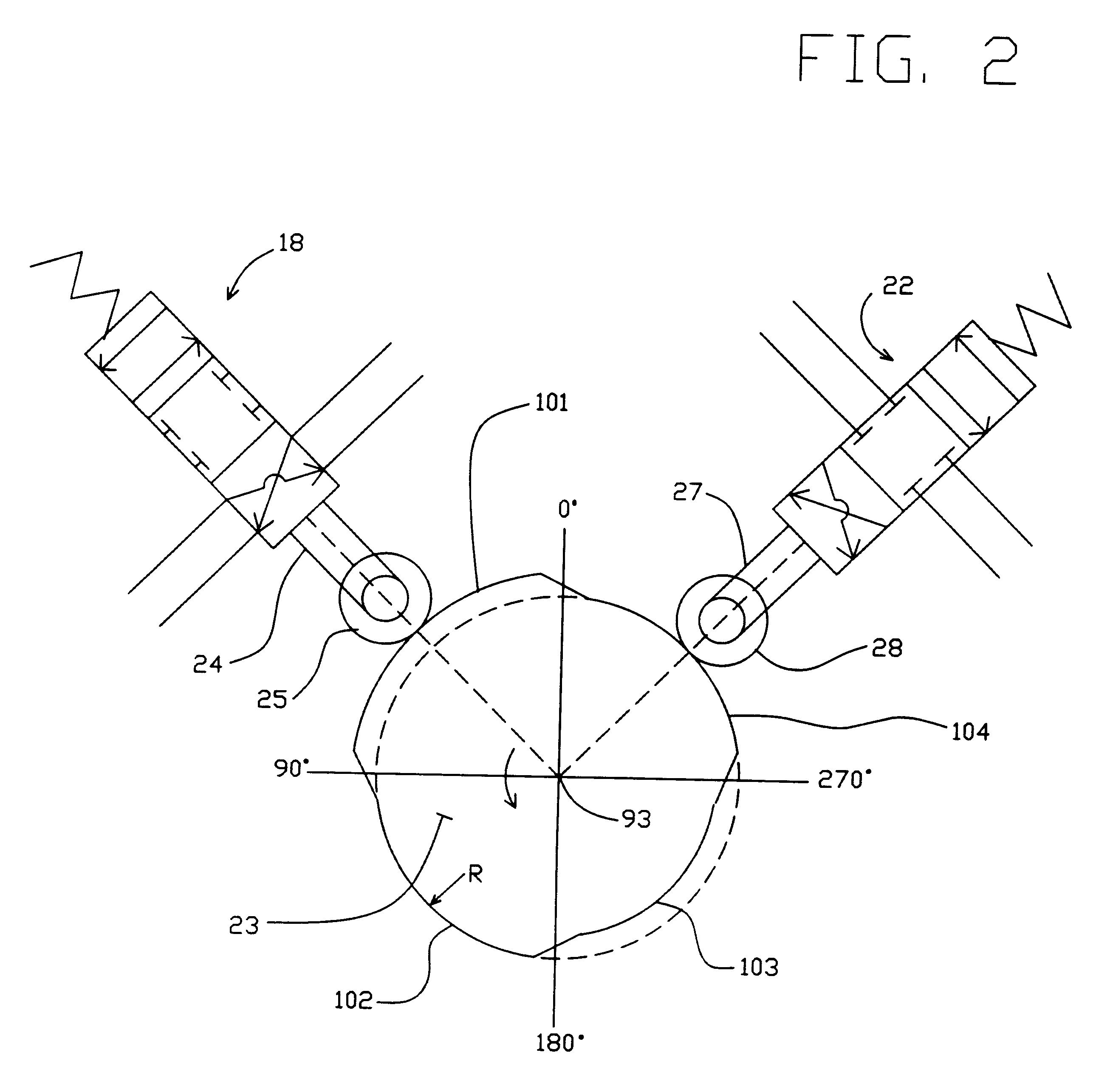
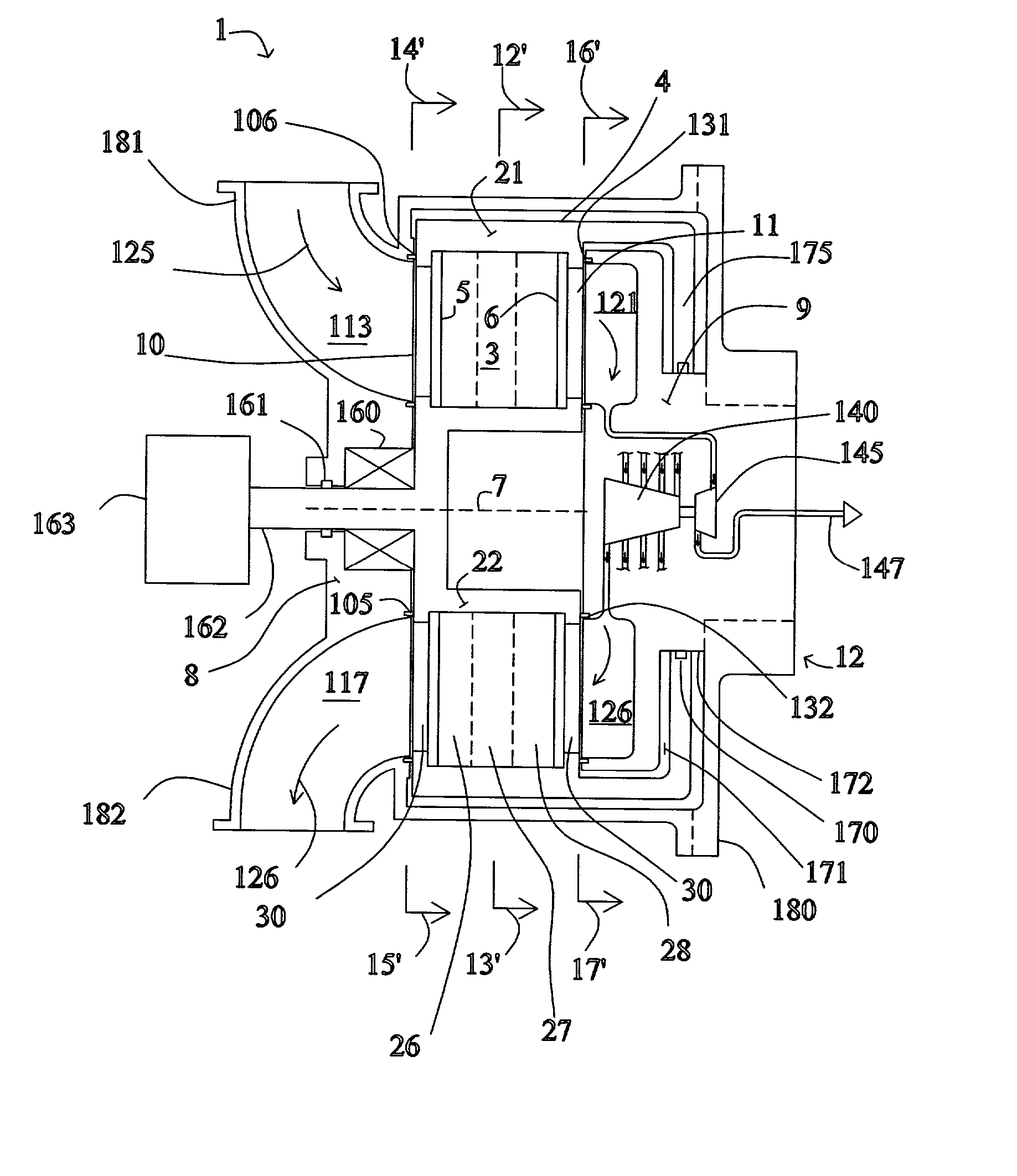
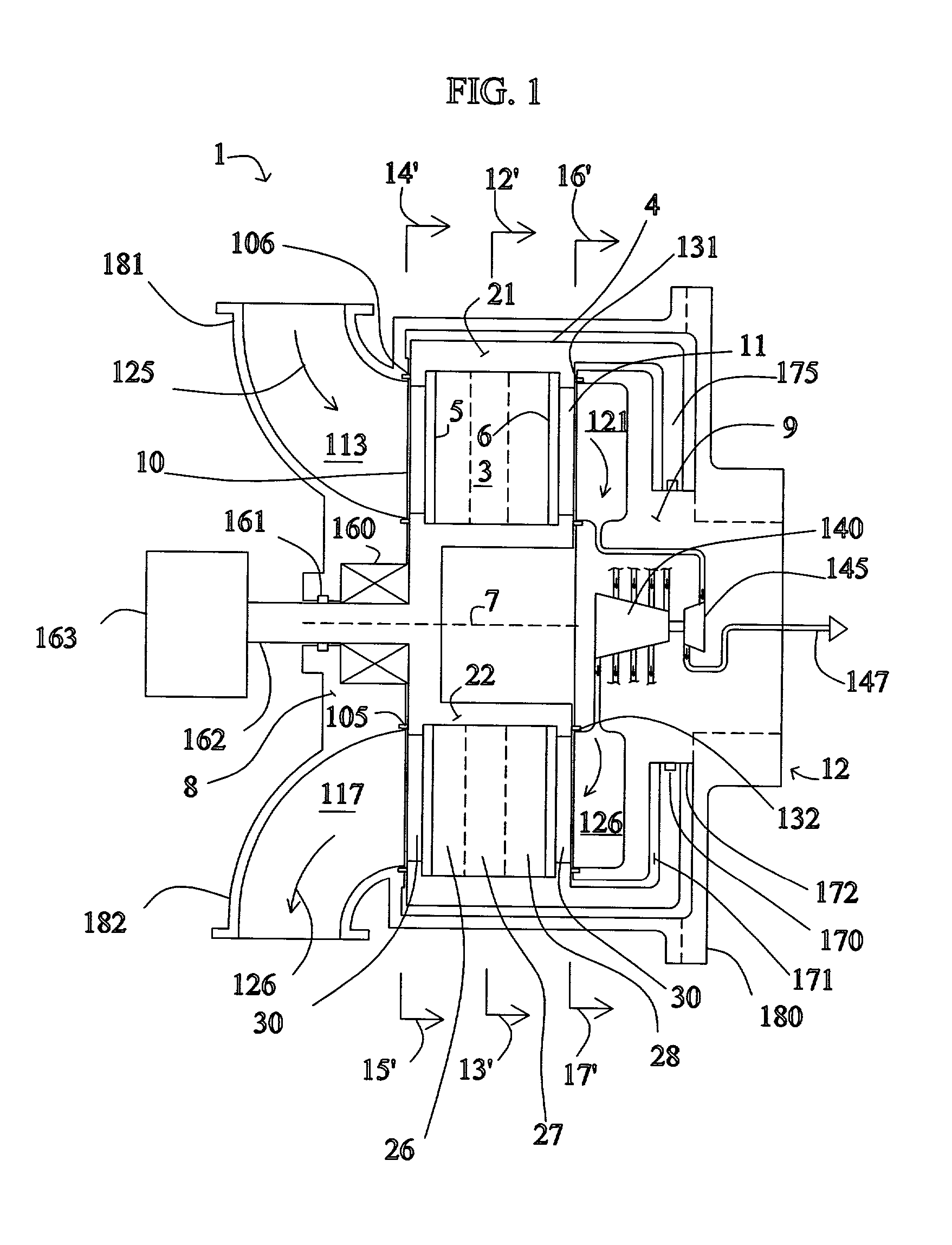
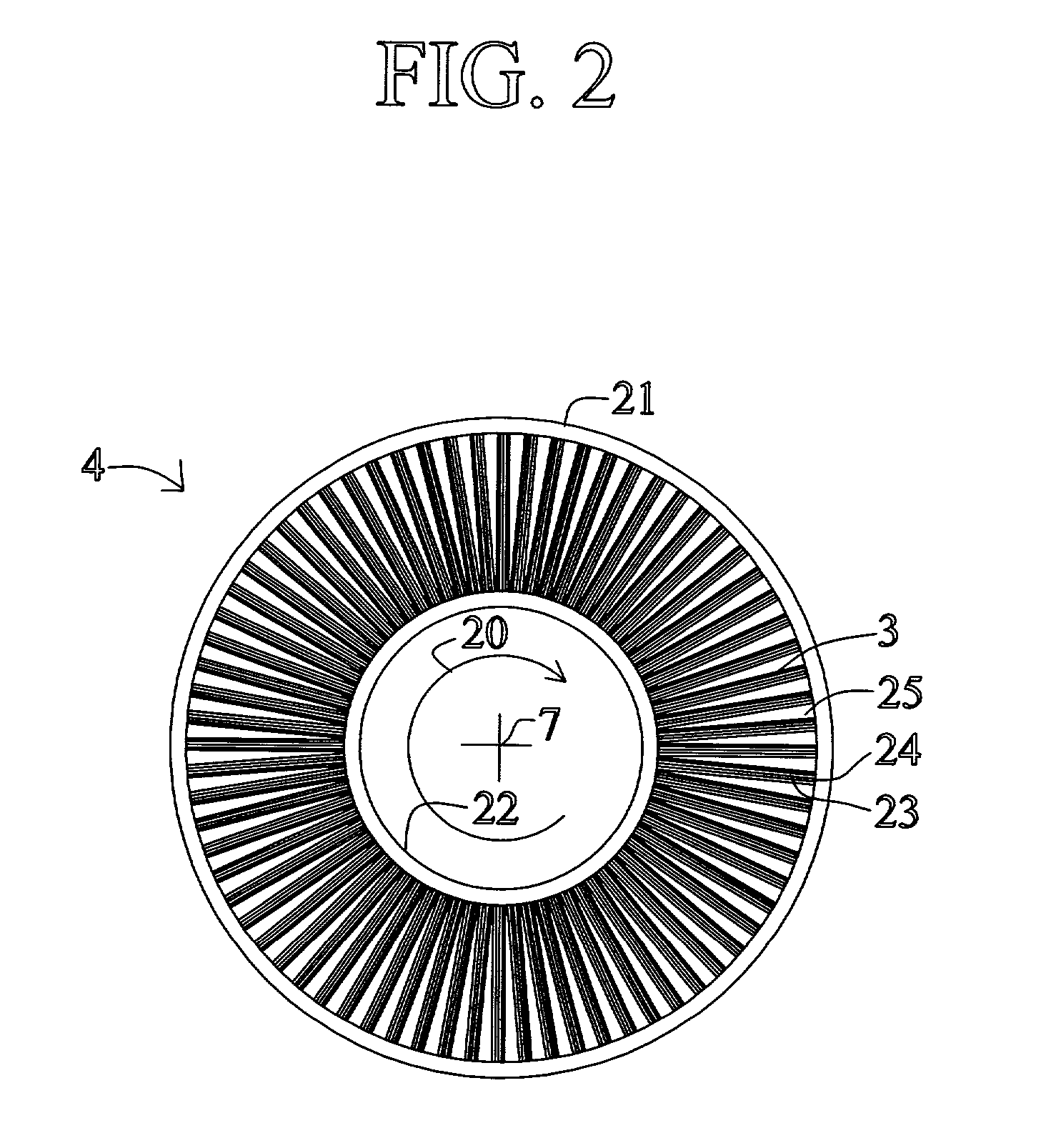
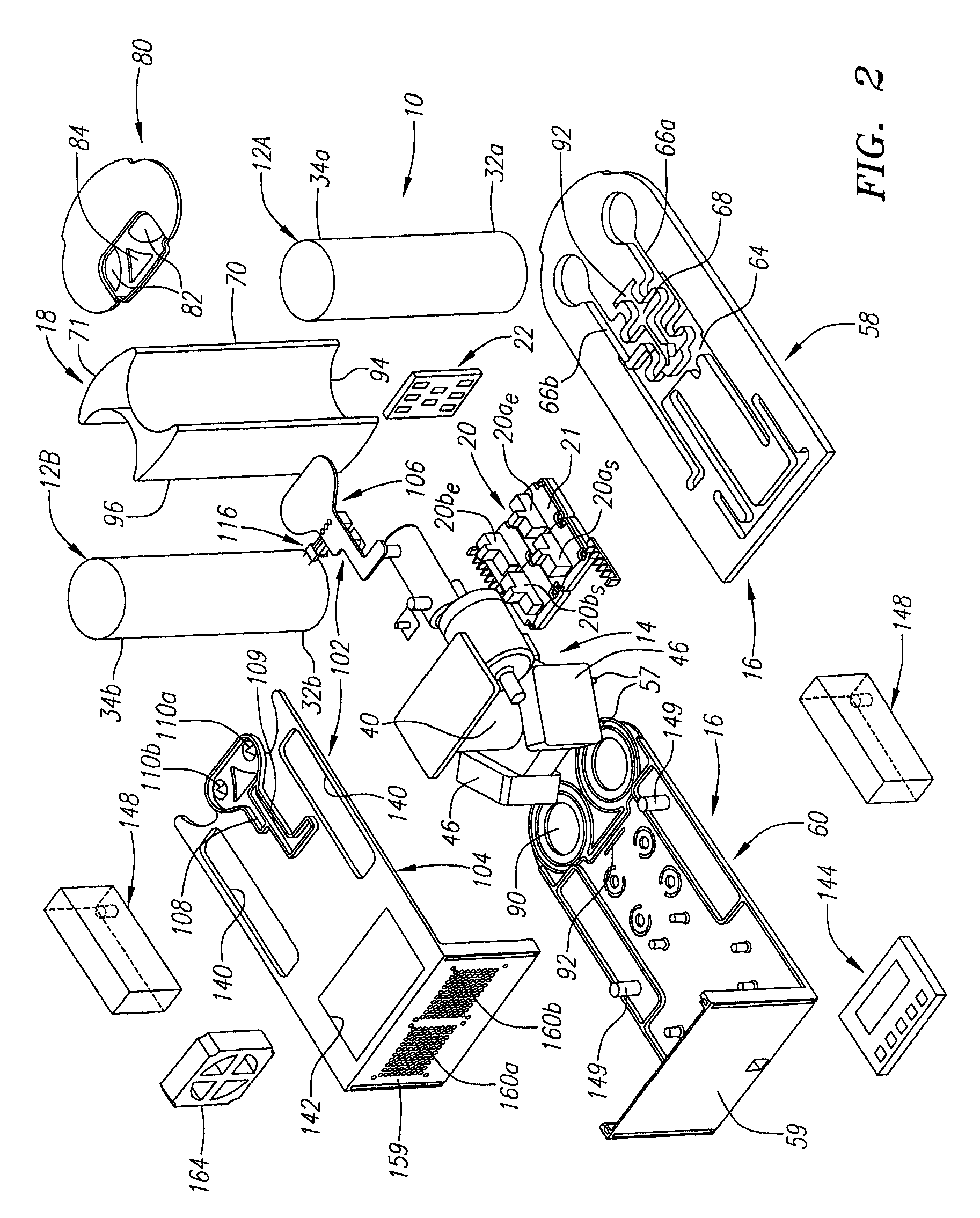
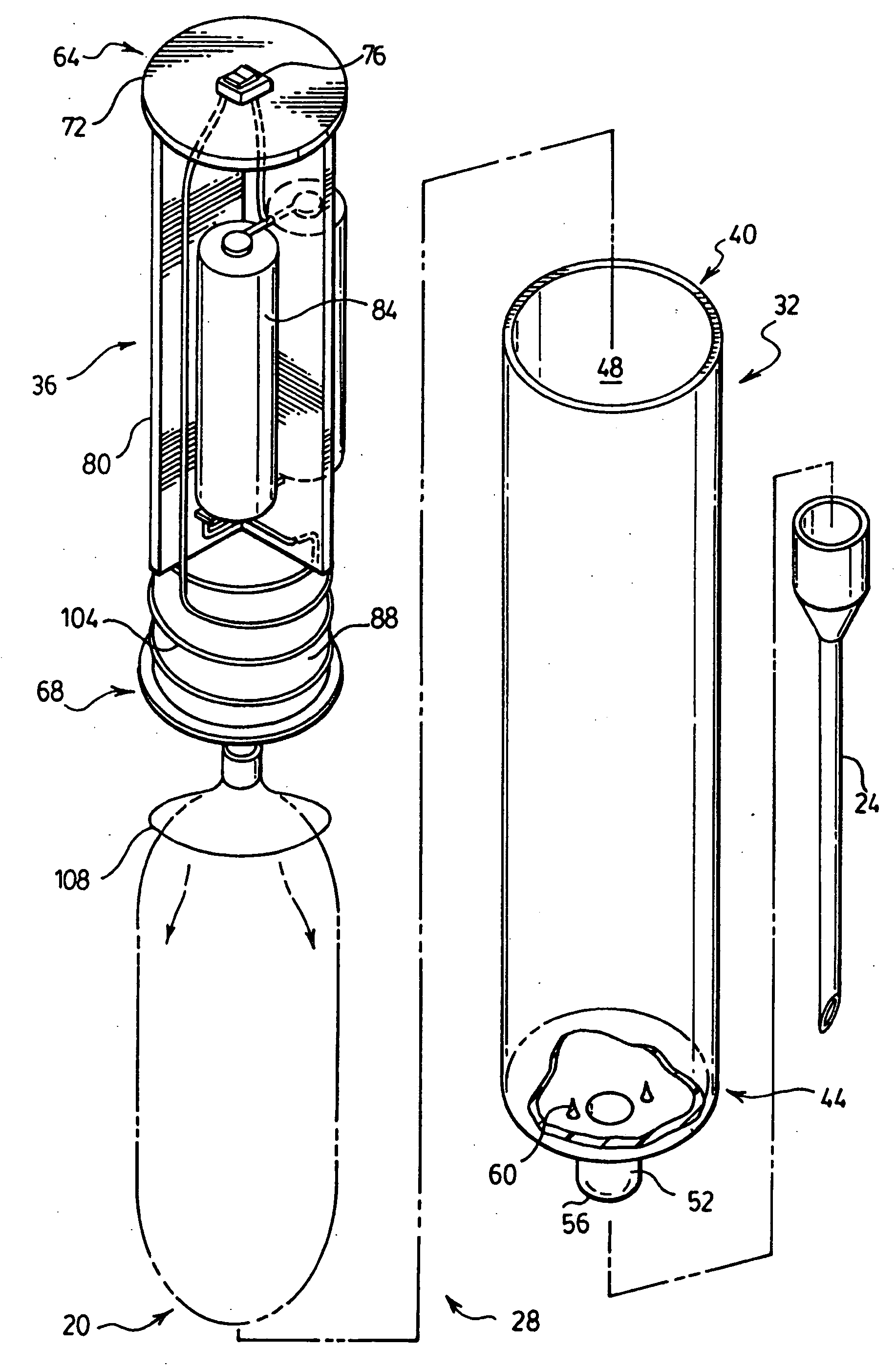
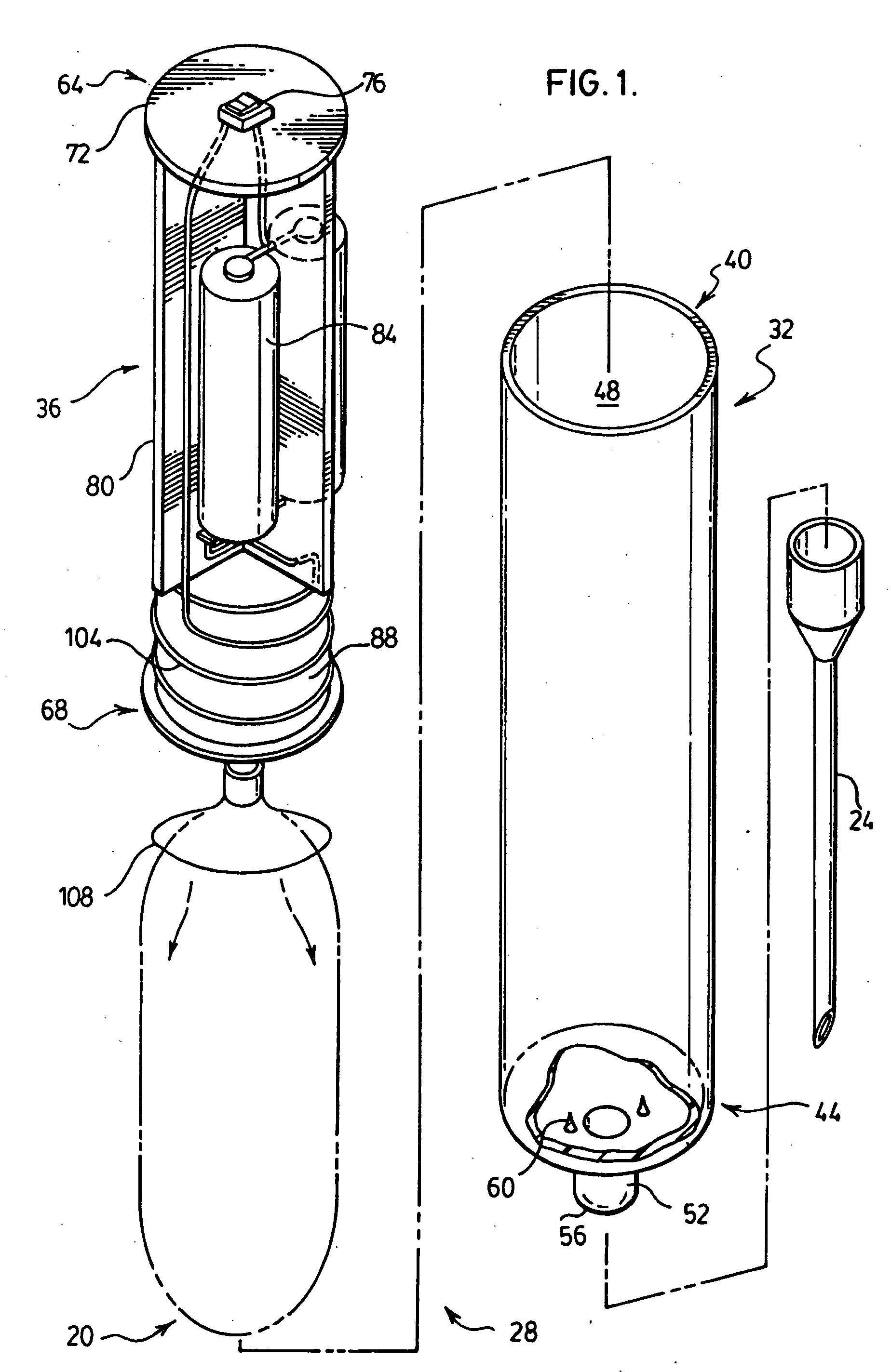
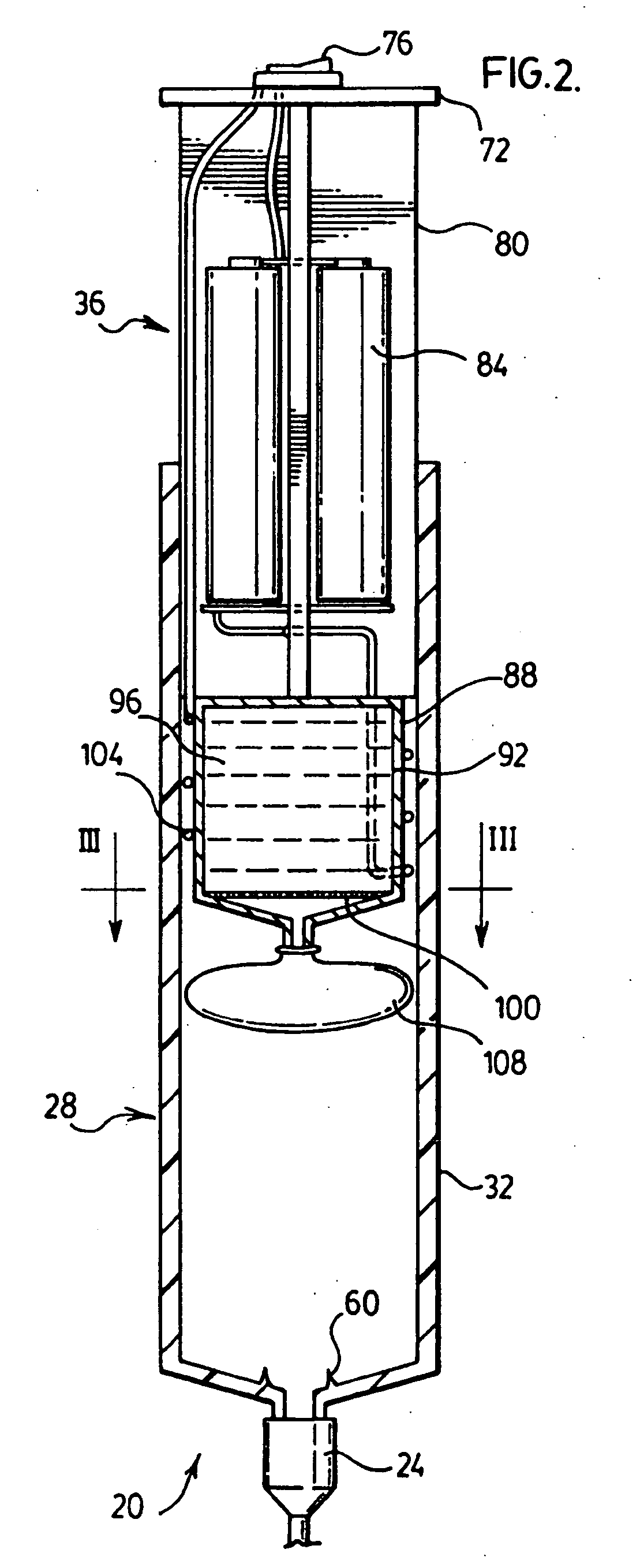
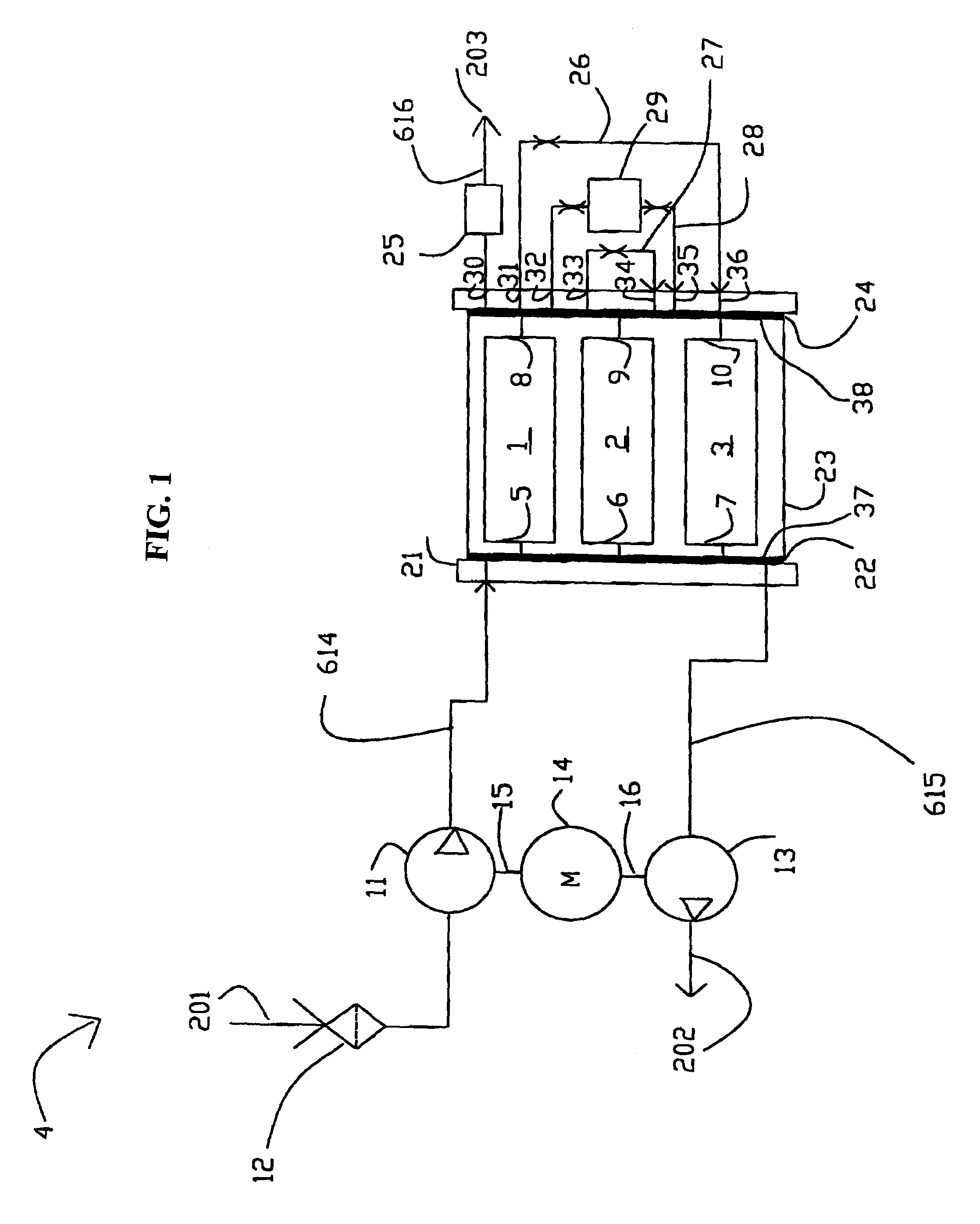
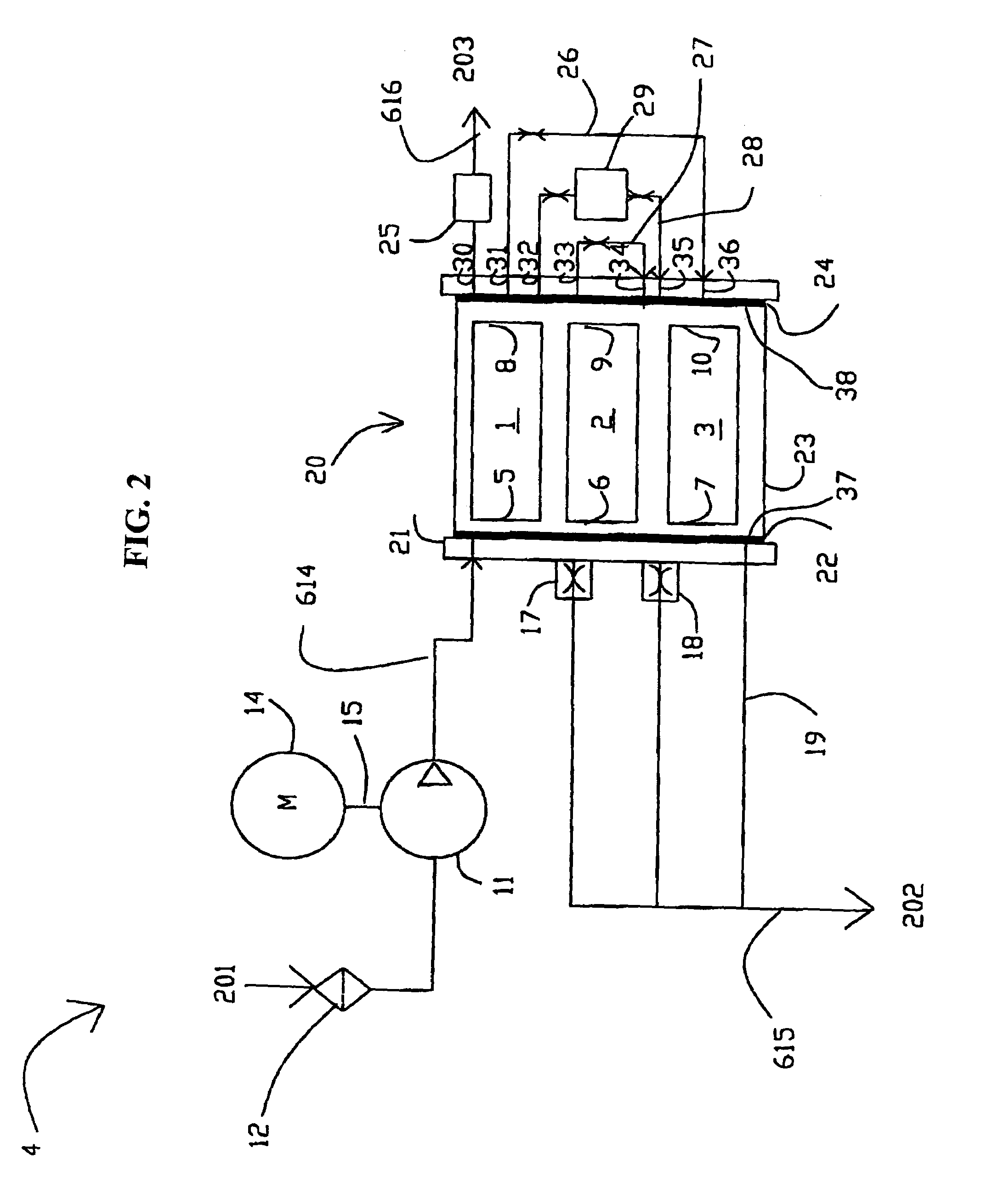
High frequency pressure swing adsorption
InactiveUS6176897B1High purityRecoverable expansion workNitrogen purification/separationGas treatmentSorbentEngineering
Pressure swing adsorption separation of a feed gas mixture, to obtain a purified product gas of the less strongly adsorbed fraction of the feed gas mixture, is performed in a plurality of preferably an even number of adsorbent beds, with each adsorbent bed communicating at its product end directly to a variable volume expansion chamber, and at its feed end by directional valves to a feed compressor and an exhaust vacuum pump. For high frequency operation of the pressure swing adsorption cycle, a high surface area layered support is used for the adsorbent. The compressor and vacuum pump pistons may be integrated with the cycle, reciprocating at twice the cycle frequency. Alternative configurations of the layered adsorbent beds are disclosed.
Owner:AIR PROD & CHEM INC
Energy efficient gas separation for fuel cells
InactiveUS20020142208A1Improve efficiencyReduce the ratioFuel cell heat exchangeFused electrolyte fuel cellsEngineeringDelivery system
An electrical current generating system is disclosed that includes a fuel cell operating at a temperature of at least about 250° C. (for example, a molten carbonate fuel cell or a solid oxide fuel cell), a hydrogen gas separation system or oxygen gas delivery system that includes a compressor or pump, and a drive system for the compressor or pump that includes means for recovering energy from at least one of the hydrogen gas separation system, oxygen gas delivery system, or heat of the fuel cell. The drive system could be a gas turbine system. The hydrogen gas separation system or the oxygen gas delivery system may include a pressure swing adsorption module.
Owner:AIR PROD & CHEM INC
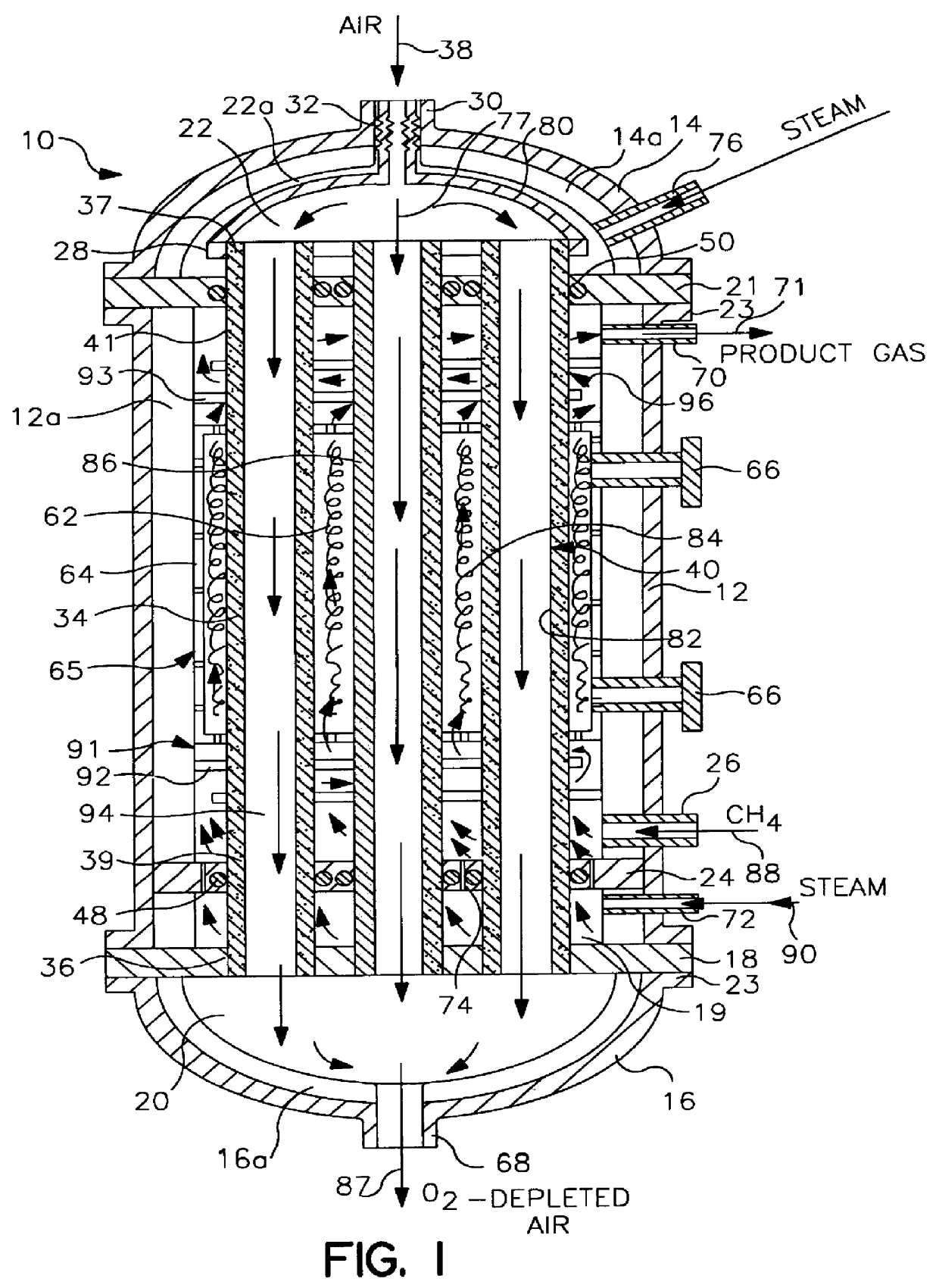
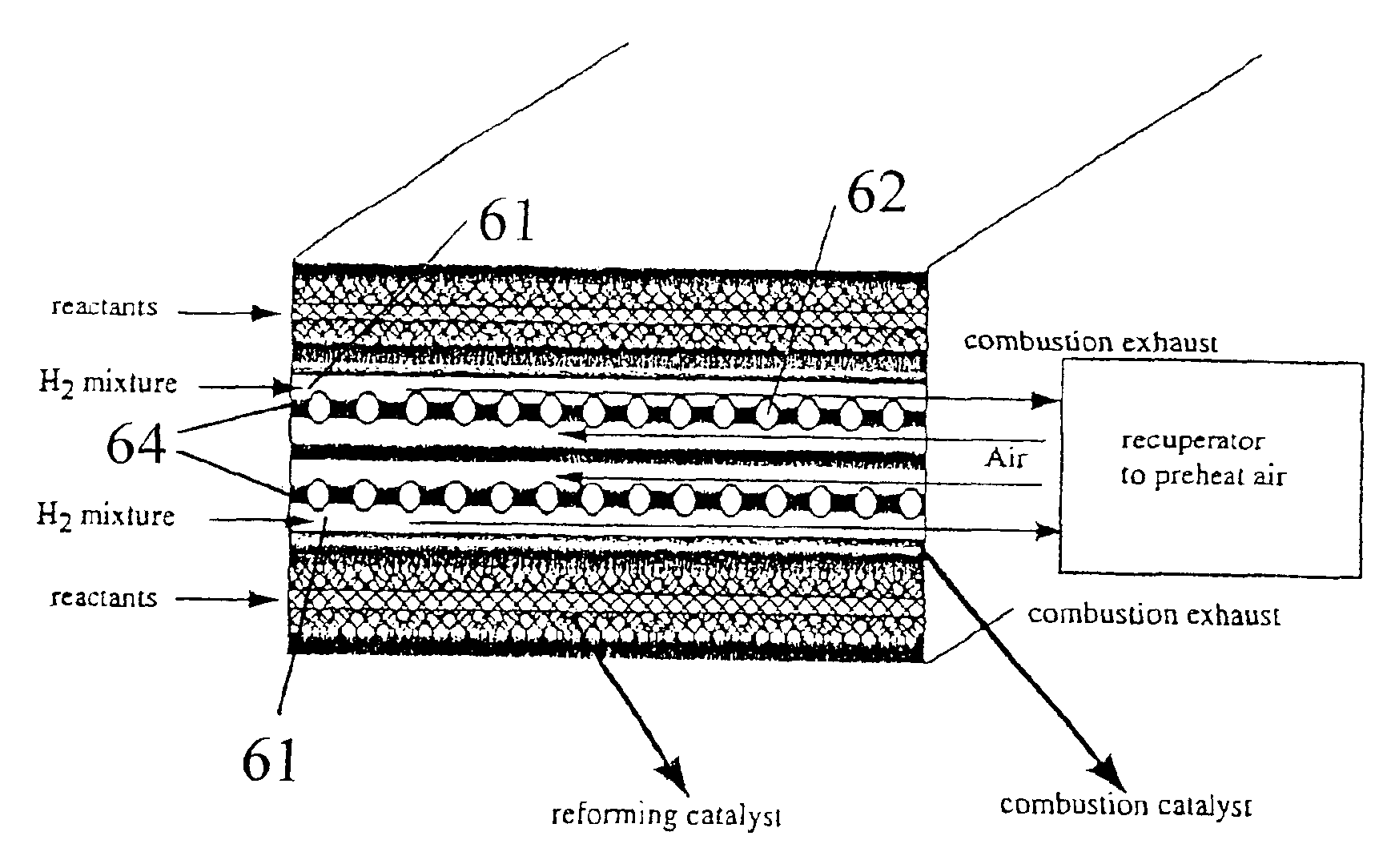
Tube and shell reactor with oxygen selective ion transport ceramic reaction tubes
InactiveUS6139810AIncrease oxygen fluxDecreasing anode side partial oxygen pressureIsotope separationHydrogen/synthetic gas productionPtru catalystElectrical conductor
A reactor comprising: a hollow shell defining a hermetic enclosure; a plurality of tube sheets disposed within said hermetic enclosure, a first one of said plurality of tube sheets defining a first chamber; at least one reaction tube each having a first end and an opposing second end, said first end being fixedly attached and substantially hermetically sealed to one end of said plurality of tube sheets and opening into said first chamber, the second end being axially unrestrained; each of said reaction tubes is comprised of an oxygen selective ion transport membrane with an anode side wherein said oxygen selective ion transport membrane is formed from a mixed conductor metal oxide that is effective for the transport of elemental oxygen at elevated temperatures and at least a portion of said first and second heat transfer sections are formed of metal; each of said reaction tubes includes first and second heat transfer sections and a reaction section, said reaction section disposed between said first and second heat transfer sections; a reforming catalyst disposed about said anode side of said oxygen selective ion transport membrane; a first process gas inlet; a second process gas inlet; and, a plurality of outlets.
Owner:STANDARD OIL CO +1
Mixed-metal oxide particles by liquid feed flame spray pyrolysis of oxide precursors in oxygenated solvents
Liquid feed flame spray pyrolysis of solutions of a metal oxide precursor which is an alkoxide or C1-6 carboxylate and at least one second metal oxide precursor and / or second metal compound dissolved in oxygenated solvent by combustion with oxygen lead to the formation of sub-micron mixed-metal oxide powders not accessible by other processes or by the pyrolysis of metal chlorides or nitrates. The powders have numerous uses in advanced materials applications including particulate solid state lasers, advanced ceramic materials, and as catalysts in organic synthesis and automobile exhaust systems.
Owner:TAL MATERIALS +1
Mixed-metal oxide particles by liquid feed flame spray pyrolysis of oxide precursors in oxygenated solvents
Liquid feed flame spray pyrolysis of solutions of a metal oxide precursor which is an alkoxide or C1-6 carboxylate and at least one second metal oxide precursor and / or second metal compound dissolved in oxygenated solvent by combustion with oxygen lead to the formation of sub-micron mixed-metal oxide powders not accessible by other processes or by the pyrolysis of metal chlorides or nitrates. The powders have numerous uses in advanced materials applications including particulate solid state lasers, advanced ceramic materials, and as catalysts in organic synthesis and automobile exhaust systems.
Owner:TAL MATERIALS +1
Electrical current generation system
InactiveUS6921597B2Facilitate pressureFacilitate ion exchangeFuel cell auxillariesHydrogen/synthetic gas productionHydrogenFuel cells
An electrical generating system consists of a fuel cell, and an oxygen gas delivery. The fuel cell includes and anode channel having an anode gas inlet for receiving a supply of hydrogen gas, a cathode channel having a cathode gas inlet and a cathode gas outlet, and an electrolyte in communication with the anode and cathode channel for facilitating ion exchange between the anode and cathode channel. The oxygen gas delivery system is coupled to the cathode gas inlet and delivers oxygen gas to the cathode channel. The electrical current generating system also includes gas recirculation means couple to the cathode gas outlet for recirculating a portion of cathode exhaust gas exhausted from the cathode gas outlet to the cathode gas inlet.
Owner:AIR PROD & CHEM INC
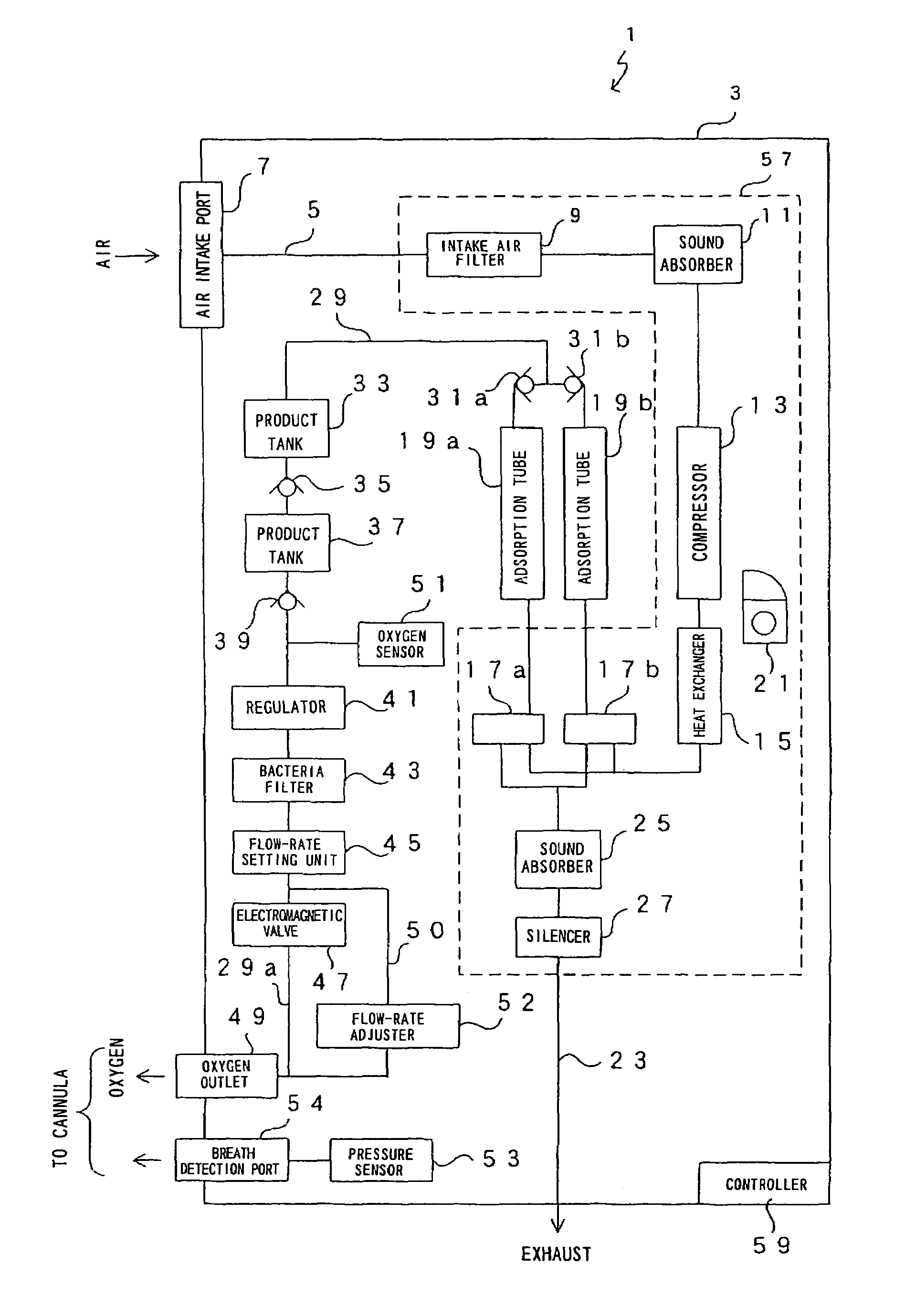
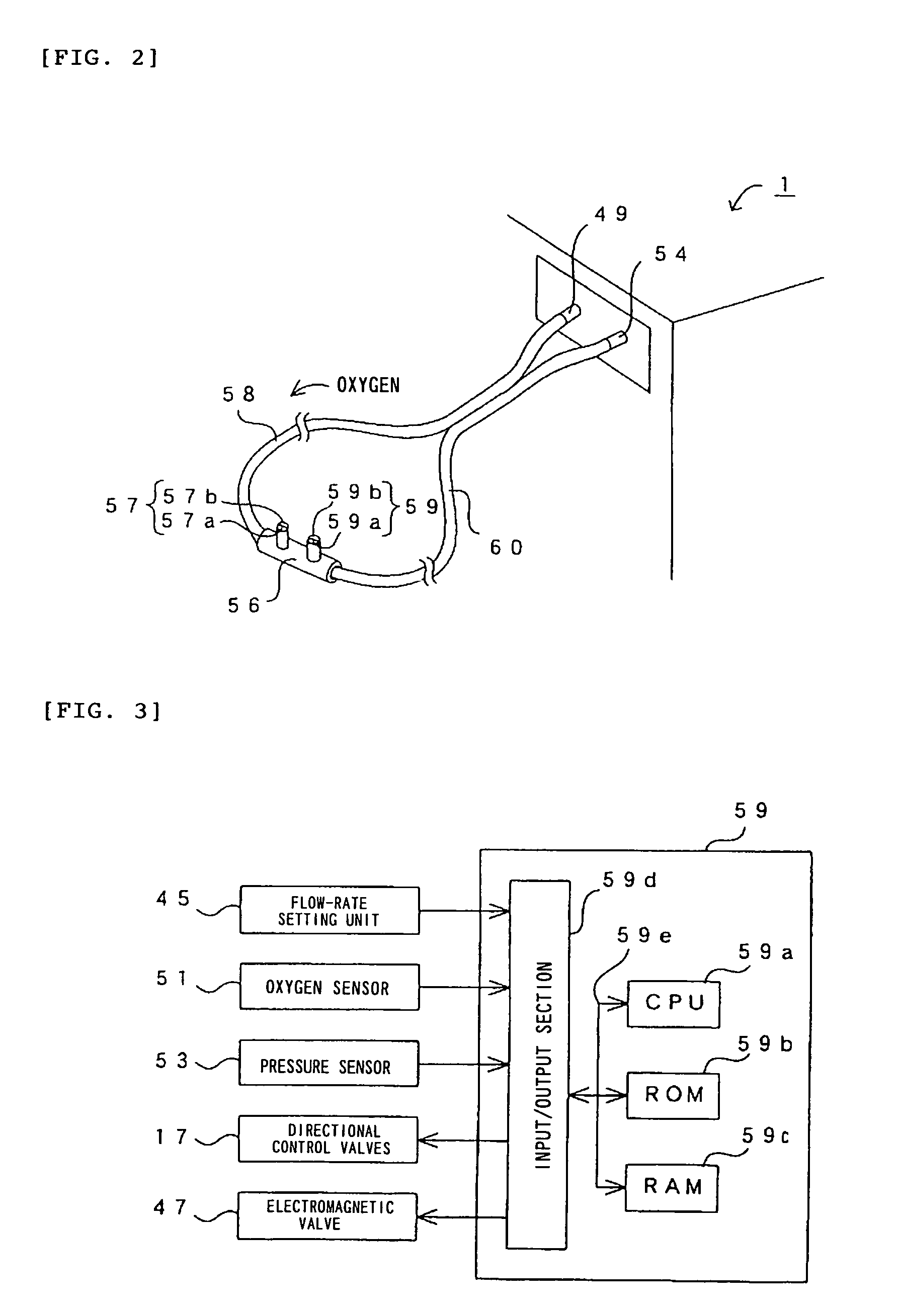
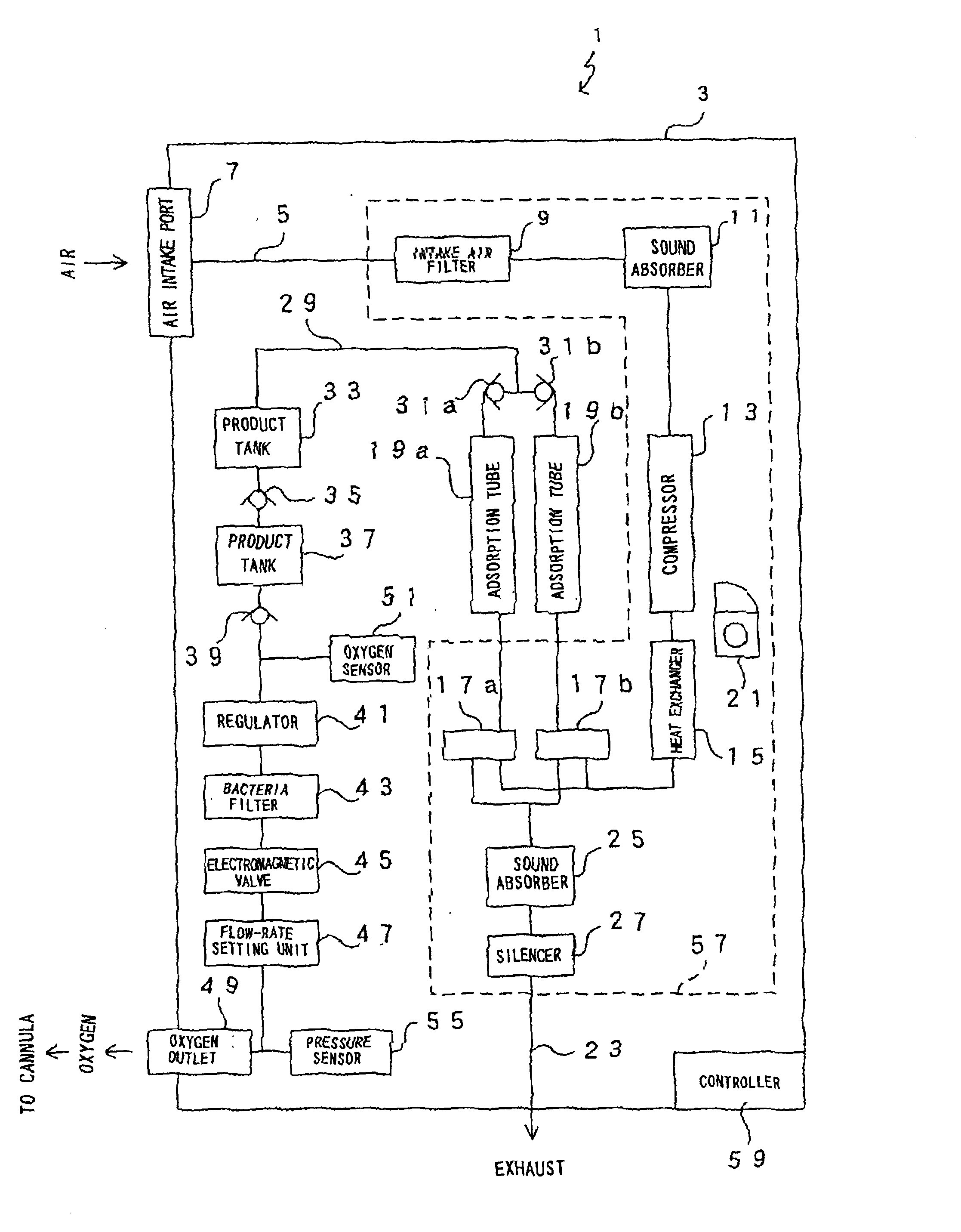
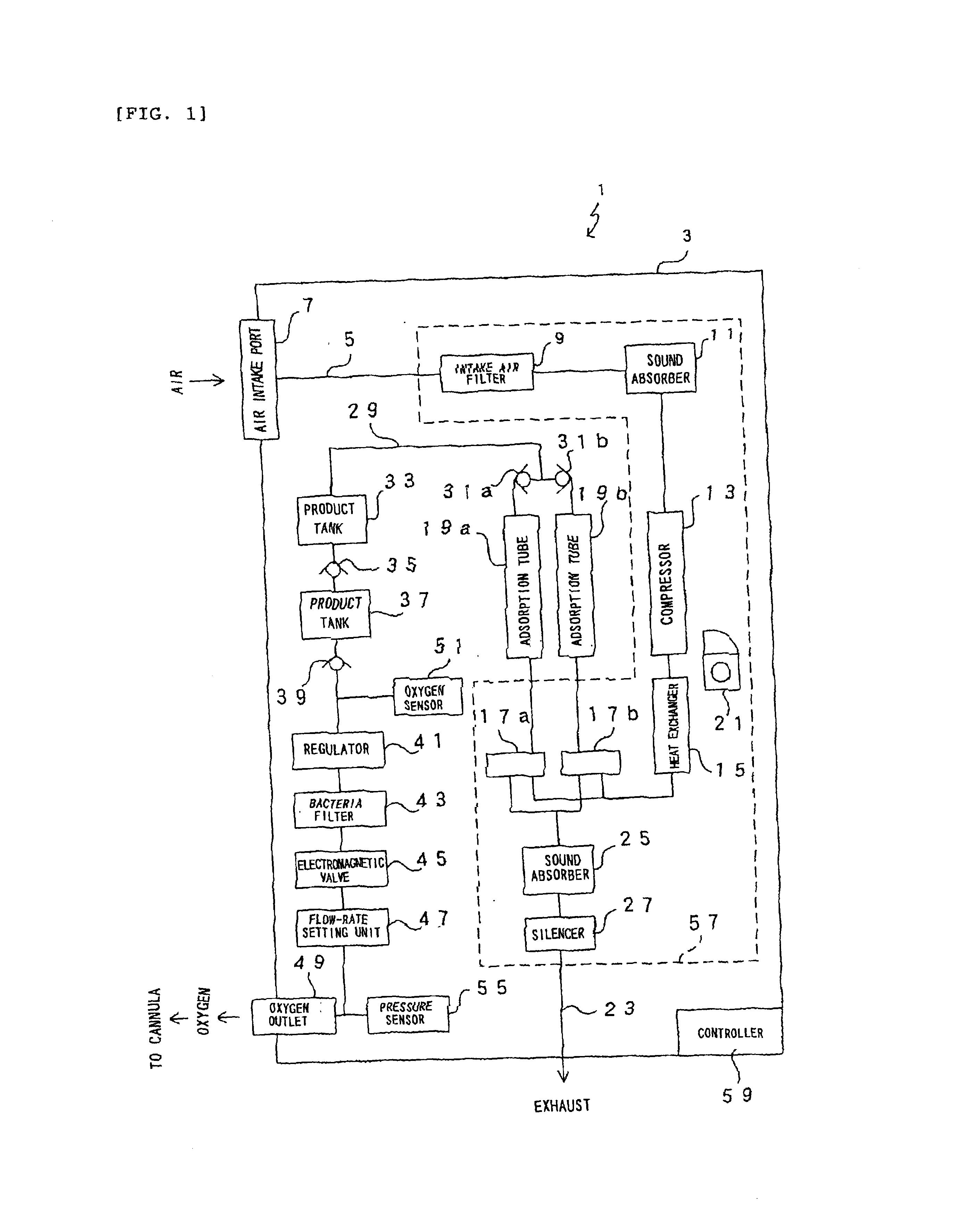
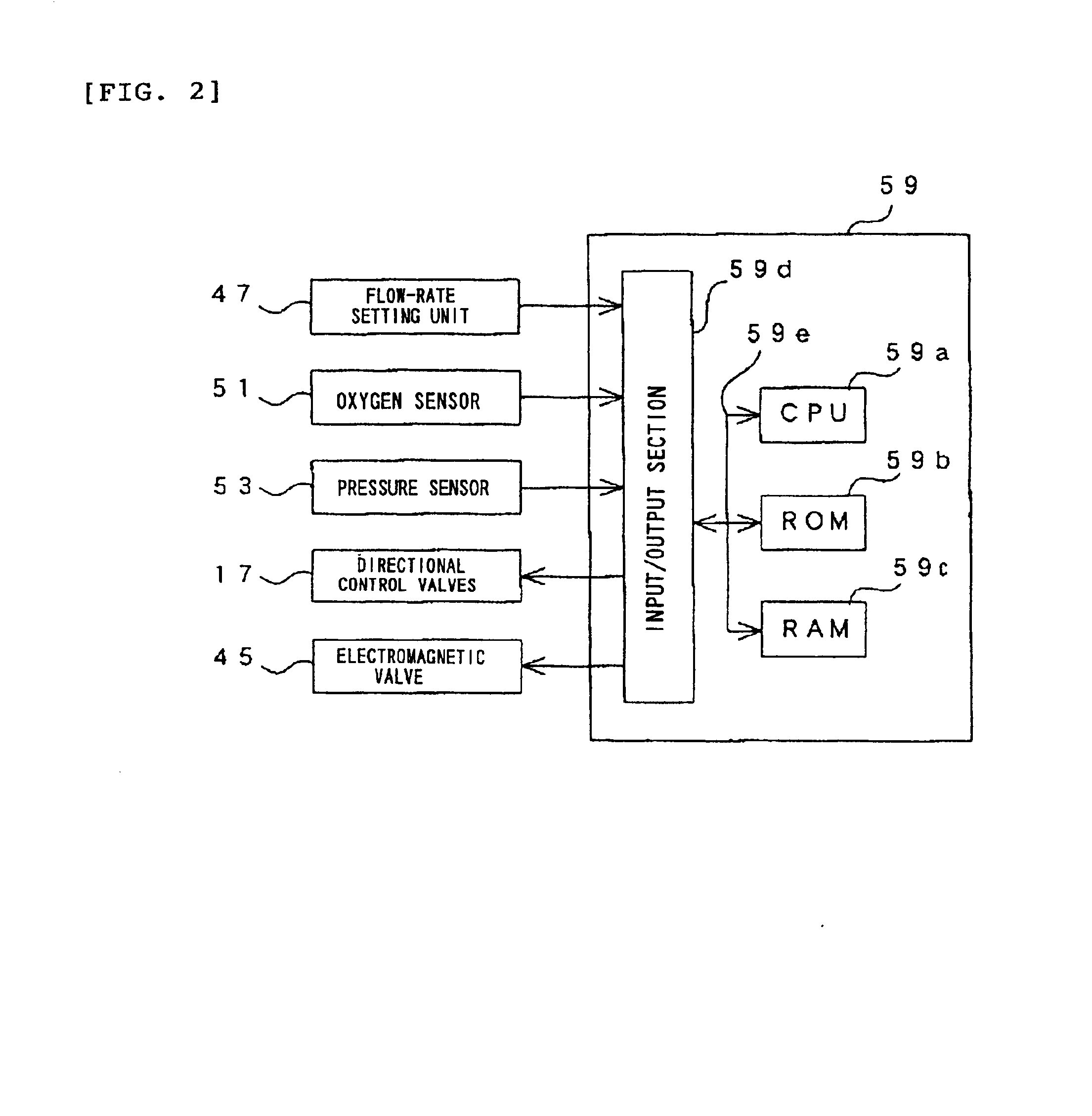
Oxygen enriching apparatus, controller, and recording medium
InactiveUS7077133B2Increase flow rateImprove securityOperating means/releasing devices for valvesRespiratory masksInhalationProcess engineering
A small oxygen enriching apparatus which can supply oxygen-enriched gas at high flow rate without imparting unnatural sensation to a user, as well as a controller and recording medium therefore. In step 100, a judgment is made as to whether a flow rate set by use of a flow-rate setting unit 45 is equal to or less than a continuous base flow rate (3 liters / min). When the set flow rate is a low flow rate of not greater than 3 liters / min, breath-synchronized operation is not performed (continuous supply is to be performed), and therefore, in step 110, oxygen-enriched gas is supplied continuously at the set flow rate. When the set flow rate is a high flow rate of greater than 3 liters / min, breath-synchronized operation is to be performed, and therefore, in step 120, the orifice is set to an opening that enables supply at 5 liters / min. In step 140, in order to perform breath-synchronized operation, control for opening and closing an electromagnetic valve 47 is performed. Through this operation, the oxygen-enriched gas is supplied at a high flow rate (5 liters / min) in the inhalation period of each breathing cycle and at a low flow rate (2 liters / min) in the exhalation period via a bypass flow passage 50.
Owner:NGK SPARK PLUG CO LTD
Process for producing a syngas
InactiveUS6402988B1Minimize the temperatures experienced by sealsMinimize pressure differenceCatalytic gas-gas reactionHydrogenSyngasReactor design
An exothermic reaction and an endothermic reaction are thermally combined in a reactor having at least one oxygen selective ion transport membrane that provides the exothermic reaction with oxygen from an oxygen-containing gas such as air. The thermal requirements of the endothermic reaction are satisfied by the exothermic reaction. Dependent on the reactor design employed, the exothermic and endothermic reactions may be gaseously combined.
Owner:PRAXAIR TECH INC +1
Temperature control in a ceramic membrane reactor
InactiveUS6010614AEnhance exchangeFine surfaceHydrogenLiquid separation by electricityTemperature controlOxygen ions
A method for maintaining the temperature of an oxygen-selective ion transport membrane within a desired temperature range includes providing an ion transport reactor with the oxygen-selective ion transport membrane. An oxygen-donating feed gas is delivered to a cathode side at a first temperature, at a first rate, and at a first oxygen partial pressure and a reactant gas is supplied to an anode side at a second temperature and a second rate. A physical condition is then established within the ion transport reactor that favors the transport of elemental oxygen through the oxygen selective ion transport membrane as oxygen ions. One or more process variables are then regulated to maintain the temperature of the oxygen selective ion transport membrane within the desired range.
Owner:PRAXAIR TECH INC
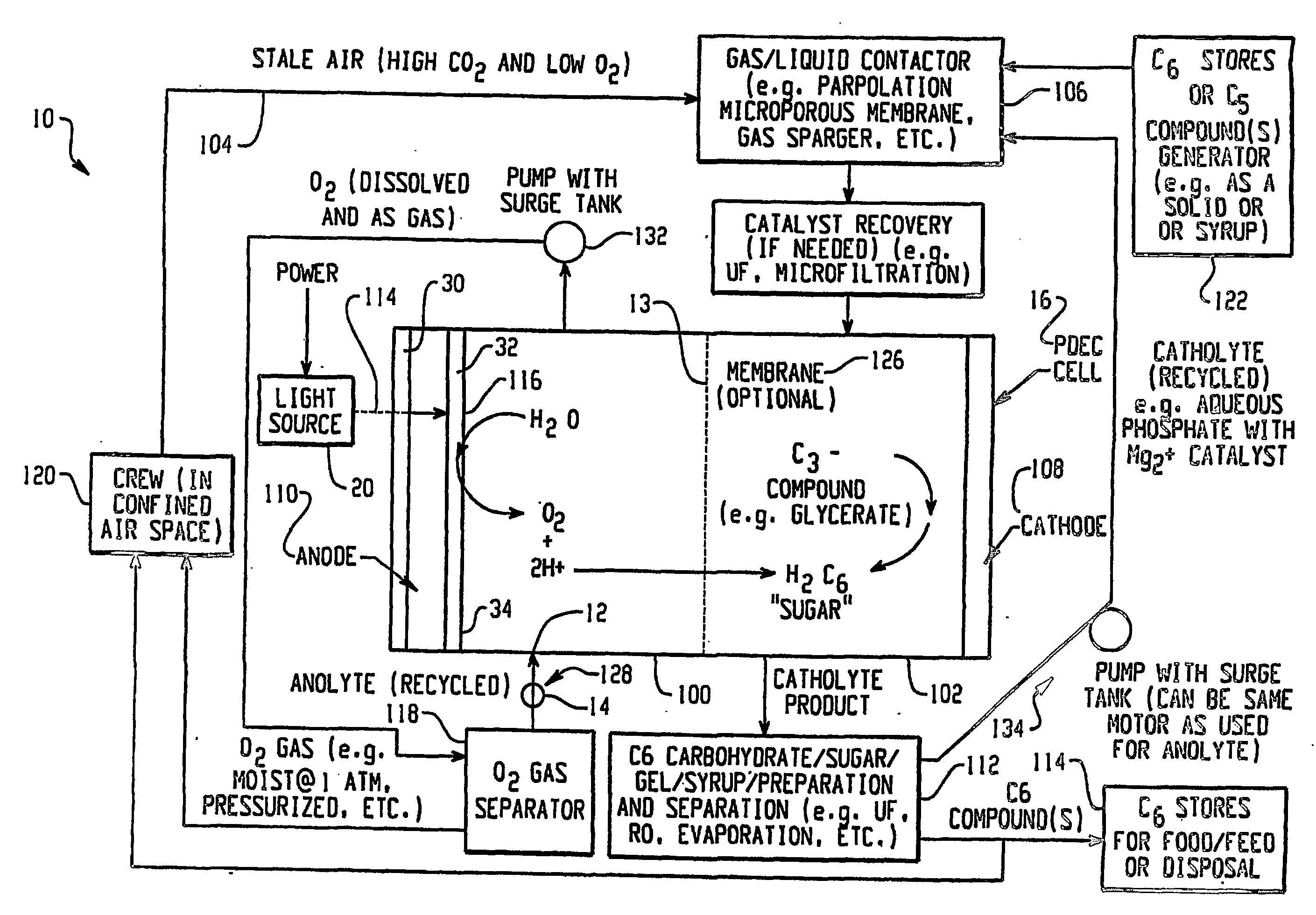
Integrated oxygen generation and carbon dioxide absorption method apparatus and systems
InactiveUS20050031522A1Improvement in mission durationLower acquisition costsFuel cell auxillariesMachines/enginesChemical speciesCo2 absorption
A method for producing oxygen and absorbing carbon dioxide in a single operation using a solution that contains an oxygen source and a redox partner that can react to form oxygen and a chemical species that can form an insoluble carbonate to precipitate and chemically store carbon dioxide. Carbon dioxide is introduced into the solution and the carbonate precipitates as the oxygen is generated. In particular, the invention uses an aqueous solution of permanganate and hydrogen peroxide that react in the presence of a catalyst to produce oxygen and manganese (II) ions. Carbon dioxide gas introduced into the solution reacts with the manganese (II) ions to precipitate manganese carbonate. Other cations capable of reacting with carbon dioxide to form an insoluble carbonate, for example calcium, barium and magnesium, may also be added to the solution to precipitate carbonate salts. Calcium permanganate may used as a source of both calcium and permanganate.
Owner:CAPITAL MANAGEMENT +1
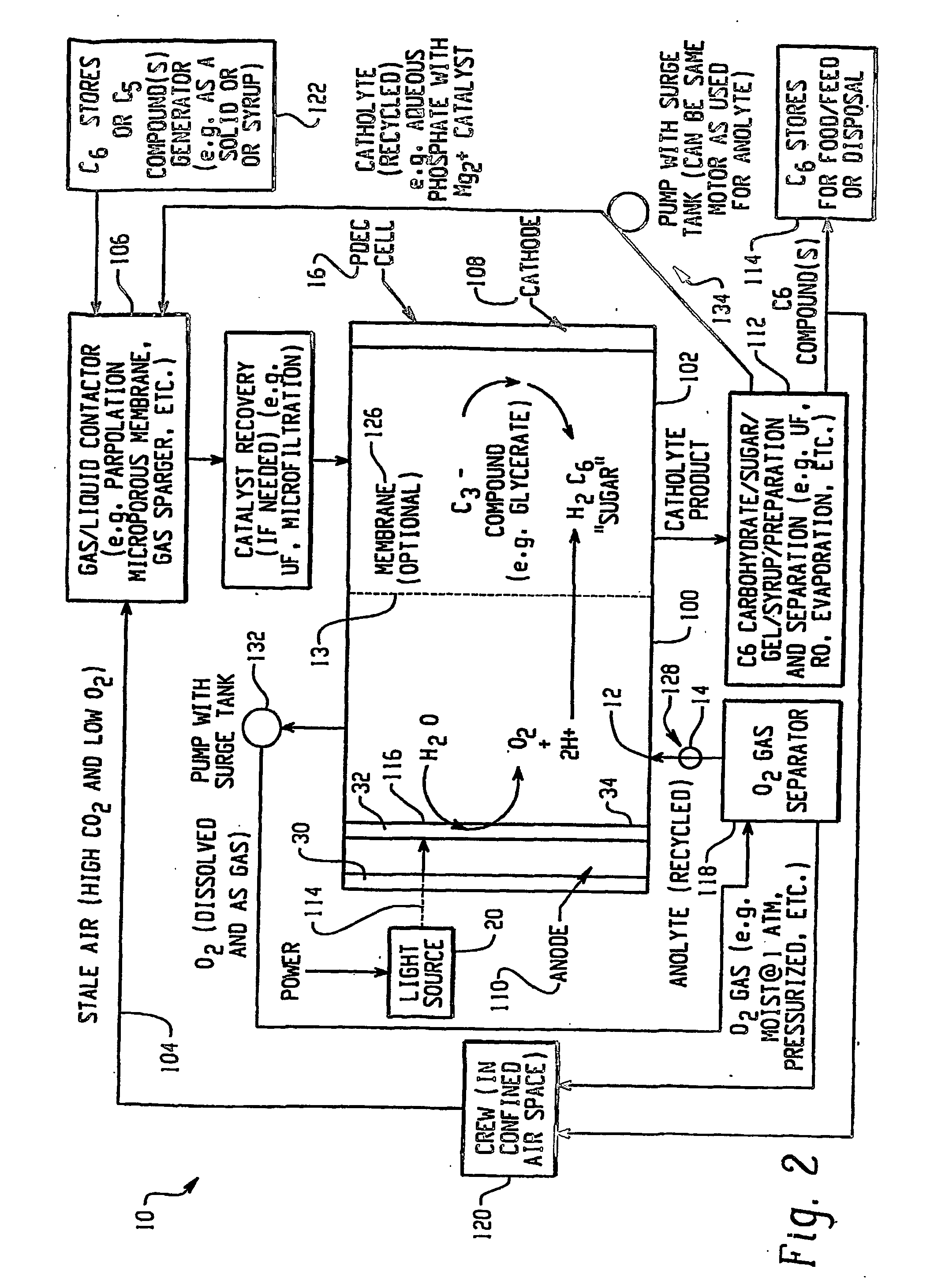
Photolytic oxygenator with carbon dioxide and/or hydrogen separation and fixation
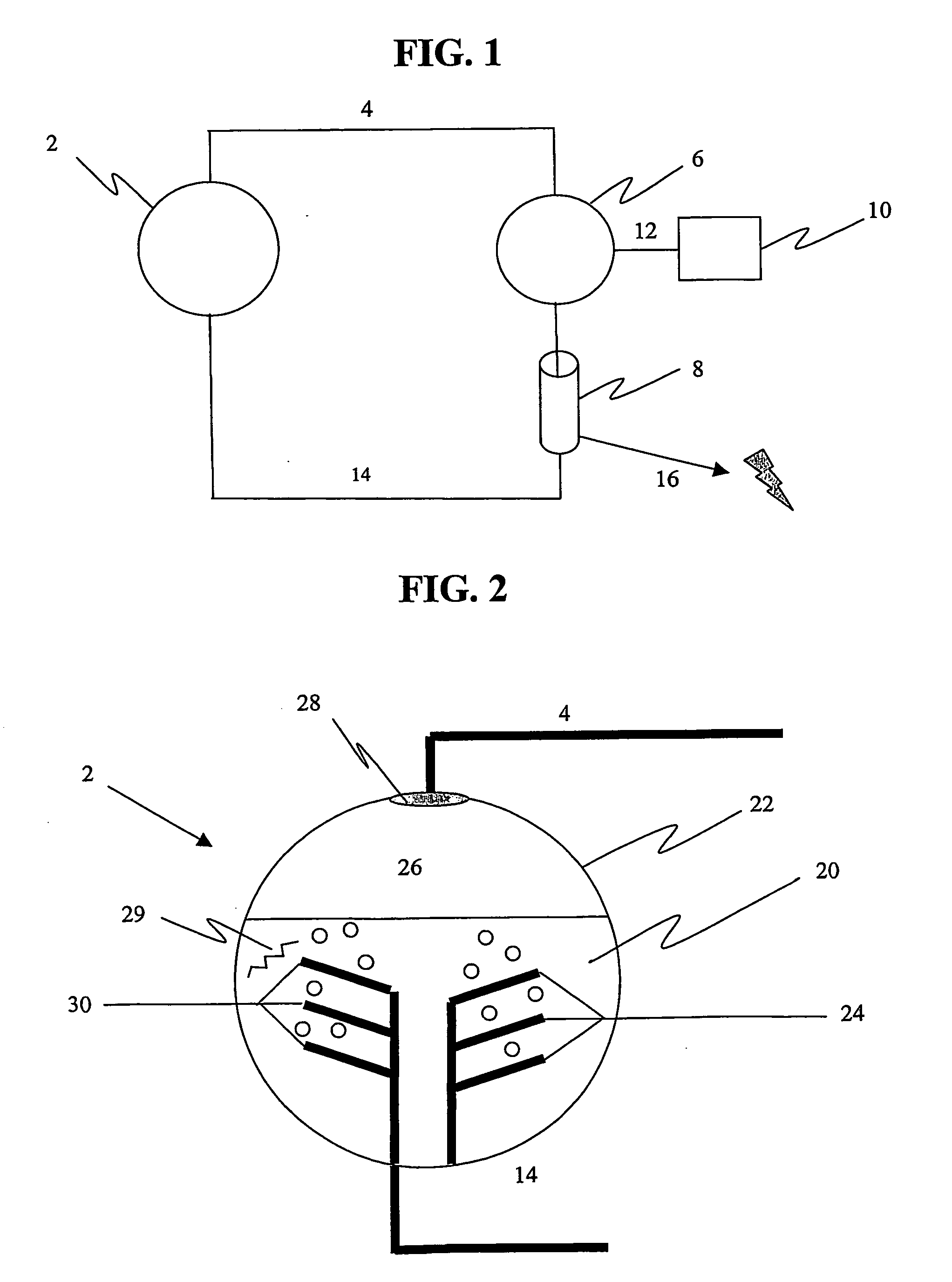
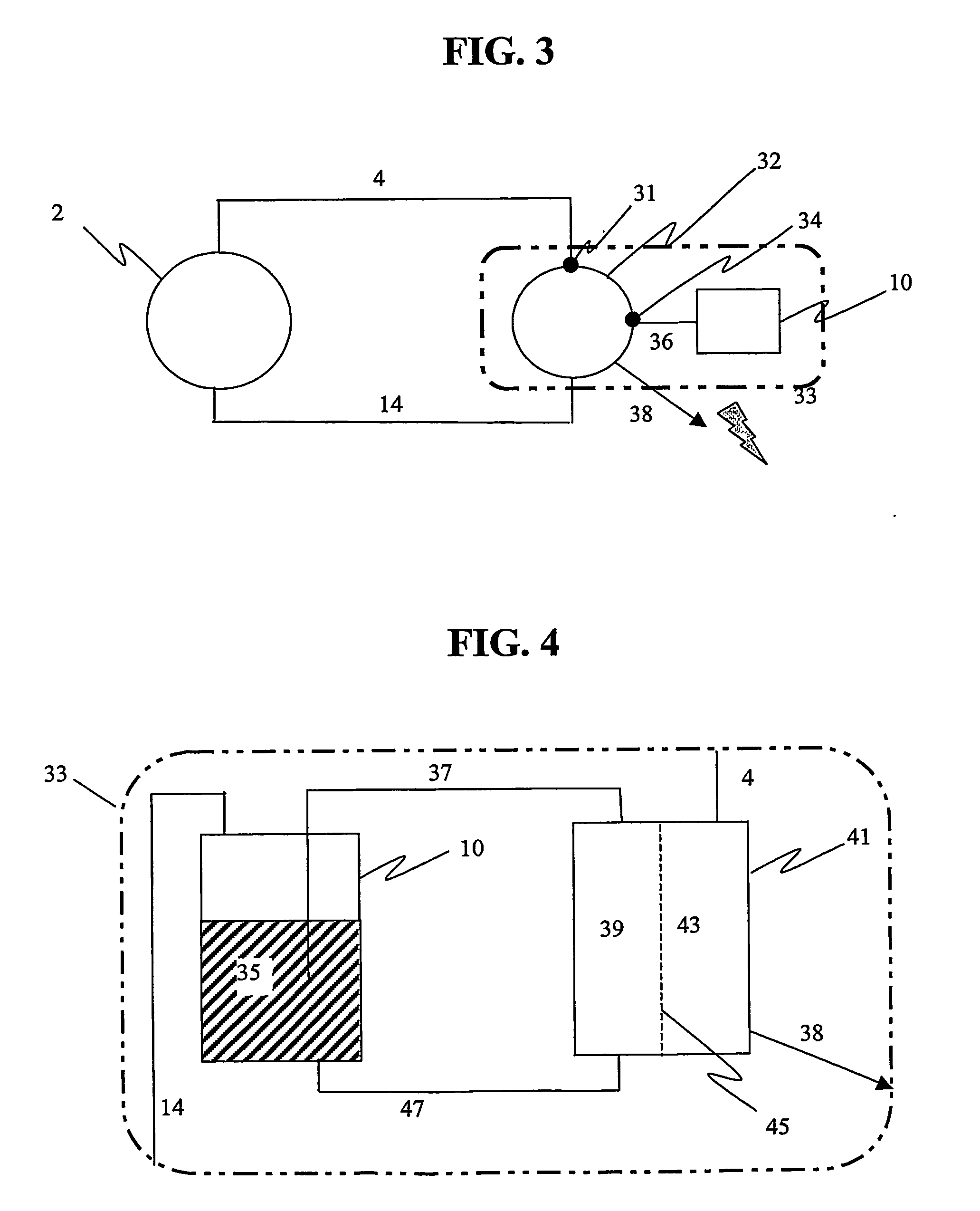
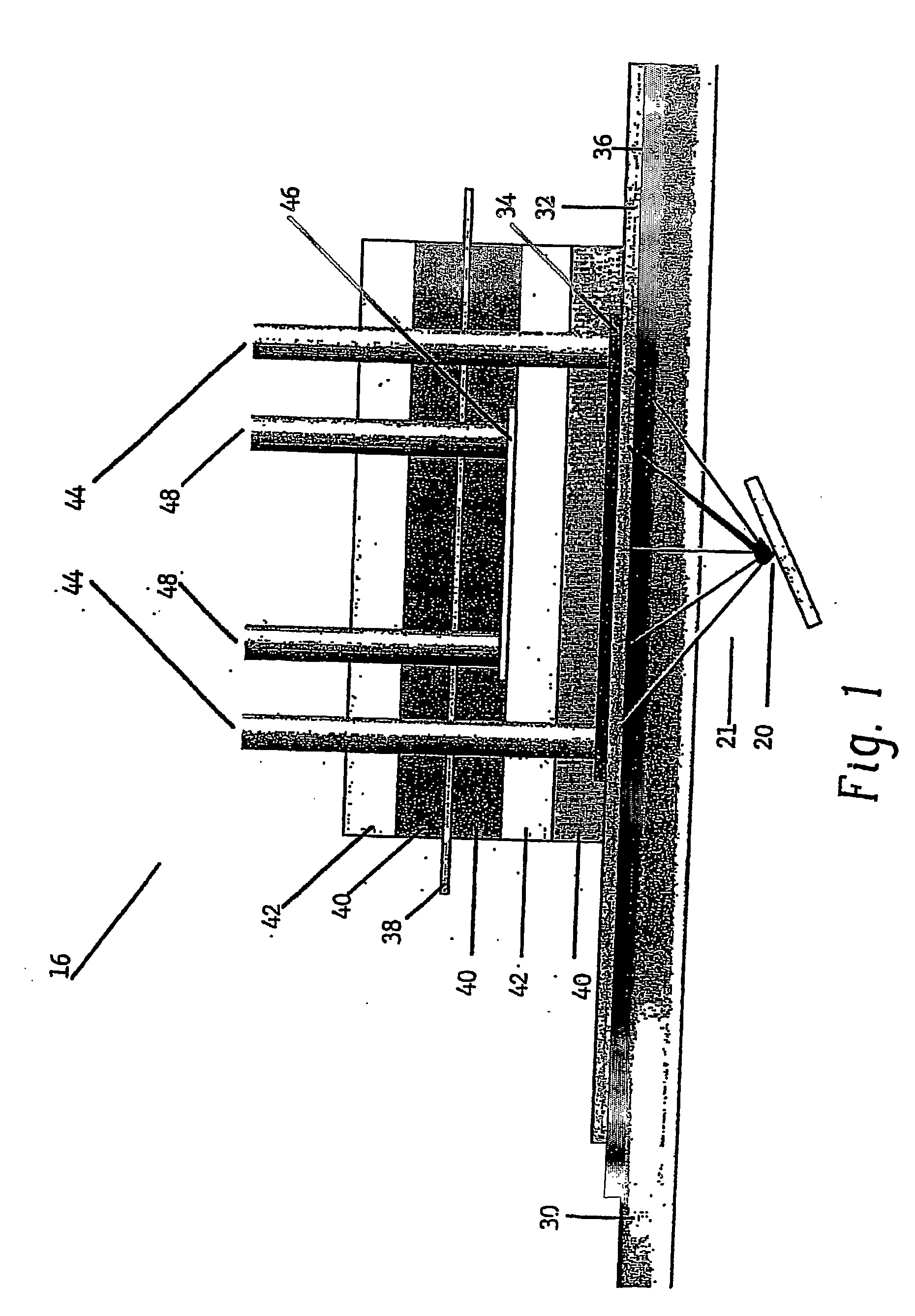
Apparatus for oxygenating an enclosed space as well as removing carbon dioxide from the enclosed space. The apparatus comprises a photolytic cell (16) having an anode compartment with a photo-active surface having the ability to convert water to oxygen; a cathode compartment having the ability to convert carbon dioxide and hydrogen to a solid or liquid medium; and a light source (20) for providing light photons (21) to said photolytic cell and activating the photo-reactive surface.
Owner:BATTELLE MEMORIAL INST
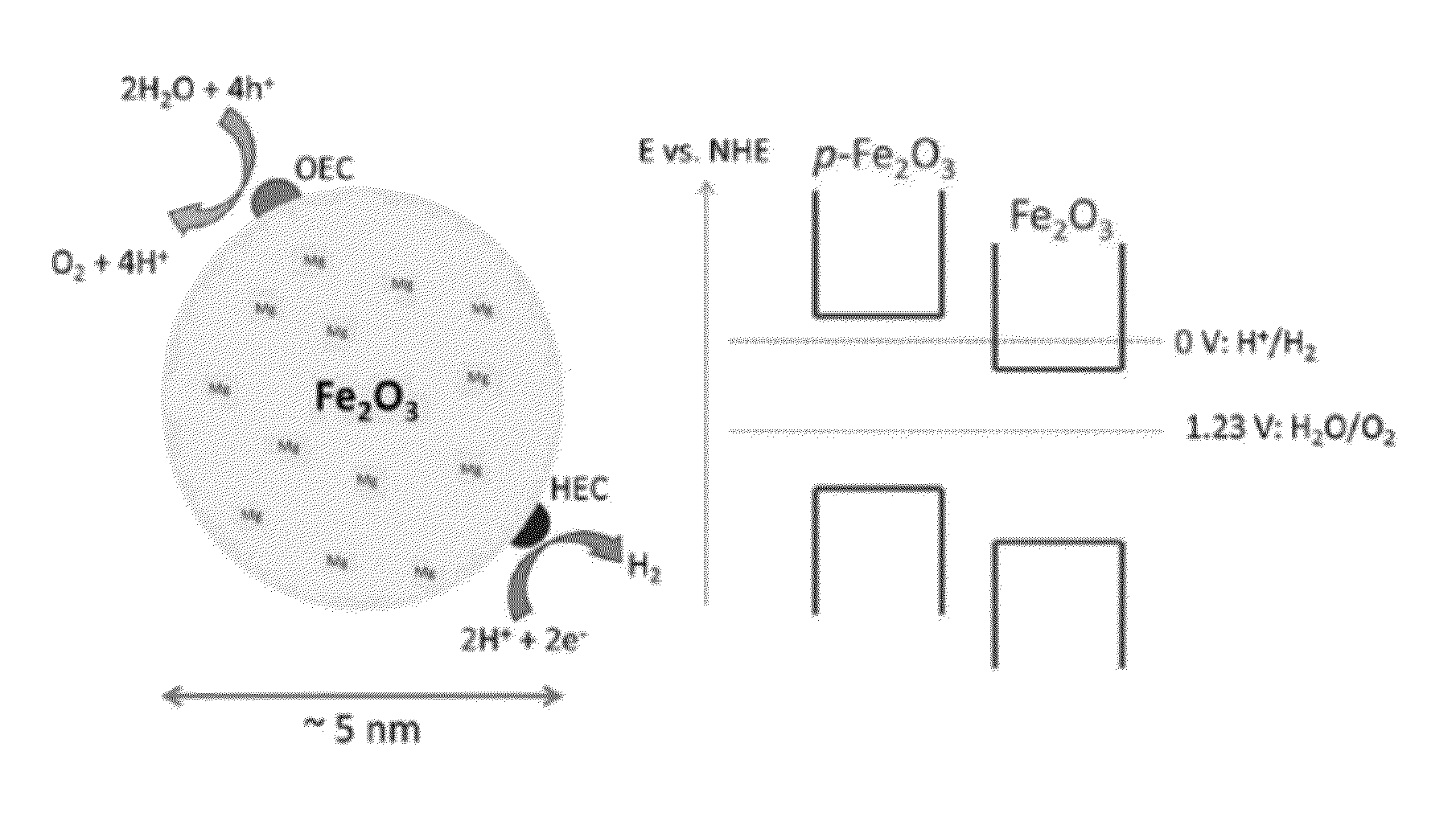
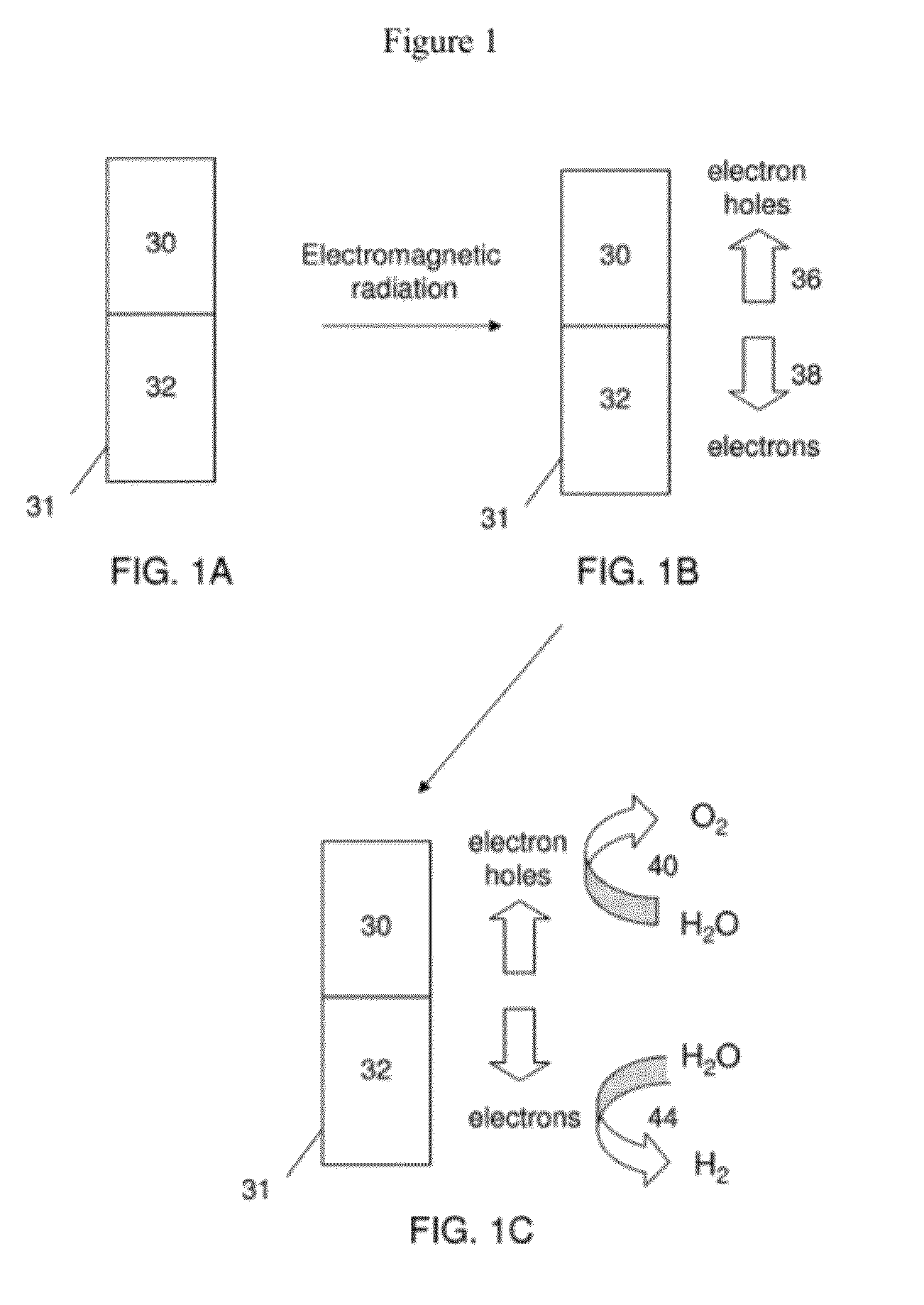
Nanostructures, Systems, and Methods for Photocatalysis
The present invention generally relates to nanostructures and compositions comprising nanostructures, methods of making and using the nanostructures, and related systems. In some embodiments, a nanostructure comprises a first region and a second region, wherein a first photocatalytic reaction (e.g., an oxidation reaction) can be carried out at the first region and a second photocatalytic reaction (e.g., a reduction reaction) can be carried out at the second region. In some cases, the first photocatalytic reaction is the formation of oxygen gas from water and the second photocatalytic reaction is the formation of hydrogen gas from water. In some embodiments, a nanostructure comprises at least one semiconductor material, and, in some cases, at least one catalytic material and / or at least one photosensitizing agent.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Portable oxygen concentrator
A portable oxygen concentrator includes a reservoir adapted to store oxygen-enriched gas, a delivery valve adapted to communicate with the reservoir, a pressure sensor adapted to measure a reservoir pressure within the reservoir and to measure a pressure drop across the delivery valve, and an input device adapted to receive a pulse does setting. A controller coupled to the pressure sensor and monitors the reservoir pressure and the pressure drop across the delivery valve. The controller is also coupled to the delivery valve to selectively open the delivery valve for pulse durations based on the pulse dose setting to deliver pulses of gas from the reservoir to a user. The controller further adjusts the pulse durations based at least partially upon the reservoir pressure and the pressure drop across the delivery valve.
Owner:RIC INVESTMENTS LLC
Apparatus and method for administering a therapeutic agent into tissue
An apparatus for administering a therapeutic is provided. In various embodiments, the apparatus includes a syringe having a barrel and a plunger and having an ozone generator associated therewith. The generator is initiated and a therapeutic gas is accumulated within the barrel, at which point it can be delivered from the barrel into a target site via a needle, thereby delivering therapeutic effects to that target site.
Owner:ACTIVEO
Methods of conducting simultaneous exothermic and endothermic reactions
InactiveUS6969506B2High yieldImprove performanceFinal product manufactureChemical/physical/physico-chemical microreactorsCombustion chamberEngineering
Integrated Combustion Reactors (ICRs) and methods of making ICRs are described in which combustion chambers (or channels) are in direct thermal contact to reaction chambers for an endothermic reaction. Superior results were achieved for combustion chambers which contained a gap for free flow through the chamber. Particular reactor designs are also described. Processes of conducting reactions in integrated combustion reactors are described and results presented. Some of these processes are characterized by unexpected and superior results.
Owner:BATTELLE MEMORIAL INST
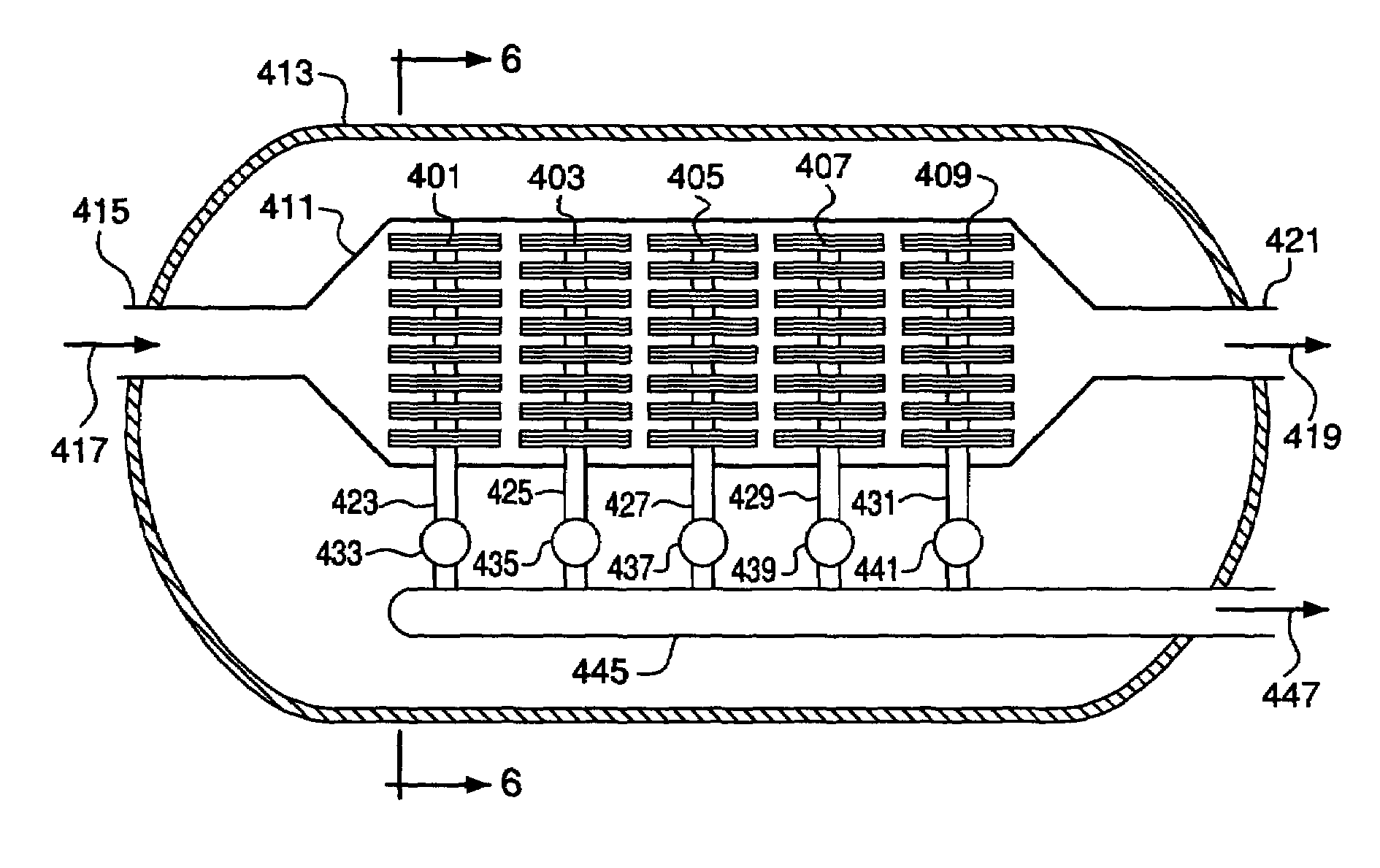
Ion transport membrane module and vessel system
An ion transport membrane system comprising (a) a pressure vessel having an interior, an exterior, an inlet, and an outlet; (b) a plurality of planar ion transport membrane modules disposed in the interior of the pressure vessel and arranged in series, each membrane module comprising mixed metal oxide ceramic material and having an interior region and an exterior region, wherein any inlet and any outlet of the pressure vessel are in flow communication with exterior regions of the membrane modules; and (c) one or more gas manifolds in flow communication with interior regions of the membrane modules and with the exterior of the pressure vessel.The ion transport membrane system may be utilized in a gas separation device to recover oxygen from an oxygen-containing gas or as an oxidation reactor to oxidize compounds in a feed gas stream by oxygen permeated through the mixed metal oxide ceramic material of the membrane modules.
Owner:AIR PROD & CHEM INC
Energy efficient gas separation for fuel cells
InactiveUS7087331B2Intuitive effectFully purifiedFuel cell heat exchangeFused electrolyte fuel cellsDelivery systemSolid oxide fuel cell
An electrical current generating system is disclosed that includes a fuel cell operating at a temperature of at least about 250° C. (for example, a molten carbonate fuel cell or a solid oxide fuel cell), a hydrogen gas separation system or oxygen gas delivery system that includes a compressor or pump, and a drive system for the compressor or pump that includes means for recovering energy from at least one of the hydrogen gas separation system, oxygen gas delivery system, or heat of the fuel cell. The drive system could be a gas turbine system. The hydrogen gas separation system or the oxygen gas delivery system may include a pressure swing adsorption module.
Owner:AIR PROD & CHEM INC
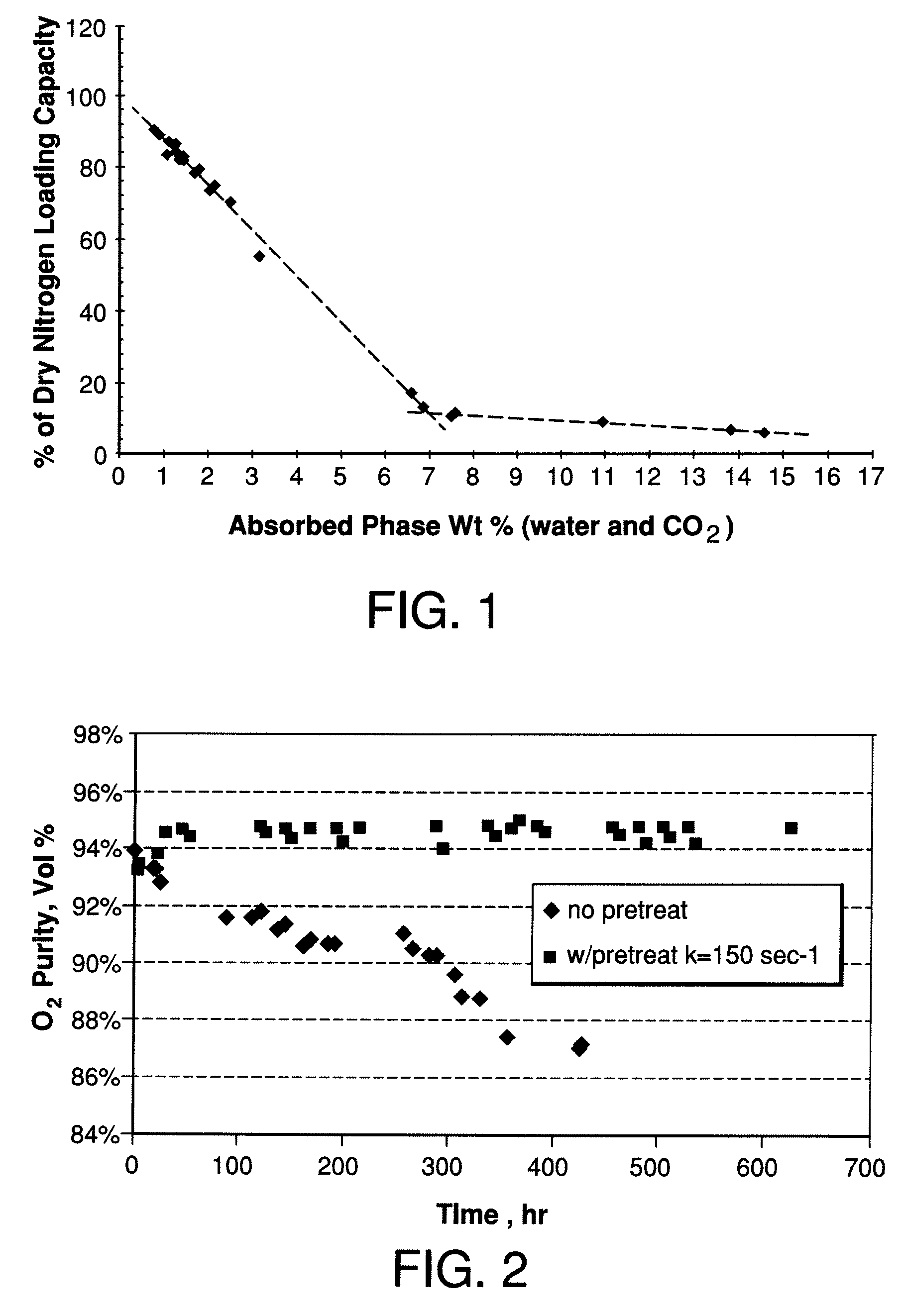
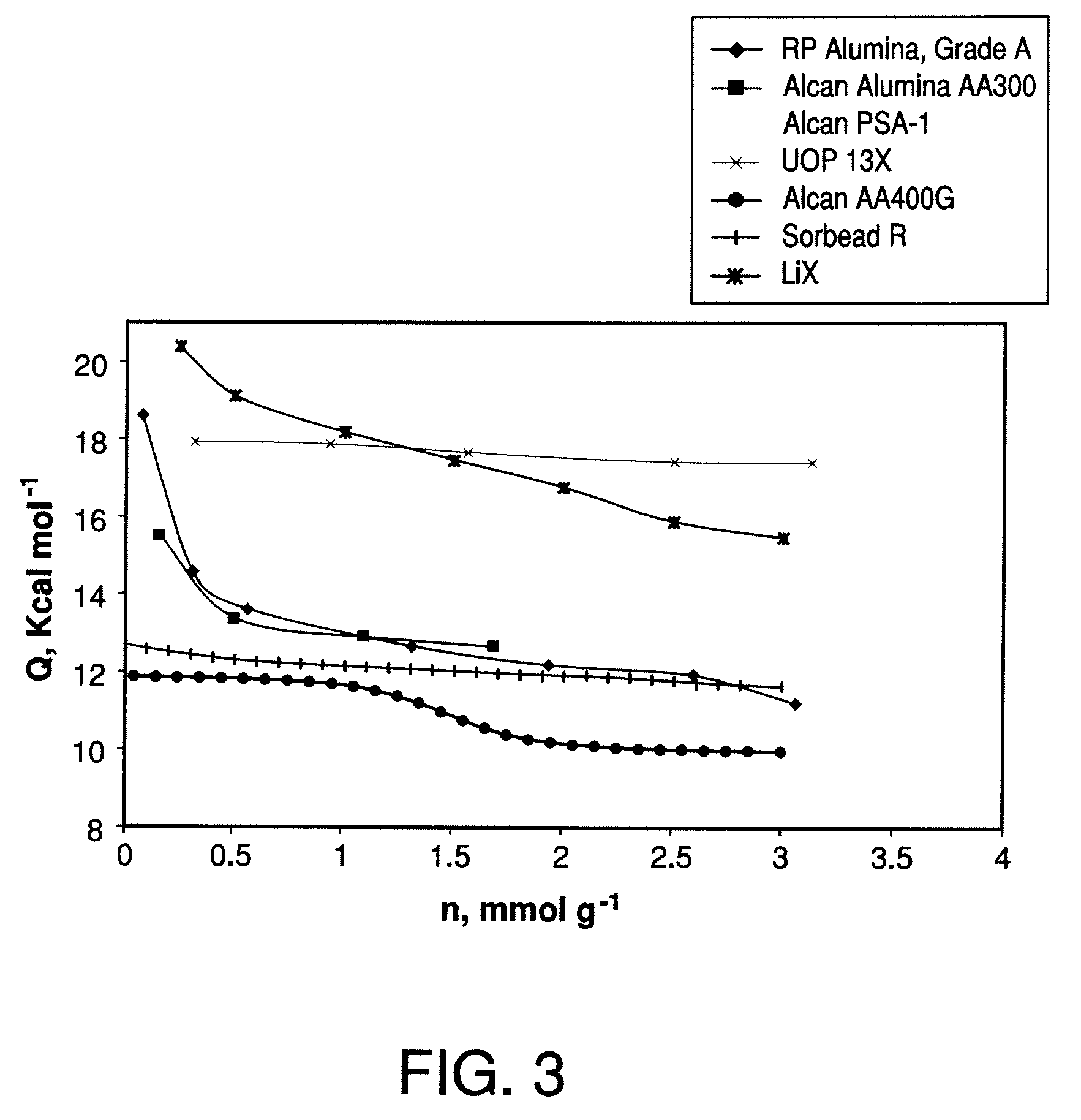
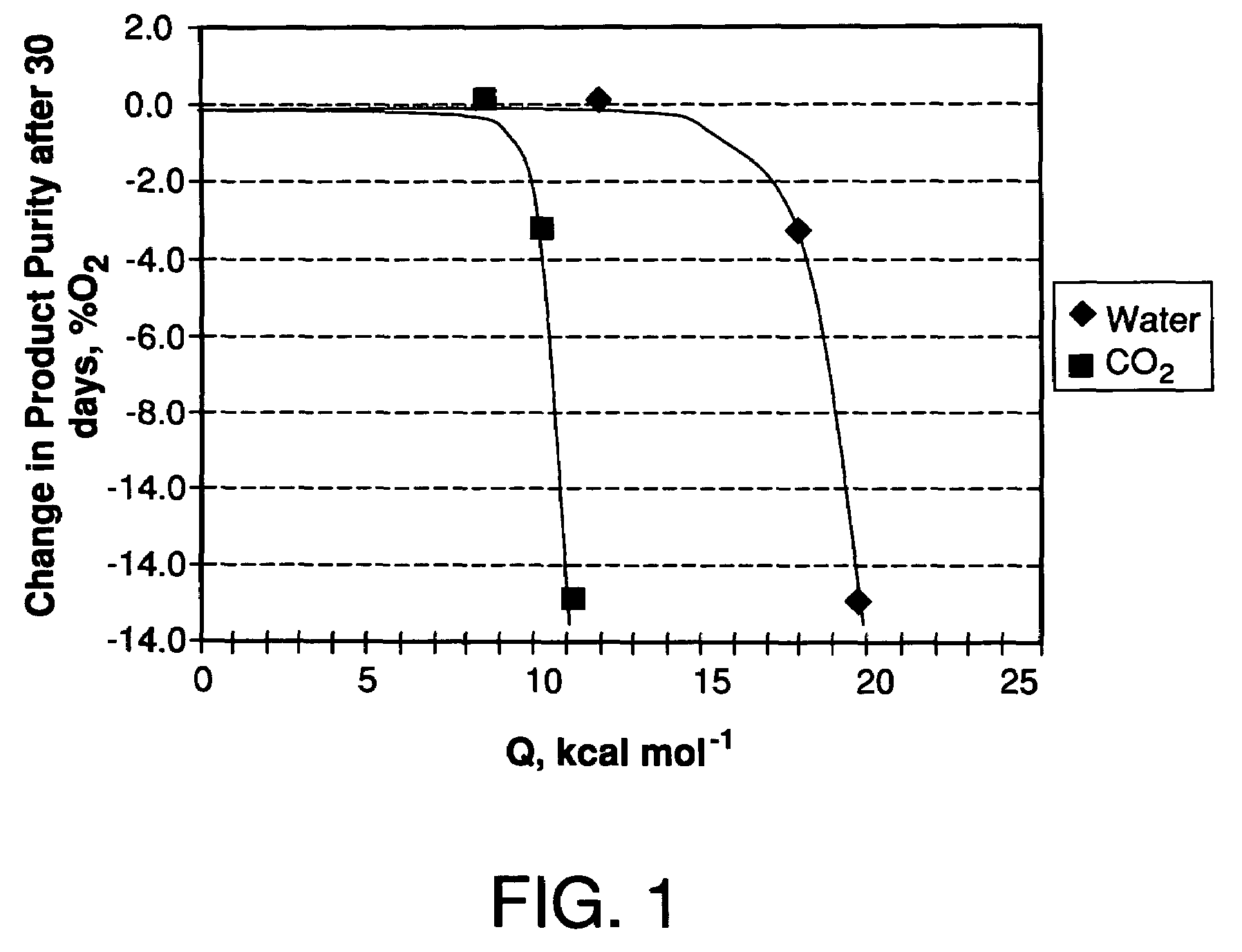
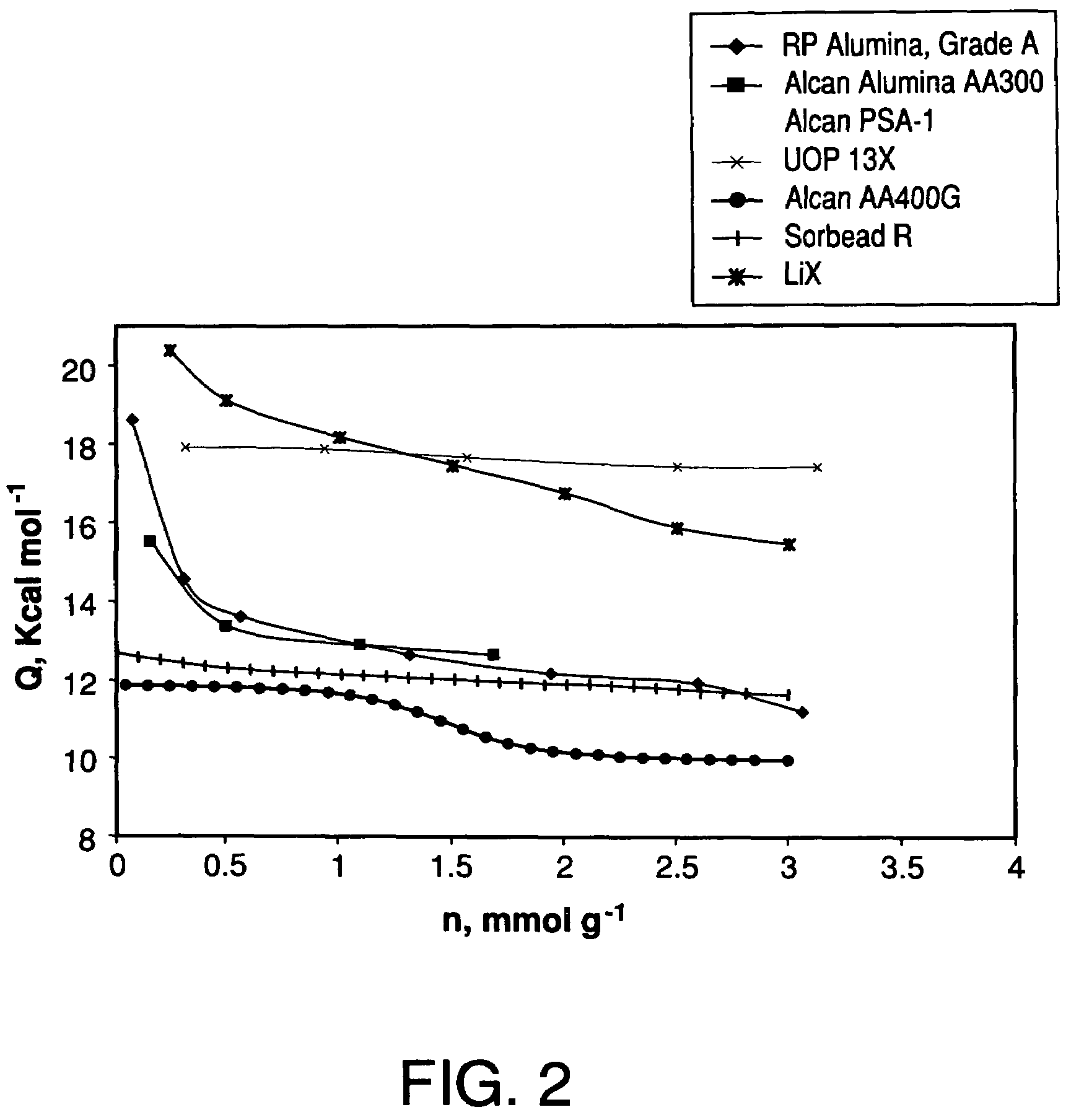
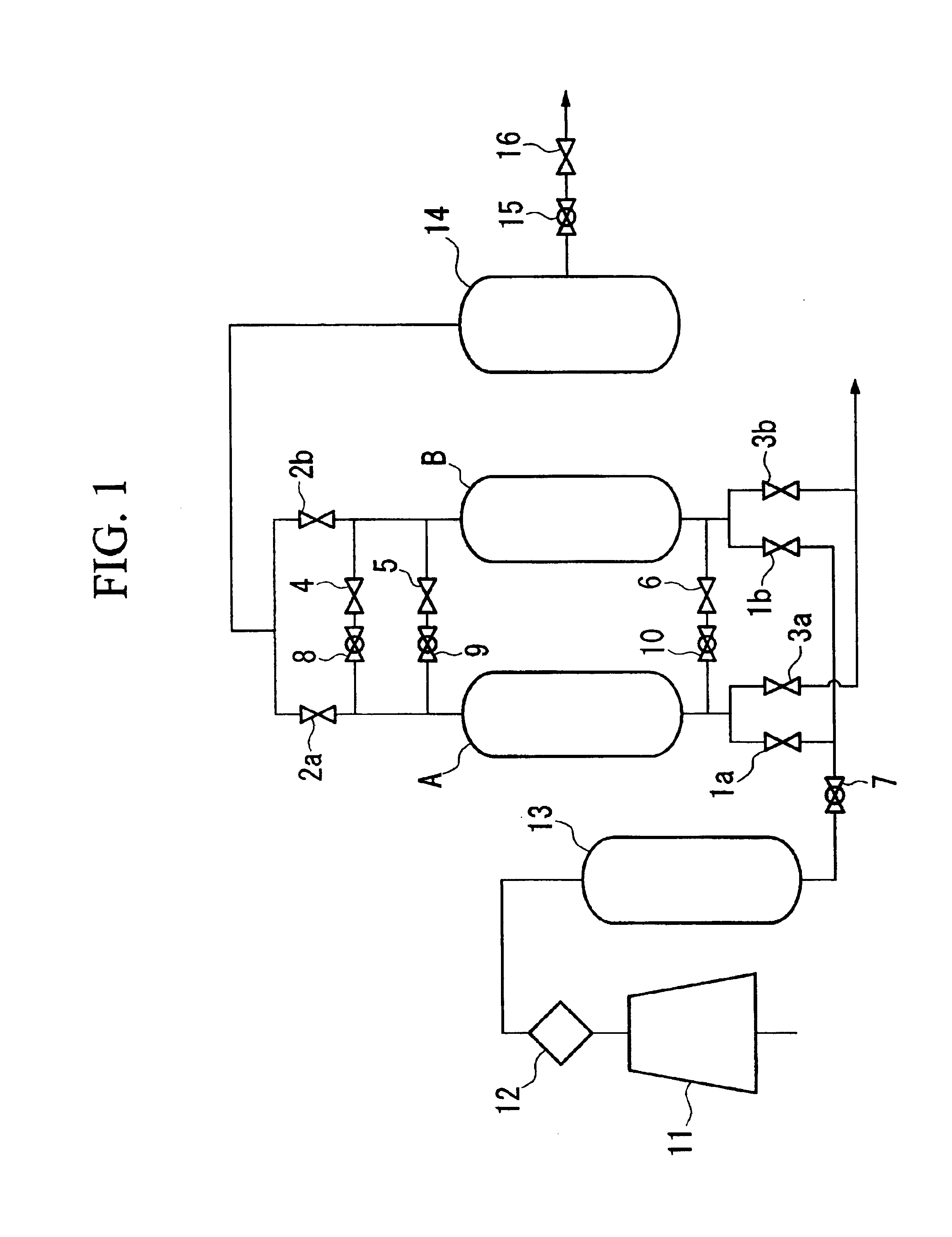
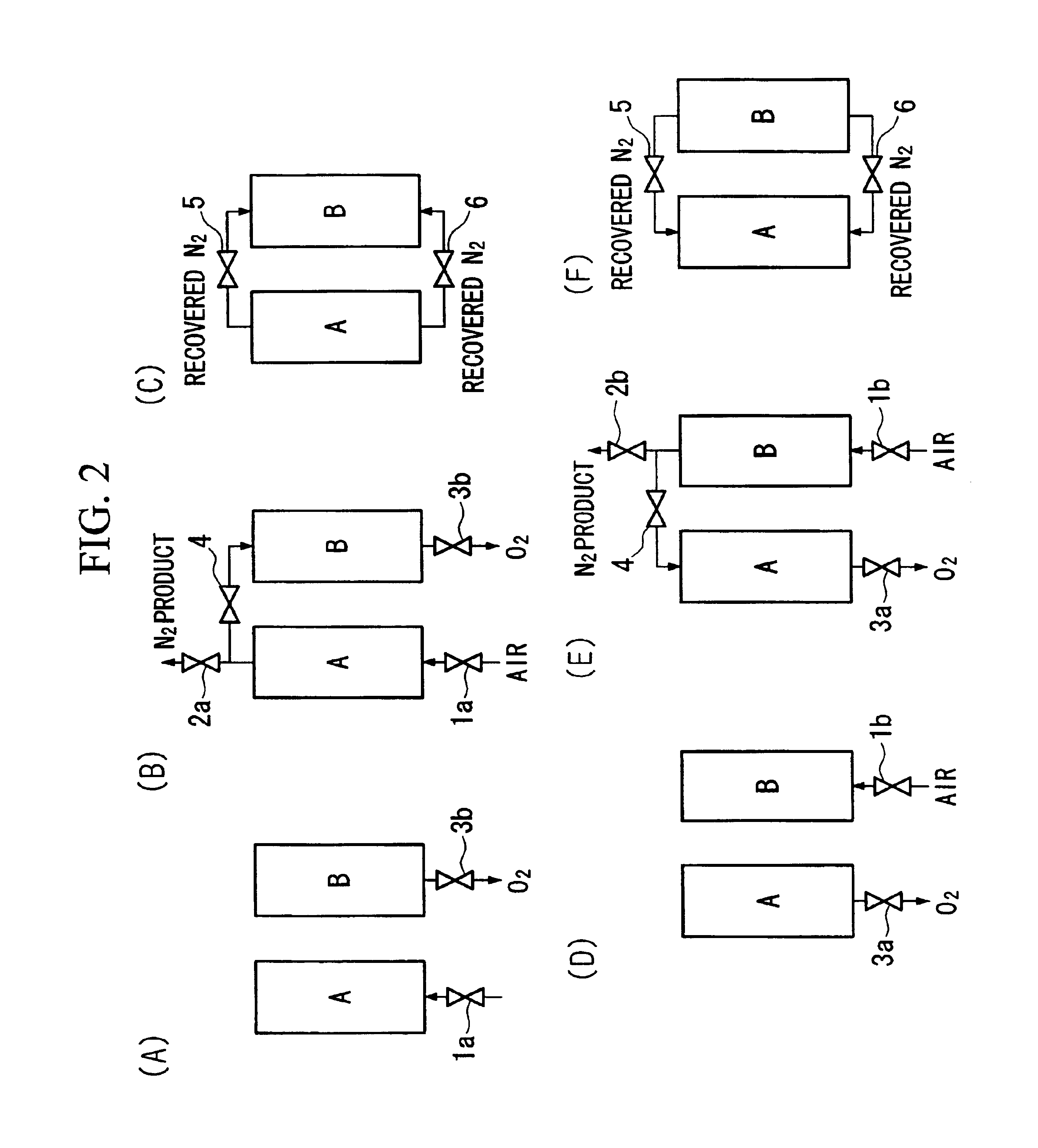
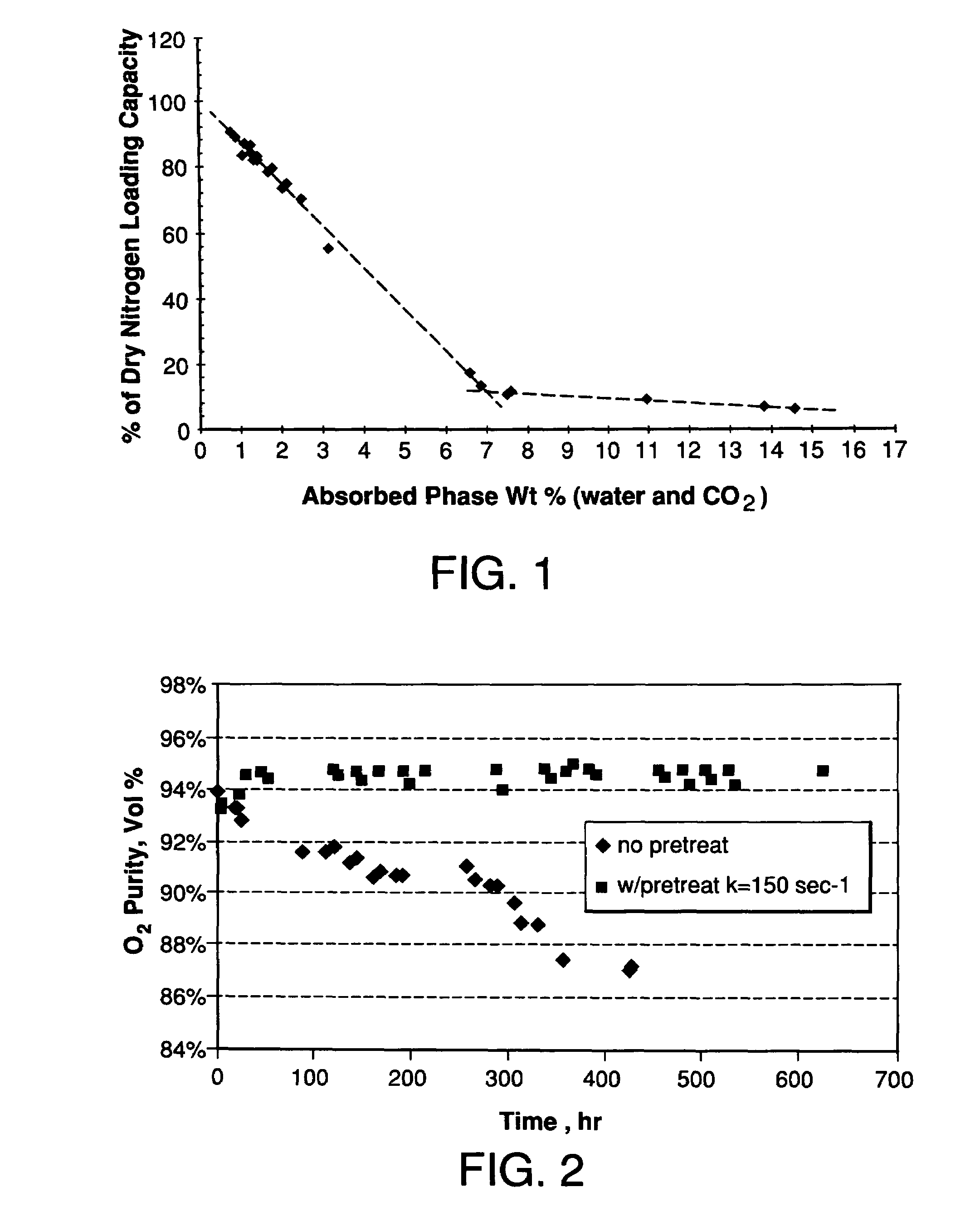
Performance Stability in Shallow Beds in Pressure Swing Adsorption Systems
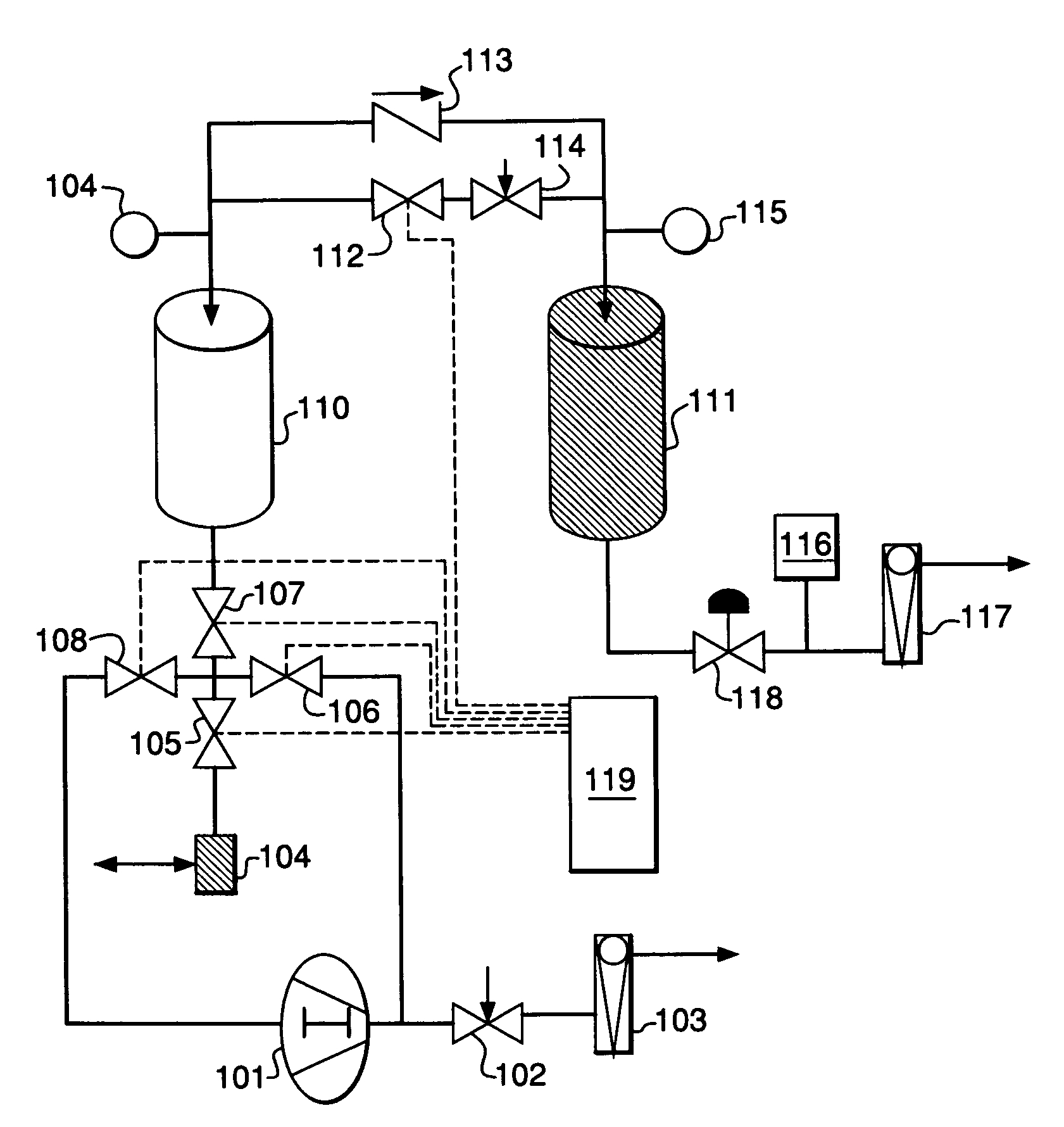
PSA process for oxygen production comprising (a) providing an adsorber having a first layer of adsorbent selective for water and a second layer of adsorbent selective for nitrogen, wherein the heat of adsorption of water on the adsorbent in the first layer is equal to or less than about 14 kcal / mole at water loadings less than about 0.05 mmol per gram; (b) passing a feed gas comprising at least oxygen, nitrogen, and water successively through the first and second layers, adsorbing water in the first layer of adsorbent, and adsorbing nitrogen in the second layer of adsorbent, wherein the mass transfer coefficient of water in the first layer is in the range of about 125 to about 400 sec−1 and the superficial contact time of the feed gas in the first layer is between about 0.08 and about 0.50 sec; and (c) withdrawing a product enriched in oxygen from the adsorber.
Owner:AIR PROD & CHEM INC
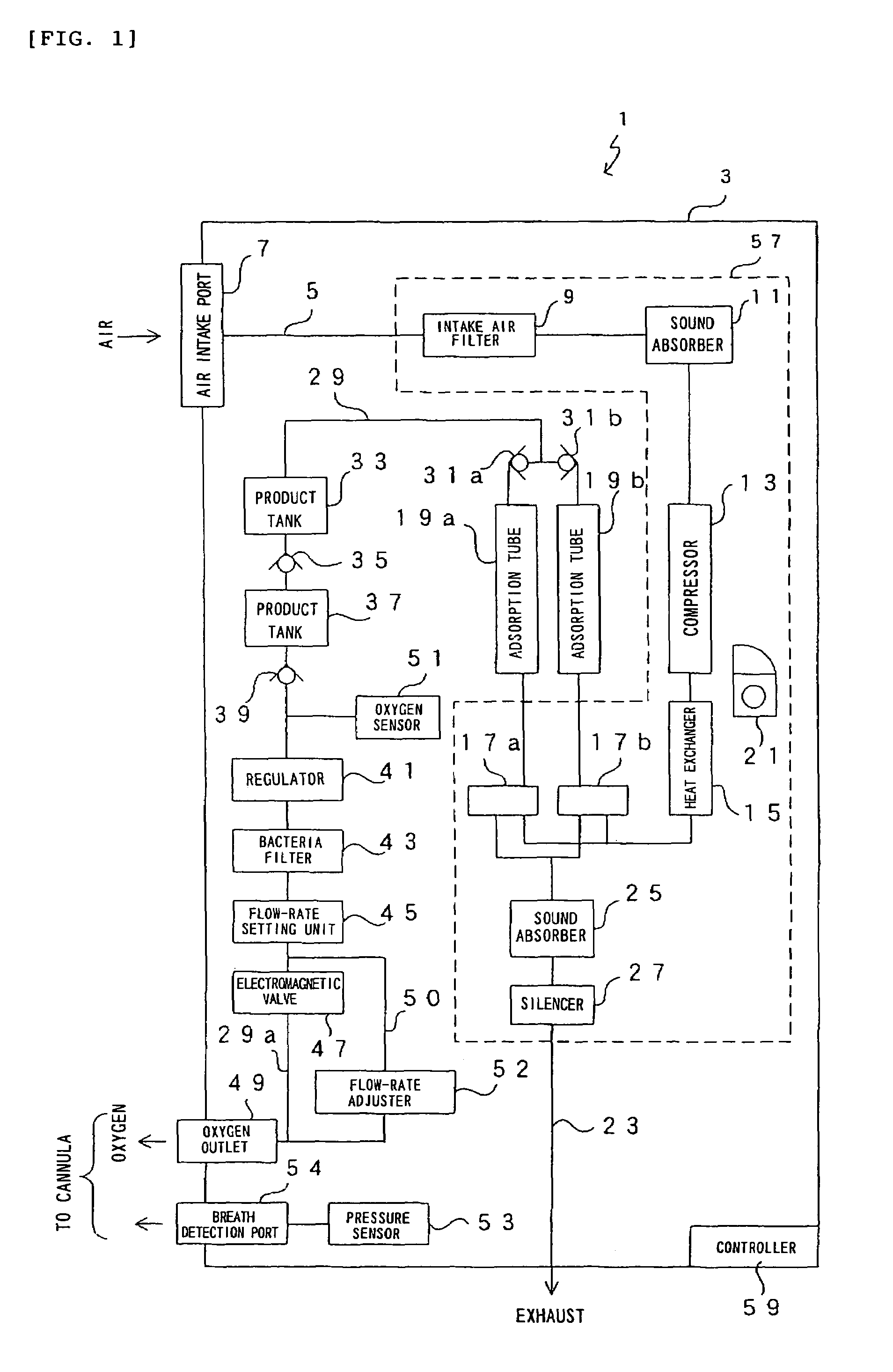
Oxygen enriching apparatus, controller for the oxygen enriching apparatus, and recording medium for the controller
InactiveUS6837244B2Increase flow rateAdverse effect of pressure can be preventedOperating means/releasing devices for valvesRespiratory masksInhalationProcess engineering
A small oxygen enriching apparatus which can supply oxygen-enriched gas at high flow rate without imparting unnatural sensation to a user, as well as a controller and recording medium therefore. In step 100, a judgment is made as to whether a flow rate set by use of a flow-rate setting unit 47 is equal to or less than a continuous base flow rate (2 liters / min). When the set flow rate is a low flow rate of not greater than 2 liters / min, breath-synchronized operation is not performed (continuous supply is to be performed), and therefore, in step 110, oxygen-enriched gas is supplied continuously at the set flow rate. When the set flow rate is a high flow rate of greater than 2 liters / min, breath-synchronized operation is to be performed (supply during the inhalation period only of each breathing cycle), and therefore, in step 120, oxygen-enriched gas is continuously supplied at the continuous base flow rate. In step 140, in order to perform breath-synchronized operation, control for opening and closing an electromagnetic valve 45 is performed. That is, oxygen-enriched gas is supplied during the inhalation period only of each breathing cycle, and supply of oxygen-enriched gas is stopped during the exhalation period.
Owner:NGK SPARK PLUG CO LTD
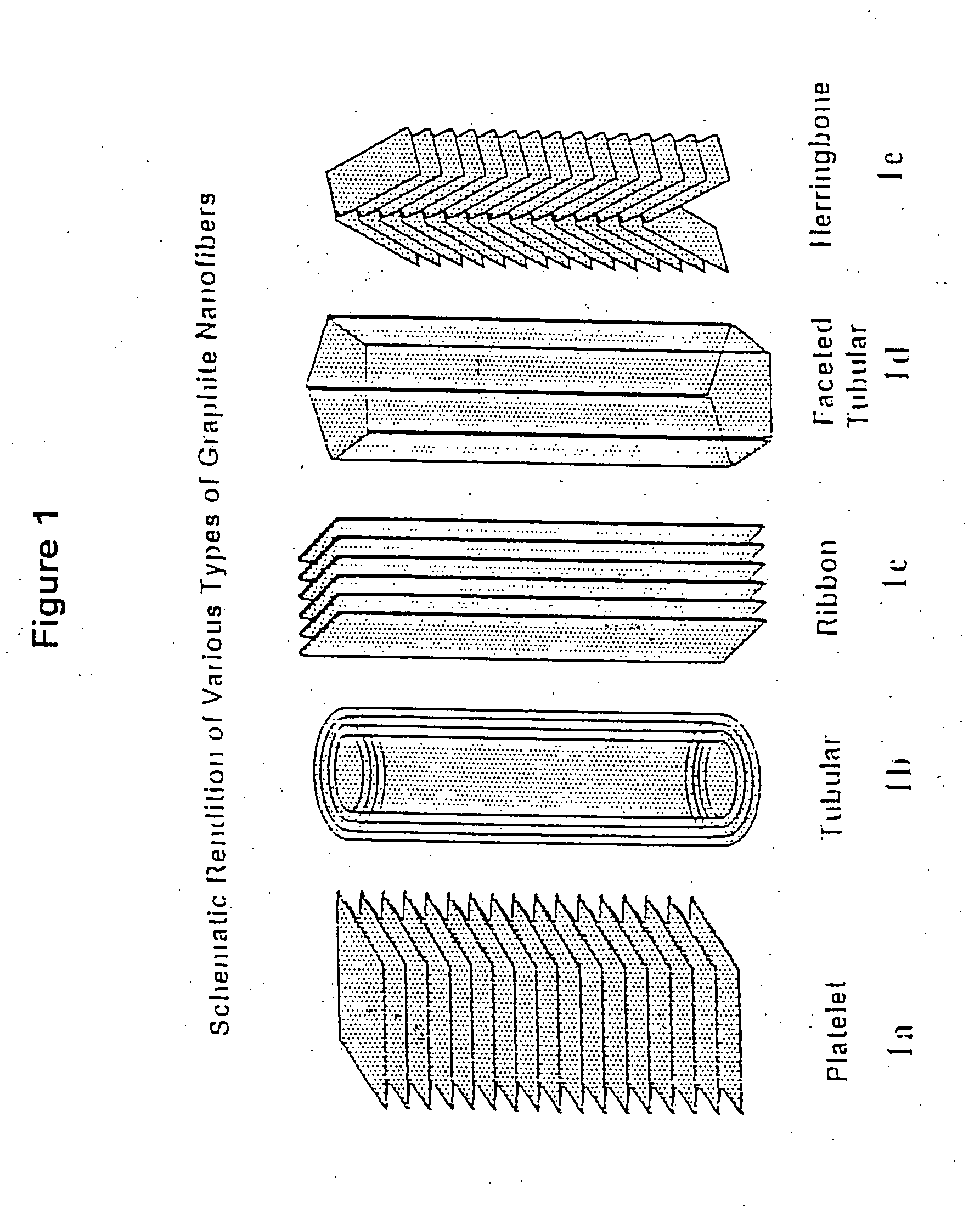
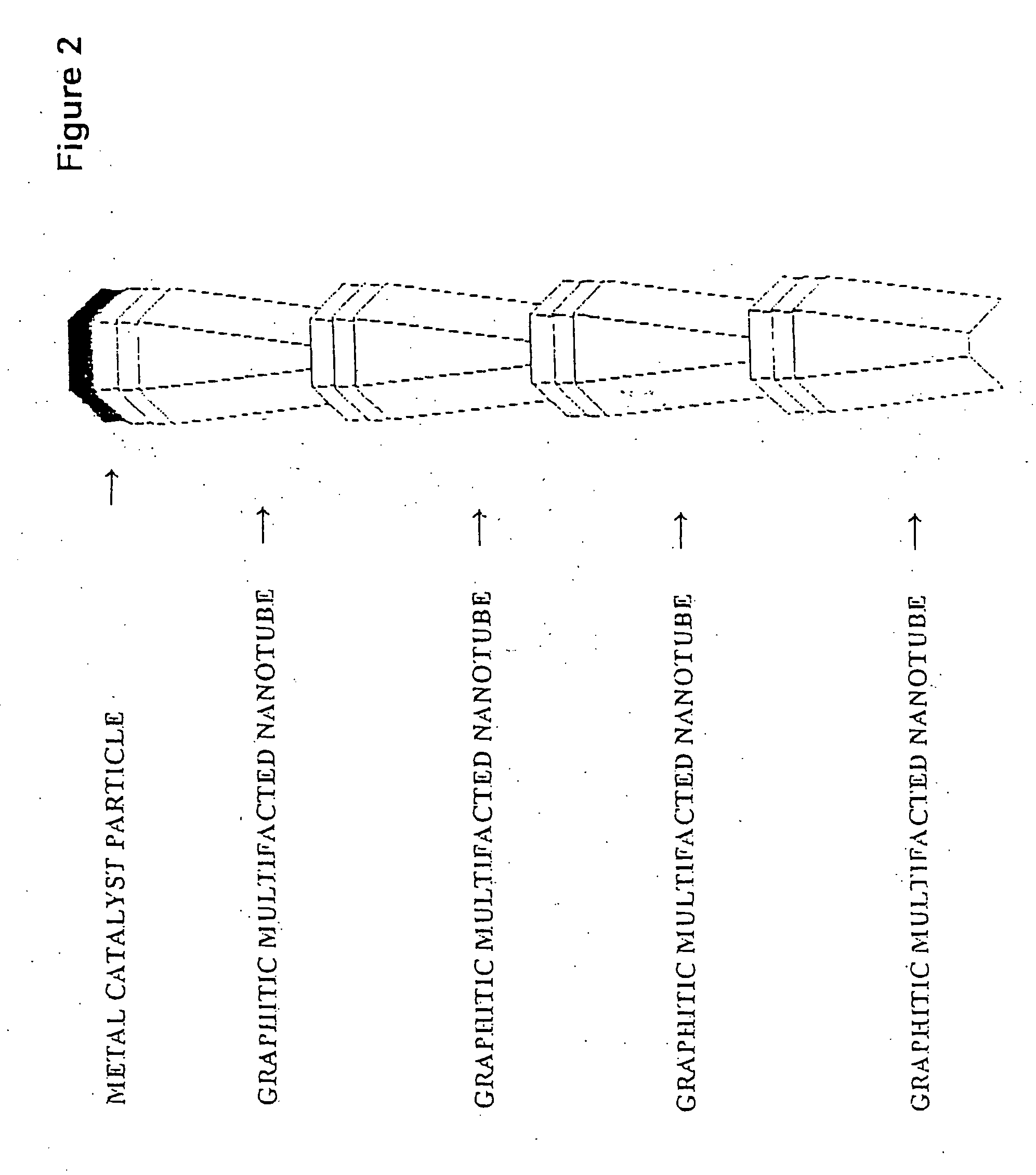
Novel graphite nanocatalysts
Novel catalysts comprised of graphitic nanostructures. The graphitic nanostructure catalysts are suitable for catalyzing reactions such as oxidation, hydrogenation, oxidative-hydrogenation, and dehydrogenation.
Owner:CATALYTIC MATERIALS
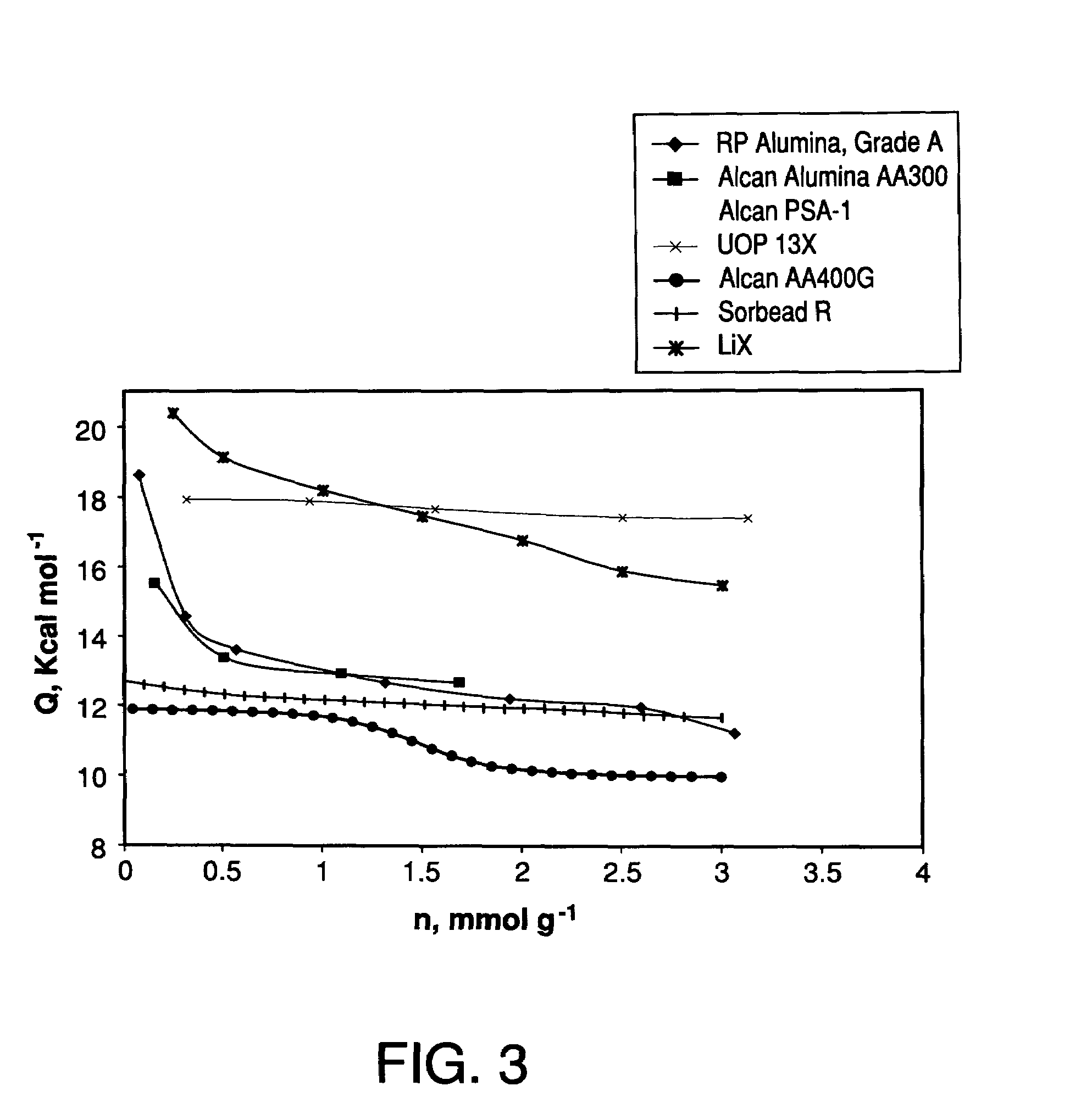
Performance stability in rapid cycle pressure swing adsorption systems
Pressure swing adsorption process for producing oxygen comprising (a) providing at least one adsorber vessel having a first layer of adsorbent adjacent the feed end of the vessel and a second layer of adsorbent adjacent the first layer, wherein the surface area to volume ratio of the first layer is in the range of about 0.75 to about 1.8 cm−1; (b) introducing a pressurized feed gas comprising at least oxygen, nitrogen, and water into the feed end, adsorbing at least a portion of the water in the adsorbent in the first layer, and adsorbing at least a portion of the nitrogen in the adsorbent in the second layer, wherein the superficial contact time of the pressurized feed gas in the first layer is between about 0.08 and about 0.50 sec; and (c) withdrawing a product gas enriched in oxygen from the product end of the adsorber vessel.
Owner:AIR PROD & CHEM INC
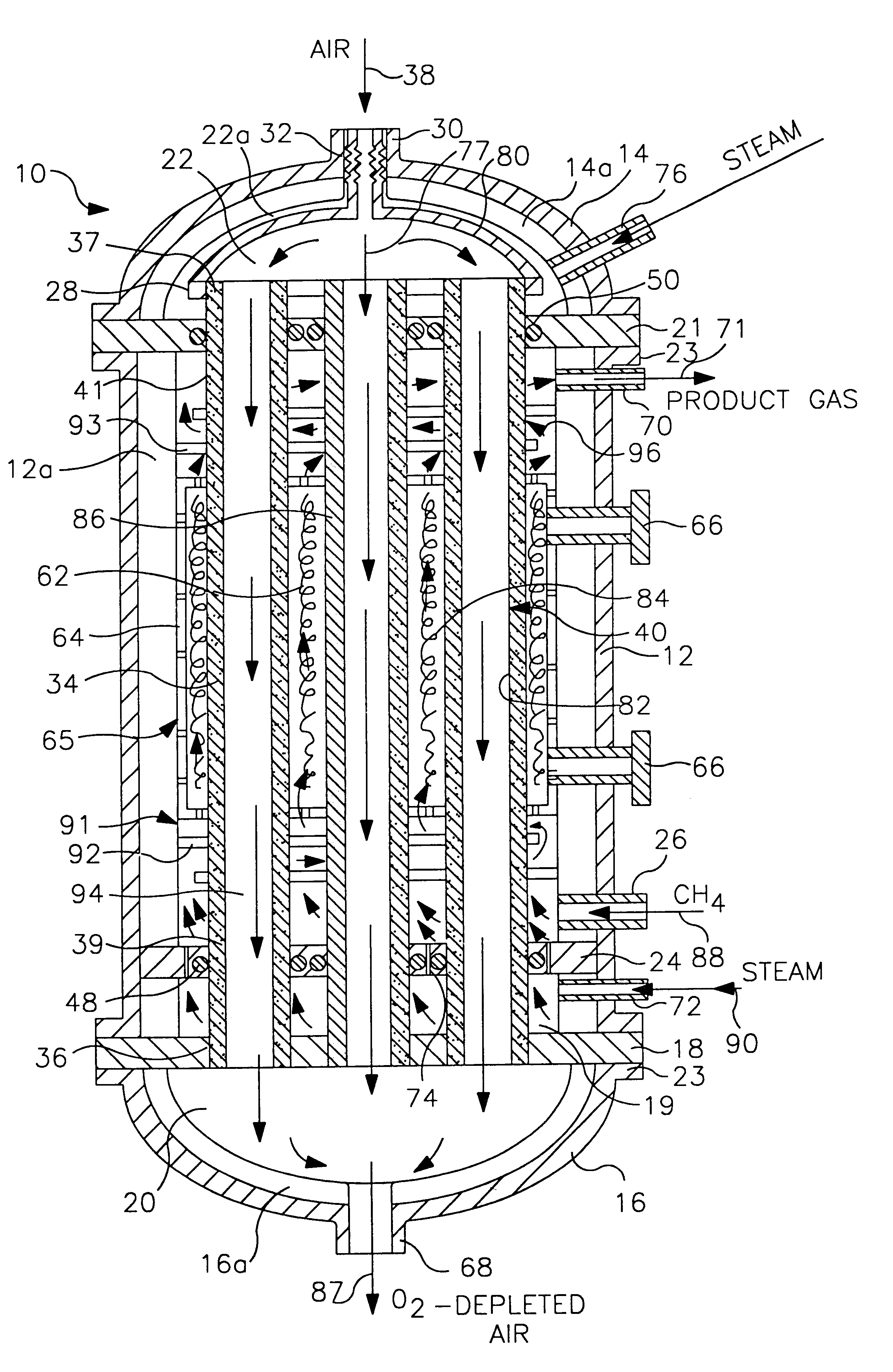
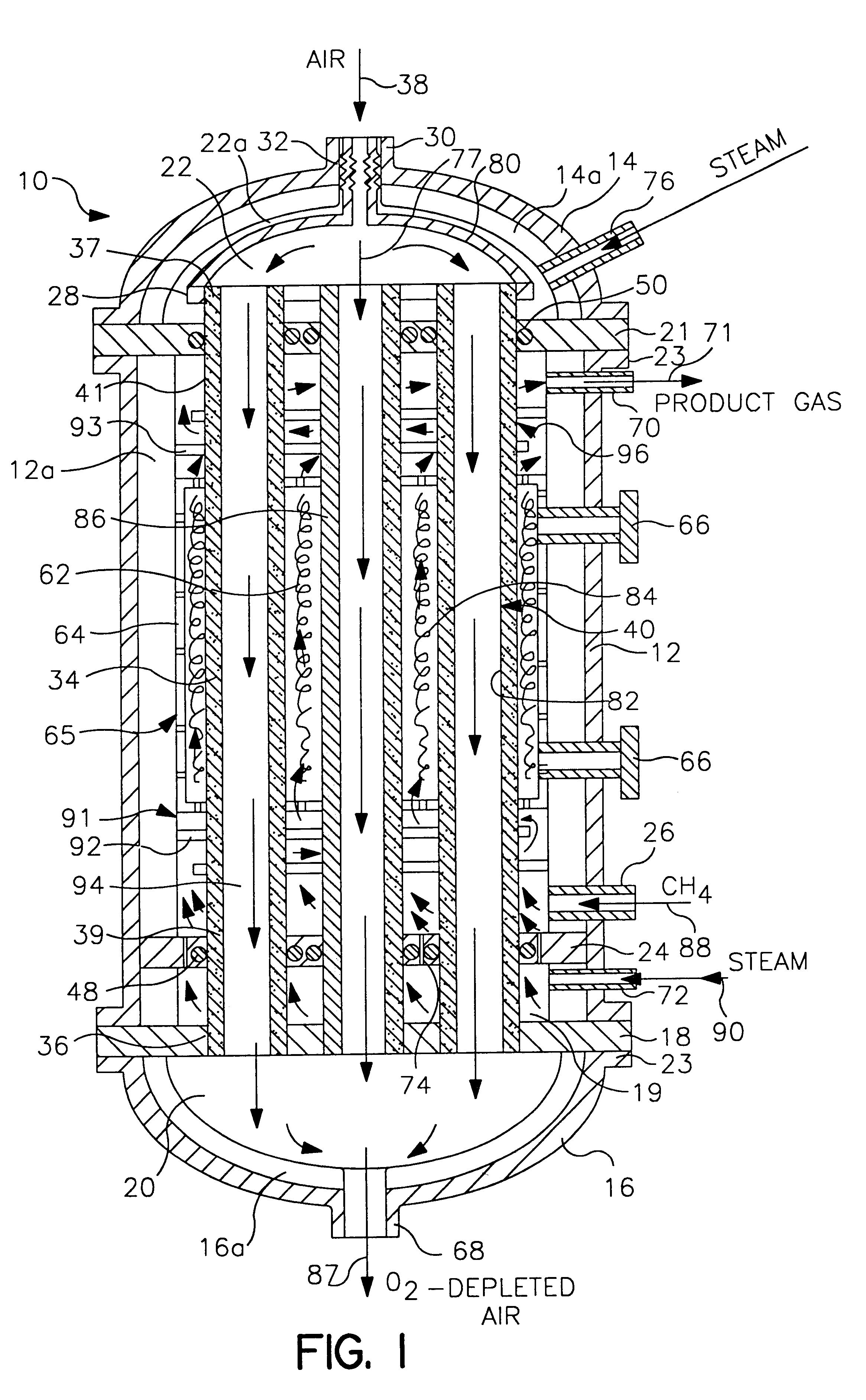
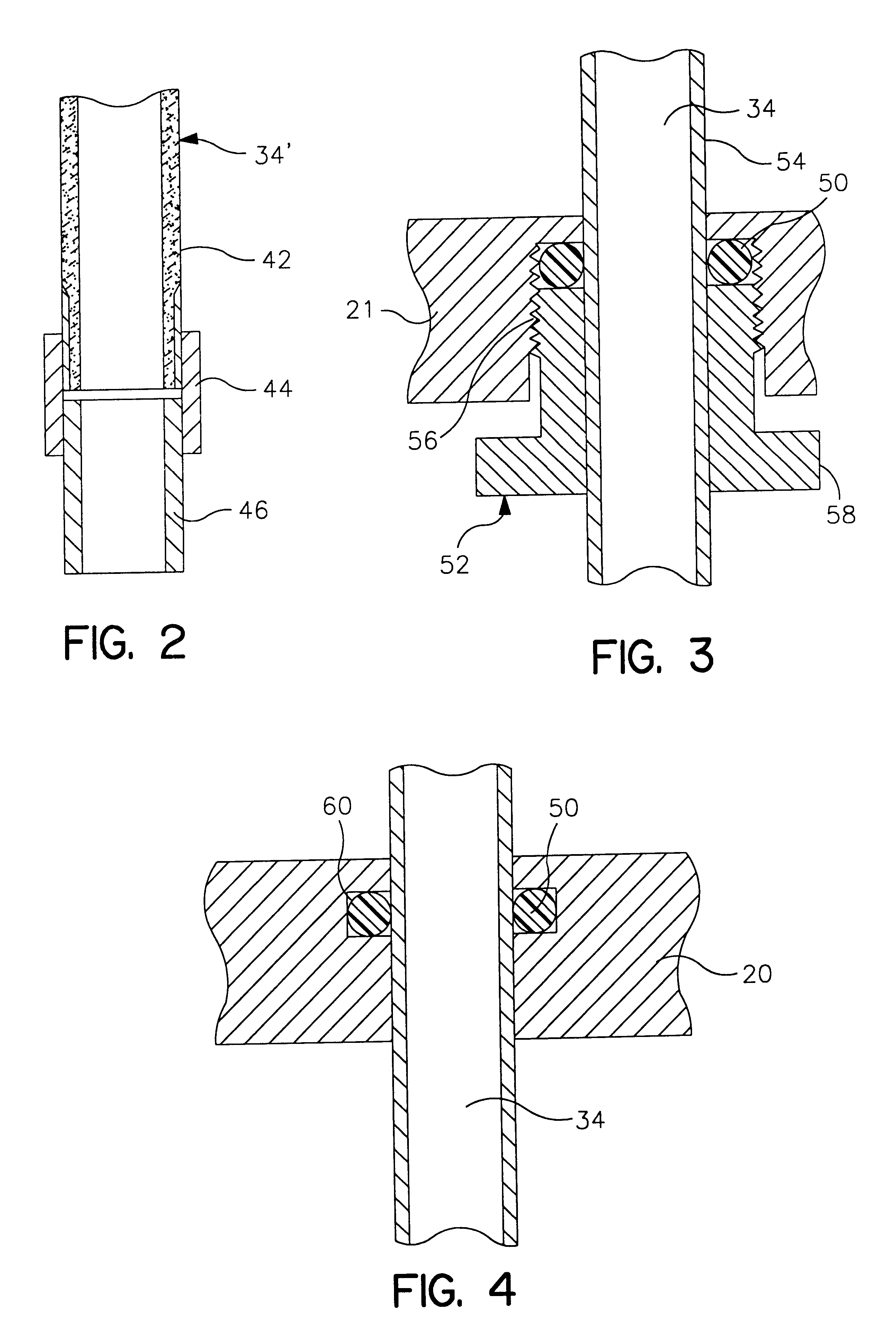
Life support oxygen concentrator
InactiveUS7250073B2High-frequency operationImprove energy efficiencyGas treatmentIsotope separationVacuum pressureProduct gas
Owner:AIR PROD & CHEM INC
Adsorbent for separating nitrogen from mixed gas of oxygen and nitrogen
InactiveUS6916358B2Minimal operating costMore productiveNitrogen purification/separationGas treatmentSorbentNitrogen
An adsorbent for separating nitrogen from a mixed gas of oxygen and nitrogen is MSC wherein an oxygen and nitrogen separation ratio α and a ratio (t95 / t50) of a time t50 required for adsorbing 50% of the oxygen equilibrium adsorption amount and a time t95 required for adsorbing 95% of the oxygen equilibrium adsorption amount satisfy the inequality(t95 / t50)<0.4×(α−24)α≧35.The amount of adsorbent used with respect to a predetermined amount of nitrogen generated can be reduced since a method is carried out which uses this adsorbent to produce nitrogen by separating nitrogen from the mixed gas of oxygen and nitrogen by means of a PSA method. By this method, it is possible to reduce the cost of the apparatus and reduce and miniaturize the scale of the apparatus, and the power consumption amount can be reduced.
Owner:NIPPON SANSO CORP
Oxygen-Producing Bandage With Releasable Oxygen Source
InactiveUS20100217177A1Easy to changeKeep dryPlastersMedical devicesLocking mechanismOxygen delivery
A device for delivering oxygen to a wound or injury includes a bandage, which includes a base defining an opening, a cover, and a locking mechanism for releasably securing the cover to the base. An oxygen source is in fluid communication with the cover, whereby oxygen is delivered to the opening in the base.
Owner:NEOGENIX LLC
Method for producing nitrogen to use in under balanced drilling, secondary recovery production operations and pipeline maintenance
InactiveUS7468173B2Offsetting costsNitrogen purification/separationDispersed particle separationElectrolysisAtmospheric air
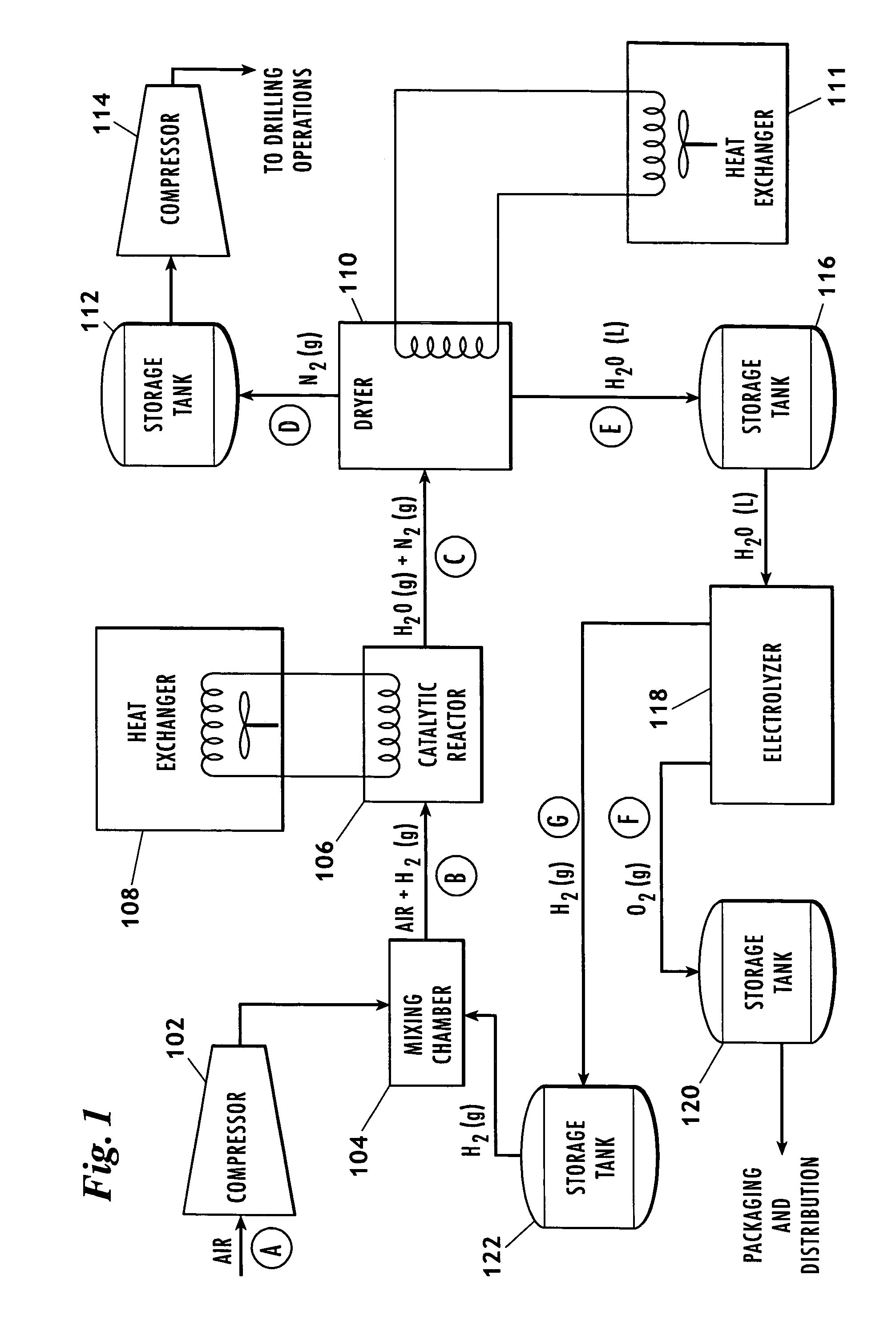
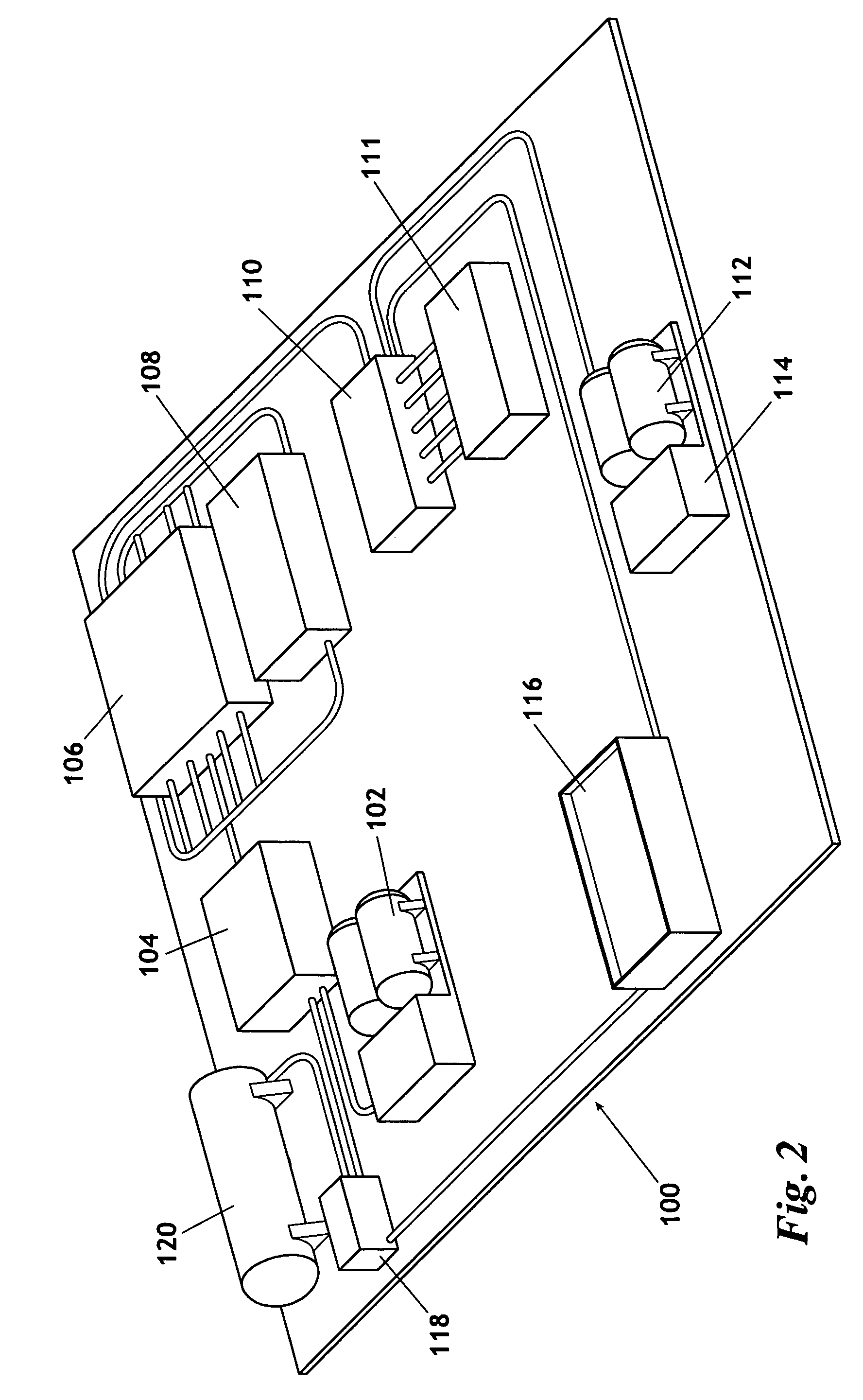
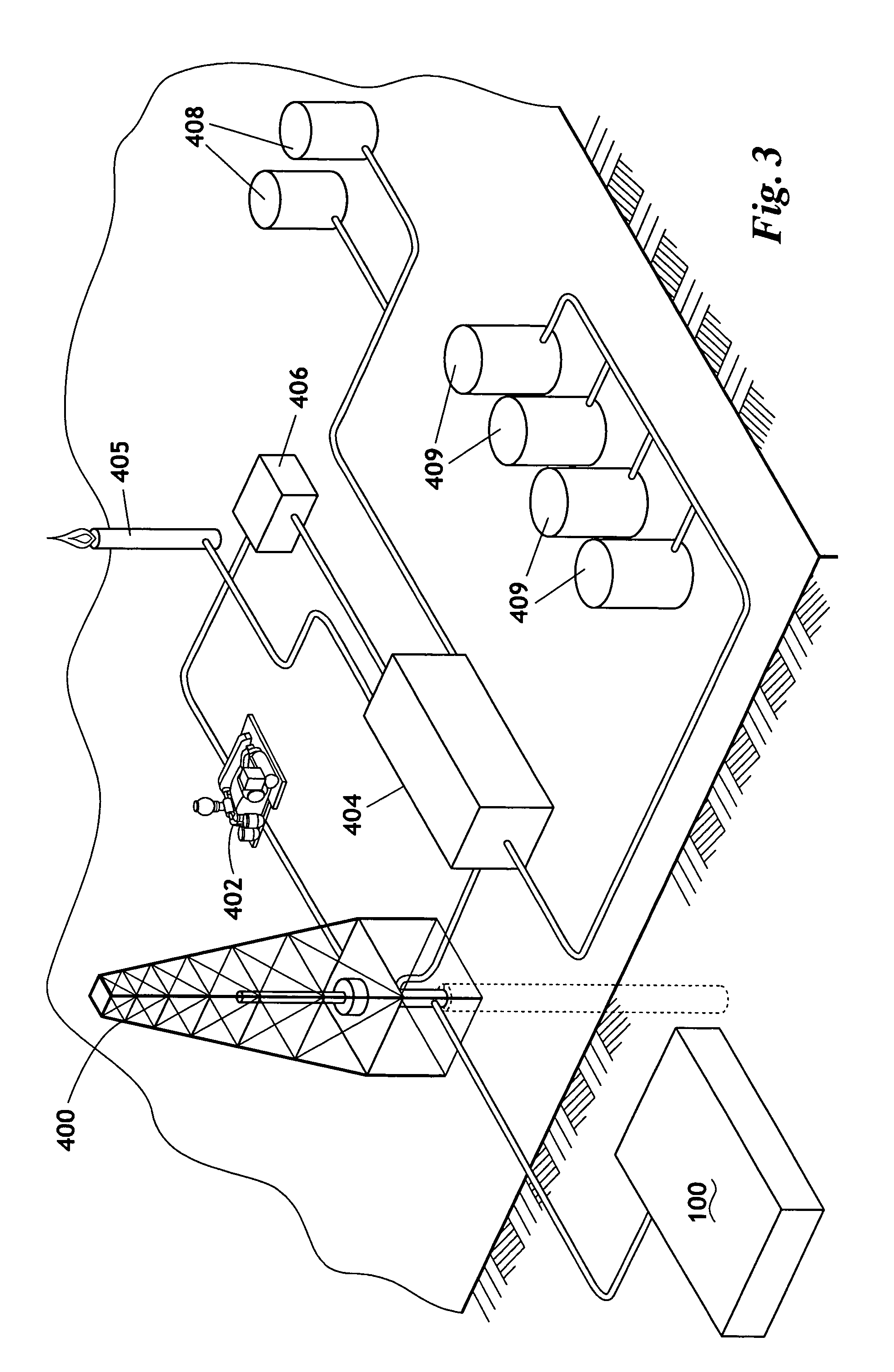
The invention uses a feed of atmospheric air and mixes the air with hydrogen. The hydrogen and air mixture is fed into a catalytic reactor where a deoxygenation reaction occurs. The deoxygenation reaction uses a platinum catalyst to produce water from oxygen and hydrogen. The nitrogen passes through the catalytic reactor without reacting with the hydrogen, the oxygen, or the water. The water is separated from the nitrogen in a dryer. The nitrogen may then be used in drilling and production operations. The water is fed into an electrolyzer where an electrolysis reaction occurs. The electrolyzer passes an electrical current through the water to produce gaseous oxygen and hydrogen. The hydrogen is recycled back to the catalytic reactor and the oxygen may be vented or sold.
Owner:SUNSTONE TECH
Performance stability in shallow beds in pressure swing adsorption systems
Owner:AIR PROD & CHEM INC
Solid state electrochemical composite
InactiveUS20050214612A1Improve power densitySolve the lack of activityFinal product manufactureCell electrodesElectrolysisConductive materials
Provided is a composite electrochemical device fabricated from highly electronically conductive materials such as metals, metal alloys, or electronically conductive ceramics. The electronic conductivity of the electrode substrate is maximized. The invention allows for an electrode with high electronic conductivity and sufficient catalytic activity to achieve high power density in ionic (electrochemical) devices such as fuel cells and electrolytic gas separation systems including oxygen generation system.
Owner:RGT UNIV OF CALIFORNIA
Agglomerated adsorbent, process for the production thereof and use thereof for the non-cryogenic separation of industrial gases
InactiveUS6652626B1Reduce the burden onReduce consumptionIsotope separationHydrogen/synthetic gas productionLithiumIndustrial gas
A description is given of agglomerates of faujasite X with an Si / Al ratio of 1, the inert binder of which, on the one hand, has been converted to active zeolite by conversion to zeolite in an alkaline liquor, and which have been subjected, on the other hand, to an exhaustive lithium exchange. These adsorbents develop a nitrogen adsorption capacity (1 bar / 25° C.) of at least 26 cm<3> / g, which makes them excellent adsorbents for the non-cryogenic separation of gases from air and for the purification of hydrogen.
Owner:ARKEMA FRANCE SA
Solar water evaporation purification and decomposition device
ActiveCN107879405AAchieve fadeAchieve purificationGeneral water supply conservationSeawater treatmentWater desalinationEvaporation Purification
The invention discloses a solar water evaporation purification and decomposition device which comprises a water supply line, a float and heat insulation layer, an evaporation layer and a photothermalconversion and solute barrier layer which are connected in sequence from bottom to top. The water supply line enables water to pass through the float and heat insulation layer, and the water is pumpedinto the evaporation layer by virtue of a capillary action; solar energy absorbed by the photothermal conversion and solute barrier layer is converted into heat, and water is heated to be vaporized into water vapor from the evaporation layer; due to pore channel volatilization of the photothermal conversion and solute barrier layer, partial water vapor is decomposed to produce hydrogen and oxygen; since the photothermal conversion and solute barrier layer contains a hydrophobic part and is not infiltrated by water, the solar energy can directly heat a heat-absorbing material only, but not heat water, so that high photothermal conversion efficiency is realized; and meanwhile, the solute in the water is prevented from being separated out on the membrane, and sea water desalination, sewage purification and water decomposition can be realized. Due to the design of a hydrophilic / hydrophobic double-layer evaporation structure, the solar water evaporation purification and decomposition device with high photothermal conversion efficiency and high stability is obtained.
Owner:XI AN JIAOTONG UNIV
Axial flow catalyst pack
InactiveUS6746651B1Promote uniform mixingEffective contactHydrogen productionChemical/physical/physico-chemical stationary reactorsCatalytic decompositionEngineering
A stack of very thin metal plates provides high surface area for catalytic decomposition of a fluid flowing axially from upstream to downstream through the stack. Each plate has flow-through passages of selected size and location to promote uniform flow and good surface contact with a catalyst surface on the plates. The downstream surface of each plate is etched to provide gaps between plates for lateral fluid flow. The plates are divided into groups separated by metering plates that promote more uniform flow from group to group.
Owner:AEROJET ROCKETDYNE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com