Method for catching sulfur dioxide by adopting halogenated carboxylic acid ion liquid
A technology of halogenated carboxylic acid and ionic liquid, which is applied in the field of sulfur dioxide absorption, can solve the problems of difficult desorption, high absorption enthalpy, poor cycle performance, etc., and achieve the effects of avoiding loss and volatilization, increasing absorption, and speeding up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
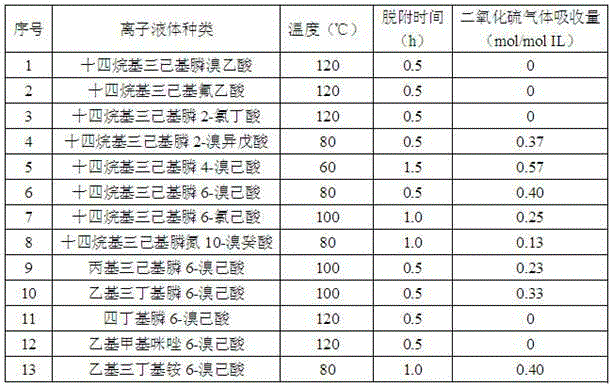
[0013] Select different kinds of tetraalkylphosphine halides, dialkylimidazolium halide salts or tetraalkylammonium halides respectively to form tetraalkylphosphine hydroxides, dialkyl imidazole hydroxides or tetraalkylammonium hydroxides through anion exchange reaction. The formed hydroxides are reacted with different kinds of halogenated carboxylic acid compounds according to the molar ratio of 1:1 at normal temperature and pressure for 6-24 hours to obtain different kinds of halogenated carboxylate ions as shown in Table 1 liquid.
[0014] In a 5mL glass container with an inner diameter of 1cm, add 0.002mol of the different types of halocarboxylate ionic liquids prepared above, and then slowly introduce sulfur dioxide gas at a flow rate of 40mL / min, absorbing the gas pressure at 0.1MPa, and controlling the temperature at At 20°C, the absorption time is 0.5h for sulfur dioxide absorption. During the absorption process, the electronic analytical balance is used for weighing. ...
Embodiment 2
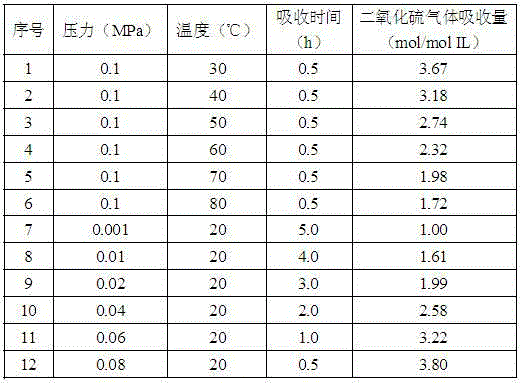
[0019] Add 0.002mol of tetradecyltrihexylphosphine 6-bromohexanoic acid into a 5mL glass container with an inner diameter of 1cm, and then slowly introduce sulfur dioxide gas at a flow rate of 40mL / min. Set different absorption gas pressure, absorption temperature and The absorption time is for the absorption of sulfur dioxide gas, and the electronic analytical balance is used for weighing during the absorption process. The absorption results of sulfur dioxide gas are shown in Table 2.
[0020] Table 2 Effects of different absorption conditions on the absorption of sulfur dioxide gas by tetradecyltrihexylphosphine 4-bromohexanoic acid
[0021]
[0022] Compared with Table 1, combined with Table 2, it can be seen that the absorption amount of sulfur dioxide gas will change significantly depending on the absorption temperature and the absorption gas pressure. The higher the absorption temperature or the lower the absorption gas pressure, the halogenated carboxyl The lower the...
Embodiment 3
[0024] Nitrogen gas is slowly introduced into the tetradecyltrihexylphosphine 6-bromohexanoic acid that has absorbed sulfur dioxide gas, the flow rate is 40mL / min, the pressure is 0.1MPa, the desorption temperature is controlled at 120°C, and the desorption time is 0.5h. Electronic analytical balance weighing shows that the sulfur dioxide gas absorbed by the halogenated carboxylate ionic liquid has been completely desorbed.
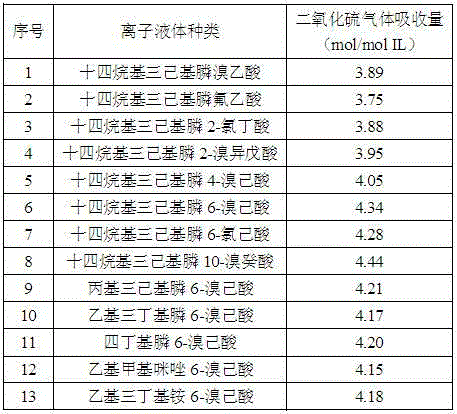
[0025] Weigh different kinds of halogenated carboxylate ionic liquids that have absorbed sulfur dioxide gas with an electronic analytical balance and add them to a 5mL glass container with an inner diameter of 1cm, and then slowly feed nitrogen gas with a flow rate of 40mL / min and a pressure of 0.1MPa. The desorption temperature and desorption time, the desorption results are shown in Table 3.
[0026] Table 3 Effects of different kinds of halocarboxylate ionic liquids on the desorption of sulfur dioxide gas
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com