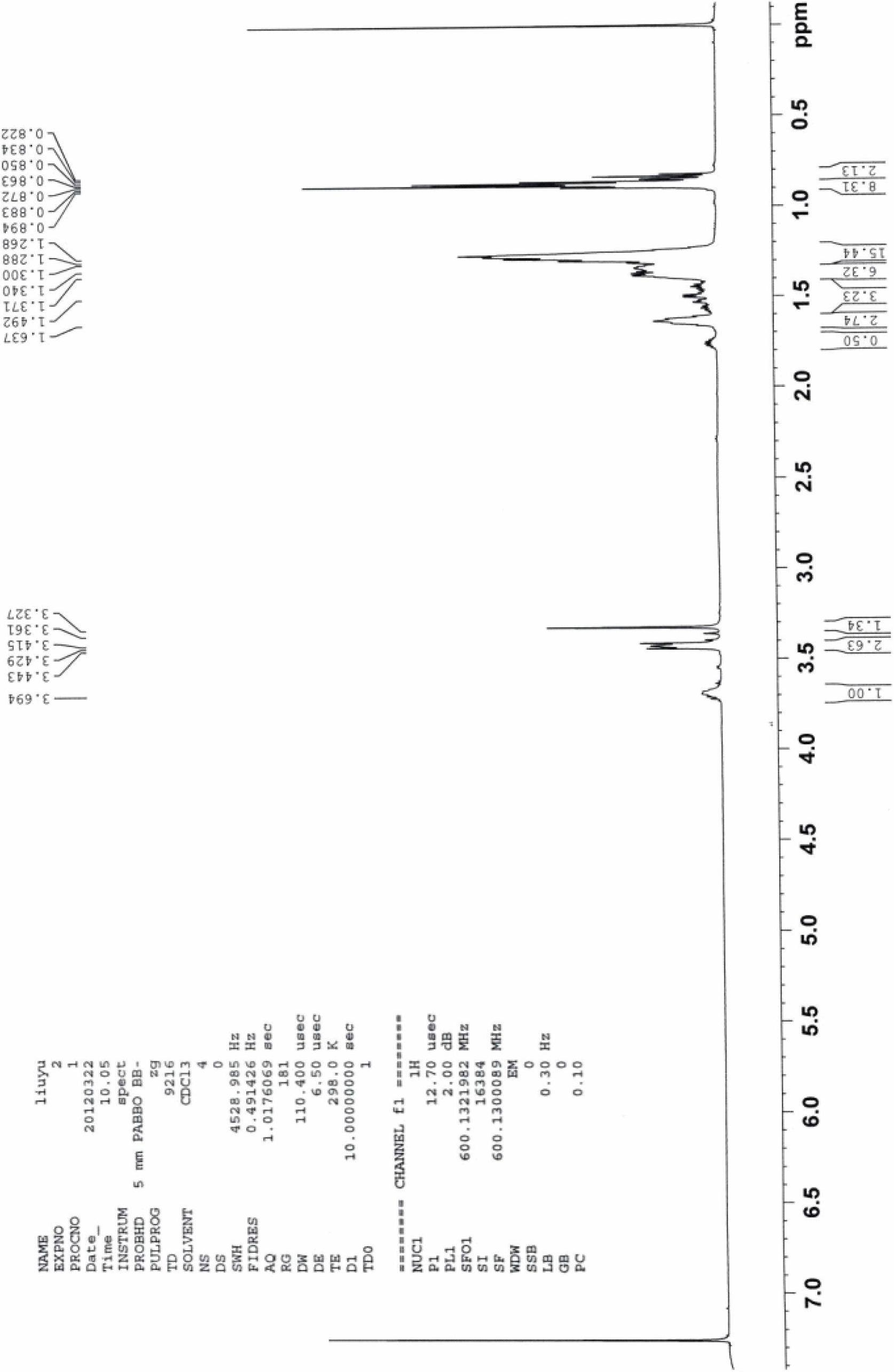
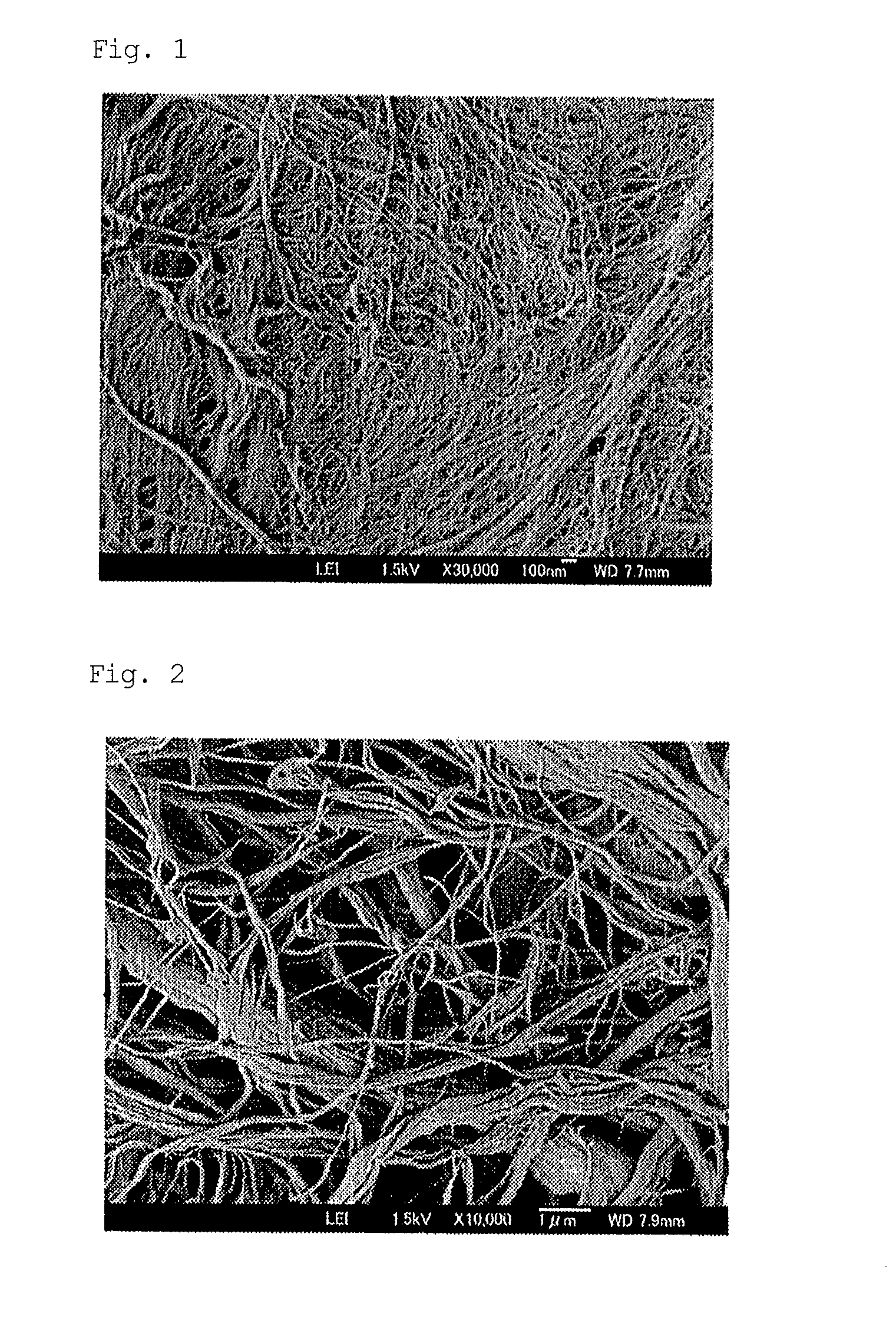
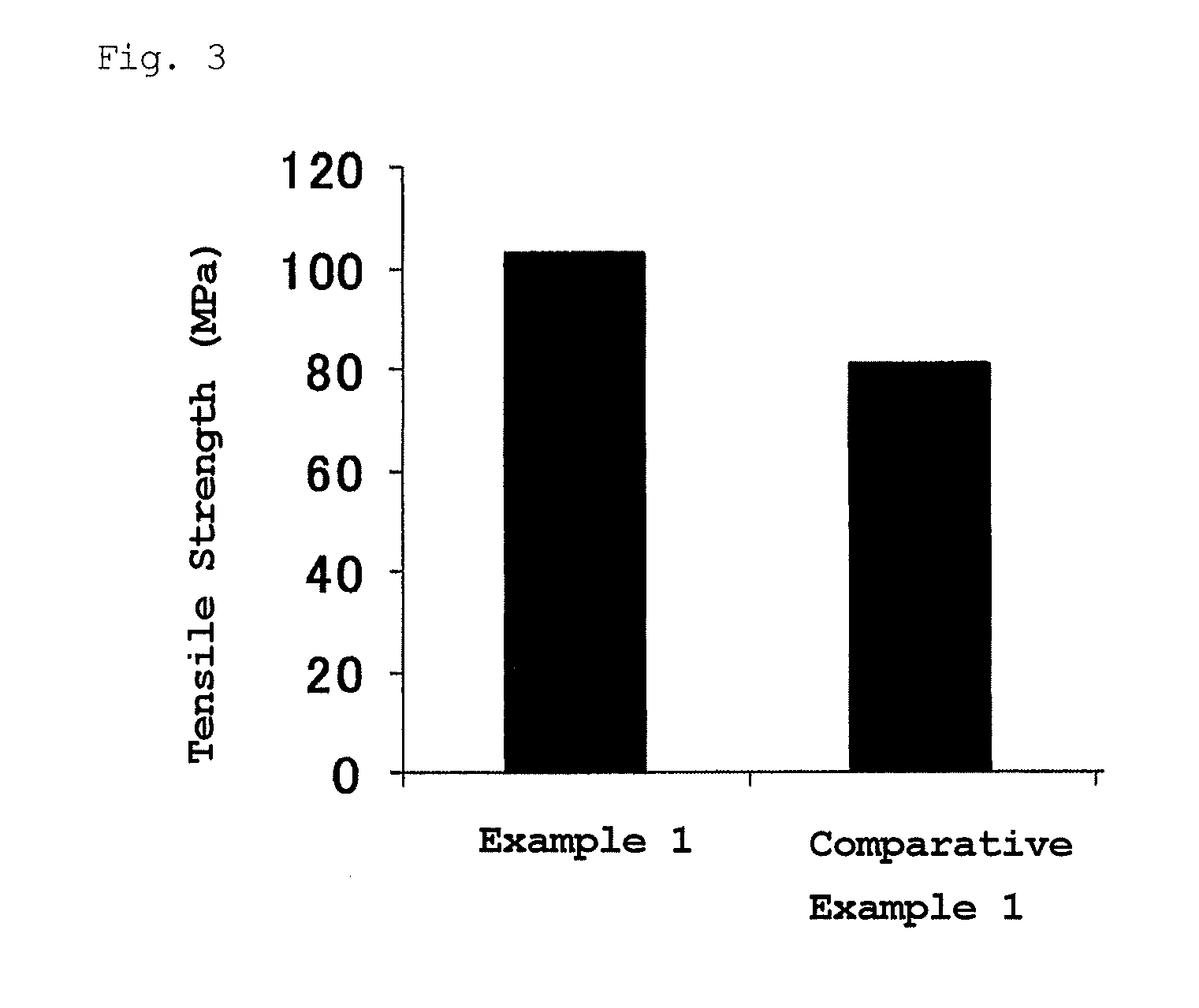
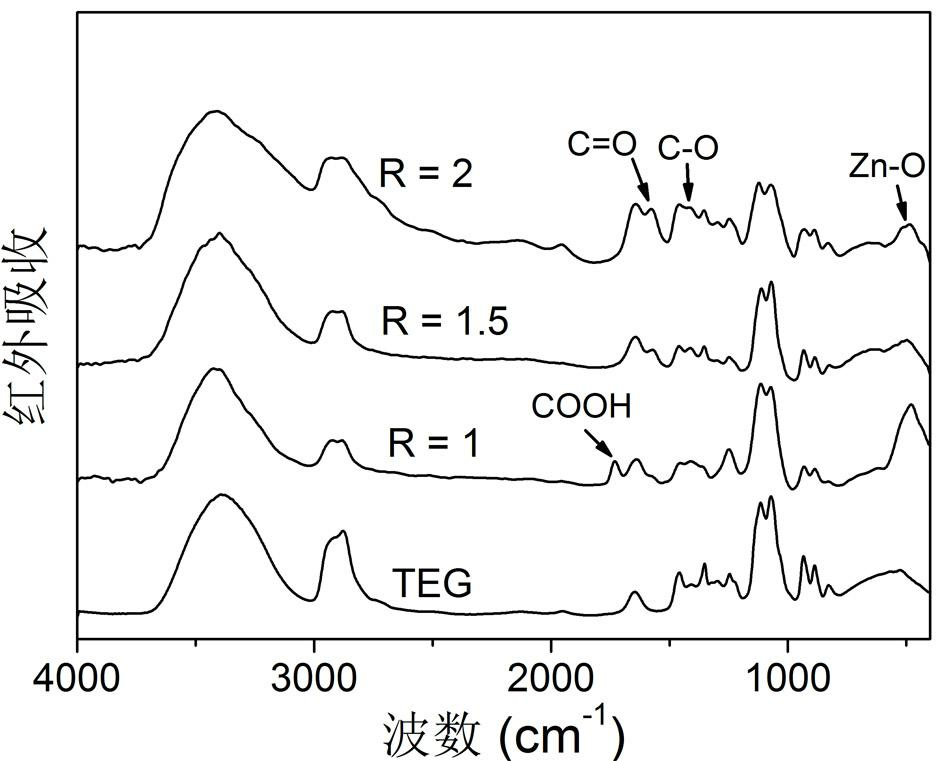
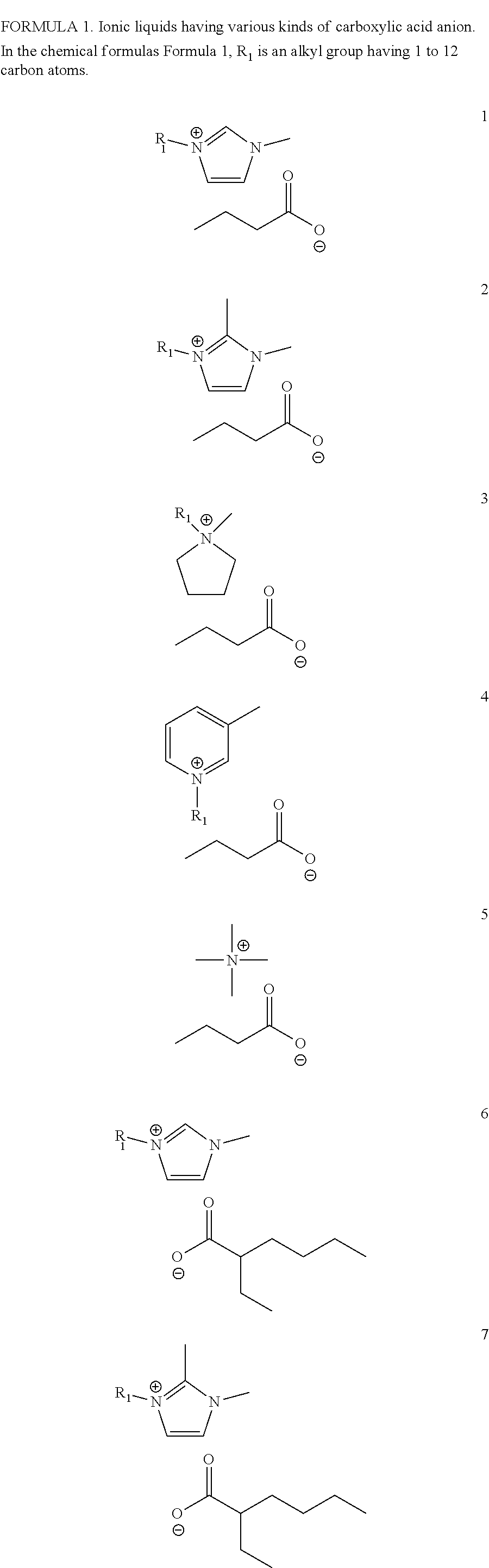
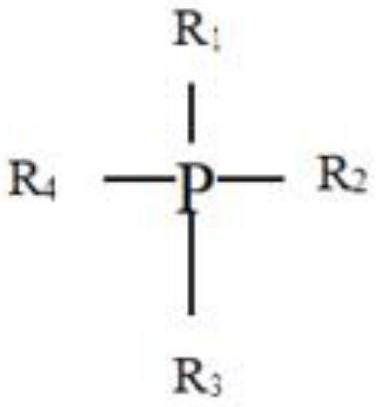
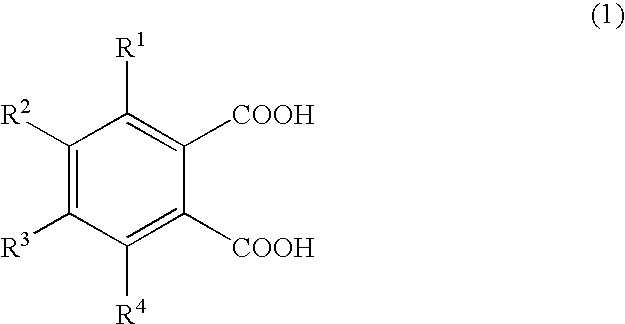
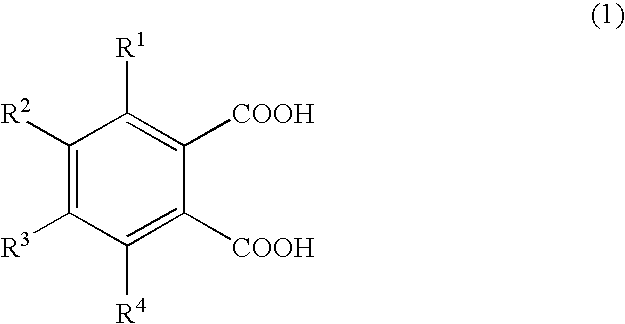
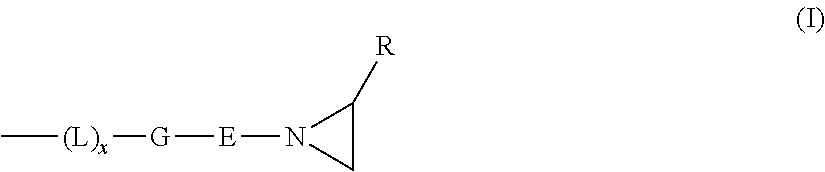Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
73 results about "Carboxylic acid anion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
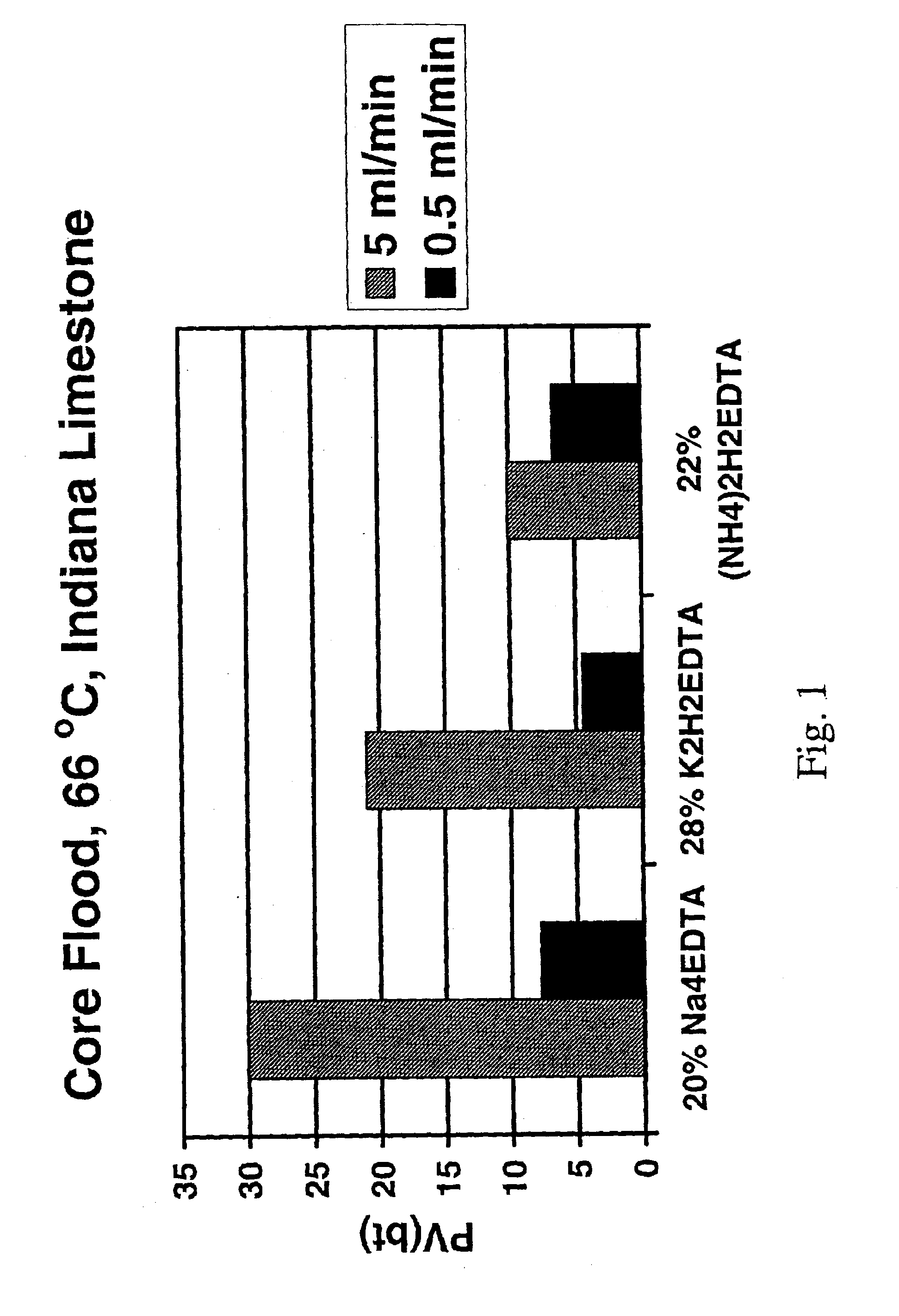
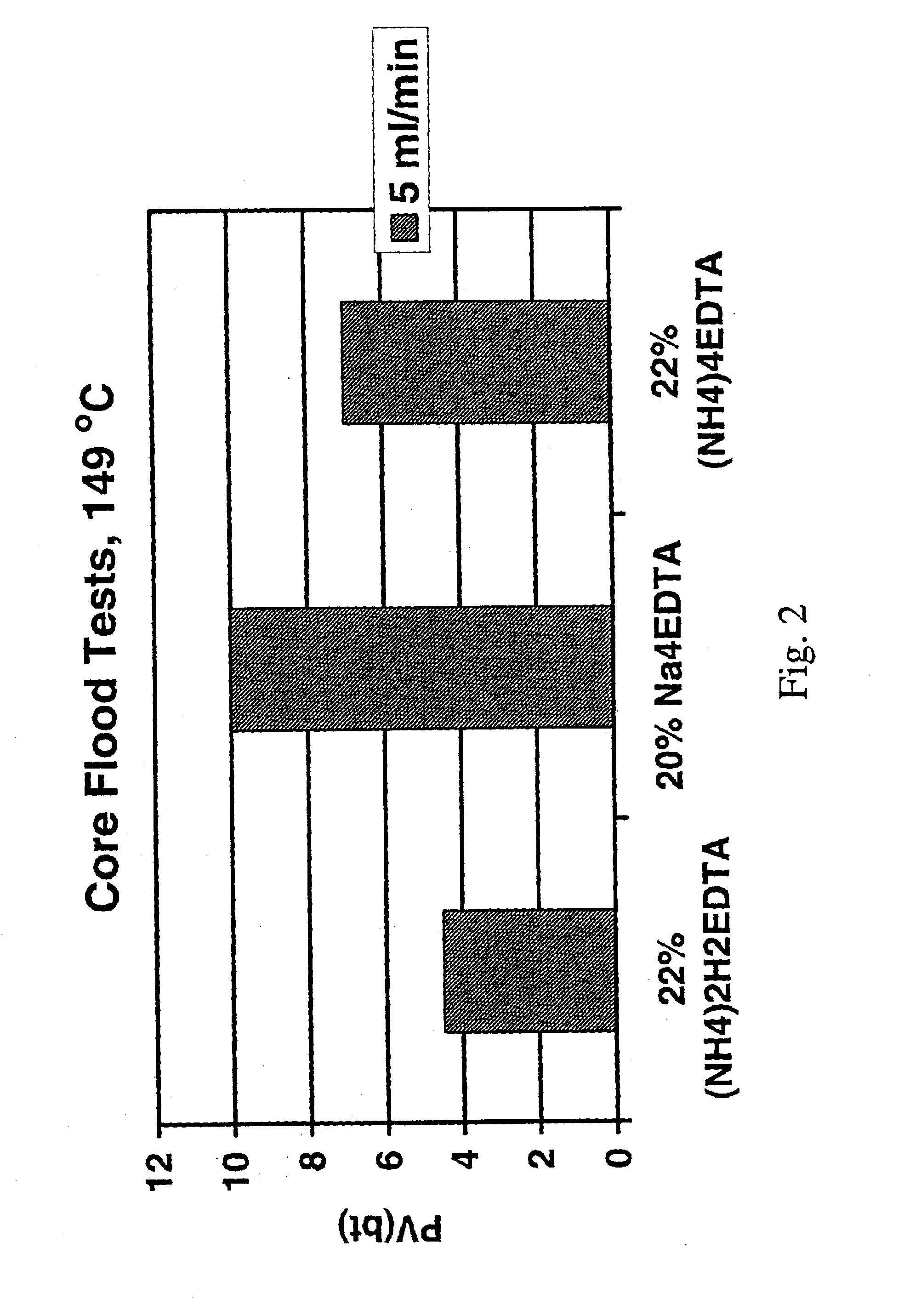
Method for treating a subterranean formation
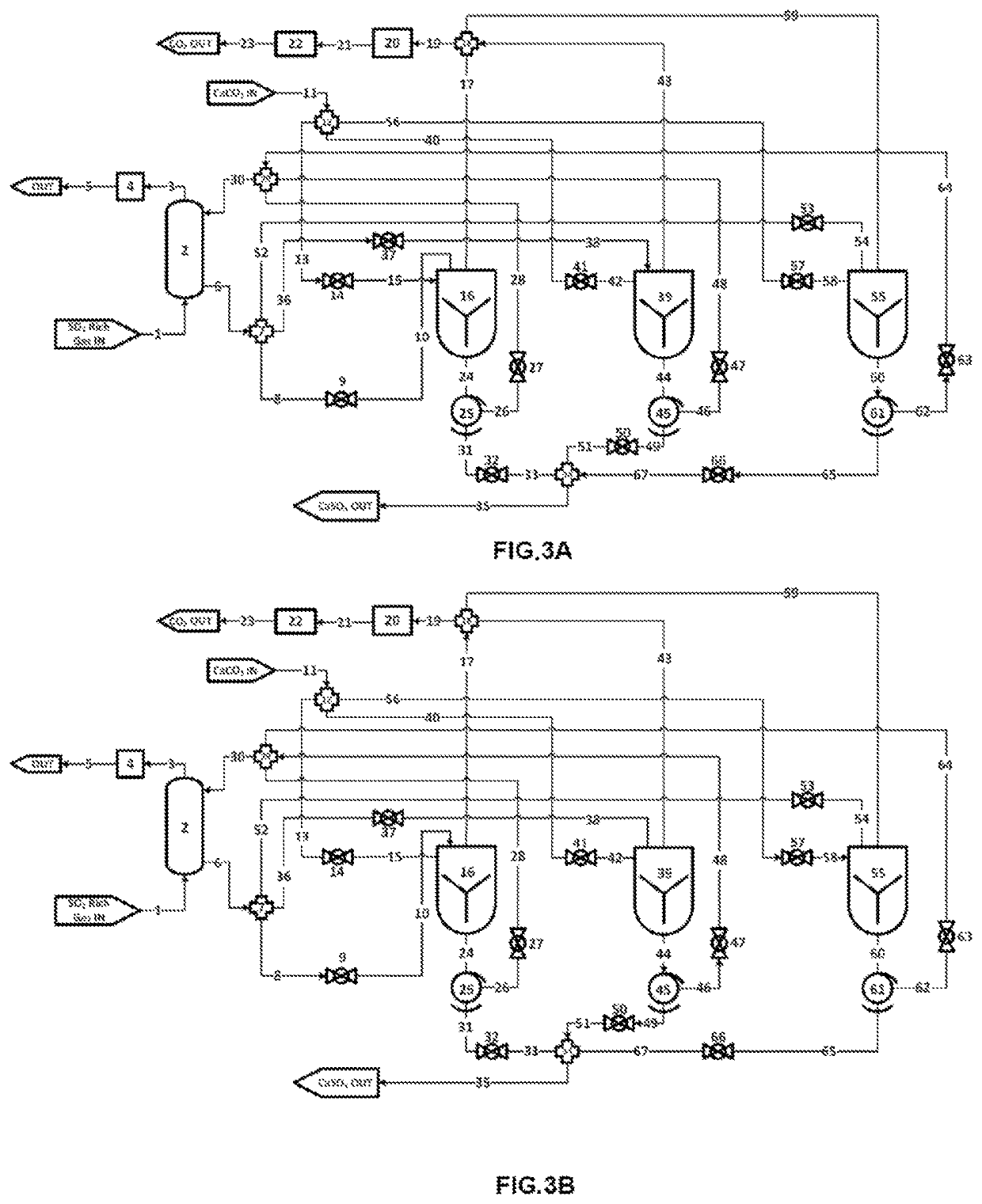
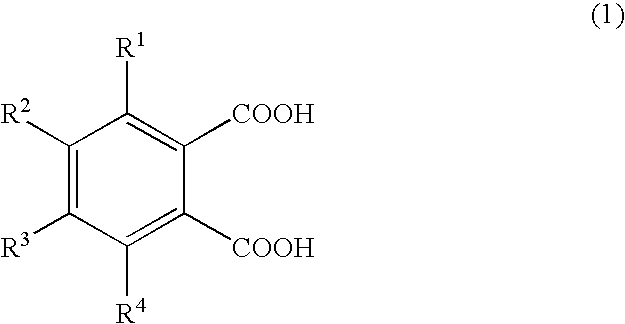
InactiveUS6911418B2Improve breathabilityMinimized volumeOther chemical processesCleaning apparatusFormation matrixCarboxylic acid anion
A method for increasing the permeability of a subterranean formation is disclosed in which an amount of an aqueous mixture, sufficient to increase the permeability of the formation, comprising specified aminopolycarboxylic anionic species, and specified cationic species, is injected into the formation matrix.
Owner:SCHLUMBERGER TECH CORP
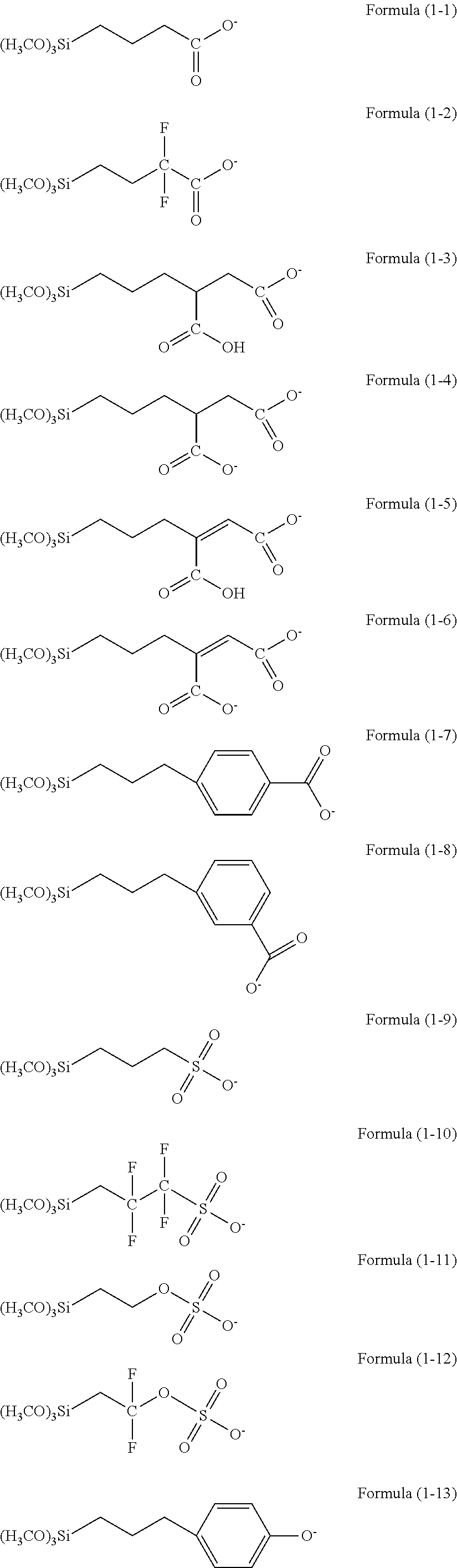
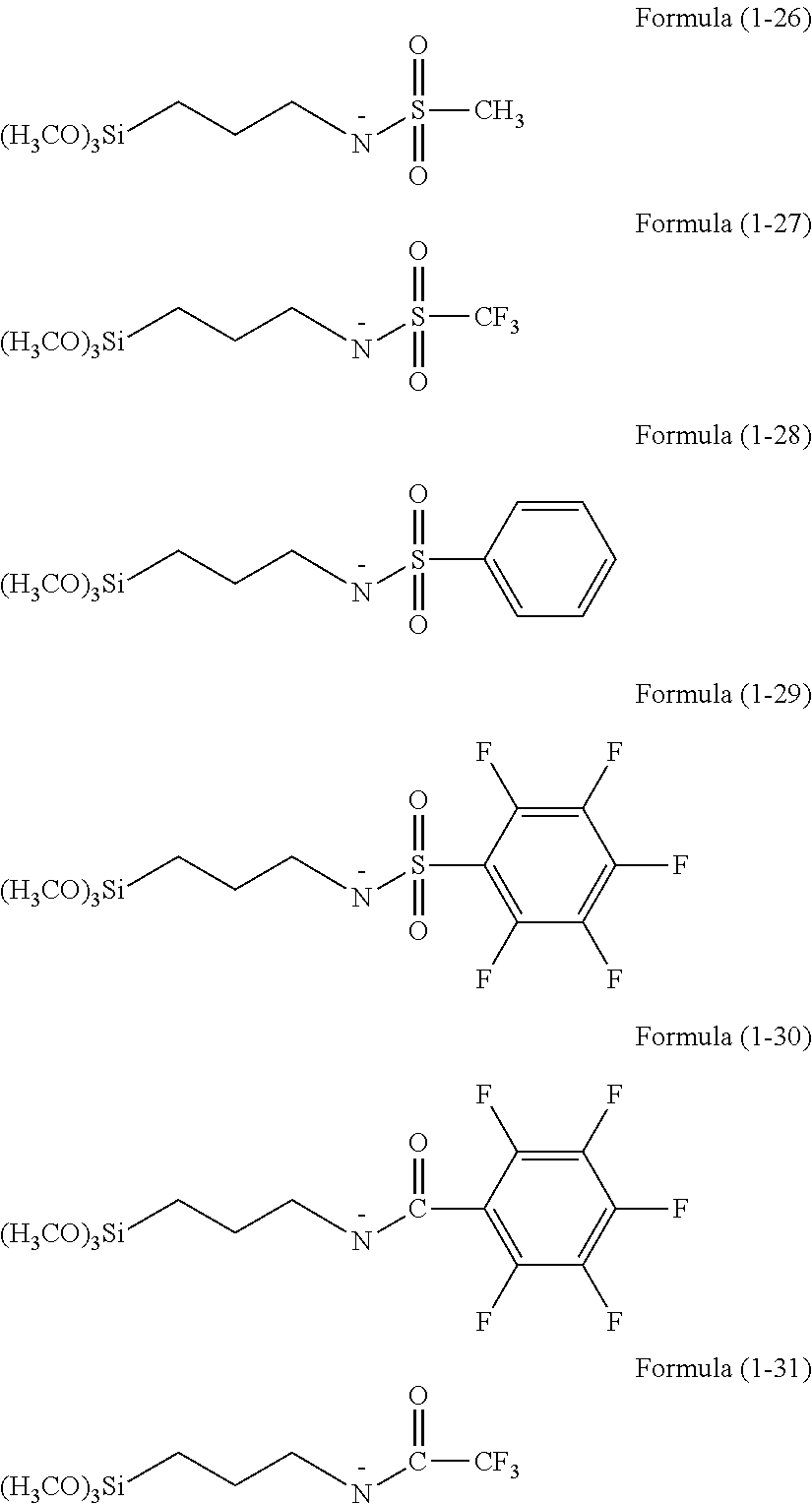
Resist underlayer film forming composition containing silicon having anion group
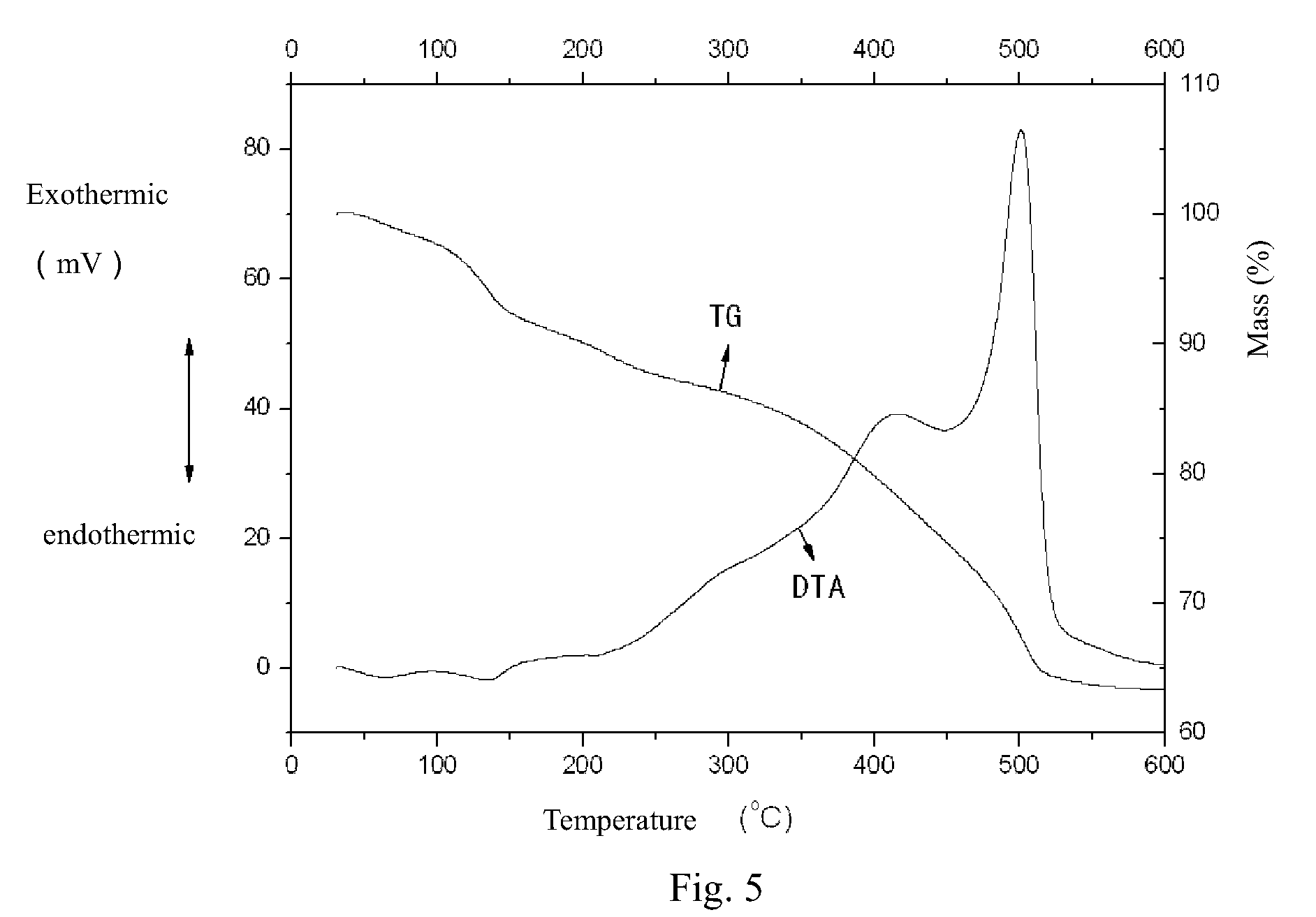
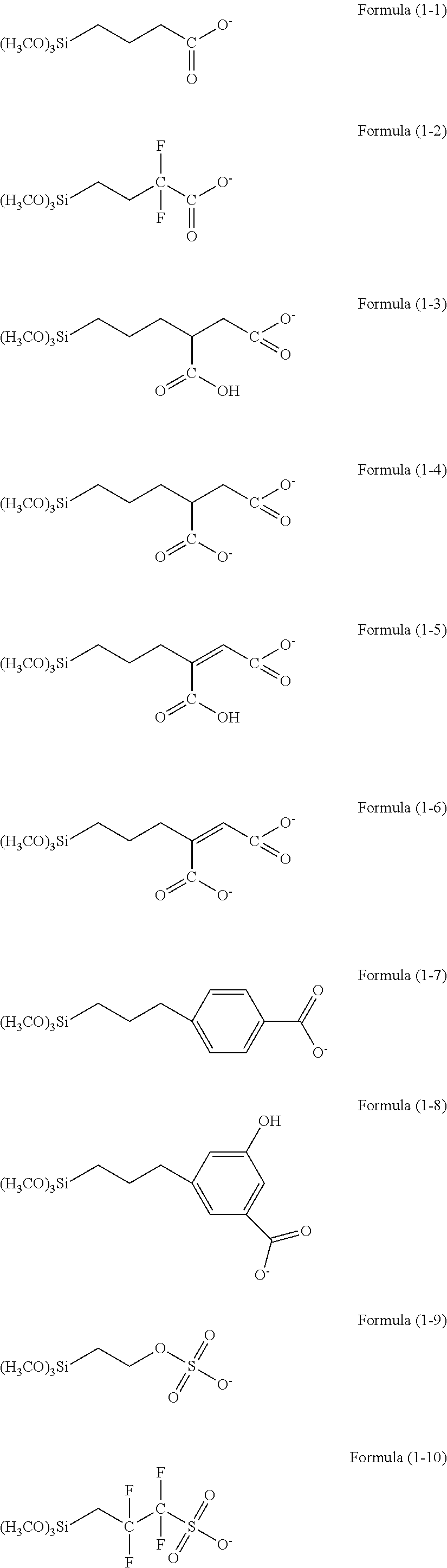
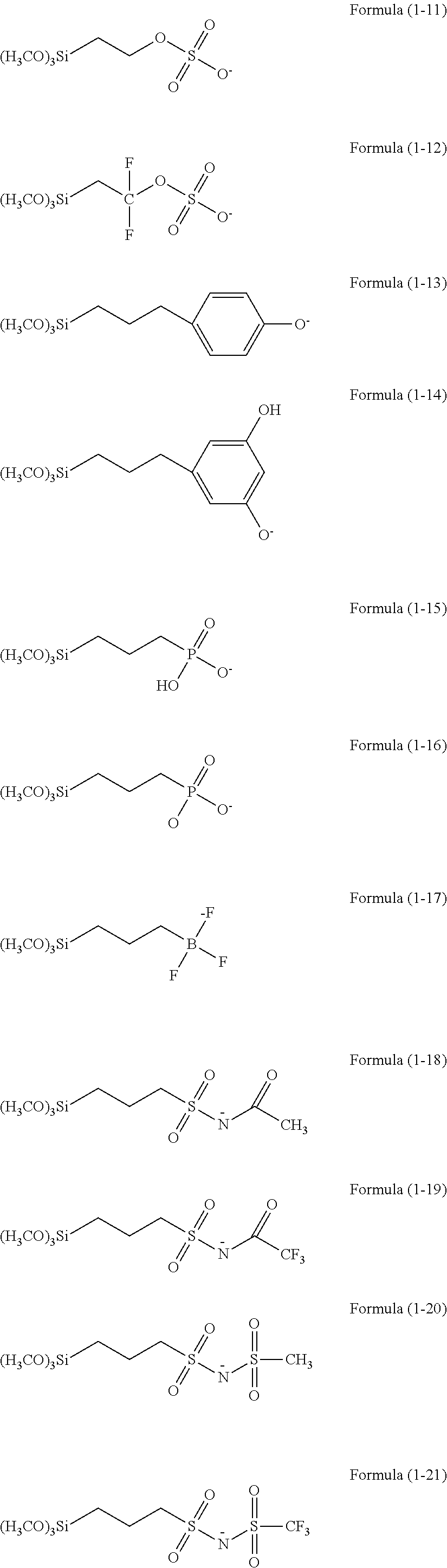
There is provided a resist underlayer film forming composition for lithography for forming a resist underlayer film capable of being used as a hardmask. A resist underlayer film forming composition for lithography comprising a silane compound containing an anion group, wherein the silane compound containing an anion group is a hydrolyzable organosilane in which an organic group containing an anion group is bonded to a silicon atom and the anion group forms a salt structure, a hydrolysis product thereof, or a hydrolysis-condensation product thereof. The anion group may be a carboxylic acid anion, a phenolate anion, a sulfonic acid anion, or a phosphonic acid anion. The hydrolyzable organosilane may be a compound of Formula (1): R1aR2bSi(R3)4−(a+b) (1). A composition comprising a mixture of a hydrolyzable organosilane of Formula (1), and at least one organic silicon compound selected from the group consisting of a compound of Formula (2): R4aSi(R5)4−a (2) and a compound of Formula (3): [R6cSi(R7)3−c]2Yb (3); a hydrolysis product of the mixture; or a hydrolysis-condensation product of the mixture.
Owner:NISSAN CHEM IND LTD
Hybrid ionic liquid hydrate, preparation process and application thereof
InactiveCN101693692ALow viscosityMass transfer rate is fastOrganic chemistryOther chemical processesProtonationWastewater
Hybrid ionic liquid hydrate consists of two types of positive ions containing nitrogen and binary or polybasic carboxylate negative ions which are different in structure and property. One type of positive ions includes protonated alkylamine positive ions, and another type of positive ions inlcudes N,N-dialkyl imidazole positive ions or N, N-dialkyl pyrrole positive ions or N-alkyl pyridines positive ions or N, N, N, N-tetraalkyl ammonium positive ions. Positive ions at one end of binary acid or polybasic acid of the hybrid ionic liquid hydrate have faintly acid while positive ions at the other end are neutral, and binary or polybasic carboxylic acid negative ions have alkalescence, thereby enabling the hybrid ionic liquid hydrate to have ultra-strong pH buffer capacity and to be capable of reversibly absorbing or adsorbing SO2 gas in an environment-friendly high-efficient circulating form. The hybrid ionic liquid hydrate which is utilized as SO2 absorbent is simple in requirements to equipment for absorbing SO2, moderate in operation condition of absorbing and desorbing SO2 and high in desulfuration efficiency without problems of waste liquid and waste water discharge and the like. Further, the invention discloses a process for preparing the hybrid ionic liquid hydrate.
Owner:NANJING UNIV
Preparation method of quaternary ammonium ionic liquid
InactiveCN102659607AImprove conversion rateEasy to purifyAmino preparation from aminesGroup 5/15 element organic compoundsChemical synthesisQuaternary ammonium cation
The invention relates to a preparation method of quaternary ammonium ionic liquid, belongs to the field of chemical synthesis and solves the problems of complicated process and long reaction period in the conventional preparation method of the quaternary ammonium ionic liquid. The method comprises the following steps: reacting quaternary ammonium salt with alkali solution, generating anion exchange reaction to generate quaternary ammonium base and generating acid and alkali neutralization reaction with corresponding phosphonic acid anions or carboxylic acid anions to generate the quaternary ammonium ionic liquid. The method has the advantages of simple preparation process, short time, high product conversion rate and easiness in purification, an alcohol solvent can be recycled through distillation, extraction and other methods. The preparation method of the ionic liquid is wide in applicable scope. Compared with the prior art, the preparation method has briefer preparation steps, and the large-scale production is easy.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Molding material and manufacturing method therefor
ActiveUS20130005866A1Easily defibratedHigh tensile strengthNon-woody plant/crop pulpPolyester coatingsFiberHalogen
The present invention relates to anionically modified microfibrillated plant fibers used for obtaining a thermosetting resin molding material having excellent mechanical strength, a method for manufacturing the same, a molding material containing the anionically modified microfibrillated plant fibers and a thermosetting resin, and a method for manufacturing the same. Specifically, the present invention provides a molding material containing anionically modified microfibrillated plant fibers that are anionically modified in the presence of a base by a carboxylic acid represented by formula (I): X—(CH2)n—COOH (I), wherein X represents halogen and n is 1 or 2, and / or by a salt thereof, and a thermosetting resin, and the molding material contains the anionically modified microfibrillated plant fibers in an amount of 10 to 900 parts by weight per 100 parts by weight of the thermosetting resin.
Owner:NIPPON PAPER IND CO LTD +2
ZnO luminous nanoparticles synthesized in polyethylene glycol and preparation method thereof
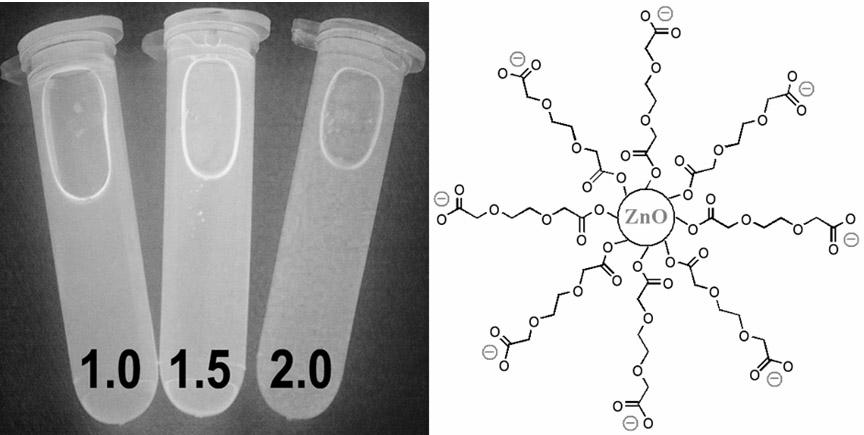
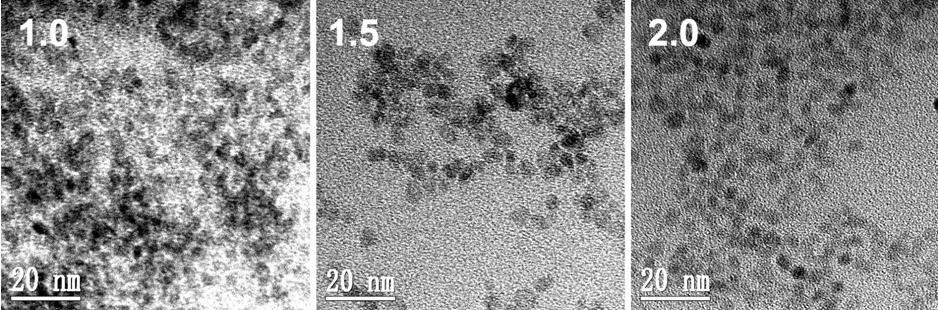
InactiveCN102321469AComposition is stableStable structureLuminescent compositionsNon solventPolyethylene glycol
The invention belongs to the technical field of nano-materials, and particularly relates to ZnO luminous nanoparticles synthesized in polyethylene glycol and a preparation method thereof. The nanoparticles consist of ZnO monocrystal particles and external polymer radicals, and have high stability and photoluminescence performance. The preparation method comprises the following steps of: dissolving zinc acetate and basic hydroxide in a solvent together with stirring; and reacting at the room temperature for a period of time. The ZnO luminous nanoparticles can be purified by adopting a non-solvent precipitation method. In the stirring reaction process, a reaction mixture is fully contacted with the air, polyethylene glycol is oxidized by the air to form a carboxylic acid anion structure under the alkali catalyzing condition, and carboxylic acid anions can be coordinated onto the surfaces of the ZnO nanoparticles to make the ZnO nanoparticles illuminate stably. The preparation method has the advantages of low cost, environmental friendliness and high yield.
Owner:FUDAN UNIV
Benzocarbazole-intercalated layered double hydroxides composite luminescent material and its preparation method
InactiveUS20100130750A1Reducing fluorescence quenchingGroup 8/9/10/18 element organic compoundsLuminescent compositionsWater bathsSlurry
The present invention discloses a benzocarbazole-intercalated layered double hydroxides (LDHs) composite luminescent material and its preparation method. The detailed procedure comprises preparing divalent and trivalent metal cation solution A and glycol solution B of sodium 2-hydroxy benzo[a]carbazole-3-carboxylate, mixing the solutions A and B to obtain solution C, slowly adding the prepared NaOH solution dropwise into the solution C, regulating pH of the resultant after dropwise addition to obtain slurry D, allowing the slurry D to react under water bath or microwave temperature-controlled heating condition, centrifuging and washing the obtained product, and drying in vacuum to obtain 2-hydroxy benzo[a]carbazole-3-carboxylate anion intercalated LDHs composite material. The method implements the immobilization of sodium 2-hydroxy benzo[a]carbazole-3-carboxylate, effectively improves thermal stability of the luminescent dye molecules, and reduces fluorescence quenching caused by aggregation of the dye molecules.
Owner:BEIJING UNIV OF CHEM TECH
Resist underlayer film forming composition containing silicon having anion group
ActiveUS20130078814A1Large dry etching rateSemiconductor/solid-state device manufacturingCoatingsResistSilane compounds
There is provided a method of making a semiconductor device utilizing a resist underlayer film forming composition comprising a silane compound containing an anion group, wherein the silane compound containing an anion group is a hydrolyzable organosilane in which an organic group containing an anion group is bonded to a silicon atom and the anion group forms a salt structure, a hydrolysis product thereof, or a hydrolysis-condensation product thereof. The anion group may be a carboxylic acid anion, a phenolate anion, a sulfonic acid anion, or a phosphonic acid anion. The hydrolyzable organosilane may be a compound of Formula (1): R1aR2bSi(R3)4−(a+b)(1).
Owner:NISSAN CHEM IND LTD
Carbon nano tube with high water-solubility and preparation method thereof
InactiveCN101037198AEasy to removeHigh purityNanostructure manufactureDispersion stabilitySolubility
The present invention discloses a high water soluble carbon nanotube and a preparation method of the same. In the present invention, the carbon nanotube experiences noncovalent bond combinative coating modification by using the pi-pi conjugation between the hydrolysis styrene-maleic anhydride copolymer and the carbon nanotube; the dispersion stability of the carbon nanotube in water is improved by using the carboxylic acid anion with good water solubility of the copolymers; so that the water solubility of the carbon nanotube is greatly improved. The water solubility carbon nanotube coated by hydrolysis styrene-maleic anhydride copolymer is obtained by using hydrolysis styrene-maleic anhydride copolymer, carbon nanotube and water as raw material, adopting a circular fashion to inject hydrolysis styrene-maleic anhydride copolymers, and using an ultra sonic dispersion method and centrifugal method. The present invention has the advantages of that the technology is simple, the obtained water solubility carbon nanotube has a solubility of 29.2 mg / ml and an excellent stability, and the styrene-maleic anhydride copolymer has a simple preparation process and a lower cost, so that the large scale preparation and the industrial application of the water solubility carbon nanotube can be greatly improved.
Owner:ZHEJIANG UNIV
Highly connected coordination polymer and preparation method thereof
InactiveCN104804024AHigh purityIncrease the number of connectionsGroup 2/12 organic compounds without C-metal linkagesLuminescent compositionsMetal clustersPolymer science
The invention discloses a highly connected coordination polymer and a preparation method thereof. The highly connected coordination polymer adopts the following chemical formula: [Zn5(3,3'-tmbpt)(btec)2(OH)2], wherein betc is deprotonated 1,2,4,5-pyromellitic acid; 3,3'-tmbpt is 1-((1H-1,2,4-triazole)methyl)-3,5-bis(3-pyridyl)-1,2,4-triazole. Carboxyl groups of polycarboxylic acid anions can coordinate with metal ions to form metal clusters with larger size; the metal clusters serve as nodes, so that connections of the nodes can be greatly increased. In addition, distances among several coordinating groups of a polydentate nitrogen-containing ligand 3,3'-tmbpt is relatively large, so that steric hindrance is relatively small during coordination, and the metal clusters can further expand easily to form the coordination polymer adopting highly connected structure. The coordination polymer can serve as a broadband semiconductor material and a molecular-based light emitting material to be further developed and applied.
Owner:HUAIYIN TEACHERS COLLEGE
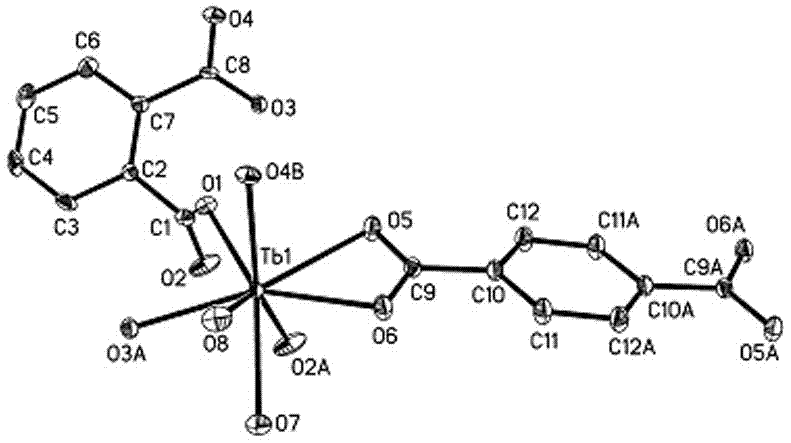
Terbium complex green fluorescent material and preparation method thereof
ActiveCN108003867AImprove luminosityGood photoluminescent propertiesLuminescent compositionsPhotoluminescenceUltraviolet lights
The invention discloses a terbium complex green fluorescent luminous material. The complex luminous material is a three-dimensional coordination polymer containing two kinds of organic polycarboxylicacid anion ligands, and the general formula of the material is [Tb(1,4-bdc)0.5(1,2-bdc)(H2O)2]n, wherein 1,4-bdc represents terephthalic acid radical divalent anions, 1,2-bdc represents phthalic acidradical divalent anions, and n is a natural number greater than 100. According to the terbium complex green fluorescent luminous material, two kinds of benzene-ring containing organic dicarboxylic acid including terephthalic acid and phthalic acid are adopted as the ligands and react with terbium perchlorate under hydrothermal conditions, a terbium complex containing the two kinds of ligands is prepared and emits green fluorescence under the excitation of 250-350nm ultraviolet light, the luminescence belongs to the characteristic fluorescence of trivalent terbium ions, and under the excitation of 300nm ultraviolet light, the luminescence quantum efficiency reaches 56%, which indicates that the complex has good photoluminescence properties. Meanwhile, the complex does not decompose at thetemperature of 300 DEG C or below, which indicates that the complex has good thermal stability.
Owner:XUCHANG UNIV
Two-part foam hair dye
ActiveUS20130125918A1Apply evenlyImprove dyeing effectCosmetic preparationsHair cosmeticsBenzeneMass ratio
A two-part foam hair dye which comprises a first part comprising an alkali agent, a second part comprising hydrogen peroxide, and a non-aerosol foamer container, wherein the first part comprises the components (A) to (D), an equivalent ratio of a anion site of the component (A) to a cation site of the component (B) (anion / cation) is more than 1, a mass ratio of a content of the component (C) to the content of the component (D) ((C) / (D)) is 5 or less, and the liquid mixture has a viscosity (25° C.) of 1 to 300 mPa·s:(A) a carboxylic acid anionic surfactant,(B) a polymer or copolymer having a mole fraction of diallyldimethyl quaternary ammonium salt monomer of 70% or more,(C) 0.5 to 1.5% by mass of an oxidation dye having a meta-dihydroxybenzene structure, and(D) 0.1 to 9% by mass of polypropyleneglycol of Mw 200 to 1200.
Owner:KAO CORP
Purification of organic acid using anion exchange chromatography
InactiveCN1292023AAdvantages VersatilityCost-effectiveFatty oils/acids recovery from wasteFatty acids production/refiningOrganic acidWaste stream
Disclosed is a cost-effective method for purifying and acidifying carboxylic acids, including organic acids and amino acids. The method involves removing impurities by allowing the anionic form of the carboxylic acid to bind to an anion exchange column and washing the column. The carboxylic anion is displaced as carboxylic acid by washing the resin with a strong inorganic anion. This method is effective in removing organic carboxylic acids and amino acids from a variety of industrial sources, including fermentation broths, hydrolysates, and waste streams.
Owner:MICHIGAN BIOTECH INST
Processes for the production of citric acid
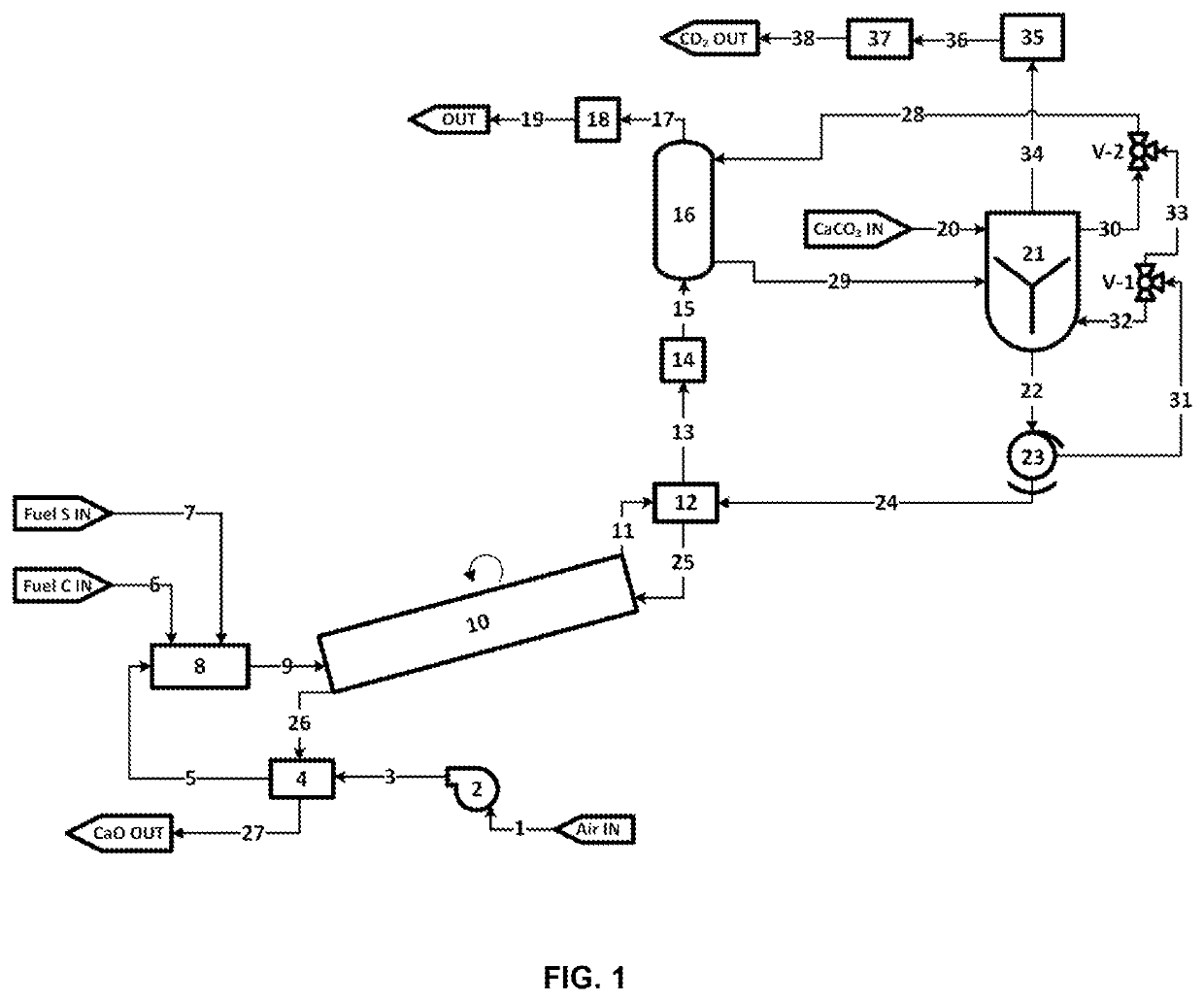
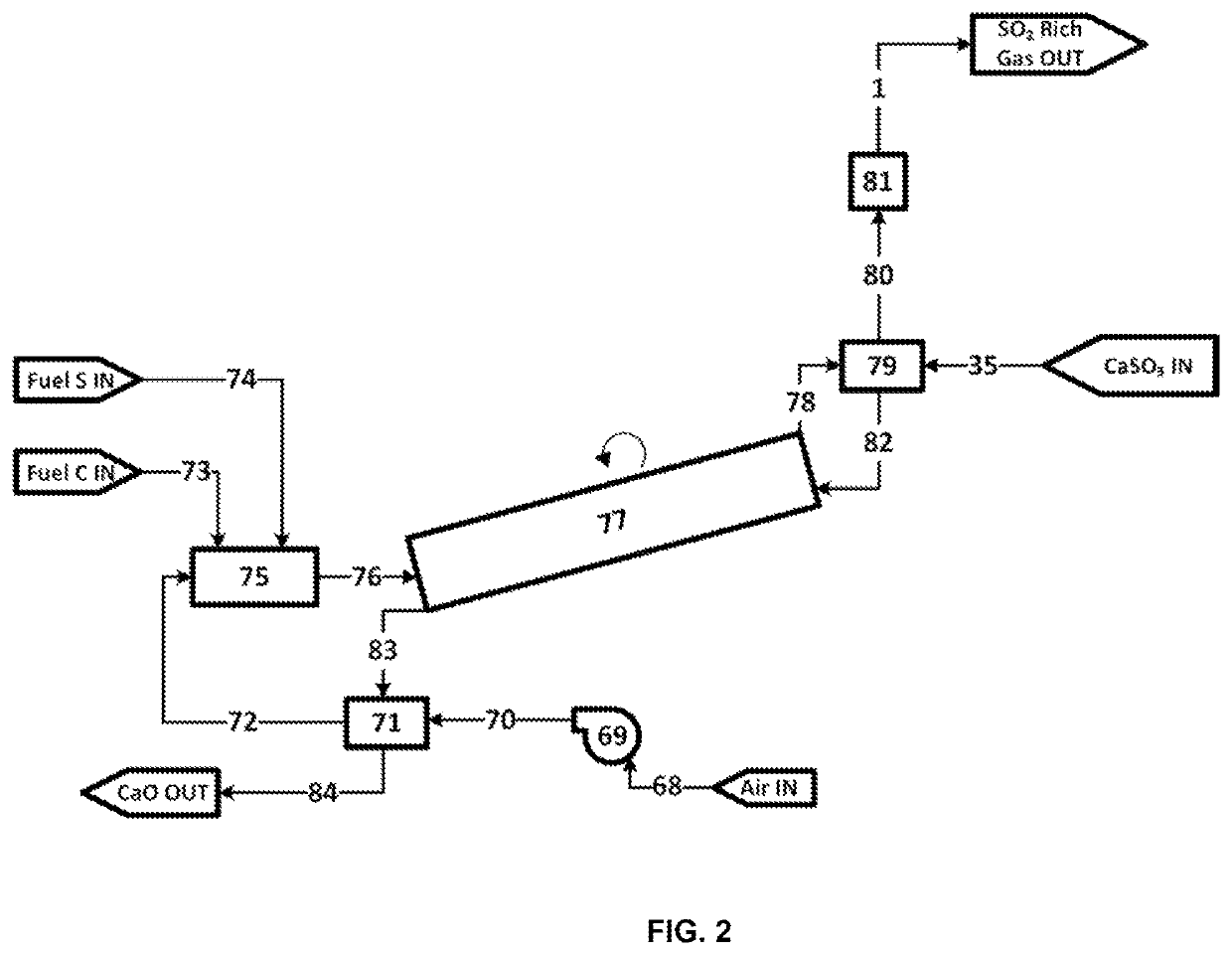
ActiveUS11236033B2High operating temperaturePrevent oxidationPreparation from carboxylic acid saltsCarbon compoundsAlkaline earth metalCarboxylic acid anion
Owner:INNOVATOR ENERGY LLC
Preparing method of titanium salt surface conditioning agent
InactiveCN105483666AImprove dispersion stabilityFor long-term storageMetallic material coating processesSodium bicarbonatePyrophosphate
The invention discloses a preparing method of a titanium salt surface conditioning agent. The preparing method comprises the following steps that firstly, 30 parts of anhydrous disodium hydrogen phosphate is prepared into a water solution with the concentration of 50%; secondly, 1-2 parts of sodium pyrophosphate, 0.8 part of titanyl sulfate, 3.5 parts of sodium pyrophosphate, 2.1 parts of sodium bicarbonate, 1.8 parts of sodium tetraborate, 3.6 parts of sodium hexametaphosphate, 0.3 part of polycarboxylic acid anion organic matter and 0.3 part of N-butyl naphthalene sulfonate are sequentially added in the water solution of disodium hydrogen phosphate, and a mixed solution is obtained; thirdly, the mixed solution is heated to 70 DEG C and fully stirred, and white powder with the water content below 2% is obtained; and fourthly, powder continues to be stirred at the temperature of 75 DEG C, 2.7 parts of sodium pyrophosphate, 1.2 parts of acetic acid, 0.7 part of methyltrimethoxysilane and 3.3 parts of magnesium sulfate are added into the powder at the same time, stirring continues, and drying is carried out till the water content is smaller than 1%. The prepared surface conditioning agent is good in scattering stability and can be stored for a long term.
Owner:常熟市虞东磷化材料有限公司
Oil-water separation method based on hydrophilic oleophobic fabric
ActiveCN112169376ASimple processMild operating conditionsGeneral water supply conservationLiquid separationFiberPolymer science
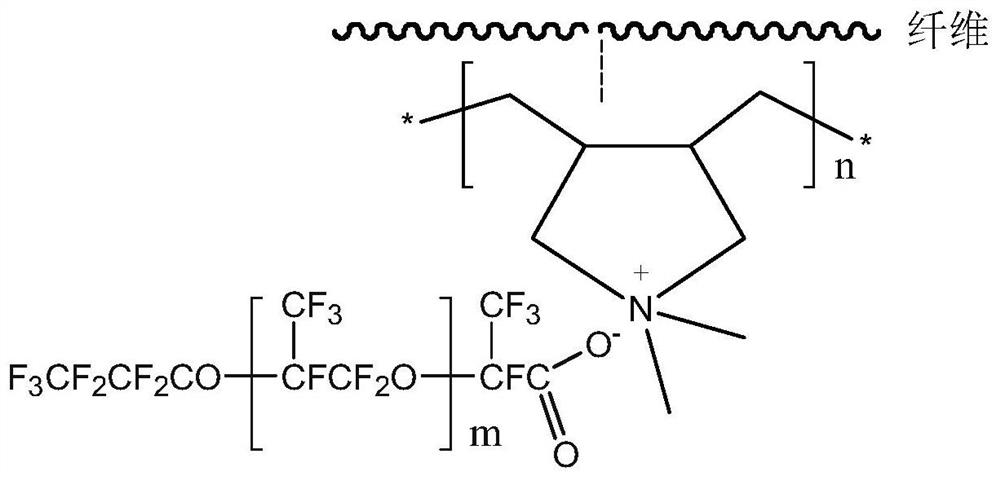
The invention discloses an oil-water separation method based on a hydrophilic oleophobic fabric. The method comprises the following steps: enabling an oil-water mixed solution to flow through the hydrophilic oleophobic fabric to finish oil-water separation; according to the method, the fiber surface is coated with quaternary ammonium salt firstly, then perfluoropolyether carboxylic acid anions andnitrogen positive ions are combined, the two steps of treatment are both achieved in an aqueous solution, perfluoropolyether carboxylic acid ammonium is generated on the fiber surface in situ, and the technical problem that perfluoropolyether carboxylic acid ammonium is hardly dissolved in all solvents and is difficult to spray or impregnate to treat the fabric is solved. According to the hydrophilic oleophobic fabric with the fiber surface coated with the fluorine-containing ammonium carboxylate polymer, hydration can be achieved when the fabric makes contact with water, the static contact angle of the fabric to water can be gradually decreased along with prolonging of the contact time and finally becomes hydrophilic, and the oleophobic function is provided when the fabric makes contactwith oil, and the method for obtaining the special wettability fabric and the oil-water separation fabric has the advantages of simple process, no use of organic solvents, mild operation conditions, and suitableness for process amplification and production.
Owner:SUZHOU UNIV
Thorium-based metal organic framework material and preparation method and application thereof
The invention discloses a thorium-based metal organic framework material and a preparation method and application thereof. The structural unit of the material is [Th6O4(OH)4(tpda)6], and tpda represents terphenyl dicarboxylic acid anions without two hydroxyl H atoms. The method comprises the following steps: uniformly mixing terphenyl dicarboxylic acid ligand, thorium nitrate tetrahydrate metal salt and tetramethylguanidine chloride ionic liquid step by step, and reacting under a heating condition through self-generated pressure to obtain crystals of the thorium-based metal organic framework material. The preparation method is simple and rapid, metal thorium is not agglomerated with the polydentate carboxylic acid ligand tpda, abundant coordination geometric structures and more leading-edge electron orbits can be formed, formation of different secondary building units and topology types is promoted, and the prepared thorium-based metal organic framework material is clear and stable in crystal structure, high in purity and suitable for industrial production. The method can be better applied to analysis and research of MOFs crystal structures, and has a wide application prospect in the field of adsorption and separation of radionuclides.
Owner:EAST CHINA UNIV OF TECH
Agent for inhibiting membrane virus reproduction, method for the production thereof, pharmaceutical composition and method for inhibiting viral infections
InactiveUS20060122276A1Lower Level RequirementsSlight water solubilityBiocidePeptide/protein ingredientsDiseaseSolubility
The present invention relates to the pharmaceutical industry and more particularly to the provision of an agent for inhibiting membrane virus replication. The object of the invention is to provide an agent based on fullerene polycarboxylic anions for suppressing the activity of membrane viruses in treating diseases caused by these viruses. For accomplishing said subject, there is proposed a group of inventions united by a common inventive concept, said group comprising a method for preparing compounds, studying the mechanisms of action, provision of pharmaceutical compositions, and developing methods of treating with their use. Said object t is accomplished by selecting such quantitative ratios of the components and reaction conditions, which ensure the preparation of polyaddition products. It has been established that in carrying out the synthesis the amount of the amino acid must exceed the amount of fullerene by more than 50 times. The product prepared by the proposed method has an unlimited solubility in water, required bioavailability, high efficiency of action on non-infected cells, low toxicity. The content of the main substance in the target product is at least 90%. The process is adaptable to streamline production and can be used in the pharmaceutical industry. Compositions of drugs for and methods of treating infectious diseases caused by human immunodeficiency virus (HIW), herpes simplex virus (HSC) and hepatitis C virus (HCV) have been developed.
Owner:RASNETSOV
Perovskite quantum dot-polymer complex, preparation method of wavelength conversion element and light-emitting device
PendingCN111196924AGood light stabilityEasy to prepareMaterial nanotechnologySolid-state devicesChemical physicsHalogen
The invention discloses a perovskite quantum dot-polymer complex, a preparation method of a wavelength conversion film and a light-emitting device. The preparation method of the perovskite quantum dot-polymer complex comprises the following steps: a step of providing precursors, including: a first precursor composed of at least one of Rb <+>, Cs <+>, NR4 <+> or [CH (NH2) 2] <+> and at least one ofcarboxylate anions or halogen anions, wherein in the NR4 <+>, R is independently a hydrogen atom or a substituted or unsubstituted C1-C10 linear or branched alkyl group; a second precursor includinga Pb halide, a Ge halide, a Si halide, a Sn halide, or a combination thereof; and a polymer; and a step of mixing the precursors at a first temperature at or above the melting point of the polymer, and then cooling the mixed precursors. According to the invention, the problem of poor stability of existing perovskite quantum dots can be effectively solved.
Owner:SUZHOU XINGSHUO NANOTECH CO LTD
Preparing method for manganese series phosphatisation surface conditioning agent
InactiveCN105463441AImprove dispersion stabilityCan be stored for a long timeMetallic material coating processesDispersion stabilitySodium bicarbonate
The invention discloses a preparing method for a manganese series phosphatisation surface conditioning agent. The preparing method includes the following steps that firstly, 35 parts of anhydrous sodium hydrogen phosphate is prepared into a water solution with the concentration being 55%; secondly, 1-2 parts of N-methyl pyrrolidone, 0.4 part of titanyl sulfate, 4.3 parts of sodium pyrophosphate, 3.8 parts of sodium bicarbonate, 0.7 part of sodium tetraborate, 2.1 parts of sodium tripolyphosphate, 0.3 part of polycarboxylic acid anion organic matter and 0.9 part of lauroyl aminoethyl sodium sulfate are sequentially added into the water solution of the sodium hydrogen phosphate, and a mixed solution is obtained; thirdly, the mixed solution is heated to 75 DEG C and sufficiently stirred, and white powder with the water content below 2% is obtained; fourthly, the powder is stirred continuously at the temperature being 80 DEG C, 0.9 part of sodium pyrophosphate, 1.2 parts of glacial acetic acid, 0.4 part of methyl triethoxysilane and 1.7 parts of magnesium sulfate are added into the powder at the same time, and stirring and drying are conducted continuously till the water content is smaller than 1%. The surface conditioning agent prepared through the preparing method is good in dispersion stability and capable of being stored for a long time.
Owner:常熟市虞东磷化材料有限公司
Polycarboxylic acid and europium complex luminescent material and preparation method thereof
ActiveCN107383072AThe synthesis method is simpleStrong fluorescenceGroup 2/12 organic compounds without C-metal linkagesLuminescent compositionsPotassiumAcid substances
The invention discloses a polycarboxylic acid and europium complex luminescent material and a preparation method thereof. The complex luminescent material is a three-dimensional coordination polymer containing two types of organic polycarboxylic acid anion ligand, and the general formula of the three-dimensional coordination polymer is [Eu2(1, 2-bdc)2(3, 5-pdc) (H2O)4]n, wherein 1, 2-bdc is phthalic acid di-anion; 3, 5-pdc is 3, 5-pyridinedicarboxylic acid di-anion; n is natural number not less than 100. The preparation method comprises the following steps: weighting 3, 5-pyridinedicarboxylic acid and potassium acid phthalate or phthalic acid, wherein the amount ratio of 3, 5-pyridinedicarboxylic acid substance to potassium acid phthalate or phthalic acid substance is 0.00008-0.00012mol to 0.00016-0.00024mol; adding deionized water to an europium compound; regulating the pH value to be 4-6; transferring into an oven to react for 48-80h; then decreasing the temperature at the speed of 1-5 DEG C per hour, wherein an europium complex obtained when the temperature is decreased to be less than 100 DEG C is a colorless crystal; then washing with absolute ethyl alcohol to obtain the complex luminescent material.
Owner:XUCHANG UNIV
Application of carboxylate compound as absorbent for capturing carbon dioxide
ActiveCN114558549ASolve corrosiveSolve volatileOther chemical processesWater contaminantsPhosphoniumQuaternary ammonium ions
The invention discloses application of a carboxylate compound as an absorbent for capturing carbon dioxide. The carboxylate compound provided by the invention is used as an absorbent for capturing carbon dioxide and / or is applied to preparation of the absorbent for capturing carbon dioxide; carboxylic acid anions in the carboxylate compound are carboxylate radicals with carbon chains with more than 3 carbon atoms, and cations are substituted quaternary ammonium ions and / or quaternary phosphonium ions. The method for efficiently trapping carbon dioxide in an energy-saving manner by adopting a carboxylate compound stable to water comprises the following steps: placing an aqueous solution of the carboxylate compound in a carbon dioxide atmosphere to absorb carbon dioxide, so as to obtain a conjugate of carboxylate and carbon dioxide separated out from water. The invention is used for solving the problems of corrosivity, volatility and high energy consumption of the existing system, and can obtain higher carbon dioxide capture capacity at high temperature, low pressure of carbon dioxide pressure and high pressure; the conjugate of carboxylate and carbon dioxide is stable to water; the carboxylate can be regenerated.
Owner:BEIJING YUTAN TECH CO LTD
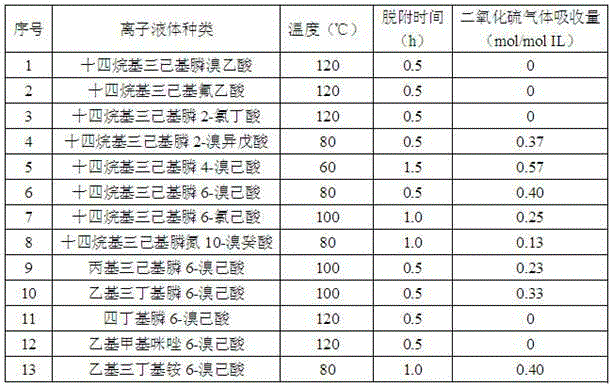
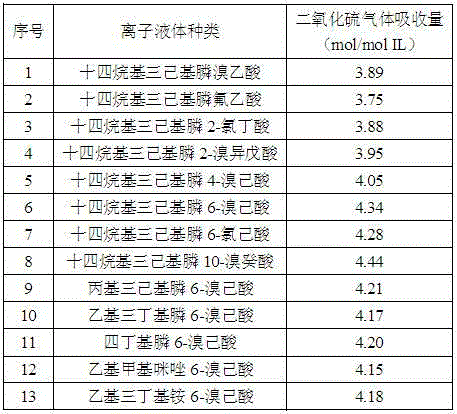
Method for catching sulfur dioxide by adopting halogenated carboxylic acid ion liquid
The invention discloses a method for catching sulfur dioxide by adopting a halogenated carboxylic acid ion liquid. According to the halogenated carboxylic acid ion liquid, halogen is introduced onto carboxylic acid negative ions of a functionalized ionic liquid, the absorbing capacity of sulfur dioxide is improved by utilization of the action of halogen reinforced onto the negative ions and sulfur dioxide, and the electron absorption property of the halogen on the negative ions is utilized to improve the desorption property of carboxylic acid ion liquid, so that efficient reversible absorption of the sulfur dioxide can be realized.
Owner:HENAN NORMAL UNIV
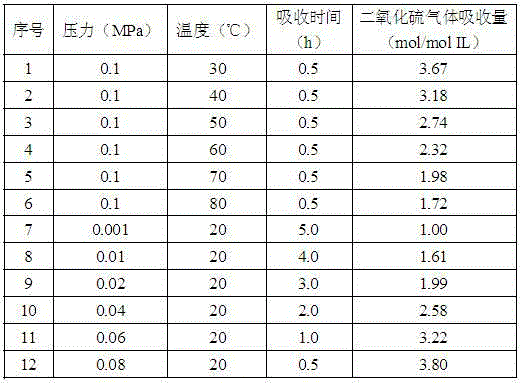
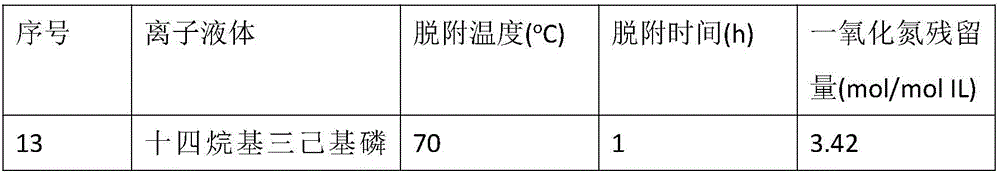
Method for capturing nitric oxide by means of carboxylic acid anion-functionalized ionic liquid
InactiveCN106422673AEasy to desorbGas treatmentDispersed particle separationAlkalinityAbsorption capacity
The invention discloses a method for capturing nitric oxide by means of carboxylic acid anion-functionalized ionic liquid. The method is characterized in that carboxylic acid anions with alkalinity are selected as a gas absorbent, and in the nitric oxide capturing process, the absorption pressure ranges from 0.0001 MPa to 0.2 MPa, the absorption temperature ranges from 20 DEG C to 100 DEG C, and the absorption time ranges from 2 hours to 16 hours; absorbed nitric oxide is easy to desorb, wherein the desorption temperature ranges from 60 DEG C to 120 DEG C, and the desorption time ranges from 0.1 hour to 40 hours. Compared with a traditional nitric oxide capturing method, the method has the main advantages that the efficiency and the capacity are high, desorption is easy, the purpose of efficient capturing is achieved through the chemical action between carboxylate radicals and nitric oxide, and the highest absorption capacity that every 1 mol of the ionic liquid absorbs 4.28 mol of nitric oxide is achieved.
Owner:ZHEJIANG UNIV
Supramolecular intercalated structure polymer antioxidant
ActiveCN102796280BImprove thermal stabilityEasy to prepareOxide/hydroxide preparationDihydrogen oxideDivalent metal ions
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing ionic liquid having carboxylic acid anion using microreactor
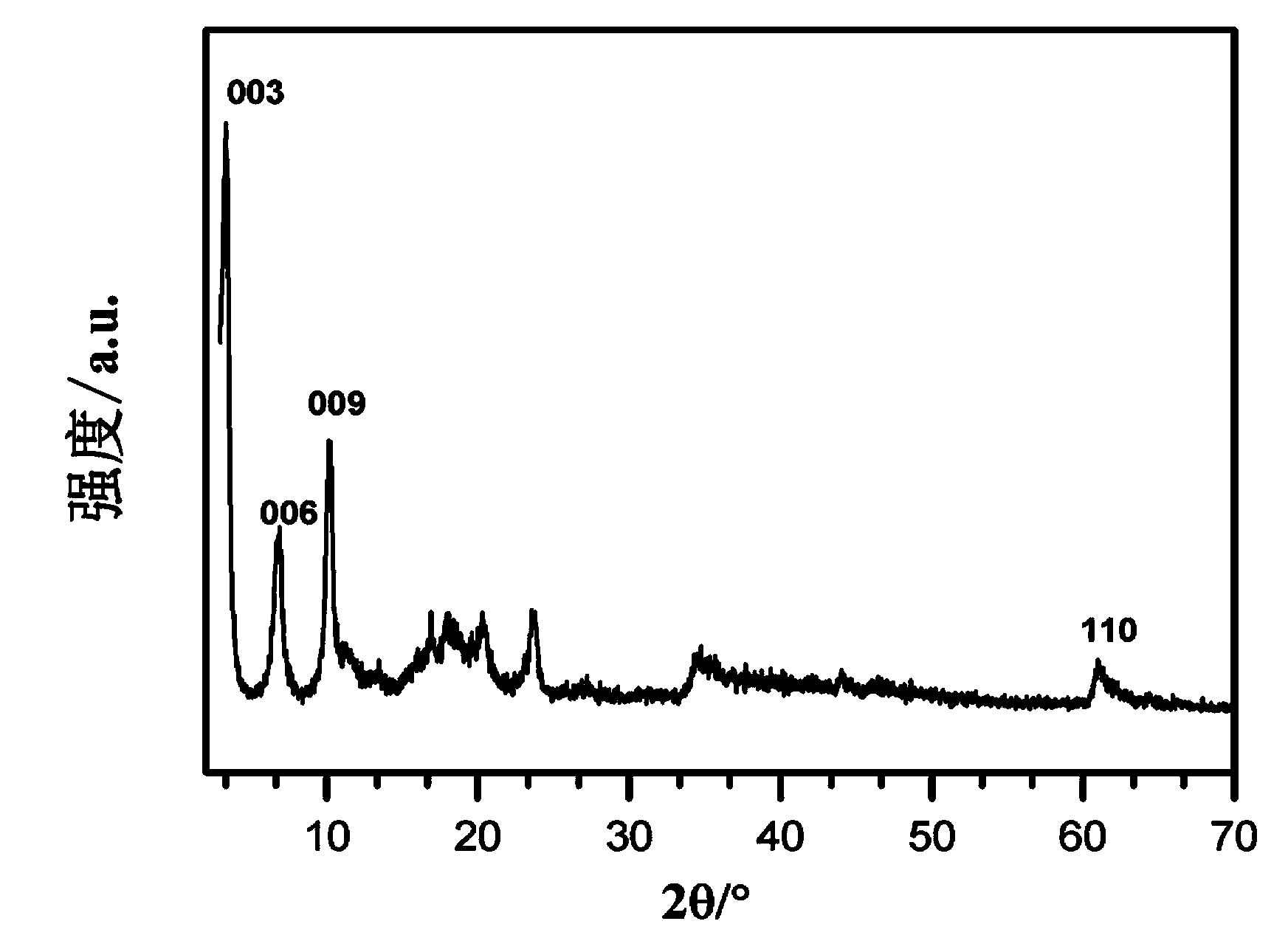
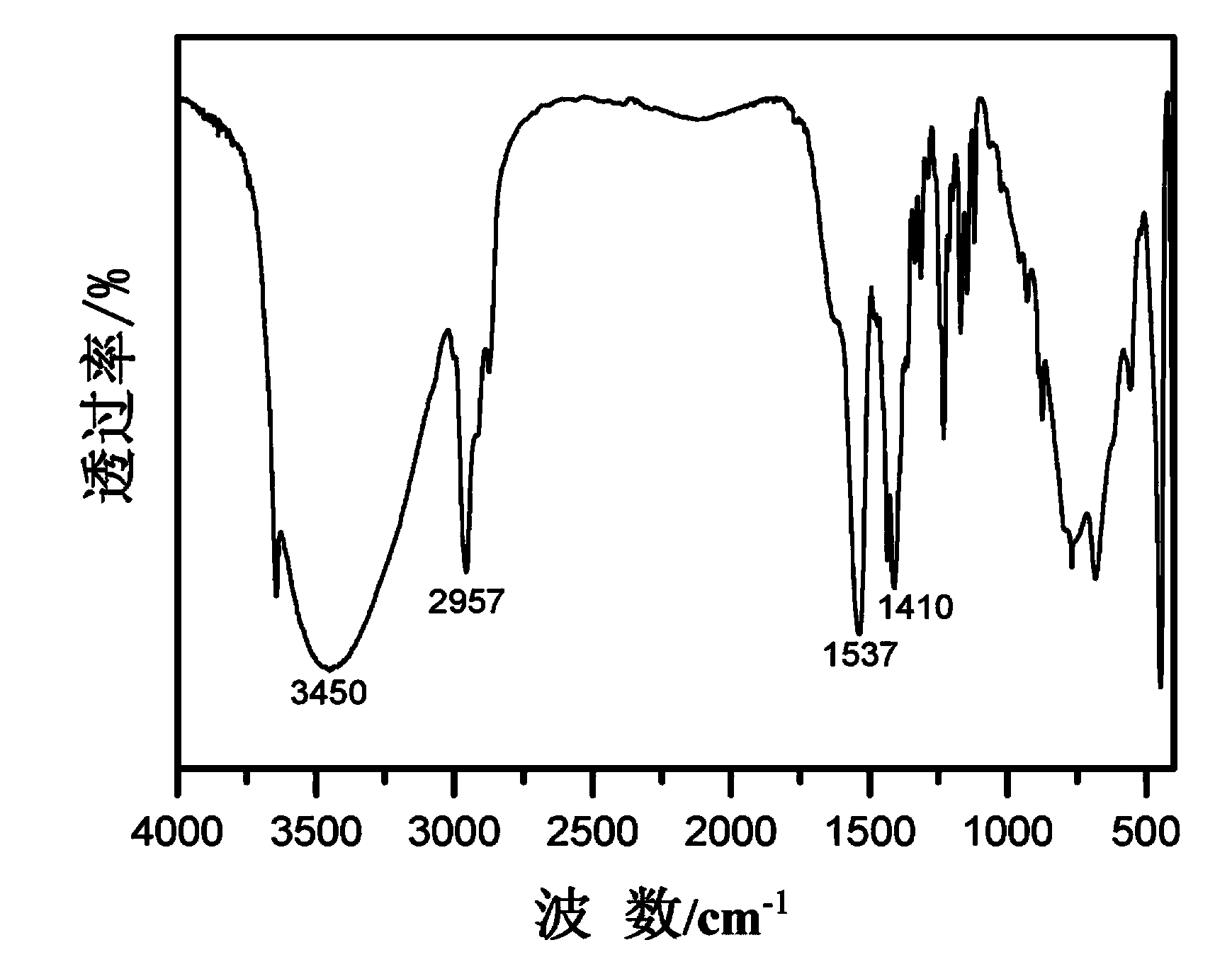
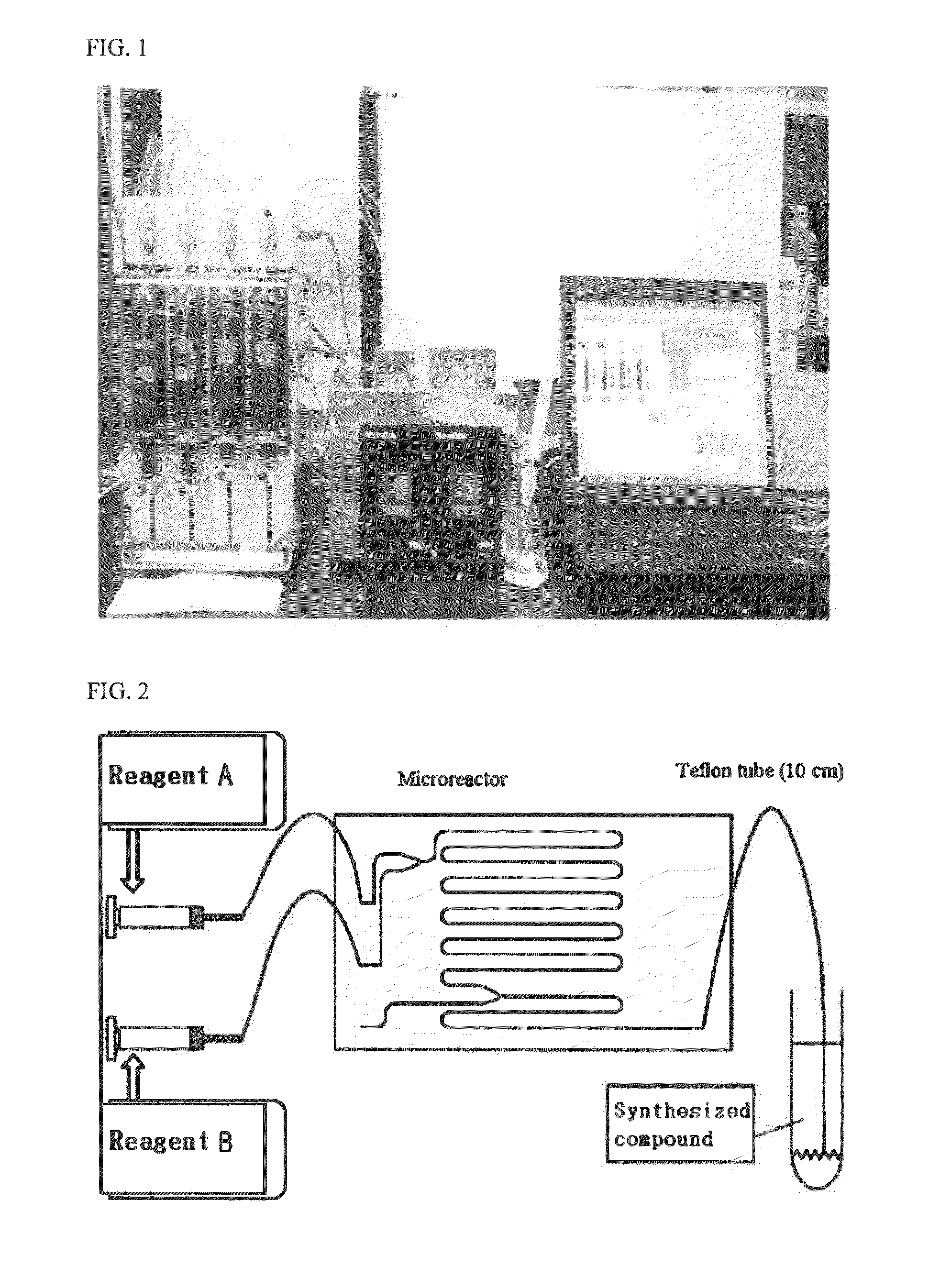
InactiveUS20160254098A1Shorten the timeProcess environmental protectionHybrid capacitor electrolytesAmino preparation from amines2-Ethylhexanoic acidSodium octanoate
The present invention relates to a method for preparing an ionic liquid having a carboxylic acid anion using a microreactor. More specifically, the present invention relates to a method for preparing, with high efficiency, an ionic liquid having a carboxylic acid anion as shown in FIG. 1, by having sodium butanoate, sodium 2-ethylhexanoate, or sodium octanoate undergo a substitution reaction with 1-alkyl-3-methylimidazolium, 1,1-alkylmethylpyrrolidinium, 1,2-dimethyl-3-alkylimidazolium, 1-alkyl-3-methylpyridinium, or tetramethylammonium, each of which being a cation. The ionic liquid prepared according to the present invention has high purity, containing residual halide at less than 10 ppm, and has high electrical conductivity, and therefore is capable of being used as an electrolyte or for a condenser.
Owner:CHEMTECH RESEARCH INC
Electrolytic capacitor
ActiveCN112582179AIncrease working voltageSolution to short lifeLiquid electrolytic capacitorsElectrolytic agentNitrobenzene
The invention provides an electrolytic capacitor. The electrolytic capacitor comprises an electrolyte and a core package soaked in the electrolyte, the sparking voltage of the electrolyte is greater than 400V, the electrolyte comprises a main solvent and a main solute, the main solvent comprises a lactone compound, and the main solute comprises imidazolinium cations and organic carboxylic acid anions; the total carbon content of the organic carboxylic acid anions is 8-26, and the structural formula of the organic carboxylic acid anions is shown in the description, wherein P is a main carbon chain of 1-25 carbons, and the main carbon chain contains or does not contain unsaturated bonds; R1, R2, R3 and R4 are respectively and independently selected from hydrogen, benzene rings, alkyl benzenerings, nitrobenzene rings, carboxyl or straight carbon chains with 1-10 carbons or carbon chains with branched chains, and one or two of R1, R2, R3 and R4 are selected from carboxyl; the core packagecomprises an anode, a cathode and electrolytic paper, the tightness of the electrolytic paper is 0.30-0.75 g / cm <3>, and the thickness of the electrolytic paper is 40-120 microns; and the working voltage of the electrolytic capacitor is 350 to 500 V. The electrolytic capacitor provided by the invention widens the working temperature range and has the characteristic of high voltage resistance.
Owner:SHENZHEN CAPCHEM TECH
Electrolysis solution and electrolytic capacitor using the same
The present invention has its object to provide an electrolyte anion which is high in decomposition temperature, in order to inhibit the electrolyte anion in the electrolyte solution for electrolytic capacitors from undergoing decarboxylation in the lead-free solder reflowing step to thereby prevent valve opening.The present invention uses an electrolyte solution comprising, as an electrolyte, the salt (A) composed of ammonium cation (a) and a polybasic carboxylic acid (b) anion, wherein the proton part charge of each carboxyl group in the polybasic carboxylic acid (b) as calculated by the quantum mechanics calculation software CAChe-based AM1 method is not higher than 0.243. Preferred is the polybasic carboxylic acid (b) represented by the general formula (1):wherein R1 to R4 may be the same or different and each represents a hydrogen atom, a functional group or a hydrocarbon group containing 1 to 3 carbon atoms, which may optionally contain a functional group, provided that at least one of R1 to R4 is an electron-donating group.
Owner:PANASONIC CORP +1
Curable composition, process of production and use thereof
ActiveUS20130157226A1Improve rheologyImprove mechanical propertiesImpression capsTeeth fillingNitrateImpressions materials
The present disclosure relates to a curable composition to be prepared by mixing a base paste and a catalyst paste, the base paste comprising (A) a hardenable compound comprising at least two aziridine moieties, and (B) a metal containing component containing anions and / or ligands, the metal containing component being present in an amount of about 0.1 to about 5 wt.-%, the metal being selected from Zn, Cu, Co, Ni, Ag and combinations thereof, the anions or ligands being selected from oxide, hydroxyl, (hydro)carbonate, sulphate, nitrate, halide, lactate, benzoate, wolframate, linear or branched aliphatic carboxylic acid anions, ligands having not more than two coordinating moieties and combinations thereof, the catalyst paste comprising (C) a Lewis acid, the composition optionally further comprising (D) a retarder, (E) filler and (F) additive(s).The present disclosure also relates to a kit of parts, the use of a metal containing component as a means for reducing the curing time of a cationically curable composition and a method of taking a dental impression.
Owner:3M INNOVATIVE PROPERTIES CO
Process for the production of metal oxides
ActiveUS11174169B2High operating temperaturePrevent oxidationCarbon compoundsCarbon captureAlkaline earth metalCarboxylic acid anion
The present application pertains to methods for making metal oxides and / or citric acid. In one embodiment, the application pertains to a process for producing calcium oxide, magnesium oxide, or both from a material comprising calcium and magnesium. The process may include reacting a material comprising calcium carbonate and magnesium carbonate. Separating, concentrating, and calcining may lead to the production of oxides such as calcium oxide or magnesium oxide. In other embodiments the application pertains to methods for producing an alkaline-earth oxide and a carboxylic acid from an alkaline earth cation-carboxylic acid anion salt. Such processes may include, for example, reacting an alkaline-earth cation-carboxylic acid anion salt with aqueous sulfur dioxide to produce aqueous alkaline-earth-bisulfite and aqueous carboxylic acid solution. Other useful steps may include desorbing, separating, and / or calcining.
Owner:INNOVATOR ENERGY LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com