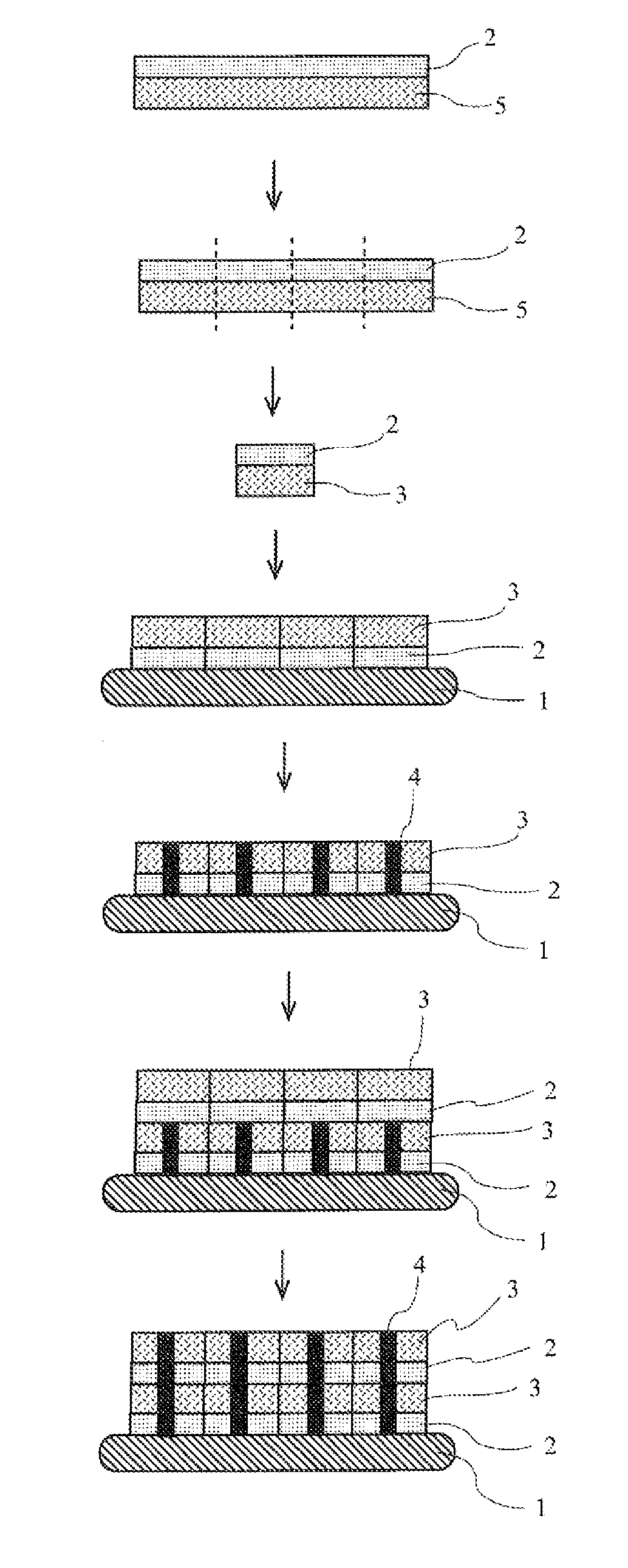
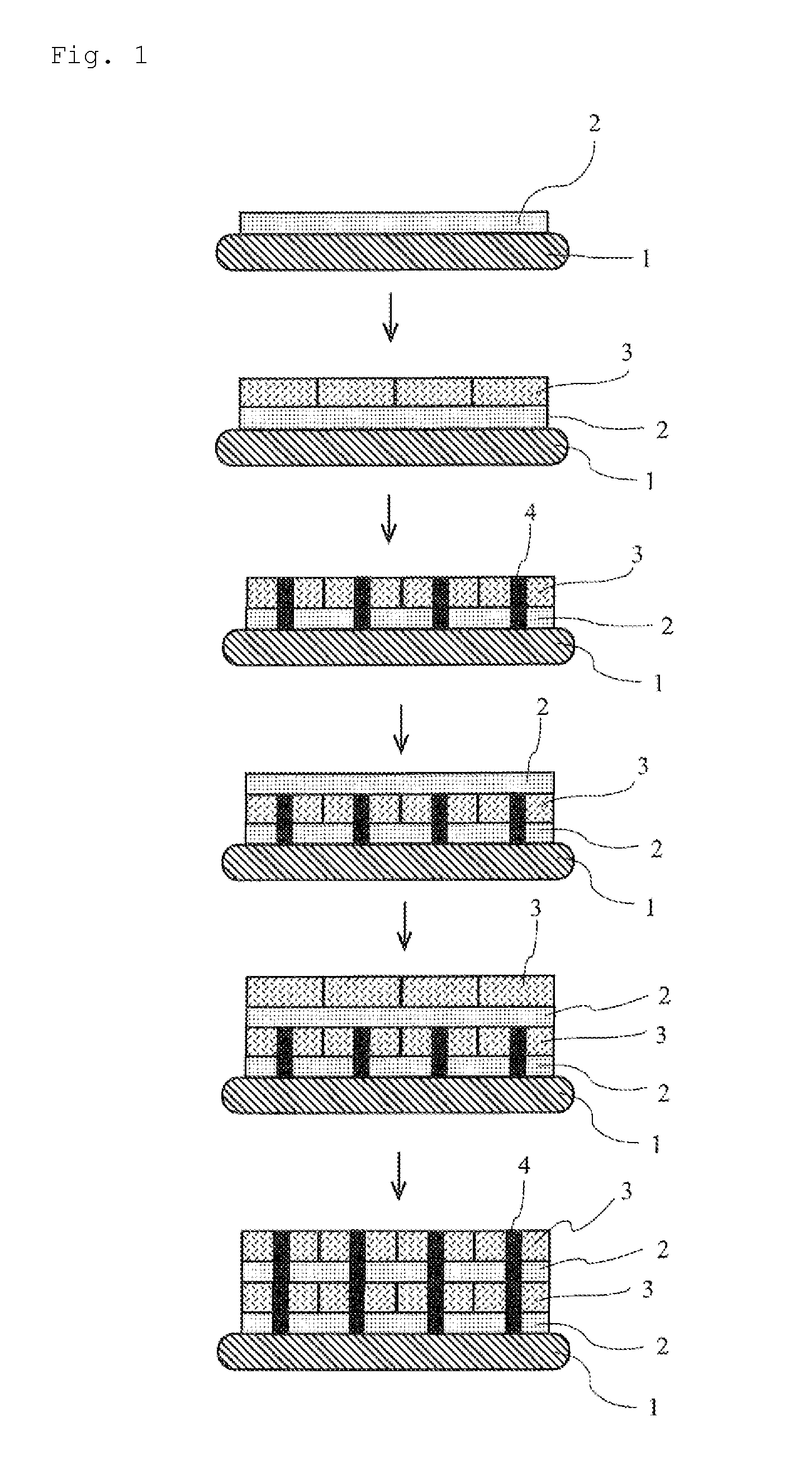
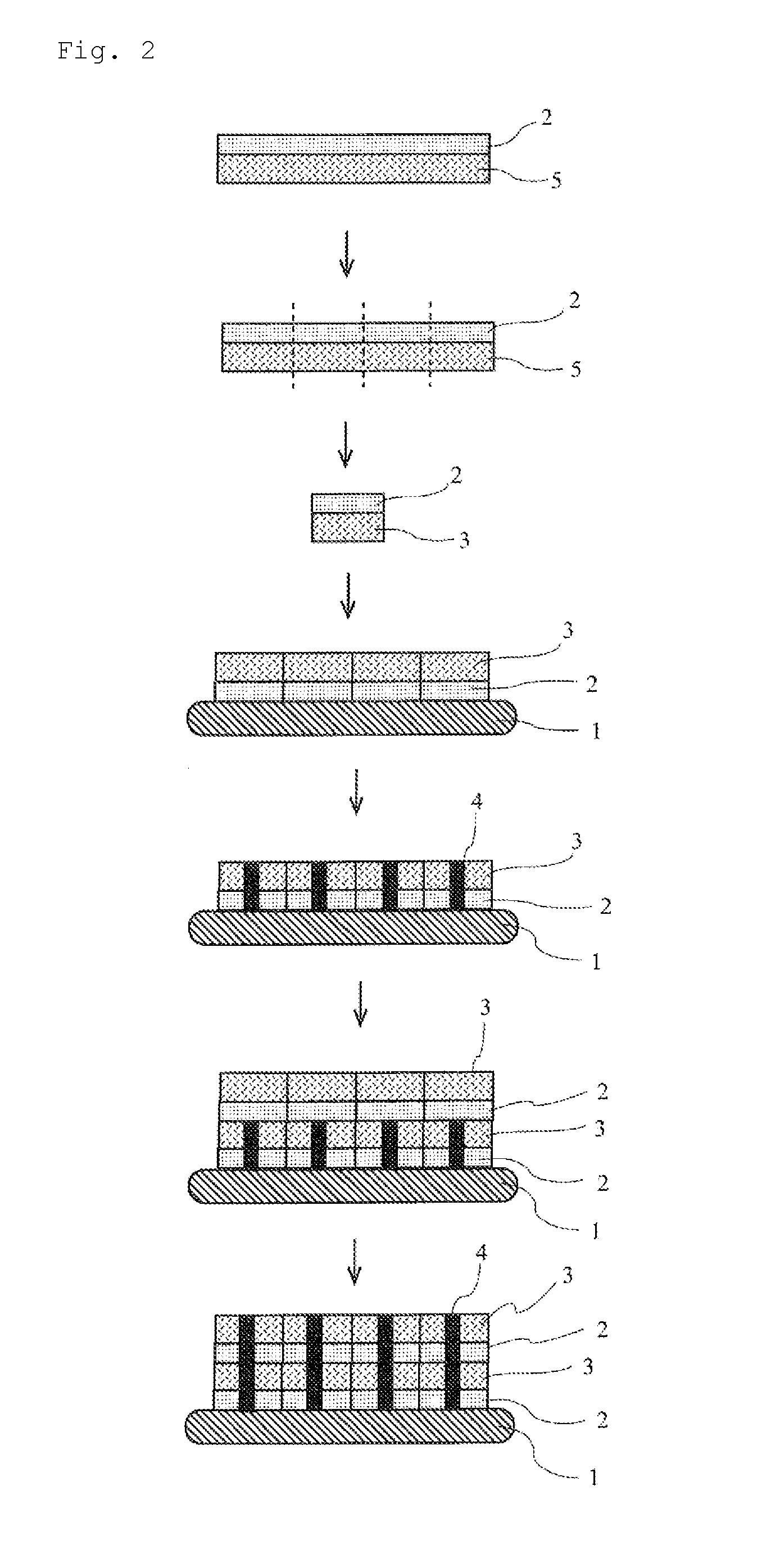
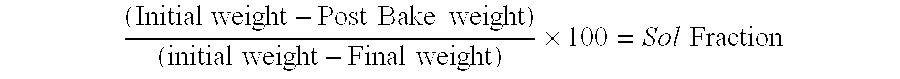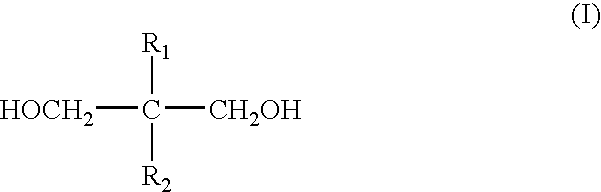Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
210 results about "Bisphenol A diglycidyl ether" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
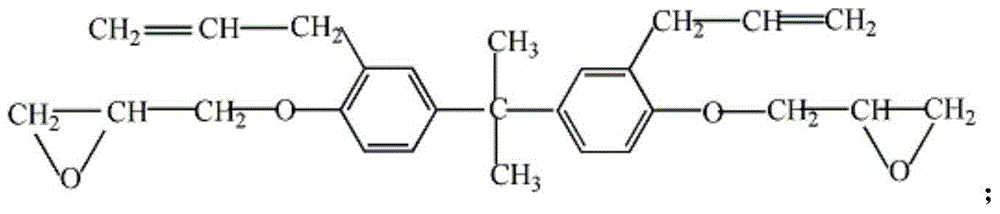
Bisphenol A diglycidyl ether (commonly abbreviated BADGE or DGEBA) is an organic compound used as constituent of epoxy resins. The compound is a colorless solid (commercial samples can appear yellow) that melts slightly above room temperature.
Bisphenol a and aromatic glycidyl ether-free coatings
ActiveUS20070036903A1Good metal substrateGood inter-coat adhesionLiquid surface applicatorsSynthetic resin layered productsPolyesterMeth-
Disclosed are Bisphenol A (BPA), Bisphenol F, Bisphenol A diglycidyl ether (BADGE), and Bisphenol F diglycidyl ether (BFDGE)-free coating compositions for metal substrates including an under-coat composition containing a polyester (co)polymer, and an under-coat cross-linker; and an over-coat composition containing a poly(vinyl chloride) (co)polymer dispersed in a substantially nonaqueous carrier liquid, an over-coat cross-linker, and a functional (meth)acrylic (co)polymer. Also provided is a method of coating a metal substrate using the BPA, BPF, BADGE and BFDGE-free coating system to produce a hardened protective coating useful in fabricating metal storage containers. The coated substrate is particularly useful in fabricating multi-part foodstuffs storage containers with “easy-open” end closures.
Owner:SWIMC LLC
BADGE- and BPA-free can coating
ActiveUS7682674B2Suitable flexibilitySuitable resistanceSynthetic resin layered productsPretreated surfacesAcrylic resinPolyvinyl chloride
The present invention relates to a composition, which is useful for producing coatings for metal sheet substrates of metal cans for storing and / or transporting food or beverages or a lid thereof, and which comprises the following components:(a) 30 to 90 wt. % of a polyvinylchloride-(PVC)-polymer,(b) 7 to 25 wt. % of an acrylic resin,(c) 3 to 40 wt. % of a crosslinking agent, which is produced from phenol, para-tert.-butylphenol, xylenol or a mixture thereof, and formaldehyde,(d) 0 to 8 wt. % additive,(e) 0 to 50 wt. % pigment and(f) a solvent-component,where all weight percentages are on the basis of the total dry weight of the coating composition (without solvents) and the composition is substantially free of bisphenol-A-diglycidyl-ether (“BADGE”) and also substantially free of bisphenol-A-resins. The composition provides metal can coatings that have suitable flexibility, scratch resistance, adherence and sterilization resistance when processed in contact with food. The coatings are suitable for three-piece cans as well as deep-drawn metal cans. In particular they are, however, useful for lids that are to be torn open due to their extraordinary flexibility and sterilization resistance.
Owner:HENKEL KGAA
Low temp. solidifeed resin emulion used for cathode electrolytic coating
InactiveCN1483772AImprove the decorative effectImprove the level ofPaints for electrolytic applicationsEpoxy resin coatingsChemical reactionPolyamine
The present invention relates to a low-temp. soldified resin emulsion for cathodic electrophoretic coating. It is made of 20-70% of modified epoxy resin, 5-50% of polyamine and 10-30% of closed polyisocyanate which can completely declosed at 120-140 deg.C, through the processes of chemical reaction, mixing, neutralizing with organic acid and dispersing in water, in which the modified epoxy resin is made up by using epoxy compound and polyhydroxylated compound and adopting epoxy resin synthesis method under the action of catalyst, its molecular weight is 300-3000, and the pure solid weight ratio of epoxy compound and polyhydroxylated compound is 5-8 / 2-5. The described epoxy compound is bisphenol A diglycidyl ether and glicidyl ether containing flexible chain or glycidyl ester, and the polyhydroxylated compound is bisphenol A and polyhydroxylated compound containing flexible chain.
Owner:CNOOC CHANGZHOU EP COATING
Phosphatized polyesters and coating compositions containing the same
InactiveUS20120301647A1Synthetic resin layered productsPretreated surfacesPolyesterBisphenol A diglycidyl ether
A coating composition comprising a resinous binder and up to 10 percent by weight of a phosphatized polyester. The compositions are useful for coating containers of all sorts such as food and beverage containers, and the phosphatized polyester provides enhanced adhesion of the coating to the container substrate. The compositions can be formulated to be substantially free of bisphenol A (BPA) and bisphenol A diglycidyl ether (BADGE).
Owner:PPG IND OHIO INC
Method for manufacturing polyesterimide enamelled wire paint
The invention discloses a method for manufacturing polyesterimide wire enamel, which comprises the steps of preparing polyesterimide resin and letdown treatment. During the preparation of polyesterimide resin, bisphenol A diisopropanol ether is used to replace part of Ethylene glycol (EG) is used as a reactant, and the imidization reaction is carried out by feeding in steps. The method of the invention can overcome the disadvantages of poor flexibility, low adhesion and small DV value of the polyesterimide enameled wire produced by the prior method. The polyesterimide wire enamelled varnish manufactured by the method of the invention has a large line coating process margin and a fast line coating speed. The method of the invention can be used to manufacture novel 180 grade high DV value polyesterimide wire enamels.
Owner:CHINESE ELECTRICAL EQUIP GRP CO LTD
Alkylphosphinate derivative fire retardant containing phosphaphenanthrene group and preparation method thereof
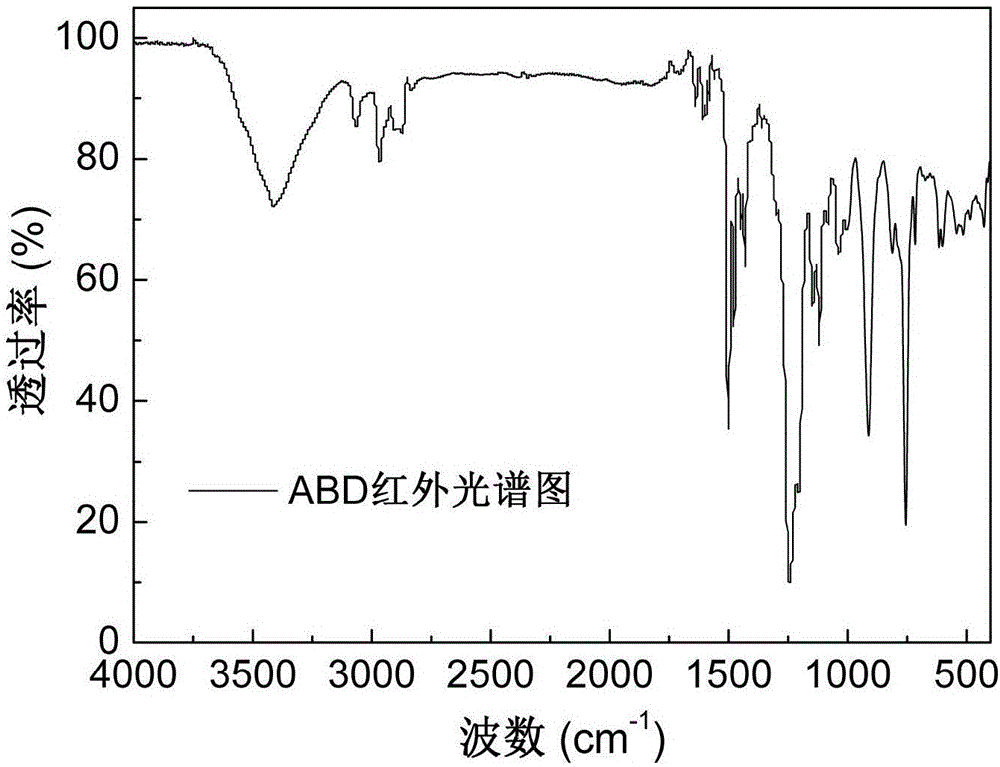
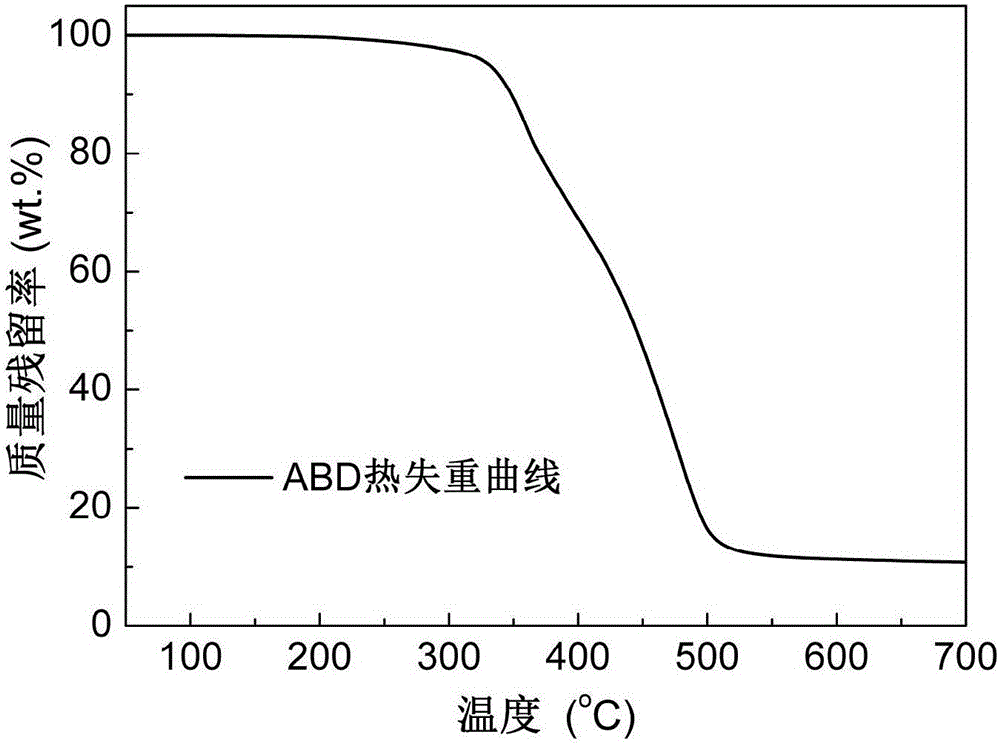
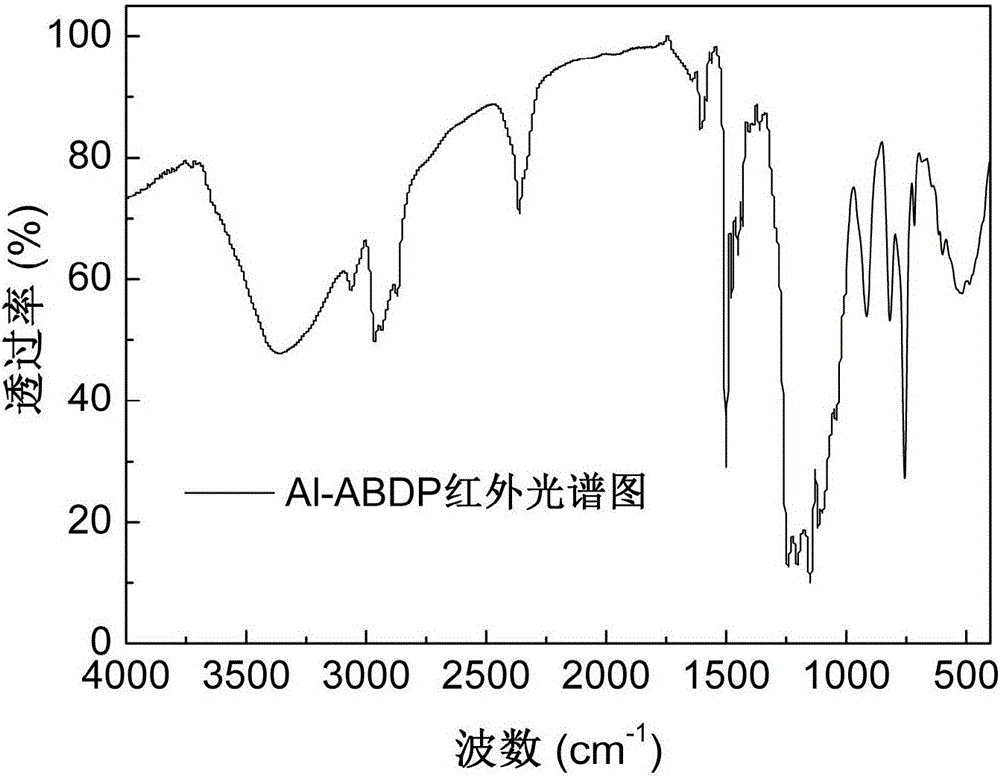
The invention discloses an alkylphosphinate derivative fire retardant containing a phosphaphenanthrene group. A preparation method of the fire retardant is as follows: fusion or solution system addition reaction is carried out for P-H bonds on 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide and epoxide groups and C=C double bonds on o-diallyl bisphenol A diglycidyl ether, in order to obtain a primary intermediate ABD; an addition reaction is carried out between hypophosphorous acid and / or P-H bond on salt thereof and residual C=C double bonds of the primary intermediate ABD initiated by free radicals, in order to obtain a secondary intermediate ABDP and / or salts thereof; metallic compounds and / or protonated nitrogen base are added, an alkaline substance is added for neutralization of acids in the reaction system, a target material is salified and separated, and alkylphosphinate M-ABDP containing the phosphaphenanthrene group is prepared. The M-ABDP has good application prospects, and can be widely applied to flame retardation modification of polyester, polyamide and thermosetting resin, and other high-molecular resin materials.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
Coating compositions with improved adhesion to containers
ActiveUS20120301645A1Improve adhesionSemiconductor/solid-state device detailsSynthetic resin layered productsPhosphoric acidEther
A coating composition comprising a resinous binder and up to 10 percent by weight of the reaction product of (i) a phosphorus acid, and (ii) a polyglycidyl ether of cyclohexane dimethanol. The compositions are useful for coating containers of all sorts, such as food and beverage containers, and the reaction product provides enhanced adhesion of the coating to the substrate. The compositions can be formulated to be substantially free of bisphenol A (BPA) and derivatives thereof such as bisphenol A diglycidyl ether (BADGE).
Owner:PPG IND OHIO INC
Coating compositions for containers
A coating composition comprising a polyether polyol having a hydroxyl functionality of 3 to 8 and the reaction product of:(i) a phosphorus acid, and(ii) a polyepoxide and / or a polyester.The compositions are useful for coating containers of all sorts, such as food and beverage containers. The compositions can be formulated to be substantially free of bisphenol A (BPA), bisphenol A diglycidyl ether (BADGE) and other derivatives of BPA.
Owner:PPG IND OHIO INC
Three-dimensional crosslinked network polymer gel electrolyte membrane, preparation method and lithium-ion battery
ActiveCN105958122AImprove mechanical stabilityImprove interface compatibilityFinal product manufactureElectrolyte accumulators manufacturePolymer scienceLithium metal
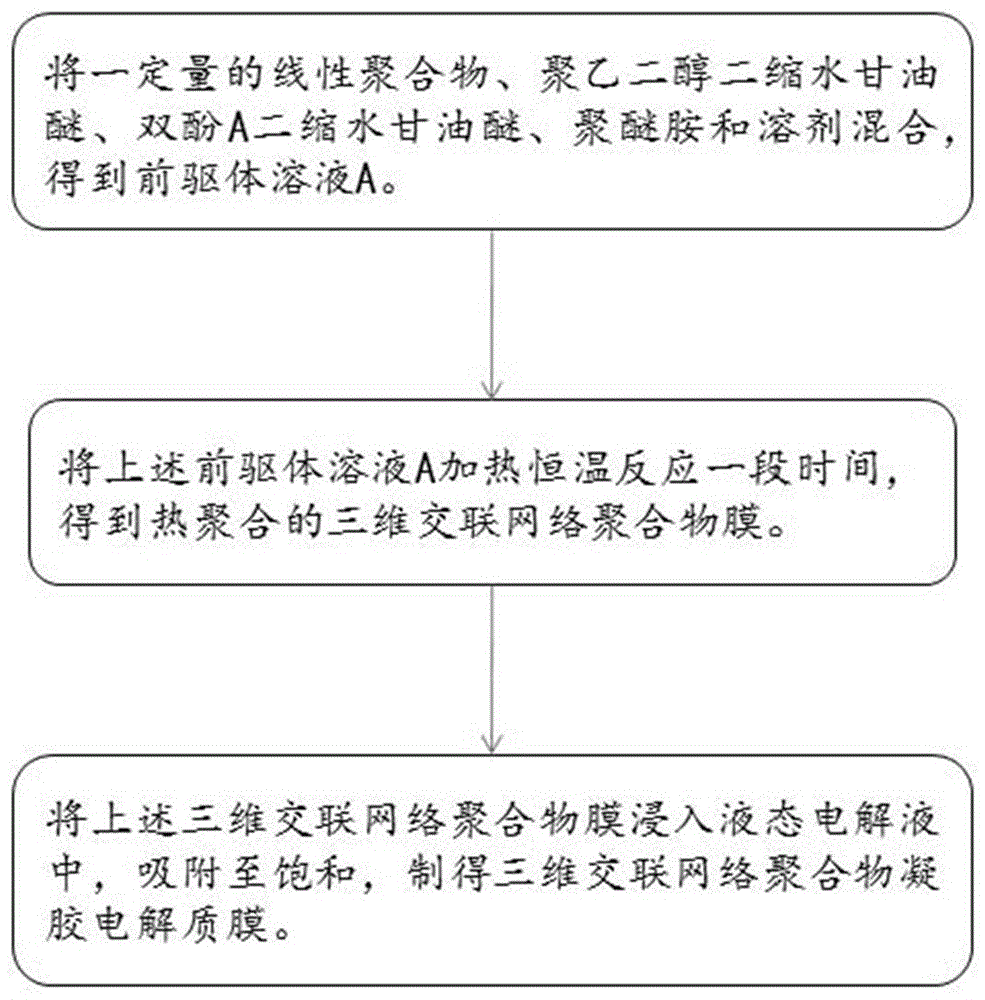
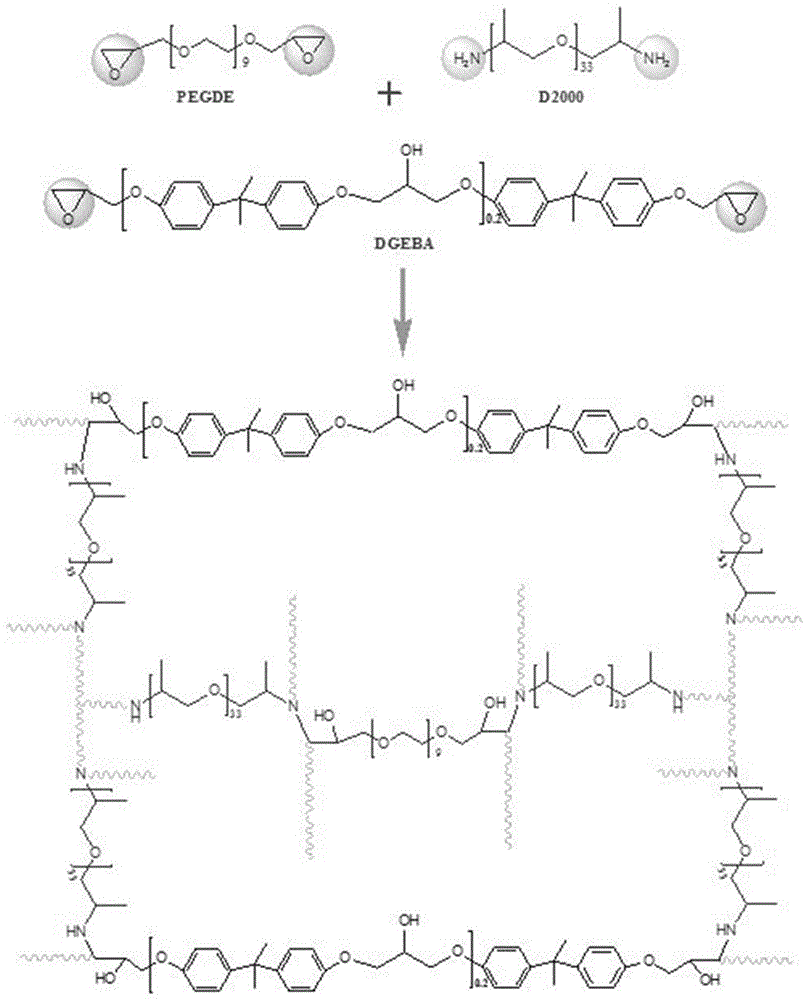
The invention provides a preparation method of a three-dimensional crosslinked network polymer gel electrolyte membrane. The method comprises the following steps of: (S1) mixing a linear polymer, polyethylene glycol diglycidyl ether, bisphenol A diglycidyl ether, polyether amine and a solvent to obtain a precursor solution; (S2) heating the precursor solution A for constant-temperature reaction for a period of time to obtain a three-dimensional crosslinked network polymer membrane; and (S3) immersing the three-dimensional crosslinked network polymer membrane into a liquid electrolyte for adsorption to saturation, thereby preparing the three-dimensional crosslinked network polymer gel electrolyte membrane. The three-dimensional crosslinked network polymer gel electrolyte membrane prepared by the method is good in mechanical stability, high in ionic conductivity and good in lithium metal interface compatibility. The invention further provides the three-dimensional crosslinked network polymer gel electrolyte membrane prepared by the preparation method, and a lithium-ion battery employing the three-dimensional crosslinked network polymer gel electrolyte membrane.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Thermosetting resin composition, and prepreg and laminated board made from thermosetting resin composition
ActiveCN104861652AImprove toughnessHigh peel strengthSynthetic resin layered productsLaminationBottleFire retardant
The invention discloses a thermosetting resin composition. The thermosetting resin composition comprises the following solids by weight: (a) a modified bismaleimide prepolymer, (b) a curing accelerator, (c) a fire retardant, and (d) inorganic fillers. A preparation method of the modified bismaleimide prepolymer comprises the following step: heating bismaleimide and a composite allyl compound in a reaction bottle, wherein the composite allyl compound is composed of a first-class allyl compound and a second-class allyl compound, the first-class allyl compound is selected from one or more of diallyl bisphenol A, diallyl bisphenol S, allyl phenoxy resin and diallyl diphenyl ether, and the second-class allyl compound is selected from one or more of diallyl bisphenol A diglycidyl ether, diallyl bisphenol S diglycidyl ether and diallyl diphenyl ether diglycidyl ether. A laminated board integrates high heat resistance, high toughness, low water absorption, excellent dielectric property and good fire resistance.
Owner:SHENGYI TECH SUZHOU
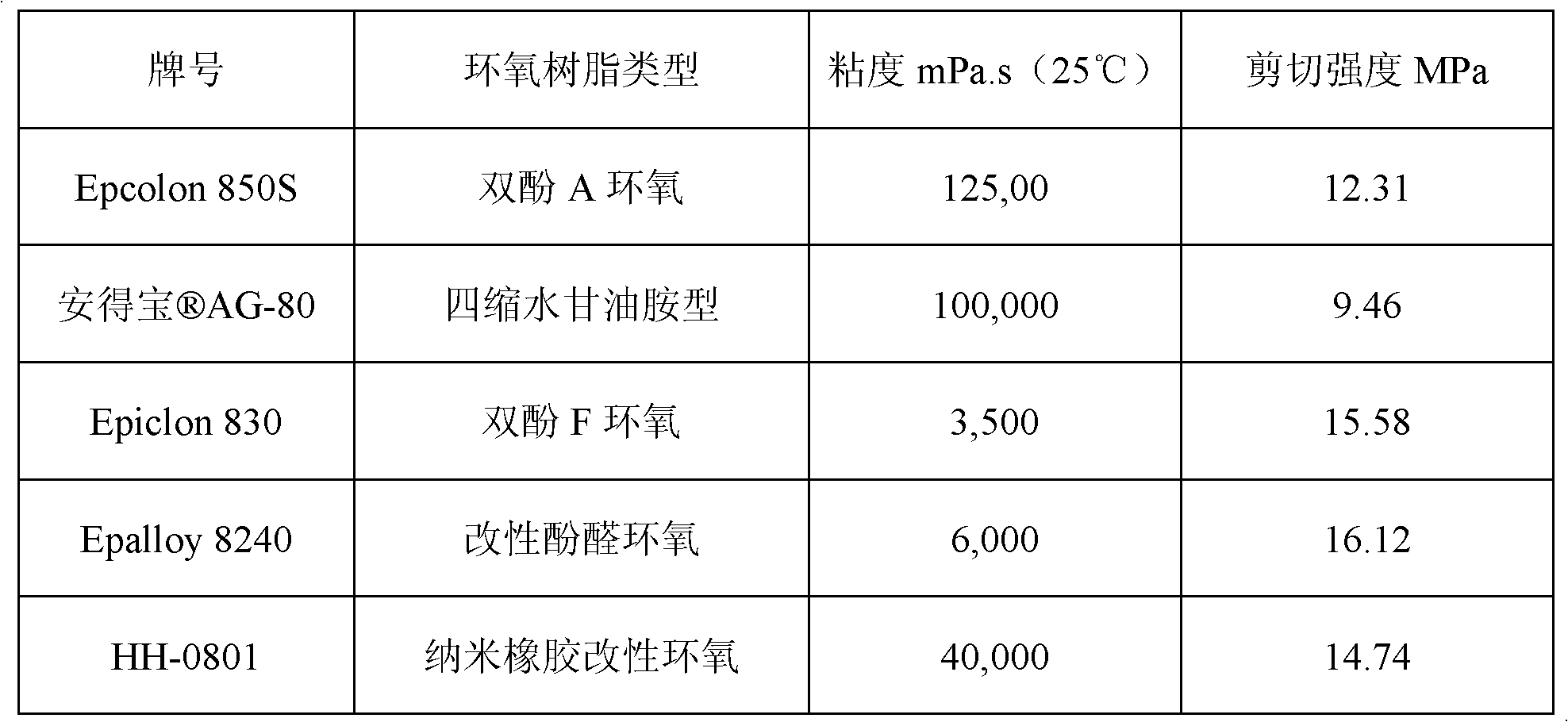
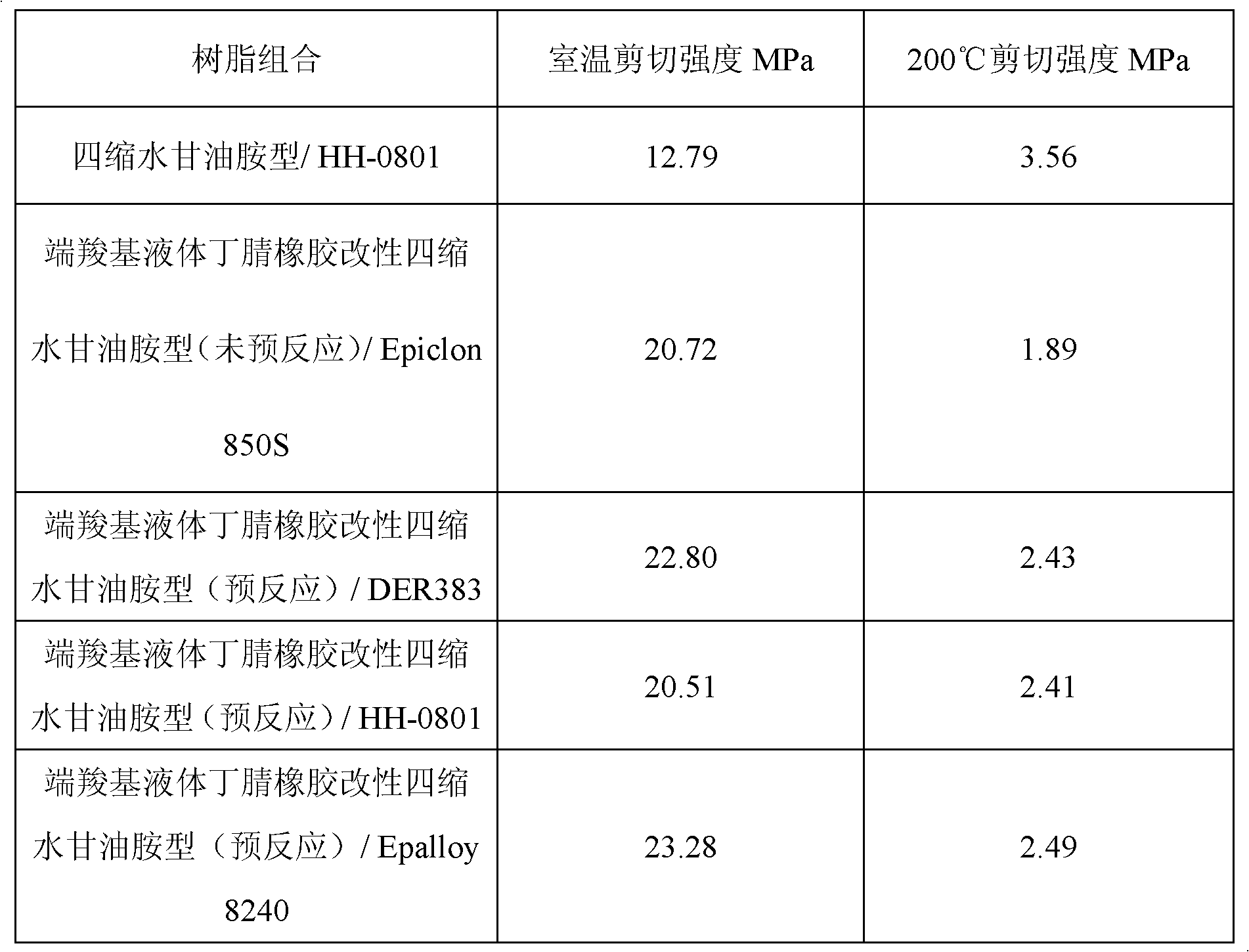
Room temperature cured high-temperature resistant epoxy adhesive
ActiveCN102850978AIncrease high temperature shear strengthNon-macromolecular adhesive additivesEpoxy resin adhesivesPolyamideRoom temperature
The invention discloses room temperature cured high-temperature resistant epoxy adhesive. The adhesive is double-component epoxy adhesive composed of resin component and curing agent component, with a weight ratio of 100:(45-60) when in use. The resin component is composed of carboxyl-terminated liquid nitrile-butadiene rubber modified tetraglycidylamine epoxy resin 100-120 weight parts and bisphenol A diglycidyl ether 25-40 weight parts; and the curing agent is prepared from (by wt%) modified amine 5.8-7.2, polyamide 18-25, 4,4-diaminodiphenylmethane 15.2-16.5, imidazole 2.3-4.8, tertiary amine catalyst 0.75-1.5, filling 16-40, and inorganic filling 1.5-3.5. The invention has curing temperature of room temperature, and shearing strength reaching 4.5 MPa at 200 DEG C.
Owner:SHANGHAI PLASTICS RES INST CO LTD
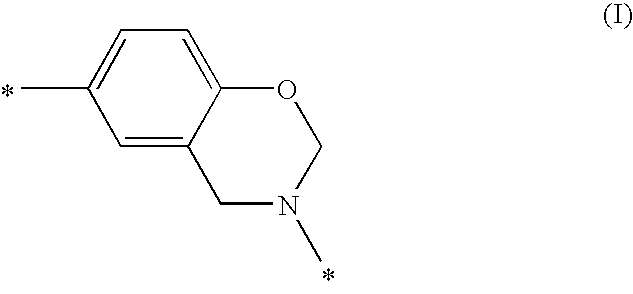
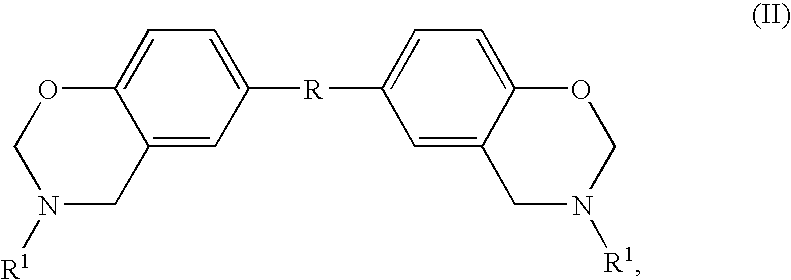
Composition Comprising Benzoxazine and Epoxy Resin
ActiveUS20080302471A1Reduce flammabilityFavorable curing conditionGroup 4/14 element organic compoundsSynthetic resin layered productsPolymer networkBisphenol AF
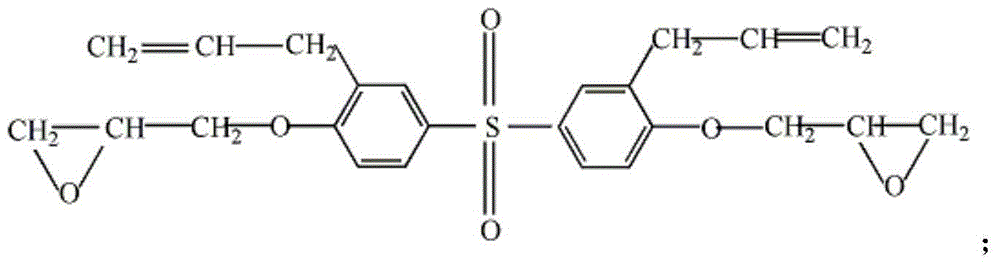
The instant invention relates to compositions comprising a benzoxazine resin and an advancement resin based on bisphenol A diglycidyl ether and bisphenol S and, optionally, ferrocene and aluminium trihydrate. Such compositions are, when cured to form polymeric networks, difficultly inflammable and resistant to high temperatures. Such compositions may especially be used in the production of printed wiring boards.
Owner:HUNTSMAN ADVANCED MATERIALS AMERICAS INC +1
Method for preparing hybrid non-isocyanate polyurethane by carbon dioxide
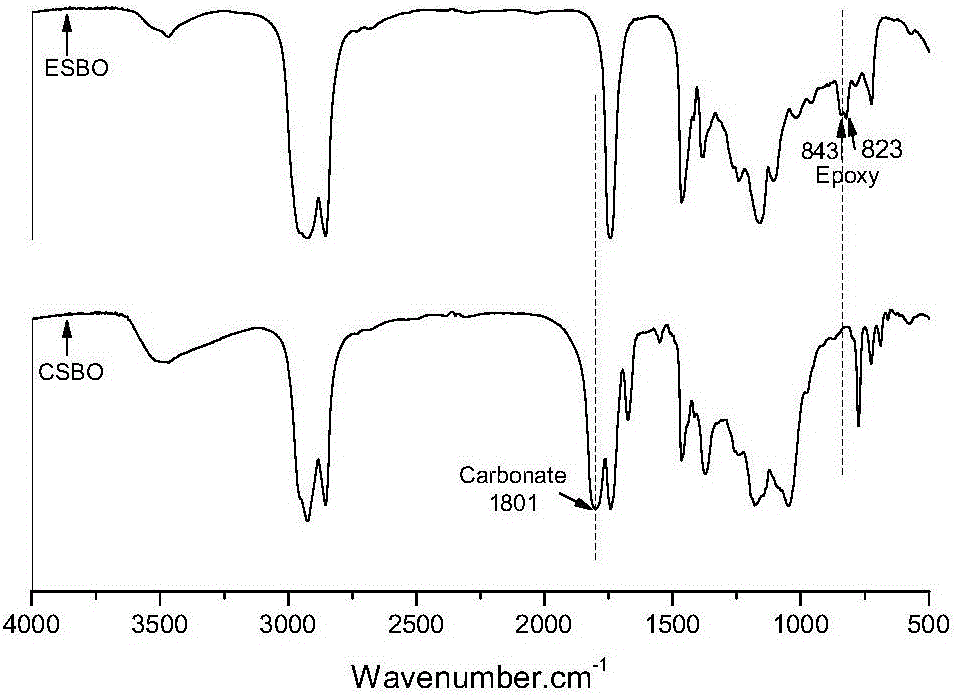
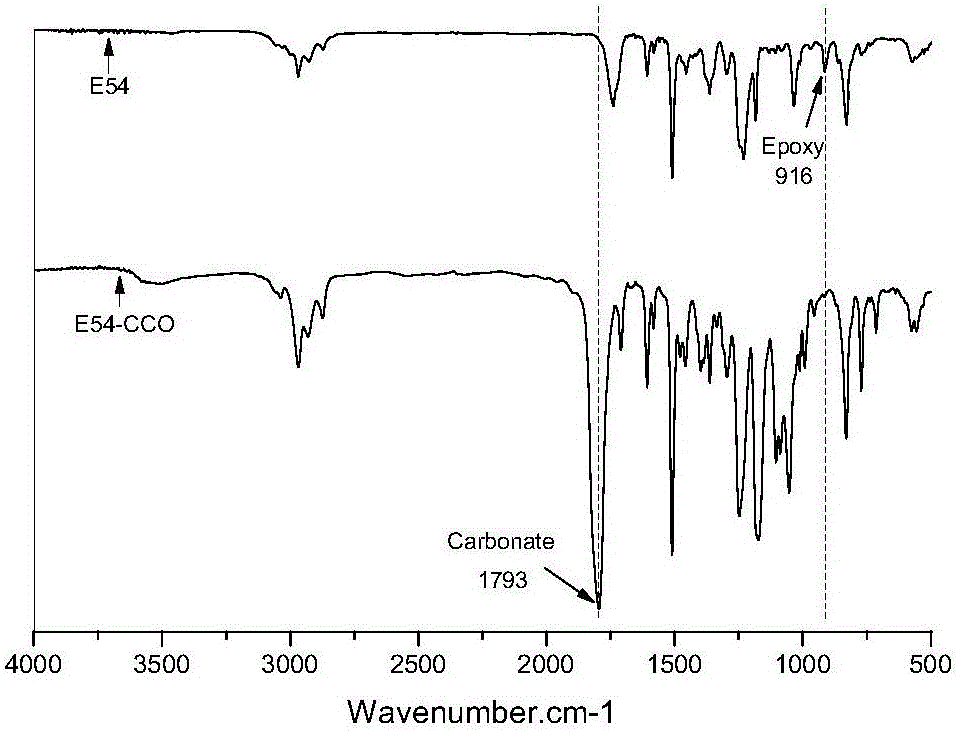
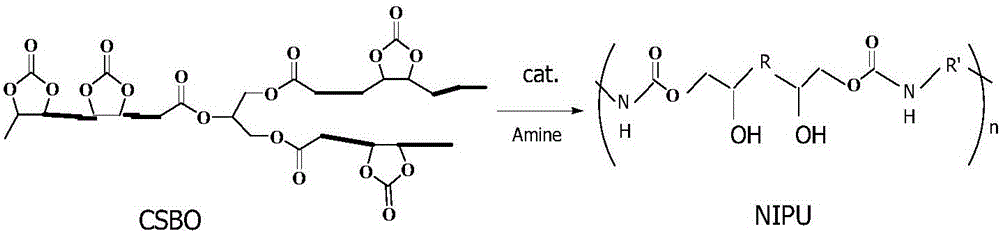
The invention discloses a method for preparing hybrid non-isocyanate polyurethane by carbon dioxide, including the following steps: first, synthesizing soybean oil based pentabasic cyclic carbonate through reaction of the carbon dioxide and epoxidized soybean oil; second, synthesizing bisphenol A-type cyclic carbonate through reaction of the carbon dioxide and bisphenol A diglycidyl ether; third, synthesizing the hybrid non-isocyanate polyurethane through reaction of the synthesized cyclic carbonates and amine. The method has the advantages of fine comprehensive mechanical properties and simplicity in preparation.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Phosphor-nitrogen halogen-free flame-retardant epoxy resin
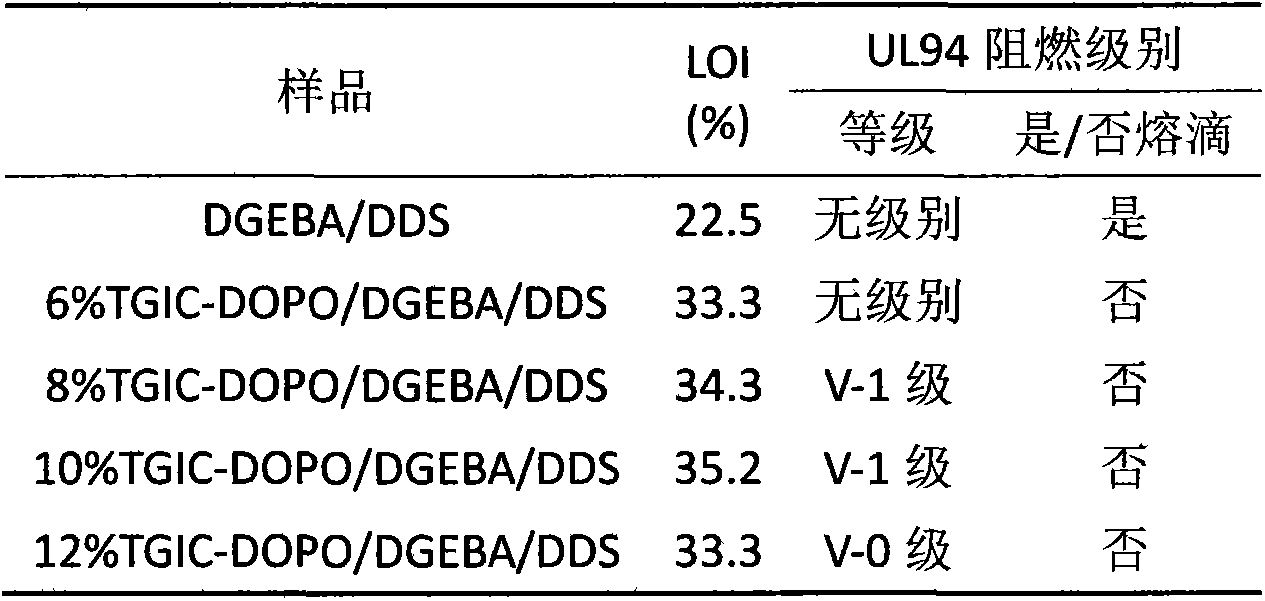
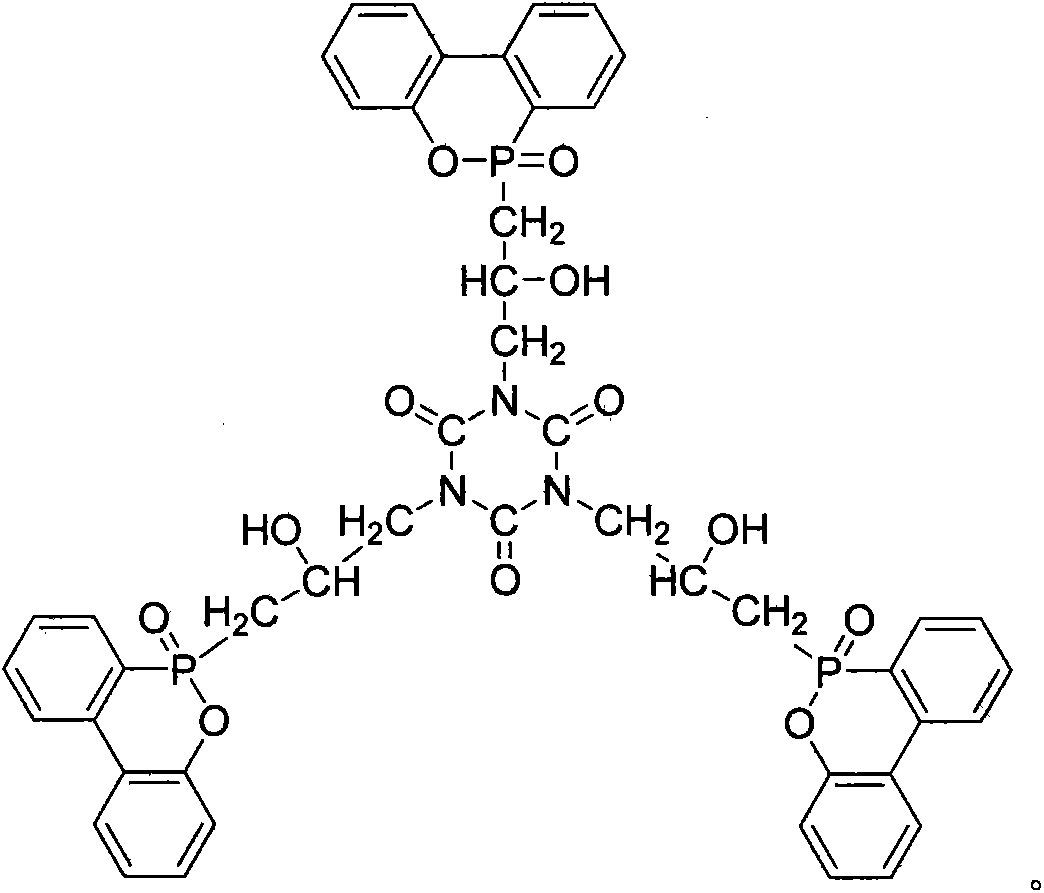
The invention relates to a phosphor-nitrogen additive type flame retardant 3-(3-DOPO-2-hydroxyl-1-propyl)-triazine triketone (TGIC-DOPO), and belongs to the technical field of preparing flame-retardant epoxy resin materials by adding specific flame-retardant components in common epoxy resin. The cured epoxy resin is prepared by carrying out melt blending on a flame retardant TGIC-DOPO and a bisphenol A diglycidyl ether type resin at a certain temperature, adding an organic solvent to prepare a flame-retardant epoxy resin, adding a curing agent into the flame-retardant epoxy resin, volatizing the organic solvent at a certain temperature and performing a curing reaction of epoxy resin. The epoxy resin disclosed by the invention can be used as a flame-retardant binding material in the field of electronic appliances.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
Transparent liquid resin material for SMT-enabled led-applications at higher temperatures and higher luminosities
InactiveUS7009008B1Great utilization temperature rangeHigh glass transition temperatureSemiconductor/solid-state device detailsSolid-state devicesVitrificationCresol
A casting resin compound as an assembly and encapsulation material for electronic and optoelectronic component parts, modules and components, for example for casting out optoelectronic components on the basis of acid anhydride-curable epoxy compounds, particularly bisphenol A-diglycidyl ether, contains multi-functional epoxy novolak resins, particularly an epoxy cresol novolak. This casting resin compound exhibits a clearly increased glass transition temperature, is suitable for mass-production, exhibits no health deteriorations, and supplies SMT-capable products that can be utilized in the automotive sector.
Owner:AVAGO TECH INT SALES PTE LTD
Halogen-free flame-retardant polyimide resin composition and prepreg and laminate made with same
ActiveCN104877134AImprove solubilityGood storage stabilityMetal layered productsHigh resistanceDiphenyl ether
The invention discloses a halogen-free flame-retardant polyimide resin composition, comprising, by weight, bismaleimide a, composite allyl compound b, and phosphorous compound c. A preparing method of the halogen-free flame-retardant polyimide resin composition includes: heating the bismaleimide, the composite allyl compound and the phosphorous compound in a reaction flask for reaction, with the composite allyl compound being composed of first allyl compound and second allyl compound. The first allyl compound is made from one or any of diallyl bisphenol A, diallyl bisphenol S, allyl phenoxy resin, and diallyl diphenyl ether. The second allyl compound is made from one or any of diallyl bisphenol A diglycidyl ether, diallyl bisphenol S diglycidyl ether, diallyl diphenyl ether diglycidyl ether. A laminate made from the halogen-free flame-retardant polyimide resin composition has the advantages of high resistance, high toughness, low water absorption rate, and excellent dielectric property and flame retardance.
Owner:SHENGYI TECH SUZHOU
Preparation method of super-hydrophobic surface of copolymer graft hollow silicon dioxide pellet
The invention discloses a preparation method of the super-hydrophobic surface of a copolymer graft hollow silicon dioxide pellet. The copolymer is a styrene-bisphenol A diglycidyl ether monoacrylate copolymer, and is prepared by virtue of synthesis of bisphenol A diglycidyl ether monoacrylate and synthesis of styrene-bisphenol A diglycidyl ether monoacrylate copolymer, wherein the mass ratio of styrene to bisphenol A diglycidyl ether monoacrylate is (6-4):1; epoxy groups in the copolymer can be grafted with the hollow silicon dioxide pellet with amino groups and also can be cured with an epoxy resin curing agent, and good adhesion can be formed with a base material; the prepared hollow silicon dioxide pellet is 50-60 nm in particle diameter and 5-10 nm in wall thickness; moreover, the obtained super-hydrophobic surface is high in mechanism strength, resistant to aging, resistant to acid and alkali, resistant to corrosion and better in transparency.
Owner:QILU UNIV OF TECH
Badge-free can coating
An exemplary coating of the invention, suitable for use as an interior coating for metal cans, comprises (a) a polyester resin; (b) a resol resin; and (c) a solvent, the coating being substantially free of bisphenol-A-diglycidyl-ether and bisphenol-F-diglycidyl ether (e.g., 'BADGE' or 'BADGE-type' components), and preferably also substantially free of polyvinyl chloride.
Owner:WR GRACE & CO CONN
BADGE- and BPA-Free Can Coating
ActiveUS20080299343A1Suitable flexibilitySuitable resistanceSynthetic resin layered productsPretreated surfacesAcrylic resinPolyvinyl chloride
The present invention relates to a composition, which is useful for producing coatings for metal sheet substrates of metal cans for storing and / or transporting food or beverages or a lid thereof, and which comprises the following components:(a) 30 to 90 wt. % of a polyvinylchloride-(PVC)-polymer,(b) 7 to 25 wt. % of an acrylic resin,(c) 3 to 40 wt. % of a crosslinking agent, which is produced from phenol, para-tert.-butylphenol, xylenol or a mixture thereof, and formaldehyde,(d) 0 to 8 wt. % additive,(e) 0 to 50 wt. % pigment and(f) a solvent-component,where all weight percentages are on the basis of the total dry weight of the coating composition (without solvents) and the composition is substantially free of bisphenol-A-diglycidyl-ether (“BADGE”) and also substantially free of bisphenol-A-resins. The composition provides metal can coatings that have suitable flexibility, scratch resistance, adherence and sterilization resistance when processed in contact with food. The coatings are suitable for three-piece cans as well as deep-drawn metal cans. In particular they are, however, useful for lids that are to be torn open due to their extraordinary flexibility and sterilization resistance.
Owner:HENKEL KGAA
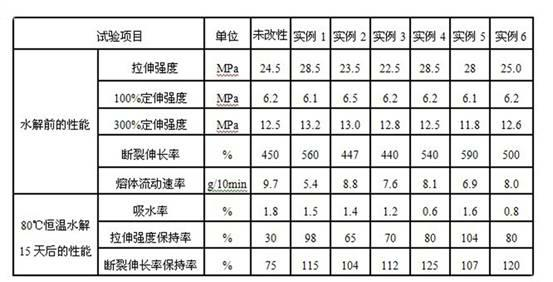
Hydrolysis-resistant thermoplastic polyurethane elastomer and manufacture method thereof
The invention discloses a hydrolysis-resistant thermoplastic polyurethane elastomer containing the following components in parts by mass: 100 parts of thermoplastic polyurethane resin, 3-20 parts of hydrolysis-resistant compound, 0.15-0.3 part of antioxidant and 0.2-0.4 part of lubricant. The thermoplastic polyurethane resin is polyester type polyurethane resin; the hydrolysis-resistant compound is a mixture of one or more of the following matters: polycarbodiimide, 1,2-epoxy-3-phenoxypropane, bisphenol A diglycidyl ether, 3-(2,3-glycidoxy)propyl-trimethoxy silane and maleic anhydride grafted styrene-ethylene / butylene-styrene block copolymer; the antioxidant is a mixture of one or both of pentaerythritol tertrakis(methyl-beta-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) and 4,4'-di-tert-octyldiphenylamine; and the lubricant is a mixture of one or both of zinc stearate and calcium stearate. The hydrolysis-resistant thermoplastic polyurethane elastomer disclosed by the invention is suitable for being taken as electric wire and cable sheath layer materials, medical materials, various hoses, shoe materials, sealing elements, wear-resistant components and the like.
Owner:SHENZHEN POLYTECHNIC
Epoxy resin adhesive for potting PVDF hollow fiber membrane and preparation method of epoxy resin adhesive
InactiveCN104371626AImprove mechanical propertiesImprove aging resistanceNon-macromolecular adhesive additivesMacromolecular adhesive additivesHydrophobic silicaSilicon oxide
The invention provides an epoxy resin adhesive for potting a PVDF hollow fiber membrane. The epoxy resin adhesive comprises a component A and a component B at a mass ratio of 100 to (15-35), wherein the component A comprises 50-70 parts of epoxy resin, 5-15 parts of a toughening agent and 1-5 parts of a defoaming agent, the component B comprise an amine curing agent, the epoxy resin comprises bisphenol A diglycidyl ether; the toughening agent is a low-molecular polyurethane or polyol glycidyl ether; and the defoaming agent is one or more selected from organic phosphonic acid, polysiloxane, fatty alcohol, fatty acid ester and hydrophobic silicon oxide. The epoxy resin adhesive provided by the invention has the advantages of excellent mechanical property, aging resistance, heat resistance, corrosion resistance, good flexibility and the like, especially, the flexibility is enhanced and the problems of breakage and soaking of a membrane fiber during the potting of the epoxy resin adhesive are solved.
Owner:HUNAN OVAY TECH CO LTD
Container coating compositions
ActiveUS20130280453A1Improve hydrolytic stabilitySynthetic resin layered productsThin material handlingPolymer sciencePolyester resin
A coating composition comprising a polyester resin binder and a phosphatized polyester. The compositions are useful for coating containers such as food and beverage containers. The compositions have excellent hydrolytic stability upon storage at high temperature. The compositions are formulated to be substantially free of bisphenol A (BPA) and bisphenol A diglycidyl ether (BADGE).
Owner:PPG IND OHIO INC
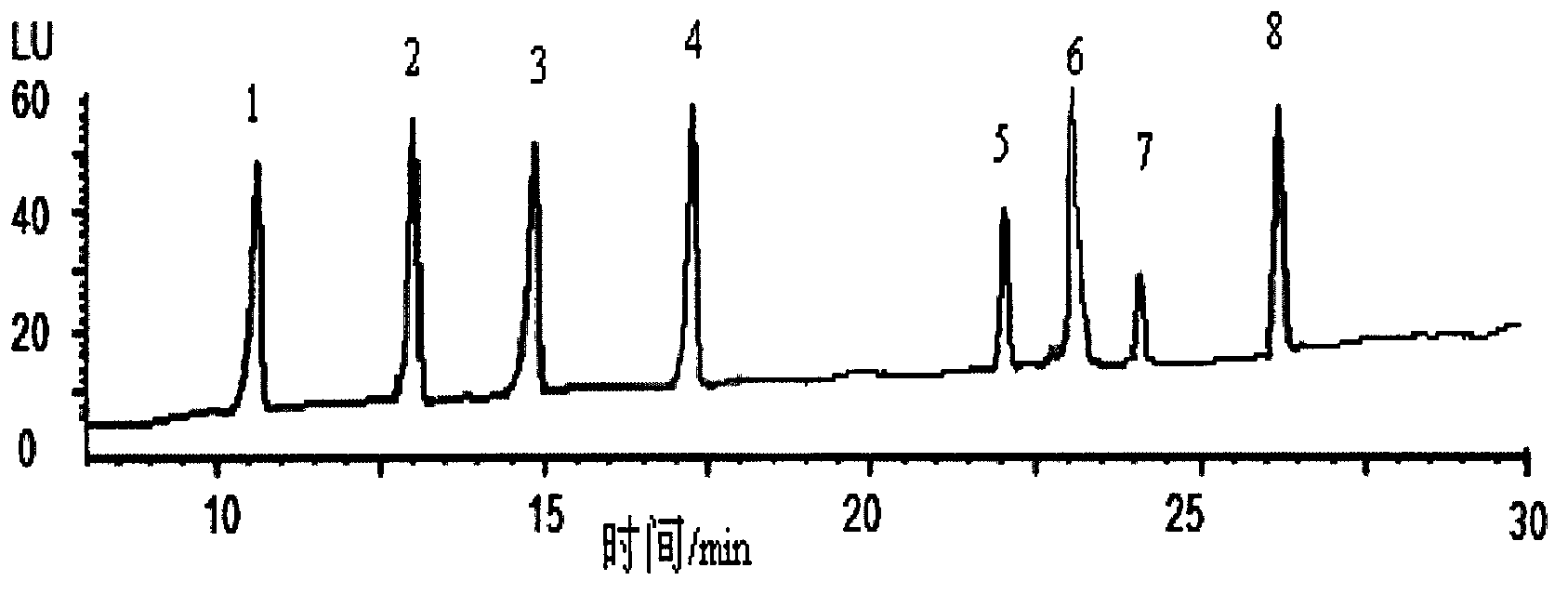
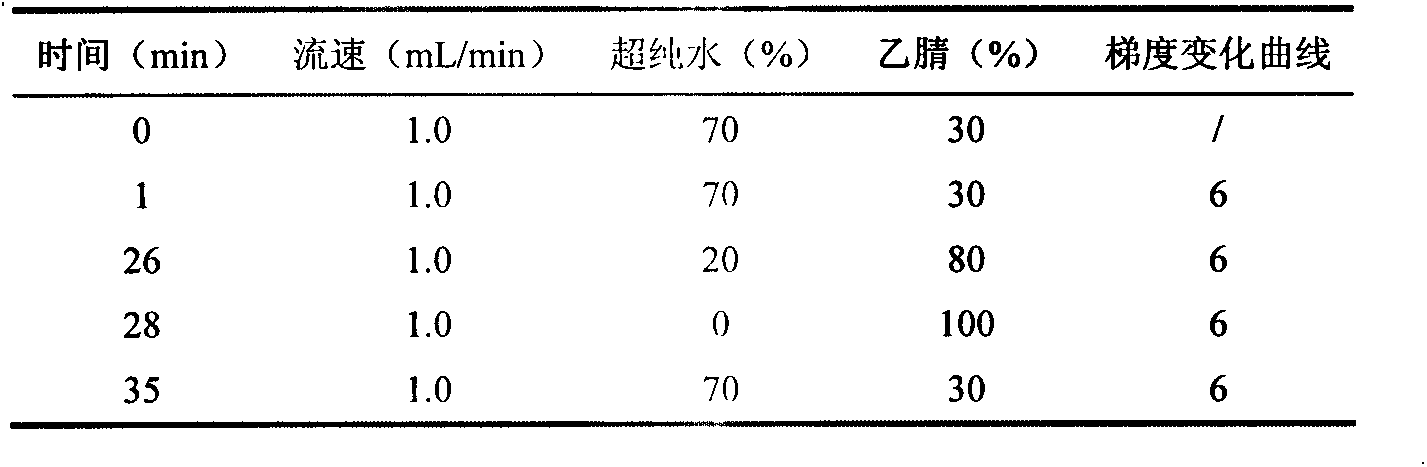
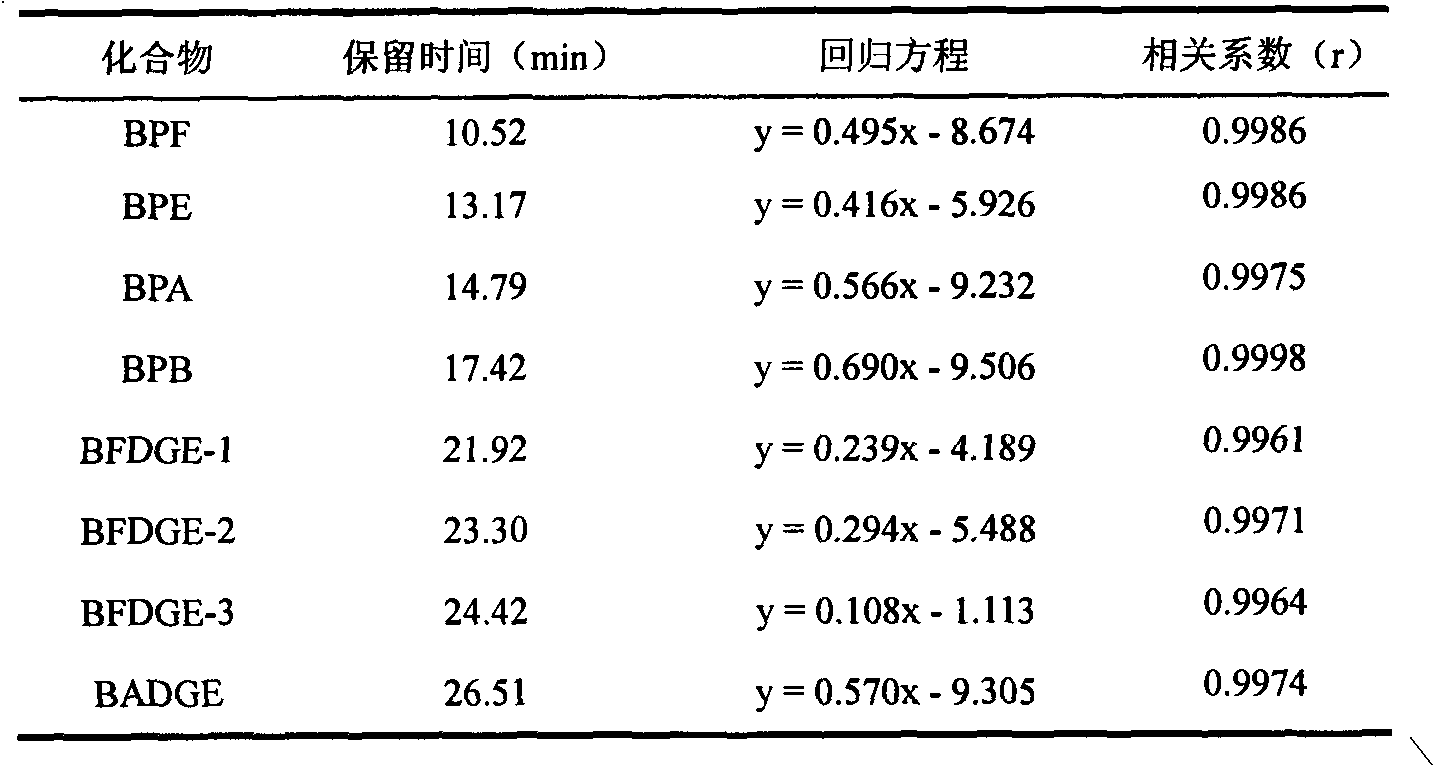
Purification enrichment pre-treatment technology for food packaging material and container transfer pollutant bisphenol substance
InactiveCN103163253AHigh recovery rateSuitable for standardizationComponent separationLiquid Chromatography-FluorescenceSolid phase extraction
The invention belongs to the technical field of purification enrichment pre-treatment for food packaging material pollutant biphenol substance. The purification enrichment pre-treatment technology comprises the following steps of: activating a C18 inverse solid phase extraction small column by methanol and ultra pure water and then loading, firstly leaching with 20% of methanol aqueous solution, then eluting with methanol, drying eluate with nitrogen in a blowing manner, redissolving residue with acetonitrile, filtering, and then carrying out liquid chromatography-fluorescence detection. By adopting the pre-treatment technology provided by the invention, recovery ratio of six biphenol substances (biphenol A (BPA), biphenol B (BPB), biphenol E (BPE), biphenol F (BPF), biphenyl A diglycidyl ether (BADGE) and biphenol F diglycidyl ether (BFDGE)) is 75.92-102.10%, relative deviation is respectivel lower than 5%, and accuracy and precision are high.
Owner:江阴市产品质量监督检验所 +1
Adhesive agent composition for multilayer semiconductor
InactiveUS20160215183A1Less progressEasy to cutSemiconductor/solid-state device detailsSolid-state devicesCationic polymerizationSemiconductor chip
Provided is an adhesive composition for multilayer semiconductors. The adhesive composition gives, when applied and dried by heating, an adhesive layer that has approximately no adhesiveness at a temperature lower than 50° C., but, when heated at such a temperature as to less cause damage to semiconductor chips, offers adhesiveness and is rapidly cured thereafter. This adhesive composition for multilayer semiconductors includes a polymerizable compound (A), at least one of a cationic-polymerization initiator (B1) and an anionic-polymerization initiator (B2), and a solvent (C). The polymerizable compound (A) contains 80% by weight or more of an epoxide having a softening point or melting point of 50° C. or higher. The cationic-polymerization initiator (B1) gives a composition having a thermal curing time of 3.5 minutes or longer at 130° C., where the composition contains 1 part by weight of the cationic-polymerization initiator (B1) and 100 parts by weight of 3,4-epoxycyclohexylmethyl (3,4-epoxy)cyclohexanecarboxylate. The anionic-polymerization initiator (B2) gives a composition having a thermal curing time of 3.5 minutes or longer at 130° C., where the composition contains 1 part by weight of the anionic-polymerization initiator (B2) and 100 parts by weight of bisphenol-A diglycidyl ether.
Owner:DAICEL CHEM IND LTD
Carbon nanotube reinforced anti-oxidation geogrid and preparation method thereof
InactiveCN105968522APromote oxidation chain reactionReduce free radical contentPolymer scienceALUMINUM HYDRIDE
The invention discloses a carbon nanotube reinforced anti-oxidation geogrid, which is composed of the following raw materials in parts by weight: 1.6-2 calcium acetylacetonate, 0.7-1 alkenyl succinic anhydride, and 1-2 barium stearate , lithium aluminum hydride 0.1‑0.3, 4‑dimethylaminopyridine 0.3‑1, antioxidant 10100.4‑1, multi-walled carbon nanotubes 10‑15, antioxidant 1680.6‑1, polypropylene imine 10‑17, acrylic acid Tert-butyl ester 6-8, high-density polyethylene 140-160, bisphenol a diglycidyl ether 0.3-1, 2-mercaptobenzimidazole 0.6-1, dibutyl maleate 4-6, cycloalkane Lithium Oxide 0.4‑1. The invention greatly reduces the content of free radicals participating in the automatic oxidation chain reaction of polymers, delays the oxidation degradation process of polymers, and improves the oxidation resistance of finished materials.
Owner:ANHUI JIEAOMAKE SYNTHETIC MATERIAL TECH
Coating composition
InactiveUS20020161106A1Sufficient volatilitySufficient amountSynthetic resin layered productsThin material handlingPolymer scienceMethyl palmoxirate
A coating composition is disclosed that is intended for application to the interior surface of a can and is free of Bisphenol A diglycidyl ether and comprises a phenoxy group-containing or amino group-containing resin, a nonaqueous carrier and a polyester resin formed from (a) isophthalic acid, (b) naphthalene dicarboxylic acid or an ester thereof, (c) trimellitic anhydride or trimethylpropane and (d) neopentyl glycol.
Owner:FLINT HILLS RESOURCES LP
Anti-yellowing hard interior wall paint and preparation method thereof
InactiveCN108003733AGood compatibilityImprove surface mechanical strengthAntifouling/underwater paintsPaints with biocidesSilicon dioxideChemistry
The invention discloses anti-yellowing hard interior wall paint. The anti-yellowing hard interior wall paint is prepared from, by weight, 1-3 parts of triglycidyl isocyanurate, 13-20 parts of nanometer silicon dioxide, 2-3 parts of N, N'-ethylene bis-stearamide, 0.8-1 part of benzotriazole, 4-6 parts of pentaerythritol, 1-2 parts of bisphenol A diglycidyl ether, 1-3 parts of ammonium octamolybdate, 0.7-1 part of trichloroisocyanuric acid, 1-2 parts of 3-(trimethoxysilyl)-1-propanamine, 46-50 parts of tetrabutyl titanate, 0.3-1 part of antioxidant 1010 and 110-120 parts of silicone modified acrylate emulsion. The interior wall paint is high in anti-yellowing performance and excellent in comprehensive performance.
Owner:桐城市桐佳装饰有限公司
Method for synthesizing bisphenol A diglycidyl ethers through halogen-free epoxidation
The invention discloses a method for synthesizing bisphenol A diglycidyl ethers through halogen-free epoxidation, which is characterized by comprising the following steps of: adding bisphenol A diallyl ethers, a solvent I and a phosphotungstic acid quaternary ammonium salt catalyst into a reactor, adding a hydrogen peroxide solution into the reactor while stirring, raising the temperature of a reactant to 30-80 DEG C, and reacting the obtained object for 5-24 hours at a temperature of 30-80 DEG C so as to obtain a reacted material; cooling the reacted material to room temperature, separating an organic phase, after the separated organic phase is subjected to distillation recycling so as to obtain an organic solvent, uniformly stirring and mixing the rest material and ethyl acetate, carrying out filtering on the obtained product, and after filter liquor is subjected to distillation recycling so as to obtain ethyl acetate, obtaining a coarse product; and carrying out column chromatography on the coarse product by using a solvent III so as to obtain bisphenol A diglycidyl ethers. After the method disclosed by the invention is adopted, no organic chlorine ion contains in the process of synthesis, a synthesized target product not only has no organochlorine heterogeneous client base, but also is low in product viscosity, good in performance, simple in process, easy to operate, high in safety, low in pollution and strong in practicability.
Owner:江苏东材新材料有限责任公司
High-strength heat-resistant corrosion-resistant polyurethane board and preparation method thereof
The invention discloses a high-strength heat-resistant corrosion-resistant polyurethane board which is prepared from diisocyanate, polycarbonate dibasic alcohol, dioctyl azelate, ethoxylated alkylphenol ammonium sulfate, toluene diisocyanate, heavy calcium carbonate, carbon black, nano attapulgite, nano silicon dioxide, bisphenol a diglycidyl ether, epoxy octyl stearate, a modified filler, methylsilicone oil, sulfur, a phenolic resin, a curing agent, melamine, hypophosphate, a flame-retardant synergistic agent, triallyl isocyanurate, dicumyl peroxide, 3,5-dimethylthiotoluylene diamine and a copper sheet. The invention also discloses a preparation method of the high-strength heat-resistant corrosion-resistant polyurethane board. The polyurethane board has the advantages of high strength, excellent heat resistance and excellent corrosion resistance.
Owner:安徽浩丰特种电子材料有限公司
Repairable cross-linked solid polymer electrolyte as well as preparation method and application thereof
ActiveCN111682261AGood thermosettingGuaranteed ionic conductivityLi-accumulatorsElectrolyte immobilisation/gelificationPolymer sciencePolythylene glycol
The invention provides a repairable cross-linked solid polymer electrolyte as well as a preparation method and application thereof, and the repairable cross-linked solid polymer electrolyte is prepared by the following method: dispersing and dissolving terephthalaldehyde, bisphenol A diglycidyl ether, polyethylene glycol diamine and a lithium salt in an acetonitrile solvent, and carrying out stirring for 2-6 hours to obtain a transparent and uniform mixed solution A; dropwise adding the mixed solution A onto a polytetrafluoroethylene mold, and volatilizing acetonitrile at room temperature to obtain a sol-like substance B; and putting the sol-like substance B into a vacuum drying oven, carrying out polymerization reaction to completely crosslink and solidify the sol-like substance B, and continuously heating to dry the sol-like substance B to prepare the polymer electrolyte. According to the invention, a dynamic imine covalent bond is introduced into the polymer electrolyte to form thesolid network-shaped polymer electrolyte, so that the solid network-shaped polymer electrolyte can be repaired in time when fracture occurs in the use process; the prepared network-like polymer electrolyte has high thermal stability and dendritic-crystal-free morphology, and has excellent electrochemical properties such as ionic conductivity and lithium ion migration number.
Owner:NANCHANG HANGKONG UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com