Preparation method of quaternary ammonium ionic liquid
A technology of ionic liquid and quaternary ammonium, which is applied in the field of preparation of quaternary ammonium ionic liquid, can solve the problems of complex process and long reaction cycle, and achieve the effect of simple preparation steps, fast reaction speed and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] A kind of preparation method of quaternary ammonium ionic liquid, comprises the steps:
[0022] 1. Dissolve the base MOH in the alcoholic solution to obtain the alcoholic solution of the base. The mass of the base and the volume ratio of the alcohol are 1g: 5-20ml, and the quaternary ammonium chloride is added dropwise in the alcoholic solution of the base, The molar ratio of the alkali in the alcoholic solution to the quaternary ammonium chloride is 1-4:1, react at room temperature for 1-3 hours, the product is filtered, and the precipitate is filtered to obtain a clear liquid R 1 R 2 R 3 R 4 N + Oh - ; The chemical formula of the quaternary ammonium salt is R 1 R 2 R 3 R 4 N + C1 - , the cation is methyl octyl ammonium ion, the anion is chloride ion, where R 1 is methyl, R 2 , R 3 , R 4 It is an octyl group; the base is lithium hydroxide, potassium hydroxide or sodium hydroxide, and the alcohol is ethanol or isopropanol;
[0023] 2. The clarified liquid...
Embodiment 1
[0038] 1. Dissolve 5.6g, 0.10mol of potassium hydroxide in 56ml of ethanol to obtain an alcoholic solution of alkali. 40g, 0.1mol of quaternary ammonium chloride [C 25 h 54 N][Cl] was added dropwise to the alcoholic solution of the above alkali, and stirred at room temperature for 1 hour, and the precipitated potassium chloride was filtered off to obtain a clear ionic liquid [C 25 h 54 N][OH], the yield is 99%, and the product is detected by ion chromatography, and the chloride ion content is 336ppm;
[0039] 2. Add 0.08mol of bis (2-ethylhexyl) phosphoric acid (P204) to the obtained 0.1mol [C 25 h 54 N][OH], stirred at room temperature for 1 hour, took the organic phase of the upper ionic liquid, washed 3 times with deionized water, then distilled under reduced pressure to reclaim the solvent ethanol, and dried the product in vacuo to obtain methyl trioctyl ammonium di(2-ethyl Hexyl) phosphate ionic liquid ([C 25 h 54 N][C 16 h 34 POO]), yield 92.5%.
[0040] The pro...
Embodiment 2
[0044] 1. Same as step 1 of embodiment 1;
[0045] 2. Add 0.08mol of 2-ethylhexyl ester mono-2-ethylhexylphosphonic acid (P507) to the obtained 0.1mol [C 25 h 54 N][OH], stirred at room temperature for 2 hours, took the upper ionic liquid organic phase, washed 3 times with deionized water, then distilled under reduced pressure to recover solvent ethanol, and dried the product in vacuum to obtain methyl trioctyl ammonium 2-ethylhexyl ester Mono 2-ethylhexylphosphonic acid ionic liquid ([C 25 h 54 N][C 16 h 34 o 2 POO]), yield 87.6%.
[0046] The product NMR characterization result that embodiment 2 obtains is:
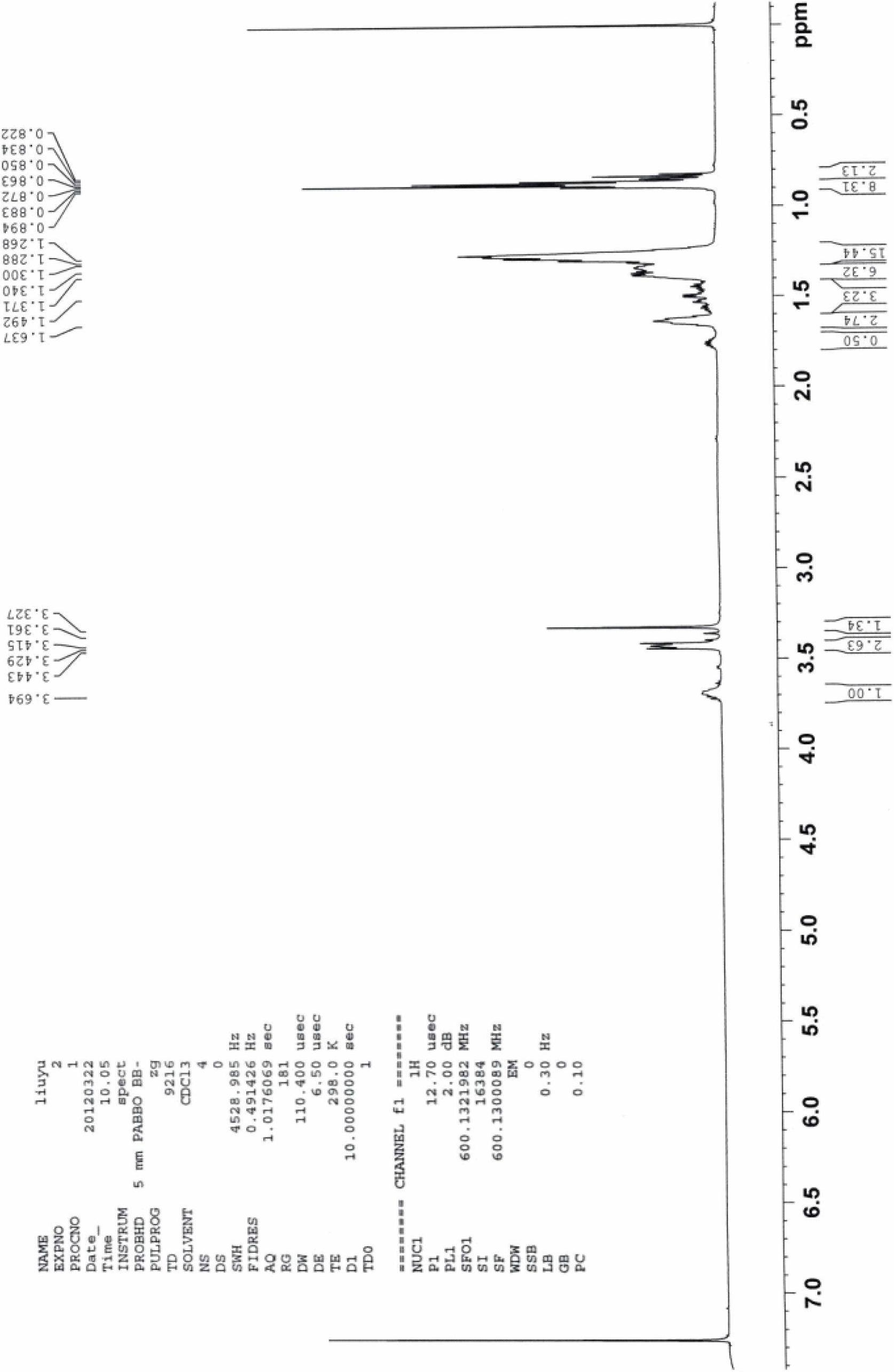
[0047] 1 H NMR: 0.822-0.850 (m, 3H), 0.863-0.894 (m, 18H), 1.268-1.300 (m, 32H), 1.340-1.371 (m, 14H), 1.492 (m, 2H), 1.637 (m, 2H), 1.780(m, 4H), 3.327(s, 3H), 3.415-30443(m, 6H), 3.694(m, 4H).
[0048] 13 C NMR: 65.87, 61.00(3C), 48.82, 40.55, 34.80, 33.75, 31.65, 31.49(2C), 30.0(2C), 29.26, 29.22, 29.07(2C), 29.02(2C), 28.99(2C), 28.87 (2C), 28.58(2C), 26...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com